Abstract
Background
Irritable bowel syndrome (IBS) is one of the most common functional gastroenterological diseases, affecting 11.2 % of people worldwide. Previous studies have shown that probiotic treatment may benefit IBS patients. However, the effect of probiotics and the appropriate type, dose, and treatment duration for IBS are still unclear. The aim of the current study was to assess the efficacy of different probiotic types, doses and treatment durations in IBS patients diagnosed by Rome III criteria via a meta-analysis of randomized controlled trials (RCTs).
Methods
Medline, EMBASE, and the Cochrane Central Register of Controlled Trials up to October 2015 were searched. RCTs including comparisons between the effects of probiotics and placebo on IBS patients diagnosed by Rome III criteria were eligible. Dichotomous data were pooled to obtain the relative risk (RR) with a 95 % confidence interval (CI), whereas continuous data were pooled using a standardized mean difference (SMD) with a 95 % CI.
Results
Twenty-one RCTs were included in this meta-analysis. Probiotic therapy was associated with more improvement than placebo administration in overall symptom response (RR: 1.82, 95 % CI 1.27 to 2.60) and quality of life (QoL) (SMD: 0.29, 95 % CI 0.08 to 0.50), but not in individual IBS symptoms. Single probiotics, a low dose, and a short treatment duration were more effective with respect to overall symptom response and QoL. No differences were detected in individual IBS symptoms in the subgroup analyses.
Conclusion
Probiotics are an effective pharmacological therapy in IBS patients. Single probiotics at a low dose and with a short treatment duration appear to be more effective in improving overall symptom response and QoL, but more evidence for these effects is still needed.
Similar content being viewed by others
Background
Irritable bowel syndrome (IBS) is characterized by abdominal pain and alterations in bowel habits. It affects 11.2 % of people worldwide [1] and is regarded as one of the most common functional gastroenterological diseases [2]. Although the exact pathophysiology underlying IBS is still not fully understood, chronic low-grade mucosal inflammation, alterations in gut epithelial and immune function, and visceral hypersensitivity caused by alterations in intestinal microbiota have been shown to be associated with IBS [3–5]. The current therapeutic options for IBS treatment include low-dose antidepressants, spasmolytics, and 5-HT3 antagonists. However, IBS patients often have variant response to these therapies, and they are also associated with several complications [6–9]. Antidepressant treatment often causes severe problems, including weight gain, and cannot be tolerated by many patients. Spasmolytics and 5-HT3 antagonists are ineffective for some people, and they may even worsen the symptoms of IBS [7]. Moreover, long-term use of these medications in IBS patients can increase the occurrence of various adverse effects [10].
New therapeutic options with the potential to alter intestinal microbiota have recently been identified and include the low fermentable, oligo-, di-, monosaccharides, and polyols (FODMAP) diet [11], antibiotics [12], and probiotics. Probiotics, defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [13], have the potential to influence the intestinal microbiota. Probiotics may affect intestinal barrier function and exert anti-inflammatory actions [10]. To date, many clinical studies have investigated the effects of probiotics in IBS patients, and more than half of these studies demonstrated that probiotic administration is effective in IBS patients [14]. Due to differences in the study designs (size of the study, duration of the treatment), probiotic doses, and strains used, clinical studies addressing the efficacy of probiotics in IBS are difficult to compare [15]. Several systematic reviews and meta-analyses on the effects of probiotics in IBS patients have been generated, and the majority of results demonstrated that the use of probiotics was beneficial in IBS patients [14, 16–20]. Despite these findings, some issues concerning probiotic treatment in IBS patients persist; specifically, the type of probiotic used in different studies varied, combination probiotics and single probiotics were both used, and the doses and treatment durations were also different between studies. Rome III criteria [21], based on Rome II criteria, applied 10 years ago, have been used more extensively than Rome I/II and Manning criteria [22–24]. The Rome II subtyping using multiple criteria was complex and difficult to use in practice. Compared with Rome II criteria, Rome III criteria require a lower frequency of IBS symptoms and focus more on recent symptom severity. The latter change may lead to increased compliance in and comparability between patients enrolled in clinical trials [2]. Previous studies also indicated that the Rome III assessment may more accurately reflect the burden of disease and epidemiological features than the Rome II criteria [25]. However, no meta-analysis based on studies using the Rome III criteria has been performed to date. Further investigations are clearly needed to establish optimal treatment regimens (the most effective probiotic species and strains, individual or mixture administration), as well as to identify subgroups of patients most likely to benefit from these treatments [26].
In this study, we conducted a meta-analysis to assess the efficacy of different types of probiotics in IBS patients with the Rome III criteria serving as the diagnostic criteria. We also analysed the effects of different doses and treatment durations in IBS patients.
Methods
Literature search
We systematically searched the Medline, EMBASE, and Cochrane Central Register of Controlled Trials databases up to October 2015 for studies that investigated the efficacy of probiotic therapy in IBS patients. We used the terms “probiotics” and “irritable bowel syndrome” both as medical subject heading (Mesh) and free text terms. The exact search strategy in Medline was ("probiotics"[MeSH Terms] OR "probiotics"[All Fields]) AND ("irritable bowel syndrome"[MeSH Terms] OR "irritable bowel syndrome"[All Fields]). All eligible studies were retrieved, and the bibliographies were manually checked to identify additional potential studies.
Inclusion and exclusion criteria
Studies were considered eligible if they met the following criteria: (1) the studies were randomized controlled trials (RCTs) that compared probiotics with placebo; (2) the diagnosis of IBS was made according to the Rome III criteria; (3) the treatment duration was >7 days; and (4) dichotomous data on the overall syndrome response to the therapy or continuous score data on the effect on individual IBS symptoms or quality of life (QoL) could be extracted or obtained from the authors. The exclusion criteria were as follows: (1) studies with only an abstract; (2) studies in which probiotics were mixed with other drugs; (3) studies in which data were still unavailable after contacting the authors; and (4) studies in which the control group received probiotics.
Data extraction
Two authors independently extracted the data from each of the eligible articles according to the inclusion and exclusion criteria. The data included the first author, publication year, country, criteria used to diagnose IBS, dose of probiotic, treatment duration and follow-up time, number of patients, mean after-treatment scores along with the standard deviation (s.d.) of individual IBS symptoms (abdominal pain and bloating), and QoL. All data were extracted for intention to treat (ITT) analysis, whereby all dropouts were assumed to be treatment failures. Only the data associated with the longest duration of therapy and largest dose were used to compare the efficacy between probiotic types.
Assessment of risk of bias
Two authors independently performed the assessment of bias risk, with disagreements resolved by discussion. The risk of bias was assessed as described in the Cochrane handbook [27] by recording the method used to generate the randomization schedule and the method used for allocation concealment, whether blinding was implemented, the completeness of follow-up, whether there was evidence of selective reporting of outcomes, and other biases.
Statistical analysis
The pooled relative risk (RR) and corresponding 95 % confidence interval (CI) were calculated in the meta-analysis to evaluate the effect of the overall symptom response of IBS patients after treatment. The standardized mean difference (SMD) and 95 % CI were used to evaluate individual IBS symptoms and QoL. The I2 statistic was calculated to quantify the proportion of the total variation due to heterogeneity, and an I2 value of > 50 % indicated significant heterogeneity among studies. A random-effects model [28] was utilized to provide a more conservative estimate of the effects of probiotic treatment, assuming heterogeneity of treatment effects across studies. Subgroup analyses according to probiotic type, dose, and treatment duration were performed for the assessment of the effects on the overall response, individual IBS symptoms, and QoL. Sensitivity analyses were performed by omitting one study at a time and analysing the remaining studies to assess whether the results were excessively influenced by any single study. The possibility of publication bias was assessed by visual inspection of a funnel plot, and the Egger test was also performed to assess the possibility of publication bias [29]. A P value of < 0.05 was considered statistically significant. All statistical analyses were conducted using Stata Statistical Software: Release 12 (StataCorp LP; College Station, TX).
Results
Studies included in the meta-analysis
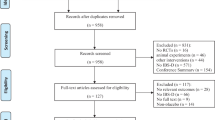
A total of 1392 publications were initially retrieved using our search strategy. Of these publications, 21 were included in the current meta-analysis [30–50]. The flow chart of search history is presented in Fig. 1. Agreements between the reviewers regarding the assessment of trial eligibility were ideal (kappa statistic = 0.88). The details of the RCTs included in the analysis are presented in Table 1.
Additional files 1 and 2 in the supplementary material demonstrates the risk of bias for all studies assessed using the Cochrane Collaboration tool. Three studies did not describe the details of the sequence generation process [35, 37, 46], and 7 studies did not describe the method of concealment [30, 33, 34, 37–39, 48], leading to an unclear risk of selection bias. The risk of blinding of participants and personnel and risk of outcome assessment were low. One study did not use ITT analysis [48], leading to an elevated risk of attrition bias. All studies exhibited a low risk of reporting bias. Wong et al. reported that the higher anxiety reported by patients taking probiotics may influence the study results, leading to an unclear risk of other bias [50].
Effects on overall symptom response in IBS patients
Sixteen RCTs [30–32, 34–38, 40–43, 45–47, 49], including 17 comparisons of the overall symptom response to probiotics versus placebo in the treatment of IBS patients, were identified. One of these RCTs, namely, the study by Lorenzo et al., examined two different dose groups [45].
The overall symptom response was the primary efficacy end-point in most studies. Overall symptom response was defined as a > 50 % reduction in IBS pain and discomfort or adequate relief of IBS symptoms for > 50 % of the time in 7 of 15 studies. Other definitions included an improvement of ≥ 50 points in the global IBS-symptom severity score (IBS-SSS), global relief of IBS symptoms, or good and excellent overall efficacy.
A total of 700 IBS patients were allocated to the probiotics group, whereas 575 IBS patients constituted the control group. The overall symptom response rate was 53.3 % in the probiotics group and 27.7 % in the control group. The RR of overall symptom response was significantly higher in the probiotics group (1.82, 95 % CI 1.27 to 2.60), and this group also had a significant degree of heterogeneity (I2 = 82.2 %, P < 0.001) (Fig. 2).
In the probiotic type subgroup, 5 single probiotic and 12 combination probiotic comparisons were performed. The RR in the single and combination probiotics subgroups was 3.54 (95 % CI 1.48 to 8.45) and 1.41 (95 % CI 1.04 to 1.91), respectively, as shown in Fig. 2. There was high heterogeneity between studies (I2 = 84.3 %, P < 0.001 and 68.5 %, P < 0.001). In the probiotic dose subgroup, 7 comparisons used a low dose (< 1010 CFU/D) and 10 comparisons used a high dose (≥ 1010 CFU/D). The RR of the low-dose group was 1.87 (95 % CI 1.28 to 2.73), and the RR of the high-dose group was 1.78 (95 % CI 1.05 to 3.01) (Fig. 3). In the duration subgroup, 10 comparisons used a short treatment duration (<8 w), whereas 7 comparisons evaluated a relatively long duration (≥8 w). The RR of the short duration was 2.23 (95 % CI 1.43 to 3.49), and the RR of the long duration was 1.31 (95 % CI 1.27 to 2.60) (Fig. 4).
Funnel plot asymmetry suggested the existence of potential publication bias (Egger test, P = 0.04) (Additional file 3).
Effects on abdominal pain in IBS patients
A total of 13 studies with 13 comparisons were included in the comparison of the effects of probiotics on abdominal pain [32, 33, 38–41, 43, 44, 46–50]. A total of 485 IBS patients were included in the probiotics group, and 404 IBS patients constituted the control group. Of the 13 included studies, 2 studies used a 100-mm visual analogue scale (VAS) measurement, 3 studies used a 7-point Likert scale, 2 studies used a 5-point Likert scale, and the remainder of the studies used another measurement or declared a lack of measurement. High heterogeneity existed between studies (I2 = 85.7 %, P < 0.001).
In the comparison of probiotics versus placebo, the overall SMD was −0.25 (95 % CI −0.62 to 0.13) (Additional file 4). Probiotic use was not associated with an improvement in abdominal pain compared with placebo. No significant differences were found for different probiotic types, doses, or treatment durations. There was no significant funnel plot asymmetry observed (Egger test, P = 0.90), suggesting no evidence of publication bias or other small-study effects (Additional file 5).
Effects on bloating in IBS patients
Bloating is another severe symptom in IBS patients, and studies have suggested that gas production may be associated with probiotic use in IBS patients [51]. Thirteen studies with 13 comparisons were included in the comparison of the effect of probiotics on bloating, with 492 individuals allocated to the probiotics group and 398 individuals allocated to the control group [32–34, 38–41, 43, 44, 46, 47, 49, 50]. The measurements used to assess bloating were the same as those used to evaluate abdominal pain. Nine studies used combination probiotics, and 5 studies used single probiotics. The overall SMD was −0.19 (95 % CI −0.45 to 0.08), with high heterogeneity (I2 = 72.2 %, P < 0.001) (Additional file 6). No statistically significant differences were detected with respect to probiotic type, dose, or treatment duration. No significant funnel plot asymmetry (Egger test, P = 0.963) was observed, suggesting no evidence of publication bias or other small study effects (Additional file 7).
Effects on QoL
IBS greatly impacts the QoL of IBS patients, and the degree of alteration in the quality of life is closely related to the severity of IBS in individual patients [52]. A recent study revealed that the QoL impact of severe IBS was similar to that of Class 3 congestive heart failure and rheumatoid arthritis [53]. Nine studies were included in the comparison of effects on QoL, with 364 subjects in the probiotics group and 265 subjects in the control group. Numeric score assessments included the SF-12, a 5-point Likert scale, and SMD was used to assess the effects of probiotics on QoL.
The overall SMD was 0.29 (95 % CI 0.08 to 0.50), and the heterogeneity was low (I2 = 36.2 %). In the probiotic type subgroup, only 1 study used a single probiotic and the remaining 8 studies used combination probiotics. The RR of the combination probiotics was 0.26 (95 % CI 0.02 to 0.50), and the RR of the single probiotics was 0.44 (95 % CI 0.09 to 0.80), as shown in Fig. 5.
In the dose subgroup, 2 comparisons used a low dose (< 1010 CFU/D) and 7 comparisons used a high dose (≥ 1010 CFU/D). The SMD of the low dose was 0.53 (95 % CI 0.22 to 0.84), and the SMD of the high dose was 0.18 (95 % CI −0.04 to 0.40) (Fig. 6).
In the treatment duration subgroup, 4 comparisons used a short treatment duration (<8 w) and 5 comparisons used a relatively long duration (≥8 w). The RR of the short duration was 0.57 (95 % CI 0.32 to 0.82), and the RR of the long duration was 0.06 (95 % CI −0.15 to 0.26) (Fig. 7).
There was no significant funnel plot asymmetry detected (Egger test, P = 0.38), suggesting no evidence of publication bias or other small study effects (Additional file 8).
Discussion
This meta-analysis indicated that probiotic use could significantly improve the overall symptom response and QoL in IBS patients compared with placebo. These results were consistent with those of previous systematic reviews that included other diagnostic criteria [14, 19, 20], which suggested that probiotics were effective in treating IBS. No significant differences were found in the relief of individual IBS symptoms (abdominal pain and bloating) between probiotics and placebo. This result is inconsistent with previous studies [14]. Previous studies suggested that combination probiotics could affect abdominal pain and bloating, but the individual probiotics Lactobacilli and Bifidobacteria were not effective [14, 19]. The larger proportion of Lactobacilli and Bifidobacteria in probiotics used in the studies included in this meta-analysis may explain the contradictory results on abdominal pain and bloating. The Rome III criteria constitute a useful tool with which to diagnose IBS, but the quantification of individual IBS symptoms is still subjective. The different diagnostic criteria and methods of quantifying individual IBS symptoms may contribute to the inconsistent reports on the efficacy of probiotic supplementation in treating individual IBS symptoms.
In the current meta-analysis, there were more studies that used combination probiotics compared with single probiotics. Only one study using single probiotics was included in the QoL assessment. Our results suggested that single probiotics appeared to be more effective in the overall symptom response (P = 0.04), but not QoL (P = 0.60), than combination probiotics. The individual IBS symptoms exhibited no improvement as a result of the administration of either single or combination probiotics. The advantages of multi- or mono-species probiotics for IBS patients are still inconclusive. Yoon et al. proposed that multi-species probiotics may produce a variety of beneficial effects on IBS symptoms because each species exerts a distinct action on the gastrointestinal tract, and two or more probiotic species in combination may exert a synergistic effect [43]. However, studies have also demonstrated that competition between ingested species or strains may occur, leading to negative effects [46]. The number of RCTs investigating the effects of single probiotics is small, and additional evidence is needed to confirm the superiority of multi-species probiotics.
In the probiotic dose subgroup, both low and high doses were associated with an improvement in the overall symptom response and QoL, but not in individual IBS symptoms. Vicente et al. compared the effects of 2 doses (the high dose, 1-3 × 1010 CFU/D, and the low dose, 3-6 × 109 CFU/D) of a new combination of probiotics on the IBS response rate and QoL and reported similar results [45]. This lack of an observed dose effect may be due to the small distinction between the high and low doses [45]. More evidence is needed to confirm the differences between high and low doses.
We also revealed that a short treatment duration (<8 w) may be more effective than a long duration (≥8 w) in improving overall symptom response and QoL. IBS is a chronic and relapsing condition, and the type and severity of symptoms may vary in the same patient over time; longer term or even continuous supplementation of probiotics may be required to detect significant alterations in symptoms [20]. A short treatment duration appeared to be more effective according to the currently available results. Roberts et al. also demonstrated greater improvement with a short duration of treatment, but the large number of dropouts in the long-duration group may have influenced these results [42].
One of the strengths of the current study is that this is the first systematic review and meta-analysis to use Rome III as the IBS diagnostic criteria. The Rome III criteria can be easily applied in clinical practice and research settings and may more accurately reflect disease burden and epidemiological features of the disorder than the Rome II criteria [25]. Another advantage of the current study is the analysis of subgroups of probiotic type, dose, and treatment duration. We also attempted to contact the authors of potential studies to gain access to all of the available data, and more than 1000 IBS patients were included in the current meta-analysis.
There are several limitations in our study. Due to the lack of available studies, neither the effects of individual probiotic species nor the effects of IBS subtypes on IBS patients were analysed. Significant heterogeneity existed due to the various outcome assessment criteria and probiotic types, doses, and treatment durations used in different studies. An appreciable placebo effect was detected in some studies, which may have minimized the effects of probiotics [54].
Conclusions
In conclusion, our results demonstrate that probiotic supplementation is an effective therapy in IBS patients. Single probiotics at a low dose and with a short treatment duration appear to be more effective in improving overall symptom response and QoL. Future studies of the effects of probiotics in IBS should focus on probiotic type, strain, dose, and treatment duration.
Abbreviations
CI, confidence interval; GIQLI, gastrointestinal quality of life index; GSRS, gastrointestinal symptom rating scale; HRQOL, health-related quality of life; IBS, irritable bowel syndrome; IBS-SSS, irritable bowel syndrome-symptom severity score; ITT, intention to treat; MMS, mean symptom composite score; QoL, quality of life; RCT, randomized controlled trial; RR, relative risk; SBDQ, standardized bowel disease questionnaire; SMD, standardized mean difference; VAS, visual analogue scale
References
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice. J Am Gastroenterol Assoc. 2012;10(7):712–21. e4.
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-1491.
Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39(10):1033–42.
Ohman L, Tornblom H, Simren M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12(1):36–49.
Hyland NP, Quigley EM, Brint E. Microbiota-host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions. World J Gastroenterol. 2014;20(27):8859–66.
Hussain Z, Quigley EM. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther. 2006;23(4):465–71.
Jarcho JM, Chang L, Berman M, Suyenobu B, Naliboff BD, Lieberman MD, et al. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: a pilot study. Aliment Pharmacol Ther. 2008;28(3):344–52.
Jones R. Treatment of irritable bowel syndrome in primary care. BMJ. 2008;337:a2213.
Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313.
Dai C, Zheng CQ, Jiang M, Ma XY, Jiang LJ. Probiotics and irritable bowel syndrome. World J Gastroenterol. 2013;19(36):5973–80.
Bohn L, Storsrud S, Liljebo T, Collin L, Lindfors P, Tornblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–407. e2.
Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32.
FAO/WHO Working Group Guidelines for the evaluation of probiotics in food. London, Ontario, Canada. Available at ftp://ftp.fao.org/es/esn/food/wgreport2.pdf.
Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547–62.
Clarke G, Cryan JF, Dinan TG, Quigley EM. Review article: probiotics for the treatment of irritable bowel syndrome - focus on lactic acid bacteria. Aliment Pharmacol Ther. 2012;35(4):403–13.
Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: updated systematic review with meta-analysis. World J Gastroenterol. 2015;21(10):3072–84.
Tiequn B, Guanqun C, Shuo Z. Therapeutic effects of Lactobacillus in treating irritable bowel syndrome: a meta-analysis. Intern Med (Tokyo, Japan). 2015;54(3):243–9.
Nikfar S, Mozafari S, Didari T, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: a systematic review with meta-analysis. Value Health. 2014;17(7):A363.
Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325–32.
Ortiz-Lucas M, Tobias A, Saz P, Sebastian JJ. Effect of probiotic species on irritable bowel syndrome symptoms: a bring up to date meta-analysis. Rev Esp Enferm Dig. 2013;105(1):19–36.
Drossman DA, Rome DDL, III. New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15(3):237.
Drossman DA. The functional gastrointestinal disorders: diagnosis, pathophysiology, and treatment: a multinational consenus: little brown. 1994.
Drossman DA. Rome II: the functional gastrointestinal disorders: diagnosis, pathophysiology, and treatment: a multinational consensus: Degnon Associates Incorporated. 2000.
Manning A, Thompson WG, Heaton K, Morris A. Towards positive diagnosis of the irritable bowel. BMJ. 1978;2(6138):653–4.
Sperber A D, Shvartzman P, Friger M, Fich A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the ‘true’prevalence of irritable bowel syndrome?. European journal of gastroenterology & hepatology. 2007;19(6):441-447.
Whelan K. Editorial: the importance of systematic reviews and meta-analyses of probiotics and prebiotics. Am J Gastroenterol. 2014;109(10):1563–5.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, et al. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53(10):2714–8.
Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC. Effect of probiotics on symptoms in Korean adults with irritable bowel syndrome. Gut Liver. 2009;3(2):101–7. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/611/CN-01044611/frame.html.
Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(10):1123–32.
Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of VSL#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1–7.
Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G, et al. Probiotic bacteria lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45(6):518–25.
Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot JM, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol. 2012;18(17):2067–75.
Ducrotte P, Sawant P, Jayanthi V. Clinical trial: lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18(30):4012–8.
Cui S, Hu Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int J Clin Exp Med. 2012;5(3):238–44.
Ki Cha B, Mun Jung S, Hwan Choi C, Song ID, Woong Lee H, Joon Kim H, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220–7.
Amirimani B, Nikfam S, Albaji M, Vahedi S, Nasseri-Moghaddam S, Sharafkhah M, et al. Probiotic vs. Placebo in irritable bowel syndrome:a randomized controlled trial. Middle East J Dig Dis. 2013;5(2):98–102.
Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbol DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48(10):1127–35.
Ko SJ, Han G, Kim SK, Seo JG, Chung WS, Ryu B, et al. Effect of Korean herbal medicine combined with a probiotic mixture on diarrhea-dominant irritable bowel syndrome: A double-blind, randomized, placebo-controlled trial. Evidence-based Complementary and Alternative Medicine. 2013;2013.
Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FD. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13(1):1.
Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol (Australia). 2014;29(1):52–9.
Abbas Z, Yakoob J, Jafri W, Ahmad Z, Azam Z, Usman MW, et al. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial. Eur J Gastroenterol Hepatol. 2014;26(6):630–9.
Lorenzo-Zuniga V, Llop E, Suarez C, Alvarez B, Abreu L, Espadaler J, et al. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol. 2014;20(26):8709–16.
Ludidi S, Jonkers DM, Koning CJ, Kruimel JW, Mulder L, Vaart IB, et al. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol Motil. 2014;26(5):705–14. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/528/CN-00988528/frame.html.
Urgesi R, Casale C, Pistelli R, Rapaccini GL, De Vitis I. A randomized double-blind placebo-controlled clinical trial on efficacy and safety of association of simethicone and Bacillus coagulans (Colinox®) in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2014;18(9):1344–53.
Rogha M, Esfahani MZ, Zargarzadeh AH. The efficacy of a synbiotic containing bacillus coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench. 2014;7(3):156–63.
Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: a liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome - a 12 week double-blind study. Aliment Pharmacol Ther. 2014;40(1):51–62.
Wong RK, Yang C, Song GH, Wong J, Ho KY. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2014;60(1):186–94.
Parkes GC, Sanderson JD, Whelan K. Treating irritable bowel syndrome with probiotics: the evidence. Proc Nutr Soc. 2010;69(2):187–94.
Coffin B, Dapoigny M, Cloarec D, Comet D, Dyard F. Relationship between severity of symptoms and quality of life in 858 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2004;28(1):11–5.
Spiegel B, Harris L, Lucak S, Mayer E, Naliboff B, Bolus R, et al. Developing valid and reliable health utilities in irritable bowel syndrome: results from the IBS PROOF Cohort. Am J Gastroenterol. 2009;104(8):1984–91.
McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30.
Acknowledgements
The authors offer their sincere gratitude to Prof. Samefko Ludidi, CK Yao, Homayoon Vahedi, Philippe Ducrotte, Simone Guglielmetti, and Reuben Wong for responding to our data queries and providing us with their original data.
Funding
This study was supported by the National Natural Science Foundation of China, grant numbers 81330012 and 81370495.
Availability of data and materials
Data are available as shown in additional files.
Author’s contributions
YZ and YQL conceived the study, participated in the study design, and drafted the manuscript. LXL and CGG collected the data and performed statistical analyses. DM and BCF collected the data and performed the statistical analyses. XLZ helped conceive the study. YZ drafted the manuscript. YQL and LXL revised the manuscript. All of the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1:
Risk of bias. (TIF 107 kb)
Additional file 2:
Risk of bias summary. (TIF 306 kb)
Additional file 3:
Funnel plot for publication bias for efficacy of probiotics on the overall symptom response. (TIF 3.38 kb)
Additional file 4:
Forest plot of effect on abdominal pain of IBS patients to probiotics: subgroup of probiotics type. (TIF 4.39 kb)
Additional file 5:
Funnel plot for publication bias for efficacy of probiotics on the abdominal pain. (TIF 3.38 kb)
Additional file 6:
Forest plot of effect on bloating of IBS patients to probiotics: subgroup of probiotics type. (TIF 3.18 kb)
Additional file 7:
Funnel plot for publication bias for efficacy of probiotics on bloating. (TIF 290 kb)
Additional file 8:
Funnel plot for publication bias for efficacy of probiotics on QoL. (TIF 3.38 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, Y., Li, L., Guo, C. et al. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol 16, 62 (2016). https://doi.org/10.1186/s12876-016-0470-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-016-0470-z