Abstract
Background
Good-quality evidence has shown that early glycaemic, blood pressure and LDL-cholesterol control in people with type 2 diabetes (T2D) leads to better outcomes. In spite of that, diseases control have been inadequate globally, and therapeutic inertia could be one of the main cause. Evidence on therapeutic inertia has been lacking at primary care setting. This retrospective cohort study aimed to determine the proportions of therapeutic inertia when treatment targets of HbA1c, blood pressure and LDL-cholesterol were not achieved in adults with T2D at three public health clinics in Malaysia.
Methods
The index prescriptions were those that when the annual blood tests were reviewed. Prescriptions of medication were verified, compared to the preceding prescriptions and classified as 1) no change, 2) stepping up and 3) stepping down. The treatment targets were HbA1c < 7.0% (53 mmol/mol), blood pressure (BP) < 140/90 mmHg and LDL-cholesterol < 2.6 mmol/L. Therapeutic inertia was defined as no change in the medication use in the present of not reaching the treatment targets. Descriptive, univariable, multivariable logistic regression and sensitive analyses were conducted.
Results
A total of 552 cohorts were available for the assessment of therapeutic inertia (78.9% completion rate). The mean (SD) age and diabetes duration were 60.0 (9.9) years and 5.0 (6.0) years, respectively. High therapeutic inertia were observed in oral anti-diabetic (61–72%), anti-hypertensive (34–65%) and lipid-lowering therapies (56–77%), and lesser in insulin (34–52%). Insulin therapeutic inertia was more likely among those with shorter diabetes duration (adjusted OR 0.9, 95% CI 0.87, 0.98). Those who did not achieve treatment targets were less likely to experience therapeutic inertia: HbA1c ≥ 7.0%: adjusted OR 0.10 (0.04, 0.24); BP ≥ 140/90 mmHg: 0.28 (0.16, 0.50); LDL-cholesterol ≥ 2.6 mmol/L: 0.37 (0.22, 0.64).
Conclusions
Although therapeutic intensifications were more likely in the presence of non-achieved treatment targets but the proportions of therapeutic inertia were high. Possible causes of therapeutic inertia were less of the physician behaviours but might be more of patient-related non-adherence or non-availability of the oral medications. These observations require urgent identification and rectification to improve disease control, avoiding detrimental health implications and costly consequences.
Trial registration
Number NCT02730754, April 6, 2016.
Similar content being viewed by others
Introduction
Managing and achieving optimal control in glycaemia (HbA1c), blood pressure and low-density lipoprotein cholesterol for people with type 2 diabetes (T2D) has been very difficult [1, 2]. Proportions of achieved treatment targets in the world for HbA1c < 7.0% (< 53 mmol/mol) were about 50%, blood pressure < 140/90 mmHg 80% and low-density lipoprotein cholesterol (LDL- cholesterol) 60% [3,4,5,6], and it is worse in lower income countries [7,8,9] and better in a higher income country [10]. In developing countries in Asia, Latin America and Eastern Europe, only 3.6% of T2D patients were able to attain all three recommended targets (blood pressure < 130/80 mmHg, LDL-cholesterol < 100 mg/dl, and HbA1c < 7%) [9]. The same rate was reported as 22% in two polyclinics in Singapore [11]. Without due clinical agility and healthcare system management for T2D, achieving and maintaining optimal treatment targets will face an uphill task and untoward consequences to all [12,13,14]. Delay in treatment intensification when hyperglycaemia, hypertension and hypercholesterolaemia are present increases the risk of diabetes-related complications [13]. This will further impacts on disease management of patients with multiple morbidity, present of impaired organ function, and when integrating diabetes care into daily life faces complex psychosocial factors from the environment, social, personal and emotional issues [14,15,16].
Clinical inertia is defined as a failure to initiate or intensify necessary treatments at a timely manner when faced with objective evidence of uncontrolled diseases in the present of clear clinical practice guidelines [17,18,19]. Therapeutic inertia, although used interchangeably with clinical inertia, focuses more on the prescribed therapies and pharmacological agents, and usually construed as the providers’ failure to increase therapy when treatment targets are not met [20, 21]. Therapeutic inertia could be due to patient-related factors [21], physician-related and healthcare system-related barriers [17, 22]. A combination of these factors may exist and compound effective and efficient diabetes care delivery and clinical consultation between the doctors and patients, patients and facility, and doctors and facility [18, 19]. Some of the principal causes of clinical and therapeutic inertia are doctors’ preference for status quo to avoid uncertainty and risk [23], and impaired communication between doctors and patients [19]. Others include a lack of infrastructure and facility for proper disease monitoring to achieve treatment goals, the mindset of ‘waiting until next visit’ in response to soft rationalizations by patients to avoid treatment intensification, overestimation of care provided, a lack of education and training of the doctors, and practice organization aimed at achieving treatment targets [24, 25].
The problems of therapeutic inertia in diabetes care or a delayed response to poor glycaemic control were about 30–40% or six months to 2 years, respectively [18]. In a recent systematic review, the median time to treatment intensification after at least one HbA1c measurement above target ranged from 0.3 to > 7.0 years [26]. The therapeutic inertia increased with the number of drugs and decreased with increasing HbA1c levels [26]. The UK Clinical Practice Research Datalink (CPRD) reported the median time of basal insulin intensification from initiation was > 4 years, and less than one-third of the eligible T2D [HbA1c ≥ 7.5% (58 mmol/mol)] had their treatment intensified (median time: 3.7 years) [27]. The corresponding therapeutic inertia for hypertension (≥ 130/80 mmHg) and dyslipidaemia (LDL-C ≥ 2.6 mmol/L or 100 mg/dl) were 46% and 40%, respectively [9].
The evidence on therapeutic inertia has been lacking at primary care setting, especially in low- and middle-income countries and in Asia [26]. Accordingly, this study aimed to determine the proportions and associated factors of therapeutic inertia of anti-diabetic, anti-hypertensive and lipid-lowering therapies when treatment targets not achieved in adults with T2D at public health clinics in Malaysia. The findings may contribute support to local initiatives in overcoming therapeutic inertia similar to the 3-year Overcoming Therapeutic Inertia initiative in the US [28].
Methods
This was a retrospective cohort study that included baseline data from a previous study in 2013 [29], together with the follow-up data from 2014 to 2016 [30]. During this period of follow-up, patients received standard diabetes care and clinical services at the respective health clinics. All methods were carried out in accordance with local clinical practice guidelines and ethical regulations.
Setting and participants
At baseline, participants were sampled consecutively as they came to the clinics over a period of six months. Inclusion criteria at baseline: age 30 years or older, a diagnosis of T2D more than one year ago, and with at least three clinic visits in the previous year. The baseline exclusion criteria were pregnancy or lactating, psychiatric/ psychological disorders that could impair judgment and memory, and participants who could not read or understand English, Malay or Mandarin [29]. The participating health clinics were chosen because they serve different sections of the local population. One health clinic (SK) is urban and is visited mainly by patients of Chinese descent, the second is a rural clinic (DK) visited by proportionally more patients of Indian descent than found in a usual public health clinic, and the third clinic (SL) is in a rural and predominantly visited by the Malays. Before answering the questionnaires in their preferred language, all participants gave written consent while waiting for a medical consultation with the clinic’s doctor. Trained research assistants interviewed participants at baseline who were not able to self-administer the questionnaires. The study protocol was approved by Medical Research Ethics Committee (MREC), Ministry of Health Malaysia.
Data collection
Baseline demographic data included age, gender, ethnicity, religion, educational level, employment status, monthly income and life event within the past six months [30]. Structured case record forms were used for data collection from the medical records. These included duration of diabetes, HbA1c, diabetes-related complications, blood pressure, lipids, number and type of medication use [29]. At follow-ups, participants were identified by an orange label on their follow-up cards and on their medical records. Follow-up data were retrieved from the medical records on glycaemic control (HbA1c), systolic (SBP) and diastolic blood pressure (DBP), LDL-cholesterol and prescribed medications [29]. These medications included oral anti-diabetic agents (ADA), insulin, oral anti-hypertensive agents (AHA) and lipid-lowering agents (LLA). Non-participants were those who lost to follow-up at the participating clinics and non-attendance for at least more than one year between 2014 and 2016. There was no other injectable ADA besides insulin at the three participating clinics during the period of the study. Every result of HbA1c and LDL-cholesterol, and every reading of SBP and DBP were retrieved. The recommended treatment targets are HbA1c < 7.0% (53 mmol/mol), blood pressure < 140/90 mmHg and LDL-cholesterol < 2.6 mmol/L, respectively [31]. Medications use was retrieved in terms of their names, dose and frequency. The medications prescribed when the annual blood tests were reviewed during the follow-up visit were considered as the index prescription. In the event of absence of an annual blood test or results, the medications prescribed in the last follow-up visit of the year were considered as the index prescription.
Data analysis
Data analyses were carried out using SPSS software version 25.0 (IBM Corp., Armonk, NY). Comparisons of mean levels were performed using the Student’s t-test or Mann-Whitney U test according to the data distribution, and the Chi-square test was used for proportionate samples between groups. Characteristics of the patients who have and did not have the required data for this study are presented as mean (SD) or median (IQR) for continuous variables, and counts and percentages for nominal variables using descriptive statistics according to the three health clinics.
Assessment of therapeutic inertia was done for year 2015 because the required medication use data were captured from year 2014, and not possible for year 2016 as the study ended in the third quarter. Index of ADA, insulin and LLA medications prescription and use of the year were compared to the previous year index prescription. Changes of medication use were classified as 1) no change, 2) stepping up: dose increment or/and replacement with a stronger medication, and 3) stepping down: dose reduction or/and replacement with a weaker medication. All the classification was verified by the author himself and the resident family physicians in SK and SL health clinics. Therapeutic inertia is defined as no change in the medication use in the present of not reaching the recommended treatment targets. Therapeutic inertia was assessed for HbA1c (no change in ADA and insulin uses separately) and LDL-cholesterol (no change in LLA use) by looking at the treatment target in the same year because medication use and change was captured after the tests were reviewed. For those who did not have results for HbA1c and LDL-cholesterol in the same year, the results in the previous year were used. This reflects the actual clinical practice at these health clinics. The therapeutic inertia assessment for blood pressure (no change in AHA use) was assessed by looking for occurrence of persistent SBP/DBP above treatment targets over two occasions with no change of AHA use in the same year. Sensitive analysis was conducted to examine the states of therapeutic inertia for AHA use in the first half and the second half of the year when at least four SBP/DBP measurements were available, and at least two consecutive SBP/DBP measurements that were above the recommended treatment targets in the respective 6-month periods. The results were essentially similar.
Possible clinic and patient’s factors on the therapeutic inertia were further evaluated using the multivariable logistic regression analysis. Besides the clinic, covariates with a P value < 0.20 from the univariable analyses were included in the final multivariable analyses. Category ‘no change’ represents therapeutic inertia and category ‘stepping up and stepping down’ was used as the reference group. The final models were re-run with ‘stepping up’ as the reference group and the results did not change the interpretations. We reported the results with the treatment targets entered as one of the covariate, with the reference category ‘stepping up and stepping down’ because this could account for all possible reasons of therapeutic changes and render the modelling clinically more meaningful [32]. Statistical significance was set at P < 0.05.
Results
Cohort characteristics
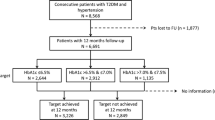
In total, 552 participants have the required follow-up data for the assessment of therapeutic inertia, representing 78.9% of the baseline sample (n=700). There were no differences in the prescribed medications between the non-participants and participants except LLA use was more among the participants (72% vs. 83%) (Table 1). Compared to the participants, the non-participants were more often treated at the rural Salak Health Clinic, had higher systolic blood pressure, and had dyslipidaemia (Table 1).
Therapeutic inertia in the three clinics
Except for ADA and LLA, there were significant differences in insulin and AHA therapeutic patterns when treatment targets were not achieved at the three health clinics (Fig. 1). DK was less likely to have insulin therapy inertia and SK less likely to have AHA inertia compared to the other health clinics. All clinics had high therapeutic inertia in ADA (61–72%) and LLA therapies (56–77%).
Anti-diabetic, anti-hypertensive and lipid-lowering therapeutic changes when their respective treatment targets were not achieved in year 2015. Sample size included: ADA, n = 367; Insulin, n = 365; AHA, n = 218; LLA, n = 206. ADA = oral anti-diabetic agents; AHA = anti-hypertensive agents; LLA = lipid-lowering agents; LDL-C = low-density lipoprotein cholesterol. Chi-square: differences in the three clinics for: ADA χ2 = 9.161, P = 0.057; Insulin χ2 = 15.410, P = 0.004. AHA χ2 = 27.953, P < 0.001; LLA χ2 = 6.634, P = 0.157
Anti-diabetics therapy inertia
Both ADA and insulin therapeutic inertia were observed, and ADA therapeutic inertia was worse than the insulin (64% versus 47%) (see Additional File Table S1) presumably because of limited dosing range and choices of the oral agents. Patients who were prescribed less medication, had shorter diabetes duration and not having dyslipidaemia were likely to experience ADA therapy inertia (see Additional File Table S1). There was no relation between ADA changes and glycaemic control (OR 1.18, 95% CI 0.77, 1.80; P = 0.454). Insulin therapeutic inertia was more likely among those with shorter diabetes duration (adjusted OR 0.9, 95% CI 0.87, 0.98) (Table 2).
Anti-hypertensive therapy inertia
AHA inertia was observed in about half (51%) of the participants with BP > 140/90 mmHg, and only 34% had their AHA therapy stepped up. This inertia happened more among the younger age group, Indians, in active employment, shorter hypertension duration and not diagnosed of hypertension (see Additional File Table S2). Those who had at least two consecutive BP > 140/90 mmHg were more likely to experience AHA therapy intensification (Table 3).
Lipid lowering therapy inertia
Therapeutic inertia in LLA was observed in 61% of those who had uncontrolled LDL-cholesterol, and this was more common among those having hypertension on top of T2D (see Additional File Table S3). However, no factor was an independent risk factor to LLA therapy inertia after adjusting for LDL-cholesterol treatment target (Table 4). Those who had not achieved LDL-cholesterol treatment target < 2.6 mmol/L were more likely to experience lipid-lowering therapy intensification.
Discussion
This study examined the therapeutic patterns of ADA, AHA and LLA in adults with T2D after three years of regular primary diabetes care at three public health clinics in Malaysia. The findings show some similarity and differences in the therapeutic changes of these medications in the three health clinics when treatment targets were not achieved. Although multivariable analyses show that treatment intensifications were more likely in the event of uncontrolled diseases, but the proportions of therapeutic inertia were high in hyperglycaemia (up to 64%), hypertension (51%) and high LDL-cholesterol (61%). These proportions were still considered high after considering the possible true contraindications and intolerance to medications of about 15 to 20% based on the authors’ experience and early studies [33,34,35].
High prevalent of therapeutic inertia in ADA in all three clinics might indicate limitation in the dosing of the oral medications when maximum doses have been prescribed and with the restricted choices of ADA [22]. The commonly available ADA are metformin and gliclazide, with additional one to two drugs from the newer classes of dipeptidyl peptidase-4 (DPP-4) inhibitors, which are restricted to the resident family physicians use. Therefore, the observed therapeutic inertia could be a result of clinical inertia in timely referral for other ADA or insulin initiation. It was also noted that patients who were relatively healthier (shorter diabetes duration, no comorbid such as dyslipidaemia and fewer number of medication) were more likely to experience ADA inertia [17]. In contrast to dose increment limitation in ADA, insulin has wider dosing possibility and this had resulted in relatively lower insulin inertia, and recorded the highest stepping-up rate among all the studied therapies in this study. The observed rate of insulin intensification was similar to the Canadian specialist treating non-insulin-requiring patients in year 2000 [36]. Patients with shorter diabetes duration of T2D were more likely to experience insulin inertia. This might be construed that both the patients and doctors preferred working harder on non-pharmacological means or ADA before insulin dose increment. Insulin therapy could be better optimized with improved HbA1c level for patients who practiced self-monitoring of blood glucose with an automated insulin dose titration advice that comes from the meter after analysing some past glucose patterns compared to health-care professional support alone [37].
AHA therapeutic patterns noted a wide variation across the three clinics, and different clinic was one of the significant factors that showed an independent effect on AHA inertia in the multivariable model. DK was most likely to have AHA inertia compared to the other clinics. These could be due to the differences in healthcare system-related factors such as having a dedicated team and consultation room at SK [22]. This might contribute to a more effective consultation with patients that improved doctor-patient communication. Other possible causes include having competent knowledge in hypertension and its treatment in T2D, and familiarity with more AHA and their use in T2D. Therapeutic inertia in AHA could also be due to the patient-related factors such as denial and refusal of treatment intensification due to non-experiencing of symptoms of hypertension or disease progression [17]. Although this study did not examine the time to anti-hypertensive treatment intensification since two consecutive BP > 140/90 mmHg had been recorded, it was likely that at least 40% experienced AHA inertia for longer than one year based on the sensitive analysis conducted.
Multivariable analysis did not reveal any significant contributing factor towards LLA inertia except that of achieving the LDL-cholesterol target. This was an assuring finding observed in this study and elsewhere [38] but the proportion of therapeutic inertia was second highest in LLA among the four therapies (highest therapeutic inertia in ADA). This might suggest that the causes were less of physician-related but more of patient-related or health system-related. The availability of LLA to the clinics’ doctors was generally restricted in the years when this study was conducted. Three types of LLA that were available were lovastatin, simvastatin and gemfibrozil, with atorvastatin and fenofibrate were further available only with endorsement by the resident family physicians with a specialist status. Patient-related causes might be statin intolerance and concern about statins worsen the glycaemic control [39]. Lessons from controlled clinical trials indicated that a combination of good patient education and support, and clear treatment strategies might reduce clinical inertia [17]. However, this may not ensure timely treatment intensification by the attending doctors who perceive time constraint in consultation [40] and concern about statin adverse effects.
Strength and limitations
Good sample size, representative samples of the participants [29] and reasonable statistical analyses rendering the study ability to produce credible answers. However, this study was subjected to several potential limitations. This study did not classify diabetes pharmacological regimen as a whole but ADA to insulin changes separately. Thus, escalation of treatment from ADA to insulin therapy in the event of HbA1c ≥ 7.0% was not recorded. Therefore, insulin therapeutic inertia provided an estimate that was closer to the actual therapeutic practices compared to the other studied therapies in the clinical management of adults with T2D. Insulin treatment was recorded from none to initiation, and to dose escalation without the constraints of medication choices when the maximum doses have been reached. Treatment targets used did not reflect risk profiles and customized target levels for the patients. This may lead to underestimation of the proportions of therapeutic inertia because majority of the adults T2D at the primary care setting were of the lower risk and in early stages of diseases, and treatment intensification should occur at lower target levels [41, 42]. Similarly, underestimation of the proportions of therapeutic inertia is likely in the participants especially in ADA and LLA since the non-participants were prescribed less of these medications, and the included cohorts were those tend to follow-up attendance at baseline. The three participating health clinics in this study are of the medium scale with resident family physicians and doctors, and situated in a developed state of Selangor across urban, sub-rural and rural regions. This limits generalizability of the results to other clinics of different scales, without resident doctors or in the more remote areas in the country that may have different healthcare systems and delivery at the meso- and micro-levels.
Conclusion
Although therapeutic intensifications were more likely in the presence of non-achieved glycaemic, blood pressure and LDL-cholesterol treatment targets but the proportions of adults with T2D who faced therapeutic inertia were high at primary diabetes care in Malaysia. Possible causes of therapeutic inertia might be due to restricted choices of oral medications and patient-related factors besides physicians’ behaviours. Therapeutic inertia usually present with complex barriers that require multisectorial efforts to overcome. This will include multidisciplinary diabetes care team, people with diabetes, advocacy, policymakers, the industries and research institutes to effect change at all levels of the care ecosystem. These require urgent identification and rectification to improve disease control [43,44,45]. The priority area may be different in different countries. Delay in treatment intensification for T2D and prolonged suboptimal control of hypertension and high LDL-cholesterol in people with T2D will lead to detrimental health implications and costly consequences.
Availability of data and materials
Participant-level datasets along with published reports from this trial is available on reasonable request to the corresponding author from whom an anonymised dataset will be shared.
References
Chew BH, Lee PY, Cheong AT, Ismail M, Shariff-Ghazali S, Goh PP. Messages from the Malaysian diabetes registries on diabetes care in Malaysian public healthcare facilities. Prim Care Diab. 2016;10(5):383–6.
Mafauzy M, Zanariah H, Nazeri A, Chan SP. DiabCare 2013: A cross-sectional study of hospital based diabetes care delivery and prevention of diabetes related complications in Malaysia. Med J Malaysia. 2016;71(4):177–85.
Non-Communicable Disease Section, Department of Public Health, National Diabetes Registry Report, Volume 1: 2009–2012 2013. Available from: https://www.moh.gov.my/index.php/pages/view/1905?mid=649. Accessed 29 Apr 2021.
Guðbjörnsdóttir S, Eliasson B, Cederholm J, Zethelius B, Ann-Marie S, Samuelsson P. Swedish National Diabetes Register Annual Report 2013. Gothenburg, Sweden: Centre of Registers, Region Västra Götaland 2014. Available from: https://www.ndr.nu/pdfs/Annual_Report_NDR_2013.pdf. Accessed 29 Apr 2021.
National Diabetes Audit: Report 1, Care Processes and Treatment Targets 2018–19, Full Report. Healthcare Quality Improvement Partnership, National Diabetes Audit, England and Wales. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-1-care-processes-and-treatment-targets-2018-19-full-report. Accessed 29 Apr 2021.
Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diab Care. 2017;40(11):1425–32.
Chan JC, Chan SP, Deerochanawong C, Go RT, Lee KO, Ma RC, et al. Diabetic dyslipidaemia in Asian populations in the Western Pacific Region: What we know and don’t know. Diabetes Res Clin Pract. 2011;94(1):1–13.
So WY, Raboca J, Sobrepena L, Yoon KH, Deerochanawong C, Ho LT, et al. Comprehensive risk assessments of diabetic patients from seven Asian countries: The Joint Asia Diabetes Evaluation (JADE) program. J Diabetes. 2011;3(2):109–18.
Chan JC, Gagliardino JJ, Baik SH, Chantelot JM, Ferreira SR, Hancu N, et al. Multifaceted determinants for achieving glycemic control: the international diabetes management practice study (IDMPS). Diab Care. 2009;32(2):227–33.
Tan NC, Koh KH, Goh CC, Koh YL, Goh SC. Asian patients with dyslipidemia in an urban population: Effect of ethnicity on their LDL-cholesterol treatment goals. J Clin Lipidol. 2016;10(2):410–9.
Goh CC, Koh KH, Goh S, Koh Y, Tan NC. Achieving triple treatment goals in multi-ethnic Asian patients with type 2 diabetes mellitus in primary care. Malays Fam Physician. 2018;13(2):10–8.
Control Group, Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–98.
Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100.
Fritzen K, Heinemann L, Schnell O. Modeling of diabetes and its clinical impact. J Diab Sci Technol. 2018;12(5):976–84.
American Diabetes Association. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2021. Diab Care. 2021;44(Supplement 1):S53–72.
Chew BH, Fernandez A, Shariff-Ghazali S. Psychological interventions for behavioral adjustments in diabetes care - a value-based approach to disease control. Psychol Res Behav Manag. 2018;11:145–55.
Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35(11):1735–45.
Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43(6):501–11.
Strain WD, Cos X, Hirst M, Vencio S, Mohan V, Vokó Z, et al. Time to do more: addressing clinical inertia in the management of type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;105(3):302–12.
Khunti K, Davies MJ. Clinical inertia-time to reappraise the terminology? Prim Care Diabete. 2017;11(2):105–6.
Khunti K, Triplitt CL. Improving outcomes of people with diabetes through overcoming therapeutic inertia. Diabetes spectrum. 2020;33(1):5–6.
Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10:1–11.
Reach G. Clinical inertia, uncertainty and individualized guidelines. Diabetes Metab. 2014;40(4):241–5.
Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825–34.
Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87.
Khunti K, Gomes MB, Pocock S, Shestakova MV, Pintat S, Fenici P, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–37.
Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diab Care. 2013;36(11):3411–7.
Gabbay RA, Kendall D, Beebe C, Cuddeback J, Hobbs T, Khan ND, et al. Addressing therapeutic inertia in 2020 and beyond: a 3-year initiative of the American Diabetes Association. Clinical Diabetes. 2020;38(4):371–81.
Chew BH, Vos R, Mohd-Sidik S, Rutten GE. Diabetes-related distress, depression and distress-depression among adults with type 2 diabetes mellitus in Malaysia. PLoS ONE. 2016;11(3):e0152095.
Chew BH, Vos RC, Stellato RK, Rutten GEHM. Diabetes-related distress and depressive symptoms are not merely negative over a 3-year period in Malaysian adults with type 2 diabetes mellitus receiving regular primary diabetes care. Front Psychol. 2017;8:1834.
American Diabetes Association. Standards of medical care in diabetes—2021. Diab Care. 2021;44(Suppl 1):S1–225.
Barton AB, Okorodudu DE, Bosworth HB, Crowley MJ. Clinical inertia in a randomized trial of telemedicine-based chronic disease management: lessons learned. Telemed J E Health. 2018;24(10):742–8.
Goudswaard AN, Stolk RP, de Valk HW, Rutten GE. Improving glycaemic control in patients with Type 2 diabetes mellitus without insulin therapy. Diabet Med. 2003;20(7):540–4.
Hosomura N, Malmasi S, Timerman D, Lei VJ, Zhang H, Chang L, et al. Decline of insulin therapy and delays in insulin initiation in people with uncontrolled diabetes mellitus. Diabet Med. 2017;34(11):1599–602.
Wan KS, Moy FM, Mohd Yusof K, Mustapha FI, Mohd Ali Z, Hairi NN. Clinical inertia in type 2 diabetes management in a middle-income country: a retrospective cohort study. PLoS One. 2020;15(10):e0240531.
Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care. 2005;28(3):600–6.
Bergenstal RM, Johnson M, Passi R, Bhargava A, Young N, Kruger DF, et al. Automated insulin dosing guidance to optimise insulin management in patients with type 2 diabetes: a multicentre, randomised controlled trial. Lancet. 2019;393(10176):1138–48.
Cebrian A, Guisasola FA, Cos FX, Quintero MR, Millaruelo JM, Beltran DO, et al. Poor glycaemic control—identifying variables associated with therapeutic inertia and lack of therapeutic adherence. Diabetes. 2018;67(Supplement 1):2407-PUB.
Cai R, Yuan Y, Sun J, Xia W, Huang R, Tian S, et al. Statins worsen glycemic control of T2DM in target LDL-c level and LDL-c reduction dependent manners: a meta-analysis. Expert Opin Pharmacother. 2016;17(14):1839–49.
Ahmad BA, Khairatul K, Farnaza A. An assessment of patient waiting and consultation time in a primary healthcare clinic. Malays Fam Physician. 2017;12(1):14–21.
Chew BH, Mastura I, Bujang MA. Comparing the disease profiles of adult patients with type 2 diabetes mellitus attending four public health care facilities in Malaysia. Malays Fam Physician. 2013;8(3):11–8.
Chew BH, Shariff-Ghazali S, Lee PY, Cheong AT, Mastura I, Haniff J, et al. Type 2 diabetes mellitus patient profiles, diseases control and complications at four public health facilities- a cross-sectional study based on the Adult Diabetes Control and Management (ADCM) Registry 2009. Med J Malaysia. 2013;68(5):397–404.
Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203–7.
Murphy ME, Byrne M, Zarabzadeh A, Corrigan D, Fahey T, Smith SM. Development of a complex intervention to promote appropriate prescribing and medication intensification in poorly controlled type 2 diabetes mellitus in Irish general practice. Implement Sci. 2017;12(1):115.
Murphy ME, Byrne M, Galvin R, Boland F, Fahey T, Smith SM. Improving risk factor management for patients with poorly controlled type 2 diabetes: a systematic review of healthcare interventions in primary care and community settings. BMJ Open. 2017;7(8):e015135.
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We thank Universiti Putra Malaysia (UPM) and the Ministry of Higher Education, Malaysia for their support in sponsoring the Ph.D. study and living allowances for Boon-How Chew. We acknowledge Prof. Dr. Guy E.H.M. Rutten from the University of Utrecht, Julius Center for Health Sciences and Primary Care and Ass. Prof. Dr. Rimke C. Vos from Leiden University Medical Center for participating in the larger EDDMQOL-Pro study. We would like to thank Professor Dr. Sazlina Sharif Ghazali, previous Head of the Department of Family Medicine UPM, Dr. Aaron Fernandez of the Psychiatry Department UPM, Dr. Dr. Noor-Hasliza Hassan at Dengkil Health Clinic, and the Petaling and Sepang District Health Offices for their assistance in this study.
Funding
This work was partly supported by the Ministry of Higher Education Malaysia (grant numbers KPT(BS)730928075371, 2013) and Universiti Putra Malaysia’s journal publication awards (award numbers UPM/TNCPI/RMC/5.1.17/F5(16)). The funding bodies had no role in the design of the study or in the collection, analysis, or interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
BHC conceived the study design, collected the data and completed the data analysis. HH and ZAS involved in the study design and assisted in data collection. BHC drafted the manuscript. All authors read, revised, and approved the manuscript critically for important intellectual content and helped to draft the final manuscript. All authors gave final approval for this manuscript to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The current study was approved by the Medical Research Ethics Committee (MREC), Ministry of Health Malaysia with the reference number of (5)KKM/NIHSEC/P16-293 on 08/03/2016. Patients who agreed to participate provided written informed consent during the screening phase.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Characteristics of patients and uncontrolled HbA1c according to the anti-diabetics therapeutic changes in 2015, n (row %) unless stated otherwise. Table S2. Characteristics of patients and at least two consecutive blood pressure not at targets according to the anti-hypertensive therapeutic changes in 2015, n (row %) unless stated otherwise. Table S3. Characteristics of patients and uncontrolled low density lipoprotein-cholesterol according to the lipid lowering therapeutic changes in 2015, n (row %) unless stated otherwise.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chew, BH., Hussain, H. & Supian, Z.A. Is therapeutic inertia present in hyperglycaemia, hypertension and hypercholesterolaemia management among adults with type 2 diabetes in three health clinics in Malaysia? a retrospective cohort study. BMC Fam Pract 22, 111 (2021). https://doi.org/10.1186/s12875-021-01472-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12875-021-01472-2