Abstract
Background
Septic shock medical treatment relies on a bundle of care including antibiotic therapy and hemodynamic optimisation. Hemodynamic optimisation consists of fluid expansion and norepinephrine administration aiming to optimise cardiac output to reach a mean arterial pressure of 65mmHg. In the prehospital setting, direct cardiac output assessment is difficult because of the lack of invasive and non-invasive devices. This study aims to assess the relationship between 30-day mortality and (i) initial pulse pressure (iPP) as (ii) pulse pressure variation (dPP) during the prehospital stage among patients cared for SS by a prehospital mobile intensive care unit (MICU).
Methods
From May 09th, 2016 to December 02nd, 2021, septic shock patients requiring MICU intervention were retrospectively analysed. iPP was calculated as the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP) at the first contact between the patient and the MICU team prior to any treatment and, dPP as the difference between the final PP (the difference between SBP and DBP at the end of the prehospital stage) and iPP divided by prehospital duration. To consider cofounders, the propensity score method was used to assess the relationship between (i) iPP < 40mmHg, (ii) positive dPP and 30-day mortality.
Results
Among the 530 patients analysed, pulmonary, digestive, and urinary infections were suspected among 43%, 25% and 17% patients, respectively. The 30-day overall mortality rate reached 31%. Cox regression analysis showed an association between 30-day mortality and (i) iPP < 40mmHg; aHR of 1.61 [1.03–2.51], and (ii) a positive dPP; aHR of 0.56 [0.36–0.88].
Conclusion
The current study reports an association between 30-day mortality rate and iPP < 40mmHg and a positive dPP among septic shock patients cared for by a prehospital MICU. A negative dPP could be helpful to identify septic shock with higher risk of poor outcome despite prehospital hemodynamic optimization.
Similar content being viewed by others
Introduction
Every year, more than 30 million people worldwide suffer from sepsis [1,2,3]. Sepsis is responsible for approximately 11 million deaths each year accounting for 20% of annual deaths [3] and almost 40% of all in-hospital deaths [4]. In 2016, the “sepsis 3” conference, the World Health Organization and the Centre for Disease Control and Prevention recommend early recognition, severity assessment and treatment instauration to decrease mortality of sepsis [5]. Indeed, during the last 40 years, sepsis overall mortality rate remains stable around 30% ranging from 15% for sepsis and 50% for septic shock, the most severe sepsis form [6,7,8].
From a pathophysiological point of view, an absolute and relative hypovolemia reflects the vascular sepsis consequences. Sepsis is characterized by the vascular tone decrease, traduced by micro, e.g., skin mottling, and macro-circulation alterations, e.g., hypotension. In order to correct both absolute and relative hypovolemia, to restore the vascular tone, and to ensure tissues perfusion [9, 10], the guidelines recommend an objective of a mean arterial pressure of at least 65 mmHg [11, 12] by fluid volume expansion within the first 3 h, and norepinephrine infusion in case of fluid expansion failure [5, 13, 14] aiming to optimise cardiac output and to ensure adequate tissues perfusion. However, undue fluid volume expansion results in a risk of fluid overload [15], independently associated with a poorer outcome, for example with septic shock mortality increase [16,17,18,19,20]. Cardiac output assessment can be performed by non-invasive approach, i.e., echocardiography, or invasive approach, i.e., Swan-Ganz catheterisation, both approaches, to date, are non-available in the prehospital daily practice. Pulse pressure (PP), e.g., the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP), is an indirect method of assessing cardiac output and an alternative for a non-invasive approach of cardiac output. Marik et al. previously reported that a PP less than 40mmHg indicates an impaired cardiac output [21].
To date most cases of sepsis (70%) occur outside hospital environment [22] with a median time to hospital admission around 60 min, to respect the treatment delay of sepsis guidelines for septic shock, the prehospital stage of care offers an opportunity to respect the delays while starting care early by prehospital caregivers [12, 23] [24]. Moreover, prompt prehospital and in-hospital hypotension correction improves septic shock survival [25,26,27,28]. Beyond sepsis origin source and antibiotic therapy, cardiac output and tissue perfusion optimization are daily questions in intensive care units in order to improve sepsis and septic shock outcome [11, 12]. In this way, PP is a parameter immediately available, non-invasive, reproductible and accessible since the prehospital setting where the resources are scarce. We hypothesized that PP, as a non-invasive surrogate of cardiac output, could be helpful for MICU physician daily practice, to early optimize cardiac output and tissue perfusion among septic shock patients.
Because, parameters variation is more informative than an isolated measure to assess the disease severity and the treatment effect, by similarity with the blood lactate clearance, for sepsis severity [29, 30] and treatment effect assessments [5, 30,31,32,33], we explored the relationship between pulse pressure variation (dPP, i.e., final prehospital PP – initial prehospital PP) and septic shock outcome, hypothesizing that, as shock index changes and lactate clearance during the prehospital stage, dPP may be an indirect tool for treatment effect assessment [5, 34, 35].
This study aims to assess the relationship between 30-day mortality and (i) initial pulse pressure (iPP) as (ii) dPP during the prehospital stage of care among patients cared for septic shock by a French mobile intensive care unit (MICU).
Methods
Patients
From May 09th, 2016 to December 02nd, 2021, patients with septic shock diagnosis presumed on clinical history, clinical signs and lactate measurement of available accordingly to the 2012 sepsis-2 conference [36] cared for by a prehospital MICU teams of one of 7 French hospital centres (Necker-Enfants malades Hospital, Lariboisière Hospital, La Pitié-Salpêtrière Hospital, Hôtel Dieu Hospital, APHP, Paris – France; The Paris Fire Brigade Paris, – France; The Toulouse University Health Centre, Toulouse – France and the Castres Hospital, Castres – France) were retrospectively included and patients care records were retrospectively analyzed in 2022. Patients younger than 18 years, and/or are pregnant, and/or with serious comorbid condition(s) with a not to be reanimated status known since pre-hospital setting were not included. Treatments management and strategy used to achieve a mean arterial pressure at the end of prehospital care were left to the MICU physician’s discretion.
Patients’ demographic characteristics, suspected prehospital origin of sepsis, initial prehospital (e.g., the first MICU contact), and final prehospital (e.g., at the end of prehospital stage) vital sign values (systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial pressure) were measured with French certified© non-invasive automated device in all centres (tool brands varied between centres), heart rate (HR), pulse oximetry (SpO2), respiratory rate (RR), body core temperature and Glasgow coma scale (GCS)), duration of prehospital care, and prehospital treatments (antibiotic therapy, fluid volume expansion, as well as catecholamine type and dose) were collected from MICU prehospital medical reports.
Hypertension, chronic cardiac failure (CCF), coronary heart disease (CHD), chronic renal failure (CRF), chronic obstructive pulmonary disease (COPD), diabetes mellitus, and history of cancer) [37] and immunosuppression defined by the existence of chronic alcoholism and/or human immunodeficiency virus infection were identified on MICU and in-hospital medical reports.
The length of stay (LOS) in the intensive care unit, in-hospital LOS, and the 30-day mortality status (alive or deceased) were retrieved from medical reports in case of in-hospital death or by patient and/or relatives phone call in case of hospital discharge. The Sequential Organ Failure Assessment (SOFA) score [38] was calculated 24 h after ICU admission.
Ethical considerations
The Society of Anaesthesia and Intensive Care ethics committee on December 12th, 2017 (Ref number: IRB 00010254-2017-026) approved the study considering that the patient consent was waived for the participation in this retrospective study.
Statistical analysis
Results are expressed as mean ± standard deviation for quantitative parameters with a Gaussian-distribution, as median with interquartile range [Q1-Q3] for parameters with a non-normal distribution and value with percentage for qualitative parameters. The main outcome was the 30-day mortality. Univariate and multivariate analyses were performed to evaluate the relationship between each covariate and 30-day mortality. Initial pulse pressure (iPP) was calculated by the difference between SBP and DBP at the first contact between the patients and the MICU team prior to any treatment. According to Marik et al. review [21], a threshold of 40mmHg was chosen to define a lowered cardiac output. Delta PP (dPP) was calculated by the difference between the final PP, the difference between SBP and DBP at the end of the prehospital stage, and iPP divided by prehospital duration (minutes). To consider cofounders, the propensity score (PS) method was used to assess the relationship between (i) iPP < 40mmHg, (ii) positive dPP and 30-day mortality. To reduce the effect of confounders on (i) iPP < 40mmHg, (ii) positive dPP and 30-day mortality, a propensity score matching was used to balance the differences in baseline characteristics between patients with (i) iPP < 40mmHg or (ii) positive dPP and those with (i) iPP ≥ 40mmHg or (ii) negative dPP. For iPP < 40mmHg, the propensity score, i.e., the probability of (i) iPP < 40mmHg was estimated using logistic regression based on potential confounders: age, sex, cancer history, CHD, CRF, diabetes mellitus, SOFA, hypertension, CCF, BMI, COPD and immunosuppression. For positive dPP, the propensity score, i.e., the probability of dPP > 0, was estimated using logistic regression based on potential confounders: antibiotic therapy administration, fluid expansion and norepinephrine administration during the prehospital setting, age, sex, cancer history, CHD, CRF, diabetes mellitus, SOFA, hypertension, CCF, BMI, COPD, and immunosuppression. Nearest neighbour matching method was used to match patients based on the logit of the propensity score [39]. The balance of covariates after matching was assessed by absolute mean differences with a considered acceptable threshold of 10% [40]. A survival analysis using Cox proportional hazards regression was used to compare 30-day mortality of patients with and without (i) iPP < 40mmHg, (ii) positive dPP in the propensity score–matched cohort. Proportional hazards assumption was verified for each Cox model variable by Kaplan Meier curve and log-rank test. Results are expressed by adjusted Hazard ratio (aHR) with 95% confidence interval [95 CI]. All tests were 2-sided.
R 3.4.2 software (http://www.R-project.org; the R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses. A p-value < 0 0.05 defined statistical significance.
Results
Patient characteristics
Five hundred and thirty patients with septic shock cared for by a prehospital MICU team of one of 7 French hospital centres were analysed. Among them, 341 patients (65%) were male, and the mean age was 69 ± 15 years old (Table 1).
Pulmonary, digestive and urinary infections were suspected in the prehospital setting for 43%, 25% and 17% patients, respectively (Table 2).
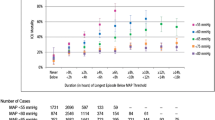
No significant difference in the prehospital stage duration, prehospital fluid expansion and antibiotic therapy was observed between patients who survived and those who died (Table 1).
Among the 132 patients (259%) who received antibiotic therapy prior to hospital admission, 74%were given 3rd generation cephalosporin among which 39% was with cefotaxime and 60% with ceftriaxone.
The median intensive care unit length of stay was 4 [2–8] days and the median length of stay in a hospital was 10 [5–18] days (Table 1).
The 30-day overall mortality reached 31%.
Bivariate analysis
Initial pulse pressure (iPP)
A significant association between 30-day mortality and the following variables: cancer, prehospital initial SBP, SDP, mean arterial pressure, RR, norepinephrine, antibiotic therapy administration, prehospital final mean arterial pressure and RR for patients with a PPi < 40mmHg (Table 3).
Delta pulse pressure (dPP)
A significant association between 30-day mortality and the following variables: prehospital initial SBP, SDP, mean arterial pressure, RR, SpO2, norepinephrine, antibiotic therapy administration, prehospital duration, prehospital final SBP and mean arterial pressure and GCS for patients with a positive dPP (Table 4).
Survival analysis
Initial pulse pressure < 40mmHg
The matched population consists of 88 controls, i.e., iPP ≥ 40mmHg and 197 cases, i.e., iPP < 40mmHg. The absolute mean differences between cases and controls after propensity score matching is depicted in Fig. 1.
Positive delta pulse pressure
The matched population consists of 77 controls, i.e., negative delta pulse pressure and 228 cases, i.e., positive pulse pressure. The absolute mean differences between cases and controls after propensity score matching is depicted in Fig. 2.
Cox regression analysis after matching showed an association between 30-day mortality and iPP < 40mmHg: aHR = 1.61 [1.03–2.51], log rank test p = 0.04. Kaplan Meier curves depict differences on 30-day survival in both subgroups after adjustment of confounders (Fig. 3).
Cox regression analysis after matching showed an association between 30-day mortality and a positive dPP: aHR = 0.56 [0.36–0.88], log rang test p = 0.01. Kaplan Meier curves depict differences on 30-day survival in both subgroups after adjustment of confounders (Fig. 4).
Discussion
An iPP < 40mmHg and a negative dPP are associated with 30-day mortality increase in patients suffering from septic shock cared for by a prehospital MICU. A negative prehospital dPP could be helpful for MICU physicians’ daily practice to identify septic shock patients with higher risk of poor outcome despite prehospital hemodynamic optimization.
The associated sepsis systemic response inflammatory syndrome results in both absolute and relative hypovolemia. Macro circulatory alterations, e.g. low blood pressure and/or cardiac output decrease, and microcirculatory alterations, e.g. hyperlactatemia or skin mottling, parameters [41] are associated with sepsis poorer outcome [42, 43]. To restore the tissues and organs’ perfusion, by restoring a sufficient cardiac output and mean arterial pressure, the international sepsis guidelines recommend early fluid expansion and norepinephrine infusion, when mean arterial pressure remains lower than 65 mmHg [5, 12, 23]. Because the negative association between the fluid resuscitation volumes, in other words the net fluid balance [15,16,17,18,19,20], and sepsis mortality is established, the optimal treatment aims to find the right equilibrium between fluid volume requirement and fluid volume overload [16,17,18,19,20, 44]. Since the prehospital and in hospital norepinephrine infusion in combination with, but not without [45], fluid resuscitation is feasible without increasing adverse effects [46]; in 2019, the Surviving Sepsis Campaign advocates the use of vasopressors even during the fluid resuscitation to reach and maintain a mean arterial pressure ≥ 65mmHg within the first hour after sepsis recognition [12]. The beneficial effects of norepinephrine are partly mediated by the cardiac output increase, mediated by the norepinephrine beta-2 agonist effect, and/or by the vascular tone increase mediated by the norepinephrine alpha-1 agonist effect [47].
Previous studies reported an association between septic shock outcome and clinical signs, biomarkers and severity scores [38, 42, 43, 48,49,50]. However, in the prehospital setting, only clinical signs, few biomarkers [51] and qSOFA, whose validity remains under debate [52,53,54,55,56,57,58], are currently available. For severity assessment, to date, lactatemia remains the best biomarker [59, 60], available in the prehospital setting [61], also allowing a dynamic approach based on lactatemia clearance for treatment effect assessment [5, 34]. To bypass biomarkers’ and qSOFA limits, capillary refill time, skin mottling score and shock index usefulness were described for septic shock severity assessment [35, 62,63,64]. iPP and dPP are in line with other clinical signs reflecting the severity of septic shock and the treatment effect of prehospital care. To the best of our knowledge, this study is the first to describe the relationship between iPP, dPP and 30-day mortality of septic shock patients cared for by a prehospital MICU.
Study limitations
Our study suffers from several limitations. From a methodological point of view, the bias from misclassification of covariates cannot be excluded as data were collected from prehospital and in-hospital medical reports. Moreover, because data abstraction was collected by a single investigator, the data accuracy can be compromised [65]. The statistical analysis performed does not allow any causal conclusion between iPP < 40mmHg, positive PP and 30-day mortality. In this study, we only included adults, consequently our conclusions are not directly transposable to a pediatric population. This is a retrospective study; because no therapeutic goal was a priori defined, we cannot define which mean arterial pressure was targeted nor when was prescribed norepinephrine administration before or after fluid expansion failure. We cannot exclude that the specificity of the French prehospital emergency medical service could affect the results’ external validity.
However, this study results suggest that iPP reflects septic shock severity and in a similar manner to lactate clearance or shock index variation. dPP could be used for treatment effect assessment and could be helpful to MICU physicians’, in their daily practice, to early optimize septic shock patients’ cardiac output and tissue perfusion.
Conclusion
An iPP < 40mmHg and a positive dPP are associated with 30-day mortality in patients with septic shock cared for by prehospital MICU. Despite prehospital hemodynamic optimization, a negative prehospital dPP may identify patients with higher risk of poorer outcome. Further studies are needed to evaluate if prehospital iPP < 40mmHg and positive dPP alone or combined with clinical scores and/or biomarkers could affect the prehospital triage decision-making process.
Data Availability
The dataset analyzed during the current study are not publicly available because their containing information that could compromise the privacy of research participants but are available from the corresponding author on reasonable request.
Abbreviations
- MICU:
-
Mobile intensive care unit
- aHR:
-
Adjusted hazard ratio
- SAMU:
-
Urgent Medical Aid Service
- SMUR:
-
Mobile Emergency and Resuscitation Service
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- HR:
-
Heart rate
- SpO2:
-
Pulse oximetry
- RR:
-
Respiratory rate
- GCS:
-
Glasgow coma scale
- LOS:
-
Length of stay
- SOFA:
-
Sequential Organ Failure Assessment
- qSOFA:
-
Quick Sequential Organ Failure Assessment
- PP:
-
Pulse pressure
- CCF:
-
Chronic cardiac failure
- CHD:
-
Coronary heart disease
- CRF:
-
Chronic renal failure
- COPD:
-
Chronic obstructive pulmonary disease
- BMI:
-
Body mass index
References
Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–72.
Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–74.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of Disease Study. Lancet. 2020;395(10219):200–11.
Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–2.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10.
Busani S, Damiani E, Cavazzuti I, Donati A, Girardis M. Intravenous immunoglobulin in septic shock: review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. 2016;82(5):559–72.
Luhr R, Cao Y, Soderquist B, Cajander S. Trends in sepsis mortality over time in randomised sepsis trials: a systematic literature review and meta-analysis of mortality in the control arm, 2002–2016. Crit Care. 2019;23(1):241.
Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, et al. Epidemiology and outcome of sepsis syndromes in italian ICUs: a muticentre, observational cohort study in the region of Piedmont. Minerva Anestesiol. 2013;79(9):993–1002.
Levy MM, Evans LE, Rhodes A. The surviving Sepsis Campaign Bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000.
De Backer D, Orbegozo Cortes D, Donadello K, Vincent JL. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5(1):73–9.
Scheeren TWL, Bakker J, De Backer D, Annane D, Asfar P, Boerma EC, et al. Current use of vasopressors in septic shock. Ann Intensive Care. 2019;9(1):20.
Chen AX, Simpson SQ, Pallin DJ. Sepsis guidelines. N Engl J Med. 2019;380(14):1369–71.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Levy MM, Evans LE, Rhodes A. The surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44(6):925–8.
Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43(1):68–73.
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–65.
Marik PE, Malbrain M. The SEP-1 quality mandate may be harmful: how to drown a patient with 30 mL per kg fluid! Anaesthesiol Intensive Ther. 2017;49(5):323–8.
Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, et al. Higher fluid balance increases the risk of death from Sepsis: results from a large International Audit. Crit Care Med. 2017;45(3):386–94.
Sirvent JM, Ferri C, Baro A, Murcia C, Lorencio C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med. 2015;33(2):186–9.
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–70.
Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
Brun-Buisson C, Meshaka P, Pinton P, Vallet B, Group ES. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in french intensive care units. Intensive Care Med. 2004;30(4):580–8.
Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49(11):e1063–e143.
Li Y, Li H, Zhang D. Timing of norepinephrine initiation in patients with septic shock: a systematic review and meta-analysis. Crit Care. 2020;24(1):488.
Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettila V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31(8):1066–71.
Dunser MW, Takala J, Ulmer H, Mayr VD, Luckner G, Jochberger S, et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35(7):1225–33.
Maheshwari K, Nathanson BH, Munson SH, Khangulov V, Stevens M, Badani H, et al. The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018;44(6):857–67.
Jouffroy R, Gilbert B, Gueye PN, Tourtier JP, Bloch-Laine E, Ecollan P, et al. Prehospital hemodynamic optimisation is associated with a 30-day mortality decrease in patients with septic shock. Am J Emerg Med. 2021;45:105–11.
Bakker J, Coffernils M, Leon M, Gris P, Vincent JL. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest. 1991;99(4):956–62.
Jones AE. Lactate clearance for assessing response to resuscitation in severe sepsis. Acad Emerg Med. 2013;20(8):844–7.
Bao L, Zhang M, Yan P, Wu X, Shao J, Zheng R. [Retrospective analysis of the value of arterial blood lactate level and its clearance rate on the prognosis of septic shock patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27(1):38–42.
Nguyen HB, Kuan WS, Batech M, Shrikhande P, Mahadevan M, Li CH, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care. 2011;15(5):R229.
Tian HH, Han SS, Lv CJ, Wang T, Li Z, Hao D, et al. [The effect of early goal lactate clearance rate on the outcome of septic shock patients with severe pneumonia]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012;24(1):42–5.
Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–8.
Jouffroy R, Gilbert B, Thomas L, Bloch-Laine E, Ecollan P, Boularan J, et al. Association between prehospital shock index variation and 28-day mortality among patients with septic shock. BMC Emerg Med. 2022;22(1):87.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228.
Salvatore F. The shift of the paradigm between ageing and diseases. Clin Chem Lab Med. 2020.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Austin PC. An introduction to Propensity score methods for reducing the Effects of confounding in Observational Studies. Multivar Behav Res. 2011;46(3):399–424.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–51.
Ait-Oufella H, Bige N, Boelle PY, Pichereau C, Alves M, Bertinchamp R, et al. Capillary refill time exploration during septic shock. Intensive Care Med. 2014;40(7):958–64.
Charlton M, Sims M, Coats T, Thompson JP. The microcirculation and its measurement in sepsis. J Intensive Care Soc. 2017;18(3):221–7.
Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625–32.
Sennoun N, Montemont C, Gibot S, Lacolley P, Levy B. Comparative effects of early versus delayed use of norepinephrine in resuscitated endotoxic shock. Crit Care Med. 2007;35(7):1736–40.
Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early Use of Norepinephrine in septic shock resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med. 2019;199(9):1097–105.
Hamzaoui O, Shi R. Early norepinephrine use in septic shock. J Thorac Dis. 2020;12(Suppl 1):72–S7.
Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37(5):801–7.
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825–31.
Le Gall JR, Lemeshow S, Saulnier F. A new simplified Acute Physiology score (SAPS II) based on a European/North american multicenter study. JAMA. 1993;270(24):2957–63.
Jouffroy R, Saade A, Tourtier JP, Gueye P, Bloch-Laine E, Ecollan P, et al. Skin mottling score and capillary refill time to assess mortality of septic shock since pre-hospital setting. Am J Emerg Med. 2019;37(4):664–71.
Askim A, Moser F, Gustad LT, Stene H, Gundersen M, Asvold BO, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality - a prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med. 2017;25(1):56.
Finkelsztein EJ, Jones DS, Ma KC, Pabon MA, Delgado T, Nakahira K, et al. Comparison of qSOFA and SIRS for predicting adverse outcomes of patients with suspicion of sepsis outside the intensive care unit. Crit Care. 2017;21(1):73.
Dorsett M, Kroll M, Smith CS, Asaro P, Liang SY, Moy HP. qSOFA has poor sensitivity for Prehospital identification of severe Sepsis and septic shock. Prehosp Emerg Care. 2017;21(4):489–97.
Harada M, Takahashi T, Haga Y, Nishikawa T. Comparative study on quick sequential organ failure assessment, systemic inflammatory response syndrome and the shock index in prehospital emergency patients: single-site retrospective study. Acute Med Surg. 2019;6(2):131–7.
Koyama S, Yamaguchi Y, Gibo K, Nakayama I, Ueda S. Use of prehospital qSOFA in predicting in-hospital mortality in patients with suspected infection: a retrospective cohort study. PLoS ONE. 2019;14(5):e0216560.
Lane DJ, Lin S, Scales DC. Classification versus prediction of Mortality Risk using the SIRS and qSOFA Scores in patients with infection transported by paramedics. Prehosp Emerg Care. 2019:1–8.
Silcock DJ, Corfield AR, Staines H, Rooney KD. Superior performance of National Early warning score compared with quick Sepsis-related Organ failure Assessment score in predicting adverse outcomes: a retrospective observational study of patients in the prehospital setting. Eur J Emerg Med. 2019;26(6):433–9.
Kushimoto S, Akaishi S, Sato T, Nomura R, Fujita M, Kudo D, et al. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med Surg. 2016;3(4):293–7.
April MD, Donaldson C, Tannenbaum LI, Moore T, Aguirre J, Pingree A, et al. Emergency department septic shock patient mortality with refractory hypotension vs hyperlactatemia: a retrospective cohort study. Am J Emerg Med. 2017;35(10):1474–9.
Leguillier T, Jouffroy R, Boisson M, Boussaroque A, Chenevier-Gobeaux C, Chaabouni T, et al. Lactate POCT in mobile intensive care units for septic patients? A comparison of capillary blood method versus venous blood and plasma-based reference methods. Clin Biochem. 2018;55:9–14.
Tseng J, Nugent K. Utility of the shock index in patients with sepsis. Am J Med Sci. 2015;349(6):531–5.
Jouffroy R, Pierre Tourtier J, Gueye P, Bloch-Laine E, Bounes V, Debaty G et al. Prehospital shock index to assess 28-day mortality for septic shock. Am J Emerg Med. 2019.
Guirgis FW, Jones L, Esma R, Weiss A, McCurdy K, Ferreira J, et al. Managing sepsis: electronic recognition, rapid response teams, and standardized care save lives. J Crit Care. 2017;40:296–302.
Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005;45(4):448–51.
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: RJ, PG. Methodology: RJ. Data curation: RJ, BG, JPT, EBL, PE, VB, JB, PG, BV. Writing-Original draft preparation: RJ, BG, PG. Investigation: RJ & PG. Supervision: RJ, PG. Validation: RJ & PG. Writing-Reviewing and Editing: RJ, BG, JPT, EBL, PE, VB, JB, PG, BV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the French Society of Anaesthesia and Intensive Care ethics committee on December 12th, 2017 (Ref number: IRB 00010254-2017-026). The French Society of Anaesthesia and Intensive Care ethics committee waived the patient consent for participation in this retrospective study. All experiments were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
None author has any competing interests. All methods were performed in accordingly to the relevant guidelines and regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jouffroy, R., Gilbert, B., Tourtier, J.P. et al. Prehospital pulse pressure and mortality of septic shock patients cared for by a mobile intensive care unit. BMC Emerg Med 23, 97 (2023). https://doi.org/10.1186/s12873-023-00864-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12873-023-00864-0