Abstract
Background
Arteriosclerosis in multiple arteries has long been associated with heightened cardiovascular risk. Acetaldehyde dehydrogenase 2 (ALDH2) and methylenetetrahydrofolate reductase (MTHFR) play an important role in the pathogenesis of arteriosclerosis by participating in the oxidation and reduction reactions in vascular endothelial cells. The purpose was to investigate the relationship of ALDH2 and MTHFR gene polymorphisms with arteriosclerosis in multiple arteries.
Methods
410 patients with arteriosclerosis in single artery and 472 patients with arteriosclerosis in multiple arteries were included. The relationship between ALDH2 rs671 and MTHFR rs1801133 polymorphisms and arteriosclerosis in single artery and arteriosclerosis in multiple arteries was analyzed.
Results
The proportion of ALDH2 rs671 A allele (35.6% vs. 30.9%, P = 0.038) and MTHFR rs1801133 T allele (32.6% vs. 27.1%, P = 0.012) in patients with arteriosclerosis in multiple arteries was significantly higher than that in arteriosclerosis in single artery, respectively. The proportion of history of alcohol consumption in patients with ALDH2 rs671 G/G genotype was higher than those in ALDH2 rs671 G/A genotype and A/A genotype (P < 0.001). The results of logistic regression analysis indicated that ALDH2 rs671 A/A genotype (A/A vs. G/G: OR 1.996, 95% CI: 1.258–3.166, P = 0.003) and MTHFR rs1801133 T/T genotype (T/T vs. C/C: OR 1.943, 95% CI: 1.179–3.203, P = 0.009) may be independent risk factors for arteriosclerosis in multiple arteries (adjusted for age, sex, smoking, drinking, hypertension, and diabetes).
Conclusions
ALDH2 rs671 A/A and MTHFR rs1801133 T/T genotypes may be independent risk factors for arteriosclerosis in multiple arteries.
Similar content being viewed by others
Introduction
Arteriosclerosis refers to the accumulation of fatty and/or fibrous material in the intima of arteries, and it remains a major killer, and has now spread globally [1, 2]. Arteriosclerosis refers to the aging and structural changes of the arterial system. In terms of pathology, arteriosclerosis is mainly manifested by elastin fatigue fracture, collagen deposition and crosslinking, and cholesterol deposition in the artery wall as the main pathological basis, leading to fibrous tissue hyperplasia and calcinosis of the affected artery, and gradually metamorphosis and calcification, resulting in thickening and hardening of the artery wall and narrowing of the vascular cavity [3,4,5]. Arteriosclerosis is an intermediate lesion connecting cardiovascular risk factors and cardiovascular events, and is the risk factor for cardiovascular and cerebrovascular diseases [6, 7]. Prevention of arteriosclerosis can delay the occurrence of cardiovascular and cerebrovascular events [8]. As a systemic disease, arteriosclerosis in multiple arteries is defined as arteriosclerosis within 2 or more arterial beds, it has long been associated with heightened cardiovascular risk [9, 10]. Arteriosclerosis often involves multiple arterial beds simultaneously and causes different clinical symptoms, and different sites of the arteries may have different degrees of arterial disease in the same patient, suggesting that risk factors for arteriosclerosis may have different degrees of effect on different sites of the arteries [11, 12].
Studies have found that the occurrence of arteriosclerosis is related to some risk factors, such as older age, male gender, hypertension, diabetes, hyperlipidemia, smoking, and drinking [13, 14], and may also be related to some genetic factors [15]. The occurrence of arteriosclerosis is associated with oxidative stress [16]. Oxidative stress at the cellular level may produce toxic aldehydes, and excess aldehydes are extremely toxic to cells [17]. Acetaldehyde dehydrogenase (ALDH) family participate in the oxidation and metabolism of aldehydes in the body [18], and ALDH2 is the most widely studied in ALDH family [19]. ALDH2 is a mitochondrial protein containing 517 amino acids, encoded by the ALDH2 gene [20], and the most common polymorphism in ALDH2 gene is Glu504Lys polymorphism (single nucleotide polymorphism (SNP) rs671, G1510A) [21]. At the SNP rs671, guanine (G) is replaced by adenine (A), resulting in the encoded amino acid from glutamic acid (Glu) to lysine (Lys). The mutation of this site changes the structure of ALDH2, and the binding of coenzyme NAD (P) + with the mutant ALDH2 is impaired, and the activity of ALDH2 is reduced due to the weakened dehydrogenation [22]. ALDH2 Glu504Lys mutation leads to the decrease of ALDH2 activity and the accumulation of aldehydes, which is associated with the occurrence of various diseases [23].
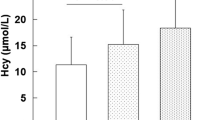
Homocysteine (Hcy) promotes programmed death of coronary endothelial cells and accelerates the occurrence of arteriosclerosis [24]. 5-10-Methylenetetrahydrofolate reductase (MTHFR) catalyses the irreversible reduction of 5-10-MTHF to 5-methylTHF, a circulatory form of folate used in the remethylation of Hcy to methionine [25]. The decrease of MTHFR activity affects the metabolic process of Hcy and leads to the increase of Hcy level that damages vascular endothelium [26]. In addition, nitric oxide (NO) can enhance vasodilatation and reducing platelet aggression and adhesion in vascular endothelium, which plays an important role in homeostasis around vascular endothelium [27, 28]. Levels of Hcy and MTHFR, play a determining role in circulating levels of NO [27]. MTHFR C677T (rs1801133) is the most common polymorphism of MTHFR gene, and the mutant allele was associated with high level of Hcy [29]. MTHFR C677T gene is mutated from cytosine (C) to thymine (T) at base 677, thereby changing codon 222 from alanine (Ala) to valine (Val). This region is the base binding site of flavin adenine dinucleotide (FAD), thus altering enzyme activity [30].
To sum up, ALDH2 and MTHFR play an important role in the pathogenesis of arteriosclerosis by participating in the oxidation and reduction reactions of vascular endothelial cells. Study has found that the mutated ALDH2 carriers were more susceptible to multi-coronary artery lesions in Chinese patients with CAD [31]. The MTHFR rs1801133 polymorphism was risk factor for carotid artery arteriosclerosis [32]. But the result of the relationship of ALDH2 and MTHFR gene polymorphisms and arteriosclerosis in multiple arteries is still unclear. The different regions, populations, lifestyles and gene polymorphisms will affect the development of arteriosclerosis [33,34,35]. In the current study, we evaluated the association between ALDH2 rs671, MTHFR rs1801133 polymorphisms and arteriosclerosis in single artery and arteriosclerosis in multiple arteries. This study will provide valuable information for guiding screening of patients at risk for multiple sites of arteriosclerosis.
Materials and methods
Study Population
A total of 882 unrelated individuals were included in this retrospective study, including 410 patients with arteriosclerosis in single artery and 472 patients with arteriosclerosis in multiple arteries, collected from Meizhou People’s Hospital, China between January 2016 and July 2019. This study was approved by the Ethics Committee of Meizhou People’s Hospital.
Arteriosclerosis is determined by tests such as angiography, magnetic resonance imaging (MRI), or computed tomography evaluated by two senior radiologists in a double-blind evaluation. In this study, arteriosclerosis was observed in coronary artery, carotid artery, cerebral artery, and limb artery. The patients’ medical records were collected from the Hospital Information System (HIS) of Meizhou People’s Hospital, including age, sex, smoking history, alcohol consumption history, hypertension, diabetes, and arteriosclerosis.
Genotyping of ALDH2 rs671 and MTHFR rs1801133
Genomic DNA was extracted from whole blood using a QIAamp DNA Blood Mini Kit (Qiagen GmbH, North Rhine-Westphalia, Germany) according to the manufacturer’s protocol. ALDH2 rs671 polymorphism and MTHFR rs1801133 polymorphism was genotyped by ALDH2 and MTHFR genotyping kit (BaiO Technology Co, Ltd, Shanghai, China), respectively. PCR was performed according to the following protocol: denaturation at 94℃ for 5 min; amplification of 35 cycles (94℃ for 25 s, 56℃ for 25 s, and 72℃ for 25 s); final elongation at 72℃ for 5 min. The PCR amplification product was hybridized with the probe fixed on the chip, and the specific hybridization signal was chromogenic by enzyme chromogenic reaction.
Statistical analysis
Data analysis was performed using SPSS statistical software version 21.0 (IBM Inc., USA). Student’s t-test or the Mann-Whitney U test was used for continuous data analysis. Genotype composition ratios and allele frequencies of groups were analyzed by the χ2 test. The χ2 test was used to test the significance of the Hardy-Weinberg equilibrium (HWE) of the ALDH2 rs671 and MTHFR rs1801133 polymorphisms in the entire data of the patients with arteriosclerosis in single artery and patients with arteriosclerosis in multiple arteries. To measure the relative risk of ALDH2 rs671 and MTHFR rs1801133 genotypes in arteriosclerosis, logistic regression analysis was performed after adjusting for sex, age, smoking history, alcohol consumption history, hypertension, and diabetes. The statistical significance level of all analysis results was defined as a P < 0.05.
Results
Patient demographics
A total of 882 patients were studied in this study, of which 308 (34.9%) were younger than 65 years old and 574 (65.1%) were ≥ 65 years old. There were 605 male patients (68.6%) and 277 female patients (31.4%). There were 410 patients with arteriosclerosis in single artery (46.5%) and 472 patients with arteriosclerosis in multiple arteries (53.5%) in this study. The differences in age distribution, sex distribution, proportions of patients with history of smoking and alcohol consumption, and proportions of patients with hypertension and diabetes between the patients with arteriosclerosis in single artery and patients with arteriosclerosis in multiple arteries were not statistically significant (all P > 0.05) (Table 1).
Frequencies of ALDH2 rs671 and MTHFR rs1801133 genotypes in patients with arteriosclerosis in single artery and arteriosclerosis in multiple arteries
The χ2 test was used to test the significance of the Hardy-Weinberg equilibrium of the ALDH2 rs671 and MTHFR rs1801133 polymorphisms in the patients with arteriosclerosis in single artery and arteriosclerosis in multiple arteries. The distributions of ALDH2 rs671 genotypes in patients with arteriosclerosis in single artery (χ2 = 0.492, P = 0.483) and patients with arteriosclerosis in multiple arteries (χ2 = 1.551, P = 0.213) were consistent with Hardy-Weinberg equilibrium, respectively. The distributions of MTHFR rs1801133 genotypes in patients with arteriosclerosis in single artery (χ2 = 0.069, P = 0.793) and patients with arteriosclerosis in multiple arteries (χ2 = 0.025, P = 0.875) were consistent with Hardy-Weinberg equilibrium, respectively. The frequencies of ALDH2 rs671 and MTHFR rs1801133 genotypes and alleles were compared between the arteriosclerosis in single artery and arteriosclerosis in multiple arteries groups. The proportion of ALDH2 rs671 A allele in patients with arteriosclerosis in multiple arteries was significantly higher than that in patients with arteriosclerosis in single artery (35.6% vs. 30.9%, P = 0.038). The proportion of MTHFR rs1801133 T allele in patients with arteriosclerosis in multiple arteries was significantly higher than that in patients with arteriosclerosis in single artery (32.6% vs. 27.1%, P = 0.012) (Table 2).
The number of the subjects with ALDH2 rs671 G/G, G/A, and A/A genotype was 395(44.8%), 385(43.7%), and 102(11.6%), respectively, with the number of the subjects with MTHFR rs1801133 C/C, C/T, and T/T genotype was 432(49.0%), 370(42.0%), and 80(9.1%), respectively. While clinical characteristics were compared among subjects stratifed by ALDH2 rs671 genotypes, the proportion of history of alcohol consumption in patients with ALDH2 rs671 G/G genotype was higher than those in patients with ALDH2 rs671 G/A genotype and A/A genotype (P < 0.001). The differences in other clinical characteristics among the different ALDH2 rs671 genotypes were not statistically significant. In addition, the differences in all clinical characteristics among the different MTHFR rs1801133 genotypes were not statistically significant (Table 3).
Association of the risk factors with arteriosclerosis in multiple arteries
To gain insight into the independent risk factors on arteriosclerosis in multiple arteries, logistic regression analysis was performed. The possible association of the ALDH2 genotypes with potential risk factors for arteriosclerosis in multiple arteries based on three genetic modes of inheritance: co-dominant model, dominant model, and recessive model. The results of univariate logistic regression showed that ALDH2 rs671 A/A genotype (A/A vs. G/G: odds ratio (OR) 1.752, 95% confidence interval (CI): 1.115–2.751, P = 0.015) may increase risk of arteriosclerosis in multiple arteries, and MTHFR rs1801133 T/T genotype (T/T vs. C/C: OR 1.775, 95% CI: 1.084–2.907, P = 0.023) may increase risk of arteriosclerosis in multiple arteries.
The results of multivariate logistic regression (adjusted for age, sex, smoking history, drinking history, hypertension, and diabetes) indicated that ALDH2 A/A genotype in the co-dominant model (ALDH2 A/A vs. ALDH2 G/G) (adjusted OR 1.996, 95% CI 1.258–3.166, P = 0.003) and ALDH2 A/A genotype in the recessive model (ALDH2 A/A vs. ALDH2 G/G + G/A) (adjusted OR 1.802, 95% CI 1.168–2.781, P = 0.008) were significant risk factors for the presence of arteriosclerosis in multiple arteries. And the MTHFR T/T genotype in the co-dominant model (MTHFR T/T vs. MTHFR C/C) (adjusted OR 1.943, 95% CI 1.179–3.203, P = 0.009), MTHFR C/T and T/T genotypes in the dominant model (MTHFR C/T plus MTHFR T/T vs. MTHFR C/C) (adjusted OR 1.387, 95% CI 1.061–1.813, P = 0.017), and MTHFR T/T genotype in the recessive model (MTHFR T/T vs. MTHFR C/C plus MTHFR C/T) (adjusted OR 1.685, 95% CI 1.042–2.723, P = 0.033) were significant risk factors for the presence of arteriosclerosis in multiple arteries (Table 4).
Discussion
The result of the relationship of ALDH2 and MTHFR gene polymorphisms and arteriosclerosis in multiple arteries is still unclear. In the current study, we evaluated the association between ALDH2 rs671, MTHFR rs1801133 polymorphisms and arteriosclerosis in single artery and arteriosclerosis in multiple arteries. The results of this study show that ALDH2 rs671 A/A and MTHFR rs1801133 T/T genotypes may be independent risk factors for arteriosclerosis in multiple arteries.
ALDH2 rs671 A/A genotype may be an independent risk factor for arteriosclerosis in multiple arteries. Study has shown that individuals with ALDH2 rs671 G/A or A/A genotype have a higher coronary artery disease (CAD) risk than individuals with G/G genotype [36]. Another study found that the mutated ALDH2 carriers were more susceptible to multi-vessel lesions [31]. In the perspective of mechanism, ALDH2 plays an important role in the development and progression of arteriosclerosis by inhibiting oxidative low-density lipoprotein (ox-LDL)-induced foam cell formation via suppressing CD36 (cluster of differentiation 36) expression [37]. It has found that the appropriate dose of ethanol reduced arterial plaque formation in ApoE-/- mice, while ALDH2 deficiency blocked the protection of ethanol against arterial plaque formation [38]. Another animal study has shown that ALDH2 inhibits transcription of a lysosomal proton pump protein ATP6V0E2 (ATPase H(+) Transporting V0 Subunit E2) to degrade ox-LDL, while ALDH2 encoded by ALDH2 rs671 polymorphism weakens this effect [39].
MTHFR rs1801133 T/T genotype may be an independent risk factor for arteriosclerosis in multiple arteries. Studies have found that MTHFR rs1801133 polymorphism was significantly associated with peripheral arterial disease (PAD) [40, 41]. The MTHFR rs1801133 polymorphism was risk factor for carotid artery arteriosclerosis [32]. The MTHFR rs1801133 polymorphism was associated with increased risk of CAD in different populations [42,43,44,45,46]. In mechanism, vascular 5-methyl-tetrahydrofolate (5-MTHF) is a key regulator of human vascular endothelial nitric oxide synthase coupling and nitric oxide bioavailability, and MTHFR rs1801133 polymorphism plays an important role in the regulation of vascular redox status by affecting the expression of vascular 5-MTHF [47].
To our knowledge, this study is the first report of the relationship of ALDH2 rs671 and MTHFR rs1801133 genotypes and arteriosclerosis in multiple arteries. Our study found that ALDH2 rs671 A/A and MTHFR rs1801133 T/T genotypes may be independent risk factors for arteriosclerosis in multiple arteries. It is of great significance for the screening and prevention of high risk individuals with arteriosclerosis in multiple arteries. Early screening for mutations in the ALDH2 and MTHFR genes should be done in people who have already found single atherosclerosis.
There are some limitations in present study. First, no information was collected about the degree or grade of arteriosclerosis of the subjects, which limited the analysis of the relationship between ALDH2 rs671 and MTHFR rs1801133 and the degree or grade of arteriosclerosis. Second, this study only analyzed the relationship between the common polymorphisms of ALDH2 and MTHFR genes and the risk of arteriosclerosis in multiple arteries. Third, deficiencies in folic acid can increase homocysteine levels, induce endothelial dysfunction, and accelerate pathological process of arteriosclerosis [48] and MTHFR is an enzyme involved in the metabolism of folic acid [49]. This study did not examine the role of MTHFR polymorphism in arteriosclerosis formation in people with low folic acid levels. So, future studies that include larger sample sizes, the degree of arteriosclerosis, the analysis of the full-length variation of ALDH2 and MTHFR genes, and analysis of folic acid levels are needed.
Conclusion
In summary, the relationship between ALDH2 rs671 and MTHFR rs1801133 polymorphisms and arteriosclerosis in multiple arteries was identified in a cohort study. After adjusting age, sex, history of smoking and drinking, hypertension, and diabetes, ALDH2 rs671 A/A and MTHFR rs1801133 T/T genotypes may be independent risk factors for arteriosclerosis in multiple arteries. Of course, further research is needed to verify our results and investigate the mechanism of the reported association.
Data Availability
The datasets used and analyzed during the current study available from the corresponding author on request.
References
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis Nat Rev Dis Primers. 2019;5(1):56.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118(4):535–46.
Willeit J, Kiechl S. Biology of arterial atheroma. Cerebrovasc Dis. 2000;10(Suppl 5):1–8.
Reimann C, Brangsch J, Colletini F, Walter T, Hamm B, Botnar RM, et al. Molecular imaging of the extracellular matrix in the context of atherosclerosis. Adv Drug Deliv Rev. 2017;113:49–60.
Gialeli C, Shami A, Gonçalves I. Extracellular matrix: paving the way to the newest trends in atherosclerosis. Curr Opin Lipidol. 2021;32(5):277–85.
Jing L, Shu-Xu D, Yong-Xin R. A review: pathological and molecular biological study on atherosclerosis. Clin Chim Acta. 2022;531:217–22.
Gutierrez J, Turan TN, Hoh BL, Chimowitz MI. Intracranial atherosclerotic stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2022;21(4):355–68.
Chen Q, Lv J, Yang W, Xu B, Wang Z, Yu Z, et al. Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis. Theranostics. 2019;9(22):6424–42.
Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128(12):1818–32.
Gutierrez JA, Aday AW, Patel MR, Jones WS. Polyvascular disease: reappraisal of the current clinical landscape. Circ Cardiovasc Interv. 2019;12(12):e007385.
Dabagh M, Vasava P, Jalali P. Effects of severity and location of stenosis on the hemodynamics in human aorta and its branches. Med Biol Eng Comput. 2015;53(5):463–76.
Chen X, Chu Y, Hou X, Han Y, Zhang C, Zhang Y, et al. Application of model-building based on arterial ultrasound imaging evaluation to predict CHD risk. Comput Math Methods Med. 2022;2022:4615802.
Zhou F, Tang J, Li P, Liao B, Qin C. Distribution of cerebral artery stenosis and risk factors in ethnic Zhuang and Han patients with ischemic stroke in Guangxi province. Ann Palliat Med. 2020;9(2):256–63.
Chi X, Li M, Zhan X, Man H, Xu S, Zheng D, et al. Relationship between carotid artery sclerosis and blood pressure variability in essential hypertension patients. Comput Biol Med. 2018;92:73–7.
Abraham G, Rutten-Jacobs L, Inouye M. Risk prediction using polygenic risk scores for prevention of stroke and other cardiovascular diseases. Stroke. 2021;52(9):2983–91.
Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019; 11(9):2090.
Rodríguez-Zavala JS, Calleja LF, Moreno-Sánchez R, Yoval-Sánchez B. Role of aldehyde dehydrogenases in physiopathological processes. Chem Res Toxicol. 2019;32(3):405–20.
Bazewicz CG, Dinavahi SS, Schell TD, Robertson GP. Aldehyde dehydrogenase in regulatory T-cell development, immunity and cancer. Immunology. 2019;156(1):47–55.
Chen CH, Kraemer BR. Annotation of 1350 common genetic variants of the 19 ALDH multigene family from global human genome aggregation database (gnomAD). Biomolecules. 2021;11(10):1423.
Raghunathan L, Hsu LC, Klisak I, Sparkes RS, Yoshida A, Mohandas T. Regional localization of the human genes for aldehyde dehydrogenase-1 and aldehyde dehydrogenase-2. Genomics. 1988;2(3):267–9.
Zeng D, Huang Q, Yu Z, Wu H. Association between aldehyde dehydrogenase 2 gene rs671 G > A polymorphism and alcoholic liver cirrhosis in southern chinese Hakka population. J Clin Lab Anal. 2021;35(7):e23855.
Zhao Y, Wang C. Glu504Lys single nucleotide polymorphism of aldehyde dehydrogenase 2 gene and the risk of human diseases. Biomed Res Int. 2015;2015:174050.
Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94(1):1–34.
Liang C, Wang QS, Yang X, Zhu D, Sun Y, Niu N, et al. Homocysteine causes endothelial dysfunction via inflammatory factor-mediated activation of epithelial sodium channel (ENaC). Front Cell Dev Biol. 2021;9:672335.
Raghubeer S, Matsha TE. Methylenetetrahydrofolate (MTHFR), the one-carbon cycle, and cardiovascular risks. Nutrients. 2021;13(12):4562.
Shivkar RR, Gawade GC, Padwal MK, Diwan AG, Mahajan SA, Kadam CY. Association of MTHFR C677T (rs1801133) and A1298C (rs1801131) polymorphisms with serum homocysteine, folate and vitamin B12 in patients with young coronary artery disease. Indian J Clin Biochem. 2022;37(2):224–31.
Yuyun MF, Ng LL, Ng GA. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc Res. 2018;119:7–12.
Gajecki D, Gawryś J, Szahidewicz-Krupska E, Doroszko A. Role of erythrocytes in nitric oxide metabolism and paracrine regulation of endothelial function. Antioxid (Basel). 2022;11(5):943.
Xuan C, Li H, Zhao JX, Wang HW, Wang Y, Ning CP, et al. Association between MTHFR polymorphisms and congenital heart disease: a meta-analysis based on 9,329 cases and 15,076 controls. Sci Rep. 2014;4:7311.
Trimmer EE. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Curr Pharm Des. 2013;19(14):2574–93.
Xu L, Zhao G, Wang J, Shen C, Li X, Lu F, et al. Impact of genetic variation in aldehyde dehydrogenase 2 and alcohol consumption on coronary artery lesions in chinese patients with stable coronary artery disease. Int Heart J. 2018;59(4):689–94.
Li A, Huang W, Yang Q, Peng L, Liu Q. Expression of the C677T polymorphism of the 5, 10-Methylenetetrahydrofolate reductase (MTHFR) gene in patients with carotid artery atherosclerosis. Med Sci Monit. 2020;26:e920320.
Kubota M, Yoneda M, Watanabe H, Egusa G. Progression of carotid atherosclerosis in two japanese populations with different lifestyles. J Atheroscler Thromb. 2017;24(10):1069–74.
Elfaki I, Mir R, Almutairi FM, Duhier FMA. Cytochrome P450: polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac J Cancer Prev. 2018;19(8):2057–70.
Liu Y, Wang X, Zhang Q, Meng G, Liu L, Wu H, et al. Relationship between dietary patterns and carotid atherosclerosis among people aged 50 years or older: a population-based study in China. Front Nutr. 2021;8:723726.
Huang L, Cai X, Lian F, Zhang L, Kong Y, Cao C, et al. Interactions between ALDH2 rs671 polymorphism and lifestyle behaviors on coronary artery disease risk in a chinese Han population with dyslipidemia: a guide to targeted heart health management. Environ Health Prev Med. 2018;23(1):29.
Wei S, Zhang L, Bailu W, Zhao Y, Dong Q, Pan C, et al. ALDH2 deficiency inhibits Ox-LDL induced foam cell formation via suppressing CD36 expression. Biochem Biophys Res Commun. 2019;512(1):41–8.
Xue L, Zhu W, Yang F, Dai S, Han Z, Xu F, et al. Appropriate dose of ethanol exerts anti-senescence and anti-atherosclerosis protective effects by activating ALDH2. Biochem Biophys Res Commun. 2019;512(2):319–25.
Zhong S, Li L, Zhang YL, Zhang L, Lu J, Guo S, et al. Acetaldehyde dehydrogenase 2 interactions with LDLR and AMPK regulate foam cell formation. J Clin Invest. 2019;129(1):252–67.
Liu F, Du J, Nie M, Fu J, Sun J. 5,10-methylenetetrahydrofolate reductase C677T gene polymorphism and peripheral arterial disease: a meta-analysis. Vascular. 2021;29(6):913–9.
Khandanpour N, Willis G, Meyer FJ, Armon MP, Loke YK, Wright AJ, et al. Peripheral arterial disease and methylenetetrahydrofolate reductase (MTHFR) C677T mutations: a case-control study and meta-analysis. J Vasc Surg. 2009;49(3):711–8.
Luo Z, Lu Z, Muhammad I, Chen Y, Chen Q, Zhang J, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17(1):191.
Bouzidi N, Hassine M, Fodha H, Ben Messaoud M, Maatouk F, Gamra H, et al. Association of the methylene-tetrahydrofolate reductase gene rs1801133 C677T variant with serum homocysteine levels, and the severity of coronary artery disease. Sci Rep. 2020;10(1):10064.
Sarikaya S, Aydin E, Ozen Y, Ozer T, Kirali K, Rabus MB. The role of genetics in coronary artery bypass surgery patients under 30 years of age. Cardiovasc J Afr. 2017;28(2):77–80.
Ramkaran P, Phulukdaree A, Khan S, Moodley D, Chuturgoon AA. Methylenetetrahydrofolate reductase C677T polymorphism is associated with increased risk of coronary artery disease in young south african Indians. Gene. 2015;571(1):28–32.
Li L, Yu H, Zhang H, Wang J, Hu W. Association between MTHFR C677T polymorphism and risk of coronary artery disease in the chinese population: meta-analysis. Herz. 2022;47(6):553–63.
Antoniades C, Shirodaria C, Leeson P, Baarholm OA, Van-Assche T, Cunnington C, et al. MTHFR 677 C > T polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation. 2009;119(18):2507–15.
Hou H, Zhao H. Epigenetic factors in atherosclerosis: DNA methylation, folic acid metabolism, and intestinal microbiota. Clin Chim Acta. 2021;512:7–11.
Jadavji NM, Emmerson JT, Shanmugalingam U, MacFarlane AJ, Willmore WG, Smith PD. A genetic deficiency in folic acid metabolism impairs recovery after ischemic stroke. Exp Neurol. 2018;309:14–22.
Acknowledgements
The author would like to thank other colleagues whom were not listed in the authorship of Center for Cardiovascular Diseases, Meizhou People’s Hospital, for their helpful comments on the manuscript.
Funding
This study was supported by the Science and Technology Program of Meizhou (Grant No.: 2019B0202001).
Author information
Authors and Affiliations
Contributions
NC and CL designed the study. NC, XG, WZ, JL, GZ, JL, JZ, and HH collected clinical data. NC and CL analyzed the data. NC prepared the manuscript. All authors were responsible for critical revisions, and all authors read and approved the final version of this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants were informed on the study procedures and goals and the study obtained written informed consent from all the participants. We confirm that all methods were performed in accordance with relevant guidelines and regulations. This study was approved by the Human Ethics Committees of Meizhou People’s Hospital.
Consent for publication
Not Applicable.
Competing interests
We declare that the authors have no competing interests as defined by Journal of BMC Cardiovascular Disorders, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cai, N., Li, C., Gu, X. et al. ALDH2 rs671 and MTHFR rs1801133 polymorphisms are risk factors for arteriosclerosis in multiple arteries. BMC Cardiovasc Disord 23, 319 (2023). https://doi.org/10.1186/s12872-023-03354-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03354-0