Abstract
This study systematically reviewed the evidence regarding differences in the neutrophil to lymphocyte ratio (NLR) level between hypertensive and normotensive individuals as well as between patients with dipper and non-dipper hypertension (HTN). PubMed, Scopus, and Web of Science databases were systematically searched up to 20 December 2021. This was done without any limitation with regard to date, publication, or language. Pooled weighted mean differences (WMD) with 95% confidence intervals (95% CI) were reported. We assessed the quality of studies based on the Newcastle–Ottawa Scale (NOS). In total, 21 studies were included in our study. There was a significant increase in NLR levels for the hypertensive group in comparison to the control group (WMD = 0.40, 95%CI = 0.22–0.57, P < 0.0001). In addition, the NLR levels were higher in the non-dipper than in the dipper group (WMD = 0.58, 95%CI = 0.19–0.97, P = 0.003). Our findings showed that hypertensive patients had higher level of NLR than normotensive individuals.
Similar content being viewed by others
Introduction
Hypertension, a globally prevalent noncommunicable disease, has gained prominence in recent years as medical researchers have discovered some of the inflammatory components that underpins its etiology. In addition, several complications of hypertension, such as retinopathy, neuropathy, and cardiomyopathy, have been linked to the inflammatory response that develops in the arterial walls over time due to consistently elevated pressures. In addition, there is a large volume of published studies reporting the elevated level of inflammatory biomarkers in HTN patients. These studies have shown that inflammation in HTN occurs not only in the arterial walls, but also throughout the whole body. HTN can be divided into two groups, including the dipper and non-dipper groups. In patients with dipper HTN, systolic and diastolic blood pressure dropped by more than 10% during sleep. This diurnal pattern is thought to be a normal variant. Patients whose blood pressure does not show this diurnal pattern are referred to as "non-dippers." Non-dippers have a higher risk of cardiovascular disease and target organ damage than dippers [1, 2]. It is very important to find responsible pathophysiological conditions which may be the cause of this risk rise. Some research teams speculated that the inflammatory process plays a role in this phenomenon; so they compared the inflammatory biomarkers between these two groups.
A large and growing body of literature has investigated the role of inflammatory biomarkers and cytokines in HTN. However, in recent years, there has been increase interest in simple hematologic biomarkers such as the neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR). Blood NLR is a simple marker for chronic low-grade inflammation that can be obtained easily from a differential blood count [3]. Neutrophils and lymphocytes are key immune system cellular components. Neutrophils are a type of innate immunity cell that can produce chemokines, cytokines, vascular endothelial growth factor (VEGF), and matrix metalloproteinase to reinforce the initial line of the immune system response. Lymphocytes, which are adaptive immunity cells, are also fine tuned controllers of this particular immune response [4]. As neutrophils and lymphocytes interact with each other, their ratio and sheer numbers have an impact on the immune response amplitude [5]. Increased neutrophil numbers, in particular, decrease lymphocyte activity [6, 7]. Recently, the NLR has emerged as an indicator of systemic inflammation in a variety of disorders including cancer [8], neurologic disorders [9], and infectious diseases [10]. It has been used as an independent prognostic biomarker in various clinical settings, predicting major mortality, morbidity, and long-term survival [11,12,13,14]. In the context of cardiovascular diseases, NLR is an emerging marker in patients with heart failure [15], acute coronary syndrome [16], stable coronary artery disease [17,18,19,20], and for patients undergoing percutaneous coronary interventions [21] or coronary artery bypass grafting [22]. In addition, there is a large volume of published studies describing the role of NLR in HTN. The majority report that hypertensive patients had elevated levels of the NLR compared to normotensive individuals and more specifically that non-dippers had an elevated level of NLR compared to dippers [23,24,25,26,27,28,29,30,31,32,33,34]. However, some studies showed no differences [35,36,37,38,39,40,41,42,43]. Although extensive research has been carried out on the role of NLR in HTN, no single study exists which reviews the available evidence in order to draw a single result from contradictory findings.
This study systematically reviewed the evidence regarding the differences in the NLR level between hypertensive and normotensive individuals as well as between patients with dipper and non-dipper HTN. The goal was to develop an understanding of the pathophysiology of HTN and explain the risk rise of cardiovascular events in dippers compared to non-dippers using the NLR.
Material and method
Search strategy and study selection
We conducted this meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [44]. PubMed, Scopus, and Web of Science databases were systematically searched up to 20 December 2021 using the following keywords: ((neutrophil AND lymphocyte AND ratio) OR neutrophil-to-lymphocyte OR NLR) AND Hypertension. No date or language restrictions were considered. In addition, we scanned the reference lists of related articles manually to find potentially missing or additional eligible studies.
The inclusion criteria based on the PICOS principle were as follows.
-
(a)
Population: Patients with HTN (either primary or secondary HTN) in first analysis AND patients with non-dipper HTN in the second analysis
-
(b)
Intervention (Exposure): High NLR
-
(c)
Control: Healthy control in first analysis AND patients with dipper HTN in the second analysis
-
(d)
Outcomes: Diagnostic role of NLR
-
(e)
Studies: case–control, cross-sectional, and cohort studies
If the study did not report the level of NLR as a mean or standard deviation (SD), Wan et al.’s method was used to calculating the estimated values [45]. In this study, they discuss different approximation methods for the estimation of the sample mean and SD and proposed some new estimation methods to improve the existing literature. They conclude their work with a summary table (an Excel spread sheet including all formulas) that serves as a comprehensive guidance for performing meta-analysis for different situations. We used this same Excel sheet in our study.
We excluded the incomplete studies and abstracts, reviews, case reports, and animal studies. Two authors independently selected the articles for final inclusion according to these criteria, and if discrepancies existed, a third author resolved any disagreements.
Data extraction
The extracted data were as follows: (1) first author; (2) country of origin; (3) year of publication; (4) study design; (5) number of cases and controls; (6) NLR level from cases and controls; (7) drug history; (8) mean age; (9) gender.
Data synthesis and analysis
Pooled weighted mean difference (WMD) with 95% confidence interval (95% CI) was used to assess the differences in NLR levels between the patients with HTN and the controls or between dipper and non-dipper HTN patients. Because different studies used similar methods to measure the NLR, the unit of NLR among included studies was recorded the same. We assessed the quality of studies based on the Newcastle–Ottawa Scale (NOS) [46], with a maximum grade of nine for each study. Heterogeneity across included studies was calculated using I 2 statistics and Q test. The I 2 values showed serious (I 2 = 75–100%), high (I 2 = 50–74.9%), moderate (I 2 = 25–49.9%), low (I 2 = 0.1–24.9%), and no (I 2 = 0) heterogeneity. Furthermore, a significant Q-statistic showed heterogeneity among studies. If heterogeneity was high or serious (I2 ≥ 50%), we used the random-effect model; otherwise, we used the fixed-effect model. In addition, we used meta-regression and subgroup analysis to explore source of heterogeneity. Subgroup analysis was stratified by sample size. The small study was defined as studies with sample size ˂ 150, and studies with ≥ 150 patients were considered large studies. Egger’s and Begg’s tests and funnel plots were used to determine the publication bias. STATA 12.0 software (Stata Corporation, College Station, TX, USA) was used in data analyses. A 2-sided P < 0.05 was considered statistically significant.
Certainty of evidence
Two authors determined the certainty of evidence using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach for two outcomes (HTN and non-dipper HTN).
Results
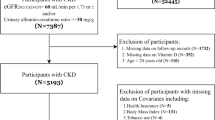
The literature search gave a total of 2165 articles. After emitting the duplicates, 1794 remained. Among them, 64 were found to be relevant in initial evaluation based on title and abstract. An additional 34 studies were excluded due to lack of data on NLR level, seven due to irrelevant outcomes, and two because they were review articles. Finally, 21 studies [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] investigating the association between NLR and HTN were included in this meta-analysis (Fig. 1).
Characteristics of the included studies
Of the 21 studies included in this meta-analysis [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], six were case–control studies [25, 33, 35, 41,42,43], and 15 were cross-sectional studies [23, 24, 26,27,28,29,30,31,32, 34, 36,37,38,39,40]. Concerning document language, all of the documents were in English. Overall 2396 patients with HTN and 1016 normotensive controls were enrolled in the selected studies. The general characteristics of the included studies is shown in Tables 1 and 2. NLR levels in hypertensive patients were compared with those of normotensive controls in 17 studies. In terms of sample size, there were nine large studies [25,26,27,28,29,30,31,32, 36] and eight small studies [33, 34, 38,39,40,41,42]. In addition, eight studies compared patients with dipper and non-dipper HTN utilizing the NLR [23, 24, 26, 28, 31, 32, 37, 43].
Differences between hypertensive and normotensive individuals in NLR level
NLR level differences between HTN patients and normotensive controls were investigated in 17 studies, including 1847 patients and 1016 controls. The pooled results showed that there was a significant increase of NLR levels in the hypertensive group in comparison to the control group (WMD = 0.40, 95%CI = 0.22–0.57, P < 0.0001, Fig. 2). There was a significant heterogeneity (I 2 = 87.5%, p < 0.001); so we used random- effect model. However, the certainty of the evidence was low (Table 3).
In the subgroup analysis according to sample size, there were nine large studies [25,26,27,28,29,30,31,32, 36], including 1438 hypertensive and 741 normotensive individuals. There were eight small studies [33, 34, 38,39,40,41,42] with 409 hypertensive and 275 normotensive individuals. Patients with HTN had higher levels of NLR in either small (WMD = 0.20, 95%CI = -0.01–0.40, P = 0.06) or large studies (WMD = 0.55, 95%CI = 0.32–0.78, P < 0.001) in comparison to normotensive individuals (Fig. 3).
In the metaregression analysis, there was no significant effect of total sample size (B < -0.001, adjusted R2 = -8.89, p = 0.68), publication year (B = -0.08, adjusted R2 = 4.06, p = 0.18), NOS score (B = 0.16, adjusted R2 = 0.0%, p = 0.95), gender (B = -8.21, adjusted R2 = 87.12, p = 0.83), mean age of cases (B = 0.001, adjusted R2 = 86.17, p = 0.86), smoking (B = 0.01, adjusted R2 = 7.11, p = 0.24), diabetes (B = 0.02, adjusted R2 = 3.62, p = 0.27), NOS score (B = -0.10, adjusted R2 = -4.54, p = 0.45), or BMI (B = 0.01, adjusted R2 = -12.40, p = 0.86). In addition, use of beta-blocker (B = 0.04, adjusted R2 = -20.85, p = 0.52), calcium channel blockers (CCB) (B = 0.01, adjusted R2 = 95.35, p = 0.85), Angiotensin receptor blockers (ARB) (B = -0.02, adjusted R2 = 97.68, p = 0.50) and diuretics (B = -0.009, adjusted R2 = 97.23, p = 0.89) had no effect on the NLR; so they could not be the source of heterogeneity. However, use of angiotensin-converting enzyme (ACE) inhibitors (B = -0.01, adjusted R2 = 84.87, p = 0.44) had significant effect on NLR; so it could be the source of heterogeneity.
Differences between patients with dipper and non-dipper HTN in NLR level
The pooled result of eight studies [23, 24, 26, 28, 31, 32, 37, 43] including 655 patients with dipper HTN and 690 patients with non-dipper HTN in NLR levels showed that the NLR levels were higher in non-dipper than in the dipper group (WMD = 0.58, 95%CI = 0.19–0.97, P = 0.003, Fig. 4). However, there was a significant heterogeneity (I 2 = 92.2%, p < 0.001); so we used a random-effect model. According to GRADE method, the certainty of the evidence was low (Table 3).
In the subgroup analysis according to sample size, there were six large studies, including 655 patients with dipper HTN and 690 patients with non-dipper HTN. There were two small studies with 655 patients with dipper HTN and 690 patients with non-dipper HTN. Patients with non-dipper HTN had higher levels of NLR in large studies (WMD = 0.54, 95%CI = 0.10–0.99, P = 0.01), but not in small studies (WMD = 0.69, 95%CI = -0.48–1.87, P = 0.24) when comparing to patients with dipper HTN (Fig. 5).
In the metaregression analysis, we found that smoking (B = 0.06, adjusted R2 = 74.68, p = 0.05) and BMI (B = 0.39, adjusted R2 = 100, p = 0.004) could be a source of heterogeneity. However, there was no significant effect of total sample size (B = -0.0001, adjusted R2 = -15.53, p = 0.66), publication year (B = -0.14, adjusted R2 = 25.86, p = 0.13), NOS score (B = 0.03, adjusted R2 = -18.87, p = 0.93), gender (B = 0.06, adjusted R2 = 33.87, p = 0.08), mean age of cases (B = 0.02, adjusted R2 = 5.28, p = 0.28), smoking (B = 0.06, adjusted R2 = 74.68, p = 0.05), diabetes (B = -0.008, adjusted R2 = -18.68, p = 0.71), and BMI (B = 0.39, adjusted R2 = 100, p = 0.004) on the association between NLR and HTN; so they could not be the source of heterogeneity.
Publication bias
As seen in Fig. 6, the funnel plots are asymmetrical and suggest that publication bias may exist. However, none of the statistical methods for subgroup analysis found such differences in NLR levels between patients with HTN and normotensive controls (Egger’s test P = 0.09, Begg’s test P = 0.08), and between patients with dipper and non-dipper HTN (Egger’s test 0.13, Begg’s test P = 0.10).
Discussion
The exact etiology which underlies HTN, a known risk factor for cardiovascular disease, is still unclear [31, 47]. In this meta-analysis, we systematically reviewed papers working on the NLR level in normotensive individuals and dipper and non-dipper hypertensive patients. Our results indicate that NLR level was significantly higher in hypertensive individuals compared with normotensive individuals. Also, it has been demonstrated that non-dipper hypertensive patients have increased NLR levels in comparison to dipper hypertensive patients. On the other hand, antihypertensive agents can regulate the NLR [48]. For example, Fici et al., in their study, showed that a selective β1 blocker, nebivolol, can cause a reduction in blood pressure, vascular micro-inflammation prevention, and NLR reduction [49]. Likewise, in a study done by Karaman et al., it was found that valsartan, which is an Angiotensin II receptor blocker, reduces the NLR after 12 weeks of treatment more efficiently compared to amlodipine, a calcium channel blocker [50]. Moreover, the NLR reliably indicates the systemic inflammation status across the body [51].
The inflammation can possibly play a role in the pathophysiology of HTN through an increase in inflammatory markers like IL-1β, IL-6, and TNF-α [52]. Based on these findings, it is important to investigate the possible role of Neutrophils and lymphocytes in inflammation causing HTN.
Neutrophils, the predominant leukocyte in the blood, are polymorphonuclear granulocytes that play an important role in modulating innate and adaptive immune responses [48, 53]. In a study by Sela et al., the number of neutrophils was found to increase before the development of HTN in experimental models on mice [54]. Moreover, in another paper by Tatsukawa et al., it has been shown that the neutrophil count was remarkably high in hypotensive Japanese women compared to the control group [55]. Different studies indicate that isolated neutrophils surges are seen in arterial hypertension (AH) pre-clinical models, hypertensive individuals, and women with preeclampsia. These conditions increase levels of ROS as well as phagocytic activity. During host-defense reactions, myeloperoxidase (MPO) and NADPH oxidase activation increase. This results in the formation of neutrophil extracellular traps constituted by DNA fibers and granule proteins. The neutrophils adhere to endothelial cells, which can increase cellular permeability and cause vascular dysfunction [48]. Furthermore, Nicholls et al. illustrated that neutrophils incubated by norepinephrine had an increased release of IL-6 and MPO [56]. This suggests a possible regulatory function for neutrophils dependent on the sympathetic system [48].
A study by Morton et al. strongly suggests a direct involvement of neutrophils in the control of blood pressure. They indicate that decreased neutrophils in normotensive mice can lead to a reduction in endothelial-dependent vasoconstriction and systolic blood pressure [57].
Therefore, according to these aforementioned mechanisms, increased neutrophil counts can likely attribute to high blood pressure.
The leukocyte response seen in increased NLR ratios is lymphocyte dependent. Reduction in the number of lymphocytes results in physiologic stress and poor health status [58]. There are various subtypes of T lymphocytes that affect blood pressure by regulating cytokine release throughout the cardiovascular system [59]. Zhang et al. found that T-bet deficient mice were unable to initiate a Th1 response. These mice had sustained hypertensive responses but were protected from renal damage from chronic angiotensin II provocation [60]. The data sugges that Th1 cells can cause kidney injury that is independent of high blood pressure [59].
Secretion of IL-17, known as a proinflammatory cytokine, is primarily by Th17 cells. This release plays a role in the pathogenesis of many autoimmune diseases. The effect of Th17 lymphocytes on blood pressure is still controversial. However, the injurious effect of IL-17 or IL-23 deficiency in the DOCA/salt model of hypertension indicates a protective role for Th17 cells [59].
In opposition to the inflammatory role of Th1 and Th17 cells, regulatory T lymphocytes can modulate the anti-inflammatory cellular immune responses [58, 59]. An animal study by Barhoumi et al. showed that Treg cells by mediating the angiotensin II response [61]. Treg cells produce IL-10, which is an important cytokine. In addition to immunosuppression, endogenous IL-10 can reduce oxidative stress and vascular dysfunction by a blood pressure-independent mechanism. It has been shown that exogenous IL-10 can reduce blood pressure to the normal range and make the endothelial function normal in hypertensive pregnant mice [59, 62]. Thus, the protective effect of Treg cells mediated through the IL-10 response warrants further investigation.
Despite the fact that additional studies should be done to elucidate the role of CD8 + T Lymphocytes in modulating hypertension, it has been shown that mice deficient in transcription factor inhibitor of differentiation (Id2) have altered CD8 + T cell memory and decreased natural killer cells. These mice do not exhibit hypertension induced by angiotensin II [59].
In summary, different subtypes of T lymphocytes can induce various levels of inflammation, which can either lead to hypertension or protect against it. The protective role of Treg cells was indicated, and it was stated that Th17 might have some protective effect against HTN if appropriately regulated.
Another type of lymphocyte is B cells which are necessary for adaptive immunity. The mechanisms by which B cells can play a role in hypertension has not been explored enough. However, it has been indicated by Chan and colleagues that angiotensin infusions induce further increase in B cells as well as plasma cell activation in lymphoid tissues. On the other hand, anti-CD20 antibody administration and genetic deficiency of B cells can cause the protection of mice against the hypertensive effects of angiotensin II [63]. Finally, additional studies focusing on the role of B cells in hypertension are needed to investigate novel mechanisms.
In our study, we indicate that in hypertensive patients, the number of neutrophils is increased, and the number of T lymphocytes that have a protective role is decreased. So, the NLR will be higher in hypertensive individuals and lower in normative controls.
As it has been stated before that non-dipper hypertensive individuals have a higher cardiovascular disease risk due to myocardial infarction and target organ damage compared with dipper hypertensive patients [51, 64]. These conditions are thought to be due to high platelet activity and increased inflammation [65]. The higher NLR levels in non-dipper hypertensive patients than dipper patients can indicate an increased pro-inflammatory state [64]. Moreover, it has been illustrated that the NLR can be used to independently predict long-term mortality and myocardial infarction [51]. Bayrakci et al. showed that the platelet-to-lymphocyte ratio (PLR), which is considered an inflammatory marker, is also remarkably higher in non-dipper hypertensive patients compared to dipper ones [66]. Inflamed tissues secrete some cytokines like IL-6, which contribute to vascular dysregulation. Through the influence of the cytokines, as mentioned earlier, the liver synthesizes C-reactive protein (CRP). High CRP levels can damage vessel walls. Also, there is an association with increased serum uric acid levels. This has been increased with higher cardiovascular disease [65]. Systemic inflammation can cause bone marrow dysfunction, leading to varied red blood cell size. Increased red blood cell distribution width (RDW) may be seen in inflammation [67]. Interestingly, CRP, uric acid, and RDW values are significantly higher in the non-dipper hypertensive patients compared with the dipper hypertensive patients and control group [65].
Higher blood pressure levels in non-dipper hypertensive individuals over the night can cause endothelial damage that triggers the proinflammatory process. Furthermore, inflammation can lead to blood pressure elevation. As a result, increased inflammation and high blood pressure both can feedback on each other contributing to cellular damage [65, 68]. Finally, because non-dipper hypertensive individuals have higher blood pressure during the night, they have increased inflammation which increases mortality and morbidity [65].
Limitation
Some limitations of our study do exist. First, geographic variability is essential to consider in the context of these results. The majority of current studies on this topic were performed in Turkey. Disparities in both HTN rates as well as HTN outcomes have been shown within different geological locations. It is important to note that the results from the studies on this topic to date may not be as applicable to hypertensive patients located in different geographical regions. Thus, similar prospective and retrospective studies are warranted in wider geographic locations to characterize any potential differences between these populations. Second, heterogeneity in studies was greater than expected due to various treatment regimens, age ranges, and gender differences for included patients. Therefore, widespread validity is a concern, and future larger prospective studies are needed. Third, this review was not registered in PROSPERO. Finally, several studies are limited by bias, whether based on selection or publication, which should be considered.
Conclusion
The current study is mainly providing knowledge of pathology of hypertension. Patients with HTN had higher level of NLR than normotensive individuals. In addition, patients with non-dipper HTN had higher NLR than those with dipper HTN. NLR represents a unique inflammatory marker whose elevation in HTN provides implications regarding immune system imbalance in the pathogenesis of the disease. In evaluation of included studies, it can be concluded that there may be association between HTN and NLR. Ultimately, with the development of new biomarkers and therapeutic modalities, we can better prevent and treat delirium to decrease long-term morbidity and mortality.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Abbreviations
- NLR:
-
Neutrophil to lymphocyte ratio
- PLR:
-
Platelet to lymphocyte ratio
- WMD:
-
Weighted mean difference
- 95% CI:
-
95% Confidence interval
- CRP:
-
C-reactive protein
- HTN:
-
Hypertension
- VEGF:
-
Vascular endothelial growth factor
- MPO:
-
Myeloperoxidase
- CRP:
-
C-reactive protein
- RDW:
-
Red blood cell distribution width
- ND:
-
Not Declared
- CCB:
-
Calcium channel blocker
- ACE:
-
Angiotensin-converting enzyme
- ARB:
-
Angiotensin receptor blockers
References
Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, Kamiya Y, et al. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kidney Int. 2004;65(2):621–5.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–9.
Alkhouri N, Morris-Stiff G, Campbell C, Lopez R, Tamimi TAR, Yerian L, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2012;32(2):297–302.
Niazi S, Krogh Nielsen M, Sørensen TL, Subhi Y. Neutrophil-to-lymphocyte ratio in age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. 2019;97(6):558–66.
Faria SS, Fernandes PC Jr, Silva MJB, Lima VC, Fontes W, Freitas-Junior R, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience. 2016;10:702.
Shau H, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141(12):4395–402.
Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers J-W, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Investig. 2012;122(1):327–36.
Yodying H, Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Yamada M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(2):646–54.
Song S-Y, Zhao X-X, Rajah G, Hua C, Kang R-J, Han Y-P, et al. Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: an updated meta-analysis. Front Neurol. 2019;10:1032.
Li X, Liu C, Mao Z, Xiao M, Wang L, Qi S, et al. Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: a systematic review and meta-analysis. Crit Care. 2020;24(1):1–10.
Zhang J, Zhang H-Y, Li J, Shao X-Y, Zhang C-X. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(40):68837.
Ghaffari S, Nadiri M, Pourafkari L, Sepehrvand N, Movasagpoor A, Rahmatvand N, et al. The predictive value of total neutrophil count and neutrophil/lymphocyte ratio in predicting in-hospital mortality and complications after STEMI. J Cardiovasc Thorac Res. 2014;6(1):35.
Solak Y, Yilmaz MI, Sonmez A, Saglam M, Cakir E, Unal HU, et al. Neutrophil to lymphocyte ratio independently predicts cardiovascular events in patients with chronic kidney disease. Clin Exp Nephrol. 2013;17(4):532–40.
Liu J, Liu X, Li Y, Quan J, Wei S, An S, et al. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis. Biosci Rep. 2018;38(3):BSR20180172.
Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107(3):433–8.
Kara M, Dogru T, Genc H, Sertoglu E, Celebi G, Gurel H, et al. Neutrophil-to-lymphocyte ratio is not a predictor of liver histology in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27(10):1144–8.
Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil–lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395(1–2):27–31.
Akrami M, Izadpanah P, Bazrafshan M, Hatamipour U, Nouraein N, Drissi HB, et al. Effects of colchicine on major adverse cardiac events in next 6-month period after acute coronary syndrome occurrence; a randomized placebo-control trial. BMC Cardiovasc Disord. 2021;21(1):1–10.
Jahangiri S, Mousavi SH, Hatamnejad MR, Salimi M, Bazrafshan H. Prevalence of non-steroidal anti-inflammatory drugs (NSAIDs) use in patients with hypertensive crisis. Health Science Reports. 2022;5(1): e483.
Sarejloo S, Dehghani F, Hatamnejad MR, Jahangiri S, Ghaedian T, Salimi M, et al. Risk stratification of diabetic patients with unusual cardiac symptoms using a myocardial perfusion scan. ARYA Atherosclerosis J. 2022. in press.
Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97(7):993–6.
Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154(5):995–1002.
Demir M. The relationship between neutrophil lymphocyte ratio and non-dipper hypertension. Clin Exp Hypertens (New York, NY : 1993). 2013;35(8):570–3.
Sunbul M, Gerin F, Durmus E, Kivrak T, Sari I, Tigen K, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2014;36(4):217–21.
Belen E, Sungur A, Sungur MA, Erdoğan G. Increased Neutrophil to Lymphocyte Ratio in Patients With Resistant Hypertension. J Clin Hypertens (Greenwich). 2015;17(7):532–7.
Kiliçaslan B, Dursun H, Kaymak S, Aydin M, Ekmekçi C, Susam I, et al. The relationship between neutrophil to lymphocyte ratio and blood pressure variability in hypertensive and normotensive subjecs. Turk Kardiyoloji Dernegi Arsivi. 2015;43(1):18–24.
Yayla Ç, Canpolat U, Akyel A, GayretlYayla K, Akboğa MK, Eyiol A, et al. Association of neutrophil-lymphocyte ratio with impaired aortic elasticity in newly diagnosed and never-treated hypertensive patients. Blood Press Monit. 2015;20(3):127–31.
Kim BJ, Cho KI, Choi JH, Park DH, Yu GI, Im SI, et al. Epicardial Fat Thickness and Neutrophil to Lymphocyte Ratio are Increased in Non-Dipper Hypertensive Patients. J Cardiovasc Ultrasound. 2016;24(4):294–302.
Wang H, Hu Y, Geng Y, Wu H, Chu Y, Liu R, et al. The relationship between neutrophil to lymphocyte ratio and artery stiffness in subtypes of hypertension. J Clin Hypertens (Greenwich). 2017;19(8):780–5.
Derya MA, Demir V, Ede H. Relationship between neutrophil/lymphocyte ratio and epicardial fat tissue thickness in patients with newly diagnosed hypertension. J Int Med Res. 2018;46(3):940–50.
Bozduman F, Yildirim E, Cicek G. Biomarkers of nondipper hypertension in prehypertensive and hypertensive patients. Biomark Med. 2019;13(5):371–8.
Cetin N, Tufan AK. Platelet activation and inflammation in hypertensive children with non-dipper and dipper status. Iran J Kidney Dis. 2019;13(2):105.
Srinivasagopalane B, Andrew Rajarathinam S, Balasubramaiyan T. Clinical pertinence of neutrophil-to- lymphocyte ratio among hypertensives with different grades and duration of hypertension–an insight. Clin Exp Hypertens. 2019;41(4):394–9.
Hou M, Cao L, Ding Y, Chen Y, Wang B, Shen J, et al. Neutrophil to Lymphocyte Ratio Is Increased and Associated With Left Ventricular Diastolic Function in Newly Diagnosed Essential Hypertension Children. Front Pediatr. 2021;9: 576005.
Mehmood MS, Hussain MM, Ahmad SQ. Evaluation of neutrophil-lymphocyte ratio and arterial stiffness index in middle aged prehypertensive and hypertensive men. J Rawalpindi Med Coll (JRMC). 2014;18(2):171–4.
Unamba NN, Romokere AM, James OO. The Relationship between Neutrophil to Lymphocyte Ratio and Left Ventricular Function in Drug-naïve Asymptomatic Hypertensive Adults in Port Harcourt, Nigeria. J Adv Med Medical Research. 2017;22:1–12.
Müjgan T, Ebinc FA, Kutlugün AA, Efe FK, Cetin S, Çelebi S, et al. The Relation Between Blood Pressure Reverse-Dipping and Neutrophil to Lymphocyte Ratio in Hypertensive Patients. Osmangazi Tıp Dergisi. 2018;41(3):203–7.
Skrzypczyk P, Przychodzień J, Bombińska M, Kaczmarska Z, Mazur M, Pańczyk-Tomaszewska M. Complete blood count-derived inflammatory markers in adolescents with primary arterial hypertension: a preliminary report. Central-Eur J Immunol. 2018;43(4):434–41.
Yousif RM, Mahmood MM, Naj S. Concomitant Measurements Of Serum Annexin V and Hemato-Inflammatory Indices In Hypertensive Patients: A preliminary Study. Biochem Cell Arch. 2018;18(1):445–50.
Atmaca HU, Akbas F, Aral H. Relationship between circulating microparticles and hypertension and other cardiac disease biomarkers in the elderly. BMC Cardiovasc Disord. 2019;19(1):1–7.
Balan R, BĂlĂŞescu E, Ion DA. Inflammation and Arterial Hypertension-Pathophysiological Links and Clinical Aspects. Curr Health Sci J. 2020;46(4):383.
Berillo O, Huo K-G, Fraulob-Aquino JC, Richer C, Briet M, Boutouyrie P, et al. Circulating let-7g-5p and miR-191-5p are independent predictors of chronic kidney disease in hypertensive patients. Am J Hypertens. 2020;33(6):505–13.
Chotruangnapa C, Tansakun T, Roubsanthisuk W. Clinical risk factors and predictive score for the non-dipper profile in hypertensive patients: a case-control study. Clin Hypertens. 2021;27(1):1–11.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:372.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1–13.
Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. p. 1–12.
Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens. 2015;28(11):1339–46.
Araos P, Figueroa S, Amador CA. The role of neutrophils in hypertension. Int J Mol Sci. 2020;21(22):8536.
Fici F, Celik T, Balta S, Iyisoy A, Unlu M, Demitkol S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. 2013;62(4):388–93.
Karaman M, Balta S, Seyit Ahmet AY, Cakar M, Naharci I, Demirkol S, et al. The comparative effects of valsartan and amlodipine on vWf levels and N/L ratio in patients with newly diagnosed hypertension. Clin Exp Hypertens. 2013;35(7):516–22.
Demir M. The relationship between neutrophil lymphocyte ratio and non-dipper hypertension. Clin Exp Hypertens. 2013;35(8):570–3.
Belen E, Sungur A, Sungur MA, Erdoğan G. Increased neutrophil to lymphocyte ratio in patients with resistant hypertension. J Clin Hypertens. 2015;17(7):532–7.
Taylor S, Dirir O, Zamanian RT, Rabinovitch M, Thompson A. The role of neutrophils and neutrophil elastase in pulmonary arterial hypertension. Front Med. 2018;5:217.
Sela S, Mazor R, Amsalam M, Yagil C, Yagil Y, Kristal B. Primed polymorphonuclear leukocytes, oxidative stress, and inflammation antecede hypertension in the Sabra rat. Hypertension. 2004;44(5):764–9.
Tatsukawa Y, Hsu W-L, Yamada M, Cologne JB, Suzuki G, Yamamoto H, et al. White blood cell count, especially neutrophil count, as a predictor of hypertension in a Japanese population. Hypertens Res. 2008;31(7):1391–7.
Nicholls AJ, Wen SW, Hall P, Hickey MJ, Wong CH. Activation of the sympathetic nervous system modulates neutrophil function. J Leukoc Biol. 2018;103(2):295–309.
Morton J, Coles B, Wright K, Gallimore A, Morrow JD, Terry ES, et al. Circulating neutrophils maintain physiological blood pressure by suppressing bacteria and IFNγ-dependent iNOS expression in the vasculature of healthy mice. Blood. 2008;111(10):5187–94.
Saylik F, Sarıkaya R. Can Systemic Immune-Inflammation Index Detect the Presence of Exxaggerated Morning Blood Pressure Surge in Newly Diagnosed Treatment-Naive Hypertensive Patients? Clin Exp Hypertens. 2021;43(8):772–9.
Zhang J, Crowley SD. Role of T lymphocytes in hypertension. Curr Opin Pharmacol. 2015;21:14–9.
Zhang J-D, Patel MB, Song Y-S, Griffiths R, Burchette J, Ruiz P, et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circul Res. 2012;110(12):1604–17.
Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T Regulatory lymphocytes prevent angiotensin ii–induced hypertension and vascular injury. Hypertension. 2011;57(3):469–76.
Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Reg Integr Comp Physiol. 2010;298(3):R713–9.
Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev. 2017;97(3):1127–64.
Sunbul M, Gerin F, Durmus E, Kivrak T, Sari I, Tigen K, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2014;36(4):217–21.
Tosu AR, Demir S, Selcuk M, Kaya Y, Akyol A, Ozdemir M, et al. Comparison of inflammatory markers in non-dipper hypertension vs. dipper hypertension and in normotensive individuals: uric acid, C-reactive protein and red blood cell distribution width readings. Postępy Kardiol Interwencyjnej. 2014;10(2):98.
Bayrakci N, Ozkayar N, Akyel F, Ates I, Akyel S, Dede F. The platelet-to-lymphocyte ratio as an inflammation marker in non-dipper hypertensive patients. Hippokratia. 2015;19(2):114.
Özcan F, Turak O, Durak A, İşleyen A, Uçar F, Giniş Z, et al. Red cell distribution width and inflammation in patients with non-dipper hypertension. Blood Press. 2013;22(2):80–5.
Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, Sasaki S, et al. Circadian variation of blood pressure and endothelial function in patients with essential hypertension: a comparison of dippers and non-dippers. J Am Coll Cardiol. 2002;40(11):2039–43.
Acknowledgements
Not applicable.
Funding
No funding was received to undertake this systematic review.
Author information
Authors and Affiliations
Contributions
ShKh contributed to the conception of the study and performed the data analyses; ShS searched the articles and reviewed all identified articles for eligibility; AG reviewed all identified articles for eligibility and assessed the quality of included studies; MKh revised the manuscript; BLW revised the manuscript; MD Assisted in judging disputed articles and assessed the quality of included studies; MF helped perform the analysis with constructive discussions. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sarejloo, S., Dehesh, M., Fathi, M. et al. Meta-analysis of differences in neutrophil to lymphocyte ratio between hypertensive and non-hypertensive individuals. BMC Cardiovasc Disord 23, 283 (2023). https://doi.org/10.1186/s12872-023-03304-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03304-w