Abstract
Background
Coronary physiology-guided PCIs are recommended worldwide. However, invasive coronary physiology methods prolong the procedure, create additional risks for the patients, and prolong the fluoroscopy time for an interventional cardiologist. Otherwise, there is a noninvasive coronary physiology evaluation method, QFR, that can be safely used even in STEMI patients.
Methods
A total of 198 patients admitted with STEMI and at least one intermediate (35–75%) diameter stenosis other than the culprit artery between July 2020 and June 2021 were prospectively included in this single-center study. All patients were randomized into one of two groups (1 - QFR-guided PCI; 2 - visual-estimation-only guided PCI). A 12-month follow-up with echocardiography, exercise stress test, and quality of life evaluation was performed in all included patients. For the QOF evaluation, the Seattle Angina Score Questionnaire was chosen. Statistical analysis was performed using the Kolmogorov–Smirnov test, Student’s t-test, Mann–Whitney U test, Pearson’s chi-squared test and Kaplan–Meier estimator.
Results
Ninety-eight (49.5%) patients were randomized to the first group, and 100 (50.5%) patients were included in the second group. Statistically, significantly more patients had a medical history of dyslipidemia (98 vs. 91, p = 0.002) and slightly better left ventricular ejection fraction (42.21 ± 7.88 vs. 39.45 ± 9.62, p = 0.045) in the QFR group. Six fewer patients required non-culprit artery revascularization within the 12-month FU in the QFR group (1.02% vs. 6%, p = 0.047). Survival analysis proved that patients in the Angio group had a more than 6-fold greater risk for death within a 12-month period after MI (OR 6.23, 95% CI 2.20-17.87, p = 0.006), with the highest mortality risk within the first two months after initial treatment.
Conclusion
Using QFR in non-culprit lesions in patients with ST-elevation myocardial infarction reduces mortality and revascularization at the 12-month follow-up and improves the quality of life of the patient.
Trial registration
The study was approved by the Regional Bioethical Committee and conducted under the principles of the Helsinki Declaration and local laws and regulations.
Similar content being viewed by others
Background
When treating patients who have suffered an ST-segment elevation myocardial infarction (STEMI), the initial percutaneous coronary intervention (PCI) should be performed as quickly as possible, and it is recommended that only infarct-related artery (IRA) PCI be performed [1]. On the other hand, more than half of patients diagnosed with acute myocardial infarction have multivessel disease, which is defined as a diameter stenosis of at least two coronary arteries that is greater than or equal to 50% [2]. As a result, the majority of patients need staged PCI in arteries that were not the culprit. In spite of the fact that it is advised that a second procedure be performed as soon as it is feasible to do so, the urgency of staged PCI is ultimately up to the discretion of the attending physician and is primarily determined by the degree of the lesion. At this time, methods of physiological evaluation are used to assist in determining the significance of lesions.
Despite all improvements, fractional flow reserve (FFR), an invasive hyperemic hemodynamic physiology evaluation method, remains the gold standard for physiological evaluation [3]. However, this method requires extra time for pressure wire manipulation and adenosine to induce hyperemia. All of these factors prolong the procedure time [4] and may trigger complications and stress for patients [5]. Furthermore, the FFR is a highly disorderable method, and its values may be underestimated in patients who consumed caffeine within 24 h, despite requiring a higher dose of adenosine [6]. The use of FFR in patients with ACS remains limited, as during the acute phase and the period up to 6 months, microcirculation dysfunction can determine the reaction of pharmacological vasodilatation [7] and can be present throughout the entire myocardium [4, 8, 9]. These changes may misrepresent the FFR values, increasing them [10, 11]. Ntalianis et al. were the first researchers to confirm FFR compatibility on non-culprit vessels in ACS [12]. The results were similar in both groups [12]. However, the average time between FFR measurements was 35 4 days, which could have an impact on not fully recovered microcirculation, resulting in incorrect FFR values in both [4, 12]. Moreover, Van Belle et al. showed that FFR in the ACS reclassified 38% of revascularization strategies and 39% of strategies in the non-ACS [13]. The FAMOUS-NSTEMI clinical trial demonstrated that FFR should be used to confirm treatment strategy in patients with NSTEMI [14]. The study showed a significantly reduced interventional treatment strategy in the FFR group compared to the initial decision [14]. Additionally, Hakeem et al. demonstrated that an FFR value < 0.84 during ACS was the cut-off for stenoses, which would significantly reduce major adverse cardiovascular events (MACEs) in the future [15]. They concluded that FFR measured between 0.8 and 0.85 in ACS patients should be confirmed before planning treatment [15].
Furthermore, compared to angiography-guided PCI, FFR-guided PCI significantly reduces reinfarction and mortality rates [16]. FFR-guided PCI can also be cost-effective up to 21% at one year and up to 22% at three years compared to IRA-PCI alone in STEMI patients [17].
The instantaneous wave-free ratio (iFR) is an additional adenosine-free physiology evaluation method that has been introduced in clinical practice because adenosine-caused hyperaemia is very uncomfortable and creates an additional risk for patients. When compared to FFR, iFR is responsible for 27.7% fewer adverse procedural reactions [18].
Two extensive clinical studies compared iFR- and FFR-based PCI in patients with stable coronary artery disease or non-STEMI with a 1-year follow-up [18, 19]. The results were similar and without significant differences [18, 19]. Hoeven et al. compared iFR values in STEMI patients 1 month later. The trial found no significant changes in iFR values during this time period, but it did find significant changes in FFR values in relation to microcirculation dysfunction and hyperaemia [20]. Another trial, however, discovered promising results between iFR classification agreement and time after STEMI [21]. The agreement between acute and within 5 days follow-up iFR classification was 89%, but only 70% between acute and > 16 days follow-up [21]. The physiological changes during STEMI may impact the classification disagreement in the more extended follow-up period [21]. In STEMI patients, it was discovered that iFR could overestimate the severity of stenoses while FFR could underestimate it in the acute and subacute phases [22]. Theoretically, the iFR could be used in ACS patients [23], but it still prolongs procedure time, requires a pressure wire, and makes the procedure costly.
Since physiology evaluation is underused worldwide, it was believed that the wireless physiology evaluation method may become a game changer. Even though it can be used offline, it is suitable for physiological evaluation in hemodynamically unstable patients with cardiogenic shock, in contrast to wire-based methods, which significantly extend the procedure time [12, 20, 21, 24]. One of the options is a novel noninvasive physiological evaluation method known as the quantitative flow ratio (QFR). The index strongly correlates with other methods, especially FFR [25,26,27]. Retrospective QFR analysis in STEMI patients revealed that if revascularization was performed on a QFR 0.8 rather than angiography-based PCI, 62.9% could avoid the primary endpoints in 5 years [28]. Furthermore, QFR-based non-infarct-related artery PCI avoids 10.5% of the primary endpoints at 12 months compared to IRA revascularization alone [29]. Lauri et al. recommend using QFR as the first-choice physiological assessment method, switching to FFR only in debatable cases [30].
Despite all novelties, physiology-guided PCIs, especially in ACS patients, remain underused worldwide. Therefore, this study was designed to compare the cardiovascular outcomes of visually estimated only-guided PCI versus noninvasive physiology assessment-guided PCI. As a result, the purpose of the study was to investigate the differences between the non-culprit lesion significance evaluation methods in terms of the quality of life (QOL), the rate of rehospitalization, and the rate of revascularization within the first 12 months following the initial treatment.
Methods
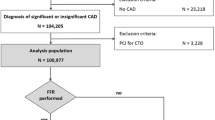
We prospectively enrolled 198 multivessel diseased STEMI patients who were admitted to our center from July 2020 to June 2021 and had a non-culprit stenosis (35–75%).
After giving their written consent, all included patients were randomized into one of two groups: (1) QFR-guided PCI (named QFR) and (2) visual estimation-only guided PCI (named Angio). Patient data, medical history, and medical treatment were collected from their medical records.
All coronary artery angiographies (CAGs) were performed in compliance with the recommendation for QFR analysis as described in previous publications [31].
Visual estimation was performed by a CAG performing doctor and discussed at the interventional cardiologists’ meeting. If any additional physiological evaluation was needed upon meeting the judgment, the patient was excluded from the study and treated following European Society of Cardiology (ESC) guidance.
The quantitative flow ratio is an innovative method to evaluate the hemodynamic significance of coronary stenoses. The evaluation is based on two different angiographic projections. Special software was used to calculate the pressure differences between pre-stenosis and post-stenosis. In this study, stenoses were observed as hemodynamically significant when the QFR was less than 0,8. For those who were randomized to the QFR group, QFR analyses were performed using the same software - Medis Medical Imaging, Medis QFR® 20.0. All of the QFR analyses were performed offline twice by an experienced and internationally certified QFR observer and averaged. If those two measurements were not matching (the difference between the two measurements was > 0.02), the third measurement was performed, and the three were then averaged. None of the patients were unsuitable for QFR analyses; therefore, none of them were excluded from this group.
For the QOL evaluation, the Seattle Angina Score Questionnaire (SASQ) was chosen (Additional File 1). According to SASQ, the physical limitation and angina frequency were classified as minimal (scores 75–100), mild (50–74), moderate (25–49), and severe (0–24).
A 12-month follow-up was performed in all included patients as a phone call if there were any adverse events or complaints within this period. If the patient complained of any new or exacerbated cardiac symptoms, an in-person meeting and additional examination were scheduled.
Patients who could not be reached by phone were mailed a letter addressing them or their relatives as provided on a written consent form. If any response within one month was obtained, they were checked on our hospital’s digital system for adverse events or death.
The primary outcome involved in this study was mortality. Secondary outcomes: rehospitalization for culprit artery and non-culprit artery revascularization within the 12-month follow-up; and physical activity limitations (according to the SASQ) within the 12-month follow-up.
Statistical analysis was performed using SPSS 28.0 software. The Kolmogorov-Smirnov test was used to test quantitative parameters; if they were normally distributed, differences between two groups were evaluated using the Student’s t-test and expressed as the mean with standard deviation (SD); otherwise, they were evaluated using the Mann-Whitney U test and expressed as the median with interquartile range (IQR). Differences between categorical parameters were tested using Pearson’s chi-squared test. Survival analysis was performed using the Kaplan–Meier estimator and expressed as a Kaplan-Meier survival curve. The chosen level of significance was p < 0.05.
The study was approved by the Regional Bioethical Committee and was done according to the principles of the Helsinki Declaration and local laws and rules.
Results
Of all, 98 (49.5%) patients were randomized to the QFR-guided PCI group, and 100 (50.5%) patients were included in the angiography-guided PCI group.
Significantly more patients had a medical history of dyslipidemia and slightly better left ventricular ejection fraction (LVEF) (Table 1; Fig. 1) in the QFR group. PCI in anamnesis was more common in the Angio group (Table 1). There were no other differences between the two groups (Table 1).
During the 12-month follow-up (FU) period, 6 (6%) patients required additional non-culprit MI artery revascularization, which was decided not to treat according to visual estimation in the Angio group (Table 2). At the 12-month follow-up, the majority of the patients continued dual antiplatelet therapy (DAPT) with aspirin and ticagrelor, 91 (92.86%) vs. 84 (84%) in the QFR and Angio groups, respectively. The other 6 (6.12%) patients in the QFR group and 15 (15%) patients in the Angio group used DAPT with aspirin and clopidogrel. Overall, only 1 patient discontinued DAPT while remaining on aspirin only in the QFR group. All patients were on an adequate dose of other post-MI medical treatments, including beta-adrenoreceptor blockers (BABs), angiotensin converting enzyme inhibitors (ACEis), and mineralocorticoid receptor antagonists (MRAs), if needed in both groups at the 12-month FU check-up. Of all, 32 (32.65%) patients in the QFR group and 52 (52%) patients in the Angio group were on oral diuretics (p = 0.006). Patients in the Angio group had a 2.2 times greater risk for the need for oral diuretics for the extended period after MI and visual estimation only of the non-culprit artery (OR 2.23, 95% CI 1.26–3.98). Additionally, patients in the QFR group had an almost 4% better LVEF increase within 6 months after MI compared to visual estimation-only guided PCI. All summarized patient 12-month follow-up data are given in Table 2.
Within the 12-month period, 14 (7.07%) patients died, 2 (2.04%) in the QFR group and the remaining 12 (12%) in the Angio group. Survival analysis proved that patients in the Angio group had a more than 6-fold greater risk for death within the 12-month period after MI (OR 6.23, 95% CI 2.20-17.87, p = 0.006) (Fig. 2), with the highest mortality risk within the first two months after initial treatment.
Discussion
In the present study, we compared QFR-guided versus angiography-based PCI on non-culprit lesions over a 12-month follow-up period. The main difference between these groups was the method based on which further clinical decisions were made regarding whether staged PCI in non-culprit arteries was necessary. Otherwise, treatment was identical in both groups. The results showed that patients whose non-culprit arteries were judged according to the QFR values (interventional treatment performed in non-culprit lesions with a QFR ≤ 0.8) had significantly better outcomes and six times less non-culprit lesion revascularization during the follow-up period. Additionally, in the one-year period, the QFR-guided group had twice as many rehospitalizations, and their physical limitations were better (Table 2). As interventional cardiologists acknowledge, physiology-guided PCI is recommended over conventional PCI [32]. This study proved that QFR might be used as a method of choice to increase physiological guidance even in STEMI patients.
Most patients with STEMI are unstable and more fragile than those with NSTEMI. For this reason, PCI for STEMI patients should be as fast as possible and limited to hemodynamically significant stenosis, especially during the index procedure. Invasive coronary physiology methods, such as iFR and FFR, require additional time and manipulations with a pressure wire. Otherwise, QFR is a noninvasive coronary physiology method that can be performed offline, not only by the interventional cardiologist but also by a specialist who is qualified to work with the software. This fact allows interventional cardiologists to perform treatment procedures on culprit lesions while one team member evaluates QFR on non-culprit stenosis. QFR makes the procedure smoother and more accurate, and our study demonstrates that it is likely to be used in STEMI patients.
During the 12-month follow-up period, only five patients in the QFR group were rehospitalized, while twice as many in the Angio group. This shows that this noninvasive coronary physiology method can decrease the rehospitalization rate. A reduced rate of rehospitalizations is one of the main pros of using physiology-guided PCI [33]. Rehospitalizations were related to culprit or non-culprit lesions. In the QFR group, six times fewer patients were hospitalized 12 months after STEMI for non-culprit PCI than in the Angio group. In addition, only one patient required PCI when QFR showed no significant stenosis on the non-culprit artery.
Similar studies to the factors of the follow-up period involved only major adverse cardiovascular events (MACEs) or physiology-guided coronary lesion revascularizations [34, 35]. However, in this study, all patients were followed up for 12 months, and the Seattle Angina Questionnaire was used to objectively identify physical limitations. This criterion is fundamental when investigating coronary physiology methods because physiology-based PCI’s most crucial part is finding hemodynamically significant stenosis and not treating stenosis, which is not substantial. The study results showed that three times fewer patients in the QFR group had moderate physical limitations than those in the Angio group (Table 2). Nevertheless, the patients in the QFR group more often had mild or minimal symptoms of physical activity limitations, according to the Seattle Angina Score Questionnaire. Additionally, all patients in this study underwent an exercise stress test and echocardiography in the following period. All participants in the QFR group had nonpathological exercise stress test results and greater LVEF recovery within 6 months of MI than in the Angio group (Table 2).
The mortality rate is one of the essential facts of the following period. FFR demonstrated this factor’s efficiency and showed us that it is possible to reduce mortality rates using coronary physiology evaluation methods [36]. In this study, the patients in the Angio group died six times more often than patients in the QFR group. Additionally, the results showed that the majority of these patients in the Angio group died during the first two months after STEMI. Therefore, as an FFR, the QFR is efficient in reducing the mortality rate and should be used for all patients with non-culprit artery stenosis.
Conclusion
The QFR is a noninvasive coronary physiology evaluation method that is accurate for STEMI patients and can be performed by any qualified team member. The use of QFR for patients with ST-elevation myocardial infarction significantly reduced the mortality rate and revascularization at the 12-month follow-up. The patients who underwent PCI guided by the QFR had lower physical activity limitations during their daily activities.
Data Availability
The datasets generated and analysed during the current study are not publicly available due to patients’ privacy but are available from the corresponding author on reasonable request.
Change history
30 March 2023
A Correction to this paper has been published: https://doi.org/10.1186/s12872-023-03189-9
References
Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.
Brener SJ, Milford-Beland S, Roe MT, Bhatt DL, Weintraub WS, Brindis RG. Culprit-only or multivessel revascularization in patients with acute coronary syndromes: an American College of Cardiology National Cardiovascular Database Registry report. Am Heart J. 2008;155:140–6.
Stegehuis VE, Wijntjens GW, Piek JJ, van de Hoef TP. Fractional flow reserve or coronary flow reserve for the assessment of myocardial perfusion: implications of FFR as an imperfect reference standard for myocardial ischemia. Curr Cardiol Rep. 2018;20.
Zuk G, Ciecwierz D, Cwalina N, Gruchala M, Cuculi F. Fractional flow reserve (FFR)-based therapy in patients presenting with acute coronary syndrome: current data and everyday practice. Cardiol J. 2017;24:426–35.
Layland J, Carrick D, Lee M, Oldroyd K, Berry C. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc Interv. 2014;7:581–91.
Nakayama M, Chikamori T, Uchiyama T, Kimura Y, Hijikata N, Ito R, et al. Effects of caffeine on fractional flow reserve values measured using intravenous adenosine triphosphate. Cardiovasc Interven Ther. 2018;33:116–24.
Cuculi F, De Maria GL, Meier P, Dall’Armellina E, De Caterina AR, Channon KM, et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894–904.
Wyatt HL, Forrester JS, da Luz PL, Diamond GA, Chagrasulis R, Swan HJC. Functional abnormalities in nonoccluded regions of myocardium after experimental coronary occlusion. Am J Cardiol. 1976;37:366–72.
Corday E, Kaplan L, Meerbaum S, Brasch J, Costantini C, Lang TW, et al. Consequences of coronary arterial occlusion on remote myocardium: effects of occlusion and reperfusion. Am J Cardiol. 1975;36:385–94.
Uren NG, Crake T, Lefroy DC, de Silva R, Davies GJ, Maseri A. Reduced coronary vasodilator function in infarcted and normal myocardium after myocardial infarction. N Engl J Med. 1994;331:222–7.
Ragosta M, Powers ER, Samady H, Gimple LW, Sarembock IJ, Beller GA. Relationship between extent of residual myocardial viability and coronary flow reserve in patients with recent myocardial infarction. Am Heart J. 2001;141:456–62.
Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interven. 2010;3:1274–81.
Van Belle E, Baptista SB, Raposo L, Henderson J, Rioufol G, Santos L et al. Impact of routine fractional flow reserve on management decision and 1-year clinical outcome of patients with acute coronary syndromes: PRIME-FFR (insights from the POST-IT [Portuguese Study on the Evaluation of FFR-Guided Treatment of Coronary Disease] and R3F [French FFR Registry] integrated multicenter registries - implementation of FFR [Fractional Flow Reserve] in routine practice). Circ Cardiovasc Interv. 2017;10.
Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J. 2015;36:100–11.
Hakeem A, Edupuganti MM, Almomani A, Pothineni NV, Payne J, Abualsuod AM, et al. Long-term prognosis of deferred acute coronary syndrome lesions based on nonischemic fractional flow reserve. J Am Coll Cardiol. 2016;68:1181–91.
Pijls NHJ, Fearon WF, Tonino PAL, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol. 2010;56:177–84.
Smits PC, Laforgia PL, Abdel-Wahab M, Neumann FJ, Richardt G, Boxma-De Klerk B et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction: three-year follow-up with cost benefit analysis of the Compare-Acute trial. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2020;16:225–32.
Davies JE, Sen S, Dehbi H-M, Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–34.
Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813–23.
Van Der Hoeven NW, Janssens GN, De Waard GA, Everaars H, Broyd CJ, Beijnink CWH, et al. Temporal changes in coronary hyperemic and resting hemodynamic indices in nonculprit vessels of patients with ST-segment elevation myocardial infarction. JAMA Cardiol. 2019;4:736–44.
Thim T, Götberg M, Fröbert O, Nijveldt R, van Royen N, Baptista SB, et al. Nonculprit stenosis evaluation using instantaneous wave-free ratio in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2017;10:2528–35.
Thim T, Götberg M, Fröbert O, Nijveldt R, Van Royen N, Baptista SB et al. Agreement between nonculprit stenosis follow-up iFR and FFR after STEMI (iSTEMI substudy).BMC research notes. 2020;13.
Choi KH, Lee JM, Kim HK, Kim J, Park J, Hwang D, et al. Fractional flow reserve and instantaneous wave-free ratio for nonculprit stenosis in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2018;11:1848–58.
Wang LJ, Han S, Zhang XH, Jin YZ. Fractional flow reserve-guided complete revascularization versus culprit-only revascularization in acute ST-segment elevation myocardial infarction and multi-vessel disease patients: a meta-analysis and systematic review. BMC Cardiovasc Disord. 2019;19.
Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70:3077–87.
Tu S, Westra J, Yang J, von Birgelen C, Ferrara A, Pellicano M, et al. Diagnostic accuracy of fast computational approaches to derive fractional flow reserve from diagnostic coronary angiography: the international multicenter FAVOR pilot study. JACC Cardiovasc Interv. 2016;9:2024–35.
Yazaki K, Otsuka M, Kataoka S, Kahata M, Kumagai A, Inoue K, et al. Applicability of 3-dimensional quantitative coronary angiography-derived computed fractional flow reserve for intermediate coronary stenosis. Circ J. 2017;81:988–92.
Bär S, Kavaliauskaite R, Ueki Y, Otsuka T, Kelbæk H, Engstrøm T et al. Quantitative flow ratio to predict nontarget vessel-related events at 5 years in patients with ST-segment-elevation myocardial infarction undergoing angiography-guided revascularization. J Am Heart Assoc. 2021;10.
Zhang J, Yao M, Jia X, Feng H, Fu J, Tang W, et al. The efficacy and safety of quantitative flow ratio-guided complete revascularization in patients with ST-segment elevation myocardial infarction and multivessel disease: a pilot randomized controlled trial. Cardiol J. 2021. https://doi.org/10.5603/CJ.A2021.0111.
Lauri FM, Macaya F, Mejía-Rentería H, Goto S, Yeoh J, Nakayama M et al. Angiography-derived functional assessment of non-culprit coronary stenoses in primary percutaneous coronary intervention. EuroIntervention. 2020;15:E1594–601.
Ziubryte G, Jarusevicius G. Fractional flow reserve, quantitative flow ratio, and instantaneous wave-free ratio: a comparison of the procedure-related dose of ionising radiation. Postepy Kardiol Interwencyjnej = Adv Intervent Cardiol. 2021;17:33.
Shinohara H, Kodera S, Kiyosue A, Ando J, Morita H, Komuro I. Efficacy of fractional flow reserve-guided percutaneous cornary intervention for patients with Angina Pectoris. Int Heart J. 2020;61:1097–106.
Li J, Elrashidi MY, Flammer AJ, Lennon RJ, Bell MR, Holmes DR, et al. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375.
Buono A, Mühlenhaus A, Schäfer T, Trieb AK, Schmeißer J, Koppe F et al. QFR Predicts the incidence of long-term adverse events in patients with suspected CAD: feasibility and reproducibility of the method. J Clin Med. 2020;9.
Piróth Z, Boxma-de Klerk BM, Omerovic E, Andréka P, Fontos G, Fülöp G, et al. The natural history of nonculprit lesions in STEMI: an FFR substudy of the compare-acute trial. JACC Cardiovasc Interv. 2020;13:954–61.
Wong CCY, Ng ACC, Ada C, Chow V, Fearon WF, Ng MKC et al. A real-world comparison of outcomes between fractional flow reserve-guided versus angiography-guided percutaneous coronary intervention. PloS One. 2021;16.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MB evaluated the QFRs, interpreted the patient data during the acute coronary syndrome phase and follow-up period, and obtained bioethics approval. GZ performed the statistical analyses, analyzed QFRs, and was a major contributor to writing the manuscript. NJ collected and analyzed the patient data during the follow-up period, performed the Seattle angina score questionnaire, and was a major contributor to writing the manuscript. RU analyzed the patient data, coordinated the study, and supervised the writing process. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by The Kaunas Regional Biomedical Research Ethics Committee No. BE-2-14, study protocol No. 1 and conducted under the principles of the Helsinki Declaration and local laws and regulations. We confirm that informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barauskas, M., Žiubrytė, G., Jodka, N. et al. Quantitative flow ratio vs. angiography-only guided PCI in STEMI patients: one-year cardiovascular outcomes. BMC Cardiovasc Disord 23, 136 (2023). https://doi.org/10.1186/s12872-023-03153-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03153-7