Abstract
Background
Beta-blockers are first-line clinical drugs for the treatment of chronic heart failure (CHF). In the guidelines for cardiac rehabilitation, patients with heart failure who do or do not receive beta-blocker therapy have different reference thresholds for maximal oxygen uptake (VO2max). It has been reported that left atrial (LA) strain can be used to predict VO2max in patients with heart failure, which can be used to assess exercise capacity. However, most existing studies included patients who did not receive beta-blocker therapy, which could have a heterogeneous influence on the conclusions. For the vast majority of CHF patients receiving beta-blockers, the exact relationship between LA strain parameters and exercise capacity is unclear.
Methods
This cross-sectional study enrolled 73 patients with CHF who received beta-blockers. All patients underwent a thorough resting echocardiogram and a cardiopulmonary exercise test to obtain VO2max, which was used to reflect exercise capacity.
Results
LA reservoir strain, LA maximum volume index (LAVImax), LA minimum volume index (LAVImin) (P < 0.0001) and LA booster strain (P < 0.01) were all significantly correlated with VO2max, and LA conduit strain was significantly correlated with VO2max (P < 0.05) after adjusting for sex, age, and body mass index. LA reservoir strain, LAVImax, LAVImin (P < 0.001), and LA booster strain (P < 0.05) were significantly correlated with VO2max after adjusting for left ventricular ejection fraction, the ratio of transmitral E velocity to tissue Doppler mitral annulus e′ velocity (E/e′), and tricuspid annular plane systolic excursion. LA reservoir strain with a cutoff value of 24.9% had a sensitivity of 74% and specificity of 63% for the identification of patients with VO2max < 16 mL/kg/min.
Conclusion
Among CHF patients receiving beta-blocker therapy, resting LA strain is linearly correlated with exercise capacity. LA reservoir strain is a robust independent predictor of reduced exercise capacity among all resting echocardiography parameters.
Clinical Trial registration: This study is a part of the Baduanjin-Eight-Silken-Movement with Self-efficacy Building for Patients with Chronic Heart Failure (BESMILE-HF) trial NCT03180320 (ClinicalTrials.gov, registration date: 08/06/2017).
Key points
-
A significant linear correlation exists between resting LA strain and exercise capacity in CHF patients receiving beta-blocker therapy.
-
LA reservoir strain is the strongest independent predictor of decreased exercise capacity among all LA strain parameters.
-
LA booster strain is also an independent predictor of decreased exercise capacity.
Similar content being viewed by others
Introduction
Beta-blockers are first-line clinical drugs for the treatment of chronic heart failure (CHF). They can reduce exercise heart rate, increase exercise fatigue, and reduce maximum oxygen uptake (VO2max) by approximately 7–15% [1, 2]. Therefore, in the guidelines for cardiac rehabilitation, patients with heart failure who do or do not receive beta-blocker therapy have different reference thresholds for VO2max [3]. Previous studies have reported that left atrial (LA) reservoir strain, conduit strain and booster strain measured by speckle-tracking echocardiography can predict the VO2max of patients with heart failure, which can be used to reflect exercise capacity [4, 5]. However, to the best of our knowledge, most existing studies have not limited the use of beta-blockers in the enrolled population. Therefore, the enrollment of a small number of patients with heart failure who did not use beta-blockers could lead to heterogeneity and influence study conclusions. In the real world, the majority of patients with heart failure use beta-blockers. No studies have reported the relationship between LA dysfunction and exercise capacity in patients with heart failure who do or do not receive beta-blocker therapy; thus, the results are unclear. Therefore, the main objectives of our study were to investigate the relationship between LA strain and exercise capacity in a CHF population who used beta-blockers.
Methods
Setting
This study involved patients from the Outpatient Clinic, Cardiac Rehabilitation Division, Ersha Branch, Guangdong Provincial Hospital of Chinese Medicine.
Study population
This was a cross-sectional study as well as a part of the Baduanjin-Eight-Silken-Movement with Self-efficacy Building for Patients with Chronic Heart Failure (BESMILE-HF) trial (NCT03180320, ClinicalTrials.gov, registration date: 08/06/2017) [6]. Patients with CHF were prospectively recruited between February 2019 and July 2022 if they fulfilled the following inclusion criteria: (1) ≥ 18 years of age; (2) met the diagnostic criteria for CHF [7]; (3) clinically stable, defined as symptoms/signs that remained generally unchanged for ≥ 1 month; (4) New York Heart Association class II or III; (5) used beta-blockers; and (6) provided informed consent [8].
The exclusion criteria were as follows: (1) patients with contraindications for exercise testing, namely, early phase after acute coronary syndrome (up to 6 weeks), life-threatening cardiac arrhythmias, acute heart failure (during the initial period of hemodynamic instability), uncontrolled hypertension (systolic blood pressure > 200 mmHg and/or diastolic blood pressure > 110 mmHg), advanced atrioventricular block, acute myocarditis and pericarditis, moderate to severe aortic valve/mitral stenosis, severe aortic valve/mitral regurgitation, severe hypertrophic obstructive cardiomyopathy, acute systemic illness, or intracardiac thrombus; (2) patients with serious acute or chronic diseases affecting major organs or with mental disorders; (3) patients with a history of cardiac surgery, cardiac resynchronization therapy, intracardiac defibrillation, or implantation of a combined device within the previous 3 months; (4) patients with a history of cardiac arrest within 1 year; (5) patients with a history of peripartum cardiomyopathy, hyperthyroid heart disease, or primary pulmonary hypertension; and (6) patients unable to perform a recumbent bicycle stress test (Fig. 1) [6].
Eligible participants underwent clinical evaluation (including history of cardiac risk factors and medications), height and weight measurements, blood testing, and electrocardiography. They then underwent a cardiopulmonary exercise test (CPET) and transthoracic echocardiography assessment at rest on the same day (Fig. 2A, B). The BESMILE-HF study[6] was approved by the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (Approval No. B2016-202-01). All of the participants provided written informed consent.
Illustration of speckle-tracking echocardiography examination (A) and cardiopulmonary exercise testing (B). Strain analysis of the left atrium in the locally enlarged apical four-chamber view and the LA strain curve throughout the cardiac cycle (C). The curves of VO2 and VCO2 with time and work rate, respectively (D). LA, left atrial; VO2, oxygen uptake; VCO2, carbon dioxide uptake; VO2max/pre, ratio of maximum to predicted oxygen uptake, WR, work rate
Cardiopulmonary exercise test
The CPET was performed using a standard ramp protocol (initial work rate of 0 W with an increase of 10% per minute of the predicted maximum work rate, which was calculated with a previously described formula[9]). The maximal exercise test was performed on an electronically calibrated recumbent bicycle (ERG 911S; Schiller, Baar, Switzerland). The patients started at 3 min of no-load cycling (0 W) and maintained pedaling with stepwise increases in the workload by 10% of the predicted VO2max every minute until they reached the maximum perceived effort [10]. Finally, the patient was instructed to ride the recumbent bicycle for 3 min without any load. The peak VO2 was averaged over 30 s as usually prescribed [3, 11]. Respiratory gas analysis was performed on a breath-by-breath basis with a metabolic cart (PowerCube, Ganshorn, Germany) and LF8 software (CARDIOVIT, CS-200 ergospirometer; Schiller AG, Switzerland). VO2max and the maximum respiratory exchange ratio (RERmax) were measured during an adequately performed test (Fig. 2B, D).
Echocardiography
Transthoracic echocardiography was performed with the patients at rest on an EPIQ 7C ultrasound system (Philips Healthcare, Amsterdam, the Netherlands) according to the guidelines of the American Society of Echocardiography (2019) [12].
Additional optimized left ventricular (LV) and LA images were acquired at a frame rate > 50 frames/s. In apical 4-, 3-, and 2-chamber views of 3 continuous cardiac cycles at rest, the early diastolic peak flow velocity of the mitral valve on pulsed-wave Doppler (E), the early diastolic velocity of the septal and lateral wall annulus on tissue Doppler (e′) and the ratio of E to e′ (E/e′) were calculated. Three-dimensional full-volume images at rest were acquired to calculate the LV volume, stroke volume and ejection fraction. All images were stored digitally. The volume and strain analyses were performed offline using commercially available software (QLab 10.8.0; Philips Healthcare). The LA maximum and minimum volume index (LAVImax and LAVImin, respectively) and the LA total empty fraction (LATEF) were evaluated from the apical 4- and 2-chamber views by the 2-dimensional quantitative speckle-tracking method [13].
Speckle-tracking echocardiography
All images were analyzed by a single investigator (S.C.) who was blinded to the participants’ characteristics and exercise performance. For the LA strain, the LA endocardium was manually traced at the end systole stage of LV, and the software automatically tracked the myocardium throughout the cardiac cycle on electrocardiography using R-to-R gating. Figure 2C shows the LA strain curve throughout the cardiac cycle. The region of interest was adjusted to the smallest LA wall thickness for tracking. The LA strain is the average value measured in the 4- and 2-apical chamber views. In the reservoir phase, as the left atrium filled and stretched, there was a positive atrial strain that peaked in systole at the end of LA filling and before the opening of the mitral valve; this was the LA reservoir strain, which was defined as the difference between the nadir and the peak of the strain curve. Elevated LAVI was defined as LAVImax ≥ 34 mL/m2, while reduced LA reservoir strain was defined as LA reservoir strain < 23% [4, 14]. Subsequent passive LA emptying with the opening of the mitral valve was observed, with negative deflection of the strain curve until a plateau was reached; this was the LA conduit strain, which was defined as the difference between the peak of the strain curve and the onset of atrial contraction following the P wave. Then, a second negative deflection in the strain curve was observed corresponding to atrial systole, which represented LA active contraction. The LA booster strain was defined as the difference between the onset of atrial contraction and the nadir of the strain curve [13, 14] (Fig. 2C).
The LV endocardium was traced at end systole in the 4-, 3-, and 2- apical views; the region of interest was selected with software and was adjusted to accommodate the thickness of the LV myocardium. The LV global longitudinal strain (LVGLS) was measured as the average strain of 17 segments.
Statistical analysis
This was an observational cross-sectional study. The predictor variables were LA strains. Potential covariates or confounders were LA volume index, LV function, right ventricular function, age, sex, body mass index (BMI), biochemical index, etc. The outcome variable was VO2max.
The independent sample t test was used to compare data with a normal distribution between groups. Data that did not conform to a normal distribution were compared using the rank sum test. Linear regression was performed using VO2max as the dependent variable. Univariate regression was used to evaluate the independent contribution of LA strain and LA volume to VO2max. Multivariate linear regression was used to combine clinical and LV function correction models.
Receiver operating characteristic (ROC) curve analysis was used to evaluate the area under the curve (AUC) for LA strain to predict impaired VO2max, which was defined as VO2max < 16 mL/min/kg. This reference value of VO2max was based on the Weber classification [15, 16]. DeLong's test was used to compare the AUC values.
Statistical analysis was performed using STATA 17.0 (STATA Corp, College Station, TX, USA) and MedCalc 20.1.0 (MedCalc Software Ltd., Ostend, Belgium). All tests were two-tailed, and P < 0.05 was considered statistically significant. Continuous variables are expressed as the mean ± standard deviation, and categorical variables are expressed as numbers and percentages.
Results
Patient characteristics
Of the 83 CHF patients treated with beta-blockers included in the study, 10 were excluded due to medical histories or clinical conditions (Fig. 1). Therefore, the final sample included 73 patients (mean age of 61 ± 10 years; 78% men). In the overall population, 35 patients (48%) had a VO2max of < 16 mL/kg/min, and 38 patients had a VO2max (52%) of ≥ 16 mL/kg/min. In general, patients with a reduced VO2max were older, were more likely to be female, had a higher NT-proBNP level, had a slightly lower hemoglobin level, and had worse renal function. There was no significant difference in comorbidities or combined medications between the two groups (Table 1).
Echocardiography parameters and VO2max
Univariate linear regression showed that E/e′ (P < 0.0001), E, left ventricular end systolic volume index (LVESVI) (above P < 0.01), left ventricular end diastolic volume index (LVEDVI), and left ventricular ejection fraction (LVEF) (all P < 0.05) were significantly correlated with VO2max (Table 2). The LV systolic function parameters LVGLS and left ventricular stroke volume index (LVSVI) and the right ventricular systolic function parameter tricuspid annular plane systolic excursion (TAPSE) were not significantly correlated with VO2max.
LA function and VO2max
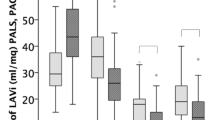
This study explored LA function. In the overall population, 22 patients (30%) had an elevated LAVI (≥ 34 mL/m2), and 36 patients (49%) showed a reduced LA reservoir strain (< 23%) [4, 14]. When VO2max decreased, LAVI increased, and the absolute value of LA strain decreased (Fig. 3). An LA reservoir strain of < 24.9% predicted a VO2max of < 16 mL/kg/min with high specificity and moderate sensitivity in this study (Table 3, Fig. 4).
Considering the results of univariate regression, clinical significance, and reducing collinearity of variables to avoid overfitting, the predictor variables of this study were LA reservoir strain, LA conduit strain, LA booster strain, LAVImax and LAVImin. Potential covariates or confounders were LVEF, E/e', TAPSE, age, sex, BMI, NT-proBNP level and hemoglobin level, which were selected based on the positive results of the univariate regression and in combination with clinical physiology and pathology[4] (Tables 1, 2). The outcome variable was VO2max. Therefore, we used three multivariate regression models to examine the independent effect of LA strain. The results showed that LA reservoir strain, LAVImax, LAVImin (P < 0.001), and LA booster strain (P < 0.05) were significantly correlated with VO2max in the 3 models (Table 4).
In our study, the lower the LA reservoir strain was, the lower VO2max was, and the larger LAVImax was (Fig. 5). The VO2max of most patients with increased LAVImax and decreased LA reservoir strain was less than 16 mL/kg/min (Fig. 5, lower left quadrant); the VO2max of most patients with normal LAVImax and normal LA reservoir strain was greater than 16 mL/kg/min (Fig. 5, upper right quadrant).
Bubble chart of LA reservoir strain < or ≥ 24.9% and normal/dilated LAVImax in patients with chronic heart failure. The size of the bubble: LAVImax. The x-axis of constant: LA reservoir strain = 24.9%. The y-axis of constant: VO2max = 16 mL/kg/min. LA, left atrial; LAVImax, maximum left atrial volume index; VO2max, maximum oxygen uptake
Discussion
Beta-blockers are first-line drugs for the treatment of heart failure, and they are administered to the majority of heart failure patients [7]. In cardiac rehabilitation guidelines, patients with heart failure who receive beta-blockers and those who do not receive beta-blockers have different reference thresholds for VO2max [3]. To avoid a heterogeneous effect of beta-blockers on assessing the association between LA dysfunction and reduced exercise capacity in patients with CHF, our study assessed only patients with CHF who received beta-blockers. To the best of our knowledge, our study is the first to confirm that in CHF patients receiving beta-blocker therapy (1) a linear correlation exists between resting LA strain and exercise capacity; (2) among all LA strain parameters, LA reservoir strain is the strongest independent predictor for detecting decreased exercise capacity and for identifying patients with VO2max < 16 mL/kg/min; notably, LA reservoir strain has high sensitivity and moderate specificity, with a critical value of 24.9%; and 3) LA booster strain was also an independent predictor of decreased exercise capacity.
Effect of beta-blockers on maximal oxygen uptake
As widely used drugs for the treatment and management of patients with CHF [7], beta-blockers have a significant impact on the regulation of the heart and peripheral blood vessels and can reduce the mortality and hospitalization rate in CHF patients [17]. When the β-receptors of the heart are blocked, the exercise heart rate is 20–30% lower than that of patients not taking beta-blockers, but the stroke volume increases by 5–23% to maintain sufficient cardiac output [18]. Due to the increase in stroke volume, myocardial work efficiency increases, myocardial oxygen consumption increases, and VO2max slightly decreases [1, 19]. In addition, β-receptor blockade causes peripheral vasodilation, pulmonary bronchoconstriction, and increased muscle and liver glycogenolysis; furthermore, this phenomenon affects the oxygen transport and work efficiency of skeletal muscles [20]. These conditions exist with both selective and nonselective beta-blockers.
Exercise capacity and maximal oxygen uptake in CHF patients
Cardiopulmonary dysfunction and skeletal muscle atrophy are the main causes of reduced exercise capacity in patients with heart failure [21]. With the progression of CHF, cardiac systolic and diastolic dysfunction leads to decreased exercise capacity, which is caused by impaired cardiac output, hemodynamic changes, and myocardial remodeling [22]. In addition, oxidative stress and proinflammatory conditions lead to muscle atrophy associated with a decrease in CHF physical function [23]. The exercise capacity of the included patients, represented by VO2max, was generally reduced, mainly due to heart-related cardiopulmonary function impairment.
LA dysfunction and reduced exercise capacity in CHF patients
Our study of patients with CHF who received beta-blockers showed that, compared with other echocardiographic parameters, LA reservoir strain was the strongest biomarker for predicting the exercise capacity of these patients. LA reservoir strain with a cutoff value of 24.9% had high sensitivity and moderate specificity in the detection of VO2max < 16 mL/kg/min in all subjects. LA booster strain with a cutoff value of -8.0% had moderate sensitivity and high specificity in the detection of VO2max < 16 mL/kg/min in all subjects. LA strain has been considered a useful echocardiographic marker in a series of patients with heart diseases and as an independent predictor of all-cause mortality [24, 25]. In terms of exercise capacity evaluation, previous heart failure studies that did not limit beta-blockers only found that LA reservoir strain was associated with VO2max and VE/VCO2slope [26]. LA reservoir strain can specifically reflect the pathophysiological mechanism of LA, such as LA myocardial remodeling and dysfunction [27], which can increase hemodynamic stress and pulmonary vascular resistance, resulting in a decrease in VO2max [28].
A recent clinical study involving myocardial biopsy demonstrated that both LA reservoir strain and LA volume were predictors of LA fibrosis [29]. LAVI is a marker of LV systolic and diastolic dysfunction[30] and has been shown to be associated with exercise tolerance in HF patients [31]. However, an increased LA volume is unlikely to completely reflect the complex phenomenon of LA remodeling. Therefore, the introduction of LA strain can be used to more accurately evaluate the physiological and pathological LA changes in CHF patients; additionally, the predictive ability of LA reservoir strain is slightly better than that of LA volume [4, 29]. In our study, LA strain and exercise capacity were closely and independently correlated, and LA reservoir strain had the strongest predictive ability among the LA strain parameters in patients with CHF.
After adjusting for LVEF, E/e′, and TAPSE, LA reservoir strain still had a good ability to predict exercise capacity (P = 0.003). Considering that the interaction between atrial phasic function and ventricular mechanics regulates cardiovascular function, our findings are not surprising. Because the increase in preload led to more severe LA dysfunction [32], LA dysfunction in turn aggravated heart failure [33].
Advanced age, BMI and sex are important factors in exercise capacity [34]. In a healthy population, LA reservoir strain showed a decreasing trend with increasing age [35]. In our study, LA reservoir strain was linearly correlated with VO2max after adjusting for age, sex, and BMI. These results suggest that the value of LA reservoir strain for predicting exercise capacity in patients with CHF is not affected by those clinical factors.
Our study showed that LA booster strain was also an independent predictor of VO2max. In previous studies, it was unclear whether LA booster strain could independently predict VO2max [4, 36, 37]. The previously reported contradictory findings may have been related to the limitation of LA booster strain measurement accuracy due to the inclusion of some patients with atrial fibrillation. In our study, only one patient had a history of paroxysmal atrial fibrillation. Due to the inhibitory effect of beta-blockers on atrial fibrillation, this patient did not develop atrial fibrillation during the study period. Relatively accurate LA conduit strain and LA booster strain values were obtained. LA booster strain represents the LA mechanical changes during the late LV diastole [13]. After active LA contraction, the pressure gradient from the left atrium to the left ventricle increases, and the left ventricle is further filled. Inoue’s [36] study found that there was a strong correlation between LA booster strain and LA reservoir strain (r = 0.81, P < 0.001). The decrease in LA pressure after atrial contraction increases the pressure gradient from the pulmonary vein to the LA, thereby increasing LA filling [36]. Therefore, the active booster function of the left atrium is one of the determinants of LA reservoir strain. Unlu’s [38] study proved that LA booster strain is the only LA strain parameter that can independently reflect the intrinsic LA function and is not affected by changes in blood volume because in the late LV diastolic phase (LA systolic phase), the participation of the left atrium in blood volume is relatively small, and it is almost unrelated to volume load. After the use of beta-blockers, the heart rate is reduced, which is manifested as prolonged diastolic time. As a result, the LA contraction time is relatively increased. Thus, LA booster strain in CHF patients using beta-blockers is more attributable to LA function.
Most LV filling is completed in the early LV diastole, and LA conduit strain is more susceptible to the effect of LV diastolic function than LA booster strain. Therefore, in our study, we corrected the representative parameter of diastolic function, which is also the early diastolic E/e′. Consequently, LA conduit strain was no longer an independent predictor of VO2max.
Limitations
Our study had strict inclusion criteria; for example, only patients with stable New York Heart Association classification II or III CHF were included. The sample size was moderate but adequate because the VO2max of nearly half of the subjects was decreased. Second, the peak diastolic flow velocity of the mitral valve (A) or the average late-diastolic velocity of the mitral annulus (a′ave) for patients with short-term or other cardiac diseases was not reached in 23 patients (32%) in this study. Therefore, A and a'ave were not included in the univariate regression. Third, severe valvular regurgitation could be a manifestation or cause of CHF and affect LA function. To avoid these confounding factors, our study excluded patients with severe valvular regurgitation and only included patients with mild or moderate valvular regurgitation. Fourth, although there have been an increasing number of studies on LA strain, the current software package for measuring LA strain that was used to evaluate LV strain has inherent limitations. Fifth, the Jones formula we used to calculate the predicted VO2max might overestimate the predicted VO2max values. Finally, this study only included patients who took beta-blockers, and further studies are needed to compare patients who do and do not take beta-blockers.
Conclusion
This study demonstrated that in patients with CHF who received beta-blockers, LA reservoir strain is a robust independent predictor of reduced exercise capacity among resting echocardiographic parameters. LA booster strain is independently correlated with VO2max. An LA reservoir strain of 24.9% can be used to identify patients with VO2max < 16 mL/kg/min with high specificity and moderate sensitivity. LA strain is a potentially useful indicator for evaluating the efficacy of cardiac rehabilitation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CHF:
-
Chronic heart failure
- CI:
-
Confidence interval
- E:
-
Early diastolic peak flow velocity of the mitral valve
- e′ave :
-
Average early diastolic velocity of mitral annular
- E/e′:
-
Ratio of transmitral E velocity to tissue Doppler mitral annular e’ave velocity
- LA:
-
Left atrial
- LATEF:
-
Left atrial total empty fraction
- LAVImax :
-
Maximum left atrial volume index
- LAVImin :
-
Minimum left atrial volume index
- LV:
-
Left ventricular
- LVEDVI:
-
Left ventricular end diastolic volume index
- LVESVI:
-
Left ventricular end systolic volume index
- LVEF:
-
Left ventricular ejection fraction
- LVGLS:
-
Left ventricular global longitudinal strain
- RERmax :
-
Maximum respiratory exchange rate
- TAPSE:
-
Tricuspid annular plane systolic excursion
- VO2max :
-
Maximum oxygen uptake
References
Mitchell BL, Davison K, Parfitt G, et al. Physiological and perceived exertion responses during exercise: effect of β-blockade. Med Sci Sports Exerc. 2019;51(4):782–91.
Dodd S, Powers S, O’Malley N, et al. Effects of beta-adrenergic blockade on ventilation and gas exchange during incremental exercise. Aviat Space Environ Med. 1988;59(8):718–22.
Corrà U, Agostoni PG, Anker SD, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the committee on exercise physiology and training of the heart failure association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(1):3–15.
Maffeis C, Rossi A, Cannata L, et al. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Heart Failure. 2022;9(2):842–52.
Pugliese NR, De Biase N, Conte L, et al. Cardiac reserve and exercise capacity: insights from combined cardiopulmonary and exercise echocardiography stress testing. J Am Soc Echocardiogr. 2021;34(1):38–50.
Chen X, Jiang W, Lin X, et al. Effect of an exercise-based cardiac rehabilitation program “Baduanjin Eight-Silken-Movements with self-efficacy building” for heart failure (BESMILE-HF study): study protocol for a randomized controlled trial. Trials. 2018;19(1):150.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876–94.
Thomas L, Muraru D, Popescu BA, et al. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. 2020;33(8):934–52.
Jones NL, Makrides L, Hitchcock C, et al. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131(5):700–8.
Myers J, Bellin D. Ramp exercise protocols for clinical and cardiopulmonary exercise testing. Sports Med. 2000;30(1):23–9.
Pugliese NR, De Biase N, Balletti A, et al. Characterization of hemodynamic and metabolic abnormalities in the heart failure spectrum: the role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol Angiol. 2022;70(3):370–84.
Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64.
Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600.
Pathan F, D’Elia N, Nolan MT, et al. Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30(1):59-70.e58.
Guazzi M, Borlaug B, Metra M, et al. Revisiting and implementing the weber and ventilatory functional classifications in heart failure by cardiopulmonary imaging phenotyping. J Am Heart Assoc. 2021;10(5): e018822.
Smarż K, Jaxa-Chamiec T, Chwyczko T, et al. Cardiopulmonary exercise testing in adult cardiology: expert opinion of the working group of cardiac rehabilitation and exercise physiology of the polish cardiac society. Kardiol Pol. 2019;77(7–8):730–56.
Nielsen PB, Larsen TB, Gorst-Rasmussen A, et al. β-Blockers in atrial fibrillation patients with or without heart failure: association with mortality in a nationwide cohort study. Circ Heart Fail. 2016;9(2): e002597.
Head A. Exercise metabolism and beta-blocker therapy. An update. Sports Med. 1999;27(2):81–96.
Palau P, Seller J, Domínguez E, et al. Effect of β-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78(21):2042–56.
Kaiser P. Physical performance and muscle metabolism during beta-adrenergic blockade in man. Acta Physiol Scand Suppl. 1984;536:1–53.
Adachi H. Cardiopulmonary exercise test: the most powerful tool to detect hidden pathophysiology. Int Heart J. 2017;58(5):654–65.
Smarż K, Jaxa-Chamiec T, Chwyczko T, et al. Cardiopulmonary exercise testing in adult cardiology expert opinion of the working group of cardiac rehabilitation and exercise physiology of the polish cardiac society. Kardiol Polska. 2019. https://doi.org/10.33963/KP.14889.
Howden EJ, Bigaran A, Beaudry R, et al. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26(3):305–15.
Jia F, Chen A, Zhang D, et al. Prognostic value of left trial strain in heart failure: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9: 935103.
Weerts J, Barandiarán Aizpurua A, Henkens M, et al. The prognostic impact of mechanical atrial dysfunction and atrial fibrillation in heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2021;23(1):74–84.
Leite L, Mendes SL, Baptista R, et al. Left atrial mechanics strongly predict functional capacity assessed by cardiopulmonary exercise testing in subjects without structural heart disease. Int J Cardiovasc Imaging. 2017;33(5):635–42.
Benfari G, Mandoli G, Magne J, et al. Left atrial strain determinants across heart failure stages; insight from MASCOT registry. Eur Heart J Cardiovasc Imaging. 2022. https://doi.org/10.1093/ehjci/jeab289.078.
Bhatt A, Flink L, Lu DY, et al. Exercise physiology of the left atrium: quantity and timing of contribution to cardiac output. Am J Physiol Heart Circ Physiol. 2021;320(2):H575-h583.
Lisi M, Mandoli GE, Cameli M, et al. Left atrial strain by speckle tracking predicts atrial fibrosis in patients undergoing heart transplantation. Eur Heart J Cardiovasc Imaging. 2022;23(6):829–35.
Gehlken C, Screever EM, Suthahar N, et al. Left atrial volume and left ventricular mass indices in heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2021;8(4):2458–66.
Rossi A, Cicoira M, Bonapace S, et al. Left atrial volume provides independent and incremental information compared with exercise tolerance parameters in patients with heart failure and left ventricular systolic dysfunction. Heart. 2007;93(11):1420–5.
Bastos MB, Burkhoff D, Maly J, et al. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J. 2020;41(12):1286–97.
Sun M, Xing Y, Guo Y, et al. Left atrial reservoir strain is an outstanding predictor of adverse cardiovascular outcomes in patients undergoing maintenance hemodialysis: assessment via three-dimensional speckle tracking echocardiography. Clin Cardiol. 2022. https://doi.org/10.1002/clc.23815.
Roibal Pravio J, Barge Caballero E, Barbeito Caamaño C, et al. Determinants of maximal oxygen uptake in patients with heart failure. ESC Heart Fail. 2021;8(3):2002–8.
Stefani LD, Trivedi SJ, Ferkh A, et al. Changes in left atrial phasic strain and mechanical dispersion: effects of age and gender. Echocardiography. 2021;38(3):417–26.
Inoue K, Khan FH, Remme EW, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021;23(1):61–70.
Gan GCH, Bhat A, Chen HHL, et al. Left atrial reservoir strain by speckle tracking echocardiography: association with exercise capacity in chronic kidney disease. J Am Heart Assoc. 2021;10(1): e017840.
Ünlü S, Yamak BA, Sezenöz B, et al. Left atrial contractile longitudinal strain determines intrinsic left atrial function regardless of load status and left ventricular deformation. Int J Cardiovasc Imaging. 2021;37(11):3245–53.
Acknowledgements
The authors thank Yunxiang Fan (cardiology nurse) and Meifen Zhang (research assistant) at the GPHCM Department of Cardiology for assistance with data collection.
Funding
This research was funded by the General Research Fund of Traditional Chinese Medicine Science and Technology from the Guangdong Provincial Hospital of Chinese Medicine (GPHCM) (Grant No. YN2018ML02), the Clinical Research Fund of the Traditional Chinese Medicine Science and Technology (Project 1010) from GPHCM (Grant No. YN10101910) and the Research Fund from the Guangdong Traditional Chinese Medicine Health Service and Industry Development Center (Grant No. 2021YJZX014).
Author information
Authors and Affiliations
Contributions
HC, PS, and WJ designed the research study. PS, SC and HZ performed the research. YL provided help with data curation. HC analyzed the data. HC and PS wrote the manuscript. XC and HL provided advice on revising the manuscript. WL and PS provided research funding. All authors contributed to editorial changes in the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (Approval No. B2016-202-01). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with those of the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study. All procedures in this study were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, P., Cen, H., Chen, S. et al. Left atrial dysfunction can independently predict exercise capacity in patients with chronic heart failure who use beta-blockers. BMC Cardiovasc Disord 23, 128 (2023). https://doi.org/10.1186/s12872-023-03127-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-023-03127-9