Abstract
Background
Left ventricular hypertrophy (LVH) is a marker of increased risk in developing future life-threatening cardiovascular disease (CVD), however, it is unclear how CVD risk factors, such as obesity, blood pressure (BP), and tobacco use, are associated with left ventricular (LV) remodeling and LVH in urban African populations. Therefore, we aimed to identify the prevalence of LVH as well as the health factors associated with LV remodeling and LVH, within black South African adult women and their pre-pubescent children.
Methods
Black female adults (n = 123; age: 29–68 years) and their children (n = 64; age: 4–10; 55% female) were recruited from the Birth to Twenty Plus Cohort in Soweto, South Africa. Tobacco and alcohol use, physical activity, presence of diabetes mellitus, heart disease, and medication were self-reported. Height, weight, and blood pressure were measured in triplicate to determine the prevalence of obesity and hypertension respectively. Echocardiography was used to assess LV mass at end-diastole, based on linear measurements, and indexed to body surface area to determine LVH.
Results
Hypertension and obesity prevalences were 35.8% and 59.3% for adults and 45.3% and 6.3% for children. Self-reported tobacco use in adults was 22.8%. LVH prevalence was 35.8% in adults (75% eccentric: 25% concentric), and 6.3% in children. Concentric remodeling was observed in 15.4% of adults, however, concentric remodeling was only found in one child. In adults, obesity [OR: 2.54 (1.07–6.02; p = 0.02)] and hypertension [3.39 (1.08–10.62; p = 0.04)] significantly increased the odds of LVH, specifically eccentric LVH, while concentric LVH was associated with self-reported tobacco use [OR: 4.58 (1.18–17.73; p = 0.03)]. Although no logistic regression was run within children, of the four children LVH, three had elevated blood pressure and the child with normal blood pressure was overweight.
Conclusions
The association between obesity, hypertension, tobacco use, and LVH in adults, and the 6% prevalence of LVH in children, calls for stronger public health efforts to control risk factors and monitor children who are at risk.
Similar content being viewed by others
Background
Despite medical advances, cardiovascular disease (CVD) remains a global health concern affecting more than 37 million individuals [1]. CVDs are a leading cause of death globally [2,3,4]. CVD risk factors, such as hypertension, obesity, type II diabetes mellitus (T2DM), smoking, dyslipidaemia, a sedentary lifestyle, unhealthy diets, and excess alcohol intake [4, 5], are increasing in sub-Saharan Africa. South Africa is no exception, with a rising prevalence of known CVD risk factors, largely as a consequence of urbanisation and marked changes in health behaviour [6,7,8]. Thus, prevention of CVD is an important national public health goal.
Heart failure is the dominant form of CVD in South Africa, of which the main causes include hypertension, diabetes, coronary artery disease, dilated and or hypertrophic cardiomyopathy and valvular disease [9]. Unlike high-income countries, in South Africa heart failure typically affects younger working individuals and has an in-hospital mortality rate of approximately 8.4%, while 6 months post-hospital discharge, the fatality rate stands at approximately 26% [10]. As a result of the impact on young, economically active individuals, as well as the high fatality rate, heart failure in South Africa has a disproportional impact on an already fragile economy.
A key aspect of the pathological process of CVD, ultimately leading to heart failure and stroke [11, 12], is cardiac remodeling. The term is most applied to the left ventricle (LV) in which the chamber changes in size, shape, or structure [13]. Left ventricular hypertrophy (LVH), defined as an increase in muscle mass of the LV, is associated with a four-fold increase in heart failure [14,15,16,17,18,19,20]. As a result, regression of LVH is a goal of cardiovascular risk reduction [21,22,23,24]. Many of the risk factors contributing to the systemic and regional hemodynamic changes that drive the development of LVH [25] are modifiable, including obesity [26], tobacco and alcohol use [27], and elevated blood pressure and hypertension [26].
In South African adult women, there has been a sharp rise in prevalence of overweight/obesity (68%), hypertension (46%), regular alcohol use (17%) [28], and current tobacco smokers (7.6%) [29]. Given the increasing CVD rates and the restricted access to echocardiography in the public health sector [30], understanding the role of CVD risk factors in LVH development may have major implications for preventing LVH or interventions for regression of LVH. Furthermore, the growing burden of cardiovascular risk factors could lead to a growing burden of LVH [25,26,27] and potentially increase the risk of their offspring developing CVD risk factors and life-threatening CVD disease. Therefore, the study aimed to identify the prevalence of LVH as well as the CVD risk factors associated with LV remodeling and LVH, within black South African adult women and their pre-pubescent children. This could help inform public health efforts to reduce CVD risk in South Africa.
Methods
Study population
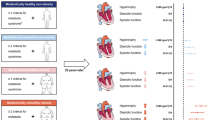
Data was collected during a cross-sectional study of vascular health in an existing birth cohort known as the Birth to Twenty Plus (BT20 +) Cohort, which started in 1990 in an urban historically disadvantaged setting, Soweto, Johannesburg, South Africa (described previously [31]), and now includes three generations. The cross-sectional study aimed to investigate the intergenerational transmission of cardiovascular health; the BT20 index female (known as 2nd generation or 2G; aged 28–29 years); their birth children or 3rd generation (3G, aged 4–10 years, female or male); and their biological mothers or 1st generation (1G, aged 43–68 years, female). For the present study, the first and second-generation participants were combined and classified as the “adult group”. Thus, 79 families, 237 participants (n = 158 adults and n = 79 children) were invited to attend for cardiovascular assessment where left ventricular remodeling and hypertrophy were assessed using echocardiography. All data were collected at the Developmental Pathways for Health Research Unit, at Chris Hani Baragwanath Academic Hospital in Soweto. Of the 237 participants recruited, n = 45 (19%) did not return to the study site and thus no echocardiography could be performed. Of the remaining 192 participants that returned to the study site, n = 5 (2.1%) was excluded due to the inability to obtain accurate linear measurements (due to excessive breast tissue), thus n = 187 (78.9%) were included in the analysis (Fig. 1). Trained researchers who spoke the participant's home language explained the study and all participants gave written informed consent (adults) or assent (children aged 7–10 years) before data collection. The Human Research Ethics Committee (Medical) of the University of the Witwatersrand approved the protocol (M190263).
CVD risk factors
Tobacco use was assessed using the Global Adult Tobacco Survey [32], alcohol use with the WHO Alcohol Use Disorders (WHO-AUDIT) test [33], and physical activity using the Global Physical Activity Questionnaire (GPAQ) [34]. Self-reported medical history (diabetes mellitus [T2DM], hypertension, high cholesterol, or previous heart disease, and current medication use) was assessed by questionnaire. Thereafter, height and weight were measured in triplicate using standardised World Health Organization measurement protocols [35]. Brachial blood pressure (BP) was measured using automated devices (MIT5 for adults; HBP-1300 for children [36]; Omron Healthcare, Kyoto, Japan). Following the International Society of Hypertension (ISH) measurement guidelines [37], participants were seated and asked to rest for at least 5 min before measurement of BP in their right arm three times in a quiet room with the monitor facing away from the participant; there was a 2 min rest interval between the measurements. The first BP measurement was discarded and the second and third measurements were averaged for analysis.
Echocardiography
Transthoracic echocardiography examination was performed by a single echocardiographer, with the Mindray DC-70 Ultrasound system (Mindray, Shenzhen China). Measurements of chamber dimensions were taken from 2D mode and left ventricular (LV) mass and relative wall thickness (RWT) were calculated. Linear measurements were made according to the American Society of Echocardiography (ASE) Guidelines: Recommendations for Cardiac Chamber Quantification in Adults [38]. LV mass was assessed at end-diastole perpendicular to the long axis of the LV. LVM was calculated according to the Devereux formula: LVM (g) = 0.8 × 1.04 ((LVDd + IVSd + LVPWd)3 – LVDd3) + 0.6 where LVDd = left ventricular diastolic diameter; IVSd = intraventricular septal diameter, LVPWd = left ventricular posterior wall thickness in diastole [38]. The left ventricular mass index (LVMI) was calculated as a ratio of LVM indexed to body surface area [38]. The RWT was defined as 2 × LVPWd/LVDd, where LVPWd = left ventricular posterior wall thickness in diastole; LVDd = left ventricular diastolic diameter [38]. RWT was used to categorise LV mass and the pattern of remodeling. Left ventricular hypertrophy (LVH) was defined as LVMI > 95 g/m2 for adult women [38] and LVMI > 95th percentile (85.6 g/m2) for children [39], and eccentric LVH (LVH with RWT ≤ 0.42) and concentric LVH (LVH with RWT > 0.42) as well as concentric remodeling (normal LVMI with RWT > 0.42) [38].
Data management
In adults, body mass index (BMI; weight (kg)/height (m)2) was categorised according to the World Health Organisation (WHO) classification, as follows: < 18.5 kg/m2 as underweight; 18.5–24.9 kg/m2 as normal; 25–29.9 kg/m2 as overweight; and ≥ 30 kg/m2 as obese [40]. In children (> 5 years), age-adjusted z-scores for BMI were calculated using the WHO reference, and children were categorised as overweight if their BMI z-score was between 1–2, and obese if their BMI z-score was > 2 [40]. While in children aged four years, age-adjusted z-scores for BMI were categorised as overweight if their BMI z-score was between 2–3, and obese if their BMI z-score was > 3 [41]. In adults, hypertension was defined as a BP ≥ 140 mmHg systolic or ≥ 90 mmHg diastolic or currently taking antihypertensive medication; in those who were not on anti-hypertensive medication, elevated BP was defined as a BP 130–139 mmHg systolic or 85–89 mmHg diastolic, following the International Society of Hypertension (ISH) Global Hypertension Practice Guidelines [37]. For children, elevated BP was defined as BP ≥ 90th percentile to < 95th percentile for height and age, while hypertension was defined as BP ≥ 95th percentile for age and height following the American Academy of Paediatrics Clinical Practice 2017 guidelines [42].
Statistical analysis
SPSS statistics 25.0 (IBM, Chicago, IL, USA) was used for statistical analysis. Non-normality of data was confirmed using visual inspection of histograms and the Shapiro–Wilk test. Continuous variables are expressed as median, interquartile range unless stated. Dichotomous variables are expressed as percentages. Multivariable logistic regression, after checking for multicollinearity by confirming a variance inflation factor of less than five [43], was conducted to examine the relationship between LVH/LV remodeling and the following health factors: age, moderate-vigorous physical activity, sedentary behaviour, obesity, hypertension, previous diagnosis of T2DM, high cholesterol, ever tobacco use and alcohol use. Statistical significance for all analyses was set at p < 0.05. Due to the low prevalence of LVH in children, regression analysis was only performed in adults.
Results
Adult women characteristics
The median age for adults was 41 years (range 28 to 68 years, n = 123) (Table 1). Median BMI was 32.5 kg/m2 (IQR: 26.6–37.7 kg/m2) with 59.3% of adults classified as obese. Forty-four out of 123 adults (35.8%) were classified as hypertensive, of whom 75% were on treatment, primarily a diuretic. Of the 123 adults, 22.8% were current or past tobacco users (self-reported smoking and smokeless tobacco), and 55.3% regularly consumed alcohol. Three adults reported having previous heart disease. A small percentage of adults reported having high cholesterol and T2DM (12.2% and 2.4% respectively). While all participants with self-reported diabetes were on treatment, only 26.7% of adults with high cholesterol were. The median LVMI for adults was 84.75 (IQR: 71.36–102.33) g/m2. Sixty out of 123 adults (48.8%) had normal LV geometry, while 19 out of 123 (15.4%) had concentric remodeling. Forty-four out of 123 women (35.8%) had LVH, of whom 33 out of 123 (26.8%) had eccentric LVH and 11 out of 123 adults (8.9%) had concentric LVH (Table 1).
Children characteristics
The median age for children was 7 years (range 4 to 10 years, n = 64, 54.7% female) (Table 1). Children had a median BMI of 15.8 kg/m2 (IQR: 14.8–17.2 kg/m2) with 6.3% of children classified as obese. Nine out of sixty-four children (14.1%) had elevated BP, while twenty-nine out of sixty-four children (45.3%) were hypertensive. The median LVMI in children was 56.29 (IQR: 47.61–69.17) g/m2. Fifty-nine out of 64 (92.2%) of children had normal LV geometry, one child had concentric remodeling, while the remaining 4 children (6.3%) had eccentric LVH. No concentric LVH was observed within the children of the current study (Table 1).
Left ventricular (LV) remodeling and left ventricular hypertrophy (LVH) in adults
Table 2 compares characteristics of adults with and without LVH. Overall, women with LVH were significantly older (48 (min: 29, max 66) vs 29 (min: 28, max: 68) years, p = 0.04), were more often obese (73% vs 52%, p = 0.04), and more often had hypertension (52% vs 27%, p = 0.01) compared to those without LVH. When considering LVH regardless of pattern, after adjusting for age, moderate-vigorous physical activity, sedentary behaviour, obesity, hypertension, T2DM, high cholesterol, ever tobacco use, and regular alcohol use, hypertension (OR: 3.39 (1.08–10.62), p = 0.04), and obesity (OR: 2.54 (1.07–6.02) were significantly associated with LVH. Obesity was the only factor significantly associated with eccentric LVH (OR: 3.46 (1.28–9.37), p = 0.02), and ever tobacco use was the only factor significantly associated with concentric LVH (OR: 5.06 (1.24–20.69), p = 0.02), although the number of adults with this condition was small. No other significant associations were found with LV remodeling in the multivariable logistic regression models (Table 3).
Figure 2 depicts those adults with LVH and the number of CVD risk factors. The prevalence of LVH in adults significantly increased (p = 0.002) with the number of CVD risk factors reported.
Percentage of South African adult women and children with left ventricular hypertrophy (LVH) according to the number of cardiovascular disease (CVD) risk factors. CVD risk factors for women included: obesity, hypertension, self-reported type II diabetes mellitus, self-reported high cholesterol, physical activity, tobacco use, and alcohol consumption. CVD risk factors for children included: obesity, hypertension, whether their mother and or grandmother use tobacco
Left ventricular (LV) remodeling and left ventricular hypertrophy (LVH) in children
Over 90% of children (median age 7 years, range 4–10, 59.3% female) had normal LV geometry despite 14.1% (n = 9) having elevated BP, 45.3% (n = 29) being hypertensive and 17.2% (n = 11) being overweight and 6.3% (n = 4) being obese (Table 1). Of the four children with LVH (all eccentric LVH), three had elevated BP and were normal weight, while the fourth child had normal BP, but was overweight. Figure 2 illustrates that in the current study, three out of the four children with LVH had a known CVD risk factor.
Discussion
In the present study, we assessed the prevalence of LVH in black South African women and their children/grandchildren and health factors associated with LVH. We found a high prevalence of LVH in South African black adult women (28–68 years), with over a quarter having eccentric LVH. Obesity and hypertension, two modifiable health factors, were associated with eccentric LVH, while self-reported tobacco use was related to concentric LVH. Of the women with LVH, half had at least three CVD risk factors. Despite the small sample size, 6% of children aged 4–10 years had eccentric LVH, of which three had elevated blood pressure (BP). The current study highlights that multiple factors significantly drive the development of LVH in adult women. In addition, although only four children had LVH, the high prevalence of elevated BP in children is concerning and supports ongoing calls for prevention efforts, particularly for youth.
In African countries, CVD is increasing rapidly. LVH is a known marker of increased risk for developing future life-threatening CVD and there is evidence to suggest that LVH is higher in black populations of African descent [44]. Various studies conducted over the past decade in Sub-Saharan Africa have reported the prevalence of LVH among women (mean age range: 43–57 years) to vary from 19.9% to 44.5% [19, 45,46,47], which is consistent with the current study’s findings (35.8% of South African women; mean age: 41.3 years). Our estimates are, however, higher than previously reported South African data by Maseko et al. and Libhaber et al. (LVH prevalence of 19.9% and 21.7% respectively, despite similar ages (44 years)) [45, 46]. This increase in LVH prevalence may be, in part, due to increased risk cardiovascular risk factors from 2013. However, although the populations in both studies were similar to that of the current study (both black South African), the methodology was different. Both studies used LVM indexed to height2.7 [45, 46], whilst the current study used LVM indexed to body surface area. However, when indexing LVM to height2.7, the current study’s prevalence increased slightly from 35.8% (n = 44) to 38.2% (n = 47) (data not shown). To date, there remains controversy around the method to index LVM to accurately identify LVH within individuals in the Southern African region. Due to the high prevalence of stunting observed within the South African population [48, 49], LVM indexed to body surface area was identified as the most appropriate method so as not to under or overestimate LVH calculated by height2.7. This, in conjunction with the guidelines from The American Society of Echocardiography Recommendations for Cardiac Chamber Quantification in Adults [38], further supports the use of body surface area over height2.7.
LVH is a compensatory response to cardiac insult through interactions between pressure and/or volume overload [50]. Increasing age, hypertension, and obesity are the three main cardiovascular risk factors that drive pressure and volume overload within the heart [50, 51]. The current study found that obesity and hypertension were associated with a two-fold and a three-fold respective risk of developing LVH (irrespective of type), and a three-fold risk of developing eccentric LVH. This is in line with results from the Framingham study that suggest a 7% increase for risk for developing LVH per one unit increase of body mass index (BMI) in women [52, 53]. Furthermore, there is a three-fold risk of developing CVD in individuals with BP ≥ 130/90 mmHg compared to individuals with BP < 120/80 mmHg [54]. In South Africa, approximately 68% of women (≥ 15 years) are overweight or obese [55] and hypertension is currently reported as one of the most common causes of CVD [19, 55]. Current understanding of hemodynamic alterations accompanying obesity and hypertension suggests that they drive an increase in LV filling and stroke volume, which specifically causes LV dilation and eccentric, rather than concentric, LVH [26, 56,57,58,59]. However, studies by Woodiwiss et al. [60] and Avelar et al. [58] found that adiposity was associated with concentric LVH, independently of BP. Our smaller sample size (n = 123, of which 11 had concentric LVH) may explain the lack of an association between obesity and concentric LVH in our study. In addition, due to the confounding effects of both hypertension and obesity, their interactions and duration within the same individual, left ventricular remodeling and hypertrophy may be different. Therefore, before definitive conclusions can be reached within this population, further studies investigating interactions of obesity and hypertension are required.
Along with obesity and hypertension, smoking prevalence in South Africa is currently on the rise [55]. There is sound evidence that smoking affects cardiac structure and function [61, 62], and results from other studies suggest significant increases in left ventricular mass index, and thus left ventricular hypertrophy, in individuals who smoke compared to those that don’t [61,62,63,64]. Similarly, although our sample of women that had concentric left ventricular hypertrophy was small, our data showed an association between smoking and concentric left ventricular hypertrophy.
The current study found a high prevalence of elevated blood pressure (59.4%) in children. Other recent studies from South Africa have also found high rates of elevated blood pressure in children [65, 66], and a stark increasing trend in paediatric hypertension was identified by a recent meta-analysis [67]. It should be noted that the present study was not designed to determine clinical prevalence, which would require confirmation of abnormal readings at multiple follow-ups and through auscultation, and we did not have data on the extent to which secondary or white-coat hypertension contributed to high blood pressure. Nevertheless, the worrying levels of paediatric elevated blood pressure require further exploration. Although only four children had LVH, of these, three had elevated blood pressure and the one child that was normotensive was overweight. Thus, following the same pattern observed in adults, rising CVD risk factors in children, namely elevated blood pressure and weight gain, are the driving factors behind CVD risk in children that also may persist into adulthood. This further support calls for earlier identification of children that are at risk and for interventions where necessary, especially within children.
A strength of this study is the indexing of LVM to body surface area, since indexing LVM to body height may result in an error in LVH classification due to the prevalence of stunting in this population [48, 49]. A limitation of the present study was the ages within the adult group. As this study recruited from within an existing birth cohort with all index children recruited at birth in 1990, these ‘children’ were all 29 years old adults at the time of the measurements, resulting in a clustering of adults around 29 years with their parents aged 45–65 years. Additionally, as we recruited participants that were from the same family (three generations), several cardiac measures may be heritable within families, which could account for the high prevalence of LVH noticed within the current study. Previous studies have shown that LV function and LVM may be heritable within European [68] and American [69] families, thus, future research is needed to identify a potential heritable component within South African families of three generations. Furthermore, we relied on self-reported data for medical history (previously diagnosed T2Dm, high cholesterol and heart disease) and CVD risk factors (tobacco and alcohol use, physical activity). We acknowledge that self-reported data may underestimate CVD risk factors and their potential association with LVH. Future studies should consider objective measures where available. Furthermore, given the potential association between self-reported tobacco use and LVH in adults, analysing biomarkers of tobacco exposure could be a useful next step in a bigger sample size. In addition, we did not have access to medical records around maternal pre-eclampsia and drug use during pregnancy, and therefore could not explore the impact of these factors on the prevalence of LVH, however, we will aim to include this in future studies. There is scant data on LVH prevalence within pre-pubescent children in Africa, thus this study has highlighted the need for additional studies to confirm the most appropriate cut-off points for identifying children with, or at risk of developing, LVH, so that appropriate intervention measures can occur to prevent the progression into severe heart disease and heart failure. As the low frequency of children with LVH prevented further statistical analysis, additional studies with larger sample sizes are needed to assess potential relationships between modifiable health behaviours and LVH.
Conclusions
In conclusion, the results have highlighted the need for specific call to action policies around obesity and hypertension in adult women and target intervention around weight management and elevated BP as well as hypertension in prepubescent children. Due to the high risk of developing LVH due to obesity and hypertension, specific interventions, before the development of obesity and hypertension (i.e. overweight and elevated BP individuals), are needed to prevent the development of LVH, and, in cases where LVH is evident, regress LVH. As obesity and hypertension have both additive and interactive effects on LVH development, both weight loss programs and BP-lowering medications (such as hydrochlorothiazide [70, 71]) are required to achieve appropriate LVH regression, particular in this population where obesity and hypertension are prevalent.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ASE:
-
American Society of Echocardiography
- BMI:
-
Body mass index
- BT20 + :
-
Birth to Twenty Plus Cohort
- BP:
-
Blood pressure
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- GPAQ:
-
Global physical activity questionnaire
- IQR:
-
Interquartile range
- ISH:
-
International Society of Hypertension
- IVSd:
-
Intraventricular septal diameter
- LVDd:
-
Left ventricular diastolic diameter
- LVPWd:
-
Left ventricular posterior wall thickness in diastole
- LV:
-
Left ventricular
- LVH:
-
Left ventricular hypertrophy
- LVM:
-
Left ventricular mass
- LVMI:
-
Left ventricular mass index
- MVPA:
-
Moderate-vigorous physical activity
- OR:
-
Odds ratio
- RWT:
-
Relative wall thickness
- T2DM:
-
Type II diabetes mellitus
- WHO:
-
World health organisation
- WHO-AUDIT:
-
WHO Alcohol Use Disorders
- 1G:
-
First generation
- 2G:
-
Second generation
- 3G:
-
Third generation
References
Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–78.
Bradshaw D, Groenewald P, Laubscher R, Nannan N, Nojilana B, Norman R, et al. Initial burden of disease estimates for South Africa, 2000 : original article. Samj. 2003.
Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006. https://doi.org/10.1056/NEJMoa051530.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update : a report from the American Heart Association. Circulation. 2015. https://doi.org/10.1161/CIR.0000000000000152.
Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009. https://doi.org/10.1161/CIRCULATIONAHA.108.815944.
Steyn K, Damasceno A. Lifestyle and related risk factors for chronic diseases. 2006.
Rayner B. Hypertension: Detection and management in South Africa. Nephron - Clinical Practice. 2010.
Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: An estimate of incidence, mortality and disability adjusted life years. BMC Neurol. 2015. https://doi.org/10.1186/s12883-015-0311-7.
Hitzeroth J, Mpe M, Klug E, Ranjith N, Sliwa K, Steingo L, et al. 2020 Heart Failure Society of South Africa perspective on the 2016 European Society of Cardiology Chronic Heart Failure Guidelines. S Afr Med J. 2020;110:13057.
Szymanski PZ, Badri M, Mayosi BM. Clinical characteristics and causes of heart failure, adherence to treatment guidelines, and mortality of patients with acute heart failure: experience at Groote Schuur Hospital, Cape Town, South Africa. South Afr Med J. 2018;108:94–8.
Bots ML, Nikitin Y, Salonen JT, Elwood PC, Malyutina S, FreiredeConcalves A, et al. Left ventricular hypertrophy and risk of fatal and non-fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Commun Health. 2002;56(1):8.
Ranganai E, Matizirofa L. An analysis of recent stroke cases in South Africa: trend, seasonality and predictors. S Afr Med J. 2020;110:92–9.
Alpert MA, Karthikeyan K, Abdullah O, Ghadban R. Obesity and cardiac remodeling in adults: mechanisms and clinical implications. Prog Cardiovasc Dis. 2018;61:114–23.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 1990;322:1561–6.
Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham heart study. Ann Internal Med. 1988;108:7–13.
Wright GA, Ang DS, Stonebridge PA, Belch JJ, Struthers AD. Left ventricular hypertrophy is present in one-half of newly-diagnosed peripheral arterial disease patients. J Hypertens. 2007;25:463–9.
Katholi RE, Couri DM. Left ventricular hypertrophy: Major risk factor in patients with hypertension: Update and practical clinical applications. Int J Hypertens. 2011;2011:1–10.
Schirmer H, Lunde P, Rasmussen K. Prevalence of left ventricular hypertrophy in a general population. Tromso Study Eur Heart J. 1999;20:429–38.
Baldo MP, Gonçalves MA, Capingana DP, Magalhães P, da Silva ABT, Mill JG. Prevalence and clinical correlates of left ventricular hypertrophy in black Africans. High Blood Press Cardiovasc Prev. 2018;25:283–9.
Brady TM. The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr Hypertens Rep. 2016;18:1–7.
Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. J Am Med Assoc. 2004;292:2350–6.
Pierdomenico S, … DL-A journal of, 2008 undefined. Regression of echocardiographic left ventricular hypertrophy after 2 years of therapy reduces cardiovascular risk in patients with essential hypertension. academic.oup.com. 2008. https://doi.org/10.1038/ajh.2008.2.
Fagard RH, Celis H, Thijs L, Wouters S. Regression of left ventricular mass by antihypertensive treatment: a meta-analysis of randomized comparative studies. Hypertension. 2009;54:1084–91.
Lønnebakken MT, Izzo R, Mancusi C, Gerdts E, Losi MA, Canciello G, et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania salute network). J Am Heart Assoc. 2017;6:e004152.
Messerli FH, Ketelhut R. Left ventricular hypertrophy: an independent risk factor - PubMed. J Cardiovasc Pharmacol. 1991;17 Suppl 4:S59–66; discussion S66–7. https://pubmed.ncbi.nlm.nih.gov/1726010/. Accessed 26 Feb 2021.
Woodiwiss AJ, Norton GR. Obesity and left ventricular hypertrophy: the hypertension connection. Curr Hypertens Reports. 2015;17:539.
Park SK, Moon K, Ryoo JH, Oh CM, Choi JM, Kang JG, et al. The association between alcohol consumption and left ventricular diastolic function and geometry change in general Korean population. Eur Heart J Cardiovasc Imaging. 2018;19:271–8.
National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC) and I, Survey H. South Africa Demographic and Health Survey 2016: Key Indicators. 2017.
Reddy P, Zuma K, Shisana O, Jonas K, Sewpaul R. Prevalence of tobacco use among adults in South Africa: Results from the first South African national health and Nutrition Examination Survey. S Afr Med J. 2015;105:648–55.
Bedeker WF, Lachman AS, Borkum M, Hellenberg D, Cupido CS. Impact of transthoracic echocardiography at district hospital level. S Afr Med J. 2015;105:817–22.
Richter L, Norris S, Pettifor J, Yach D, Cameron N. Europe PMC Funders Group Cohort Profile : Mandela ’ s children : The 1990 birth to twenty study in South Africa. Int J Epidemiol. 2007;36:504–11.
Palipudi KM, Morton J, Hsia J, Andes L, Asma S, Talley B, Caixeta RD, Fouad H, Khoury RN, Ramanandraibe N, Rarick J, Sinha DN, Pujari S, Tursan d'Espaignet E; [On behalf of the GATS Collaborative Group]. Methodology of the Global Adult Tobacco Survey - 2008-2010. Glob Health Promot. 2016;23(2 Suppl):3–23. https://doi.org/10.1177/1757975913499800.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804.
Cleland CL, Hunter RF, Kee F, Cupples ME, Sallis JF, Tully MA. Validity of the Global Physical Activity Questionnaire (GPAQ) in assessing levels and change in moderate-vigorous physical activity and sedentary behaviour. BMC Public Health. 2014;14:1–11.
de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO multicentre growth reference study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1 SUPPL. 1):15–26.
Meng L, Zhao D, Pan Y, Ding W, Wei Q, Li H, et al. Validation of Omron HBP-1300 professional blood pressure monitor based on auscultation in children and adults. BMC Cardiovasc Disord. 2016;16:1–5.
Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. International society of hypertension global hypertension practice guidelines. Hypertension. 2020;2020:1334–57.
Orsinelli DA, Armour A, De Cara J, Fey B, Frommelt P, Lopez-Mattei J, et al. The American society of echocardiography recommendations for cardiac chamber quantification in adults. J Am Soc Echocardiogr. 2015;28:1–39.
Simpson JM, Savis A, Rawlins D, Qureshi S, Sinha MD. Incidence of left ventricular hypertrophy in children with kidney disease: Impact of method of indexation of left ventricular mass. Eur J Echocardiogr. 2010;11:271–7.
WHO/Europe | Nutrition - Body mass index - BMI. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed 1 Jul 2020.
WHO. Body mass index-for-age (BMI-for-age). https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age. Accessed 17 Mar 2021.
Flynn JT, Falkner BE. New clinical practice guideline for the management of high blood pressure in children and adolescents. Hypertension. 2017;70:683–6.
Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72:558–69.
Kamath S, Markham D, Drazner MH. Increased prevalence of concentric left ventricular hypertrophy in African-Americans: Will an epidemic of heart failure follow? Heart Fail Rev. 2006;11:271–7.
Maseko MJ, Woodiwiss AJ, Libhaber CD, Brooksbank R, Majane OHI, Norton GR. Relations between white coat effects and left ventricular mass index or arterial stiffness: role of nocturnal blood pressure dipping. Am J Hypertens. 2013;26:1287–94.
Libhaber CD, Norton GR, Maseko MJ, Majane OHI, Millen AME, Maunganidze F, et al. Relationship between inappropriate left ventricular hypertrophy and ejection fraction independent of absolute or indexed mass in a community sample of black African ancestry. J Hypertens. 2013;31:169–76.
Isaac Kofi O, Emmanuel AA. Determinants of left ventricular hypertrophy in hypertensive patients seen in a teaching hospital in Ghana. J Hypertens Open Access. 2017;06:1–7.
Willey BA, Cameron N, Norris SA, Pettifor JM, Griffiths PL. Socio-economic predictors of stunting in preschool children—a population-based study from Johannesburg and Soweto. S Afr Med J. 2009. https://doi.org/10.7196/SAMJ.2652.
Nyati LH, Pettifor JM, Norris SA. The prevalence of malnutrition and growth percentiles for urban South African children. BMC Public Health. 2019;19:1–13.
Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–34.
Aronow WS. Hypertension and left ventricular hypertrophy. Ann Transl Med. 2016;5(15):310. https://doi.org/10.21037/atm.2017.06.14.
Horwich TB, Fonarow GC. Glucose, obesity, metabolic syndrome, and diabetes. Relevance to incidence of heart failure. J Am College Cardiol. 2010;55:283–93.
Hamzeh N, Ghadimi F, Farzaneh R, Hosseini SK. Obesity, heart failure, and obesity paradox. J Tehran Univ Heart Center. 2017;12:1–5.
Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–7.
National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC) and I. South Africa Demographic andd Health Survey 2016. 2019.
Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: Epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164:345–56.
Nauta JF, Hummel YM, Tromp J, Ouwerkerk W, van der Meer P, Jin X, et al. Concentric vs. eccentric remodelling in heart failure with reduced ejection fraction: clinical characteristics, pathophysiology and response to treatment. Eur J Heart Failure. 2020;22(7):1147–55.
Avelar E, Cloward TV, Walker JM, Farney RJ, Strong M, Pendleton RC, et al. Left ventricular hypertrophy in severe obesity: interactions among blood pressure, nocturnal hypoxemia, and body mass. Hypertension. 2007;49:34–9.
Aurigemma GP, De Simone G, Fitzgibbons TP. Cardiac remodeling in obesity. Circ Cardiovasc Imaging. 2013;6:142–52.
Woodiwiss AJ, Libhaber CD, Majane OHI, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008;21:1144–51.
Fidelix MP, Tanni SE, Roscani MG, Mesquita CB, Schelini KNdM, Polegato BF, et al. Vitamin D role in smoking women and cardiac remodeling. Nutrire. 2016;41:1–7.
Bullen C. Impact of tobacco smoking and smoking cessation on cardiovascular risk and disease. Expert Rev Cardiovasc Ther. 2008;6:883–95.
Leigh JA, Kaplan RC, Swett K, Balfour P, Kansal MM, Talavera GA, et al. Smoking intensity and duration is associated with cardiac structure and function: The ECHOcardiographic Study of Hispanics/Latinos. Open Heart. 2017;4:1–12.
Kaplan A, Abidi E, Ghali R, Booz GW, Kobeissy F, Zouein FA. Functional, cellular, and molecular remodeling of the heart under influence of oxidative cigarette tobacco smoke. Oxid Med Cell Longev. 2017;2017.
Boerstra BA, Soepnel LM, Nicolaou V, Kolkenbeck-Ruh A, Kagura J, Ware LJ, et al. The impact of maternal hyperglycaemia first detected in pregnancy on offspring blood pressure in Soweto, South Africa. J Hypertens. 2022;40:969–77.
Matjuda EN, Sewani-Rusike CR, Anye SNC, Engwa GA, Nkeh-Chungag BN. Relationship between high blood pressure and microalbuminuria in children aged 6–9 years in a South African population. Children. 2020;7:131.
Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. 2019;173:1154–63.
Jin Y, Kuznetsova T, Bochud M, Richart T, Thijs L, Cusi D, et al. Heritability of left ventricular structure and function in Caucasian families. Eur J Echocardiogr. 2011;12:326–32.
Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass. Hypertension. 1997;30:1025–8.
Roush GC, Abdelfattah R, Song S, Ernst ME, Sica DA, Kostis JB. Hydrochlorothiazide vs chlorthalidone, indapamide, and potassium-sparing/hydrochlorothiazide diuretics for reducing left ventricular hypertrophy: a systematic review and meta-analysis. J Clin Hypertens. 2018;20:1507–15.
Pierdomenico SD, Cuccurullo F. Risk reduction after regression of echocardiographic left ventricular hypertrophy in hypertension: a meta-analysis. Am J Hypertens. 2010;23:876–81.
Acknowledgements
Funding support from the South African Medical Research Council, Wellcome Trust, and DSI-NRF Centre of Excellence in Human Development at the University of the Witwatersrand, Johannesburg, South Africa for the Birth to Twenty Plus Cohort.
Funding
This study was supported by a Wellcome Trust Seed Award to Lisa J. Ware (214082/Z/18/Z). This Award funded the study by providing travel reimbursement and refreshments for all the participants that were enrolled into the study, and by providing salaries for the research team that carried out all the data collection for the duration of the study.
Author information
Authors and Affiliations
Contributions
AKR and LJW, conceptualised and designed the study. AKR was responsible for data management, data analysis and wrote the original draft. AKR, LMS, SHC, SN, WS, SAN, JD, LJW, contributed to interpretation of data and critical review of manuscript. LW was the Principal Investigator of the project. All authors gave final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Written informed consent was provided by all women and their children / grandchildren prior to their inclusion in the study. Trained researchers who spoke the participant’s home language explained the study and all participants provided written informed consent prior to taking part. For children, the mother of the child provided written consent that her child may take part in the study, with children older than 7 years additionally providing written assent to take part. The study was conducted according to the principles of the Declaration of Helsinki [67] and the Human Research Ethics Committee (Medical) of the University of the Witwatersrand approved the protocol with ethical clearance number M190263.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kolkenbeck-Ruh, A., Soepnel, L.M., Crouch, S.H. et al. Obesity, hypertension, and tobacco use associated with left ventricular remodeling and hypertrophy in South African women: Birth to Twenty Plus Cohort. BMC Cardiovasc Disord 22, 403 (2022). https://doi.org/10.1186/s12872-022-02837-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02837-w