Abstract
Background
Calcified plaque is thought to adversely impact outcomes after percutaneous coronary intervention (PCI). This study sought to evaluate the impact of nodular calcification in patients with acute coronary syndrome treated with primary percutaneous coronary intervention.
Methods
Using optical coherence tomography (OCT), 500 culprit plaques with calcification were analyzed from 495 acute coronary syndrome (ACS) patients on whom PCI was performed. Based on morphology, we classified calcification into two subtypes: nodular calcification and non-nodular calcification. Nodular calcification was defined as protruding mass with an irregular surface, high backscattering, and signal attenuation while non-nodular calcification was defined as an area with low backscattering heterogeneous region with a well-delineated border without protrusion into the lumen on OCT.
Results
Calcified culprit plaques were divided into nodular calcification group (n = 238) and non-nodular calcification group (n = 262). Patients with nodular calcification were older (p < 0.001) and had lower left ventricular ejection fraction (p = 0.006) compared to patients with non-nodular calcification. Minimum stent area (5.0 (3.9, 6.3) mm2 vs. 5.4 (4.2, 6.7) mm2, p = 0.011) and stent expansion (70 (62.7, 81.8) % vs. 75 (65.2, 86.6) %, p = 0.004) were significantly smaller in the nodular calcification group than in the non-nodular calcification group. Stent under-expansion was most frequent (p = 0.003) in the nodular calcification group.
Conclusion
This study demonstrate that the presence of nodular calcification is associated with a smaller minimum stent area and a higher incidence of stent under-expansion. Lesions with nodular calcification may be at risk of stent under-expansion.
Similar content being viewed by others
Introduction
Percutaneous coronary intervention (PCI) is a widely used treatment for calcified coronary lesions, which is frequently associated with increased risk of periprocedural complications and worse clinical outcomes such as target lesion revascularization (TLR) and stent thrombosis [1, 2]. Hence, the PCI approach for calcified lesions remains a challenge even in the drug-eluting stents (DES) era. Nodular calcification is defined as a protruding mass with an irregular surface, high backscattering, signal attenuation with an intact fibrous cap on optical coherence tomography (OCT) [3,4,5]. Recently, Kobayashi et al. reported that the amount and extent of coronary calcification as assessed by OCT were associated with stent expansion and stent eccentricity [6]. In addition, an OCT-based calcium scoring system was recently developed to predict stent under-expansion and to identify lesions that would benefit from plaque modification before stent implantation [7]. However, the response and impact of nodular and non-nodular calcification to stent implantation remains unclear. In this study, we sought to assess the impact of nodular and non-nodular calcification in patients with acute coronary syndrome (ACS) treated with primary percutaneous coronary intervention (PCI).
Methods
Study population
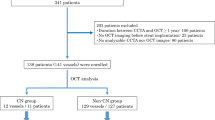
The statistics show that from a total of 1501 patients with ACS who underwent OCT-guided stent implantation between January 2016 and January 2019, 708 patients had calcified plaques at the culprit lesion. Further, among the 708 patients with calcified plaques at culprit lesion, 113 were excluded because of no post-procedural OCT imaging, 19 were excluded for incomplete culprit lesion imaging, and 81exlcuded for poor image quality. Finally, 495 patients were included in the final analysis. The study flowchart is as shown in Fig. 1, and the diagnosis of ACS includes ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation acute coronary syndrome (NSTE-ACS) [8,9,10]. STEMI is defined as persistent chest pain for at least 30 min, arrival at the hospital within 12 h from symptom onset, with a 12-lead electrocardiogram (ECG) changes (ST segment elevation > 0.1 mV in ≥ 2 continuous leads or new-onset left bundle branch block) and elevation of cardiac biomarker (creatine kinase-MB or troponin T/I). NSTE-ACS includes non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina pectoris (UAP). The NSTEMI is defined as ischemic symptoms in the absence of ST-segment elevation on the electrocardiogram with elevated cardiac marker levels. UAP is defined as the presence of newly developed/accelerating chest symptoms on exertion or rest angina within 2 weeks of presentation without biomarker release. A culprit lesion was identified based on abnormal manifestations of electrocardiographic, coronary angiography and cardiac ultrasound. Further, the demographic, laboratory, and clinical data, as well as angiographic and procedural data were evaluated. In addition, the pre- and post-procedural OCT findings were assessed. Moreover, all patients underwent primary PCI within 12 h of symptom onset. The study protocol was performed according to the relevant guidelines and regulations of the Declaration of Helsinki, and was approved by the Institutional Review Board (Ethics Committee) of the 2nd Affiliated Hospital of Harbin Medical University (Harbin, China). Also, all patients provided written informed consent to participate.
Coronary angiography analysis
The angiographic images were analyzed using a quantitative coronary angiogram analysis program (CAAS 5.10.1; Pie Medical Imaging BV, Maastricht, the Netherlands). Also, lesion location, minimum lumen diameter (MLA), reference lumen diameter (RLD), diameter stenosis (DS), and the initial thrombolysis in myocardial infarction (TIMI) flow were measured by an independent investigator who was blinded to patients’ clinical information. This is done to avoid bias in the study and to ensure accuracy.
Optical coherence tomography acquisition and analysis
OCT imaging of culprit lesions was acquired with the C7-XR/ ILUMIEN OCT system (Abbott Vascular, Santa Clara, CA, USA). Aspiration thrombectomy (Export® aspiration catheter; Medtronic, Santa Rosa, CA, USA) prior to OCT imaging was allowed in patients with large occlusive thrombus or TIMI flow grade < 1. The OCT images were digitally archived to database and analyzed in the imaging core lab by two experienced investigators (A.D and E.K) who were also blinded to patients’ information. When there is discordance between the two investigators, a consensus was obtained from experienced investigators (S.H and H.J). All measurements were performed according to previously established consensus and guidelines [11,12,13]. Also, the proximal and distal references were identified as the sites with the largest lumen area within a 10-mm to the plaque, and the mean reference lumen area was calculated. The minimum lumen area was identified along the length of the culprit lesion and cross-sectional bounded by luminal border including thrombus area. The lipid arc was measured for each 1 mm in the cross-sectional view, and then, the maximal lipid arc was calculated. Also, the minimal fibrous cap thickness (FCT) was measured three times in the thinnest place to obtain a mean value, and the culprit lesions diagnosis and identification were done using established criteria [18]. Thin-cap fibroatheroma (TCFA) was defined as a plaque with lipid content in at least two quadrants, with the thinnest part of the fibrous cap measuring less than 65 µm. Also, thrombus was defined as a mass floating in or protruding into the lumen with a dimension of at least 250 µm, and calcified plaque was identified as an area with low backscattering heterogeneous region with well-delineated border underlying the plaque. Based on morphology, we classified calcification into two subtypes: nodular calcification and non-nodular calcification. A nodular calcification was defined as protruding mass with an irregular surface, high backscattering, and signal attenuation covered by intact fibrous cap, while a non-nodular calcification was defined as an area with low backscattering heterogeneous region with well-delineated border without protrusion into the lumen [4, 5, 14, 15].
A representative OCT images of nodular and non-nodular calcification are presented in Fig. 2. The cross-sectional OCT images were quantitatively analyzed at 1-mm intervals, and the calcification depth was evaluated (the minimum distance from lumen to superficial calcium edge). The calcium edge is superficial if the distance between the lumen and the leading edge of calcium is less than 100 µm, and the edge is deep if the distance between the lumen and the leading edge of calcium is more than 100 µm [16]. Further, calcium score was specified as 2 points for maximum angle > 180°, 1 point for maximum thickness > 0.5 mm, and 1 point for length > 5 mm [7]. The postprocedural mean reference lumen area was defined as the mean of the largest lumen area within 5-mm of the proximal and distal stent edges, and minimum stent area (MSA), stent expansion, stent under-expansion, stent edge dissection (SED), stent strut malaposition, and tissue protrusion were evaluated using postprocedural OCT imaging data. MSA is the minimum area bounded by the stent border [12], and the percentage of stent expansion was defined as MSA divided by the postprocedural mean reference area. Stent under-expansion was defined as stent expansion < 80% [17]. Stent eccentricity index was defined as (maximal stent diameter minus MSD) divided by maximal stent diameter [6]. SED was defined as disruption of the vessel luminal surface with a visible flap at the stent edge or within 5-mm proximal or distal reference segments. SED was classified as major (≥ 60° of the circumference of the vessel at the site of dissection or ≥ 3 mm in length) or minor (any visible edge dissection < 60° of the circumference of the vessel and < 3 mm in length). Stent strut malposition clearly separated from the vessel wall by ≥ 0·2 mm is classified as a major stent (associated with unacceptable stent expansion [< 80%]) or otherwise minor. Also, a tissue protrusion was defined as tissue prolapsed between stent struts and extending inside a circular arc, connecting adjacent struts [17, 18].
Representative optical coherence tomography (OCT) images to define nodular calcification and non-nodular calcification. a Longitudinal OCT image of calcified plaque. (A1-A4) Cross-sectional OCT images of nodular calcification defined as protruding mass with an irregular surface, high backscattering, and signal attenuation (arrow heads). Asterisk indicates wire artefact. b Longitudinal OCT image of calcified plaque. (B1-B4) Cross-sectional OCT images of non-nodular calcification defined as an area with low backscattering heterogeneous region with well-delineated border without protrusion into the lumen (arrows). Asterisk indicates wire artefact. OCT = optical coherence tomography
Statistical analysis
Categorical data are presented as counts and percentage, and they were compared using either a chi-square test or Fisher’s exact test, as appropriate. Continuous data are presented as mean ± standard deviations when normally distributed and as median (interquartile range) when non-normally distributed by the nonparametric one sample Kolmogorov–Smirnov test. Also, the multivariable logistic regression was used to identify independent predictor of stent expansion, and all statistical analyses were performed using SPSS, Version 18.0 (SPSS, Chicago, IL, USA). To measure significance, P-values < 0.05 were considered statistically significant.
Results
Baseline clinical characteristics
Finally, 500 calcified plaques in 495 patients (238 lesions in 236 patients and 262 lesions in 259 patients) were included in the current study, and the baseline patient characteristics between the two groups are summarized in Table 1. It is observed that patients with nodular calcification were older (63.8 ± 10.1 vs. 59.6 ± 10.0 years, p < 0.001), and more likely to have a lower left ventricular ejection fraction (57.3 ± 6.5% vs. 58.5 ± 6.2%, p = 0.045) compared to patient without nodular calcification. Also, the triglyceride levels were significantly increased in non-nodular calcification group (52.1 ± 28.4 vs. 60.2 ± 39.7 mg/dL, p = 0.015), and no significant differences in clinical presentation, history, or serum cholesterol levels were observed between the two groups.
Procedural and angiographic findings
Table 2 shows the procedural characteristics of the two groups. The two groups were not significantly different regarding the scoring balloon used before stenting, and no significant difference was detected for stent length, number of stents implanted, stent diameter, post-dilation pressure and balloon size in both groups. Further, as shown in Table 3, no significant difference was found for target vessel and initial thrombolysis in myocardial infarction in both groups. To further analyze the study groups, the post-intervention minimum lumen diameter and diameter stenosis were comparable between the two groups.
Optical coherence tomography findings
The preprocedural and postprocedural analysis results are shown in Table 4. The nodular calcification group had a longer lesion length (p < 0.001) and smaller distal reference lumen area (p = 0.046) compared to the non-nodular group. As compared with non-nodular calcification, calcium depth was shallow in the nodular calcification group (p < 0.001) and the group had a higher prevalence of superficial calcification (90.3% vs. 71.8%, p < 0.001). Also, the OCT-based calcium score of 4 was more frequently observed in lesions with nodular calcification (p < 0.001). Moreover, the minimum stent area was significantly smaller in the nodular calcification group compared with non-nodular calcification group (5.0 (3.9, 6.3) mm2 vs. 5.4 (4.2, 6.7) mm2, p = 0.011), the stent expansion was significantly smaller (70 (62.7, 81.8) % vs 75 (65.2, 86.6) %, p = 0.004) and stent under-expansion was most frequent (p = 0.003) in the nodular calcification group. Representative images are shown in Fig. 3, and predictor of stent expansion is analyzed in the following section.
Post-stent optical coherence tomography (OCT) findings of nodular and non-nodular calcification. Case A. Nodular calcification are associated with small minimum stent area and stent underexpansion (54%). Case B. non-Nodular calcification are associated with good stent expansion (90%). Asterisk indicates wire artefact. OCT = optical coherence tomography
Predictor of stent expansion
Table 5 shows the results of univariable and multivariable analysis. In multivariable analysis, age, maximum post-dilation pressure, lesion length, minimum stent area, stent strut malaposition were not independent predictor of stent expansion.
Discussion
To the best of our knowledge, this is the first study to evaluate the impact of nodular calcification and non-nodular calcification in patients with acute coronary syndrome treated with percutaneous coronary intervention. The main findings in this study can be summarized as: (1) Minimum stent area and stent expansion were significantly smaller in the nodular calcification group; (2) Higher incidence of stent underexpansion was associated with nodular calcification group; (3) Nodular calcification frequently showed superficial calcium; 4) Patients with nodular calcification were older.
Percutaneous coronary intervention of calcified plaques
Smaller minimum stent area and stent underexpansion are associated with in-stent restenosis and stent thrombosis following stent implantation [19,20,21]. Calcified plaque may adversely impact the percutaneous coronary intervention (PCI) procedure by affecting the ability to effectively dilate coronary lesion and gain an acceptable lumen area. Inadequate calcified plaque preparation before stent implantation can impede stent delivery and stent expansion [22, 23]; the consequence is often incomplete stent expansion, which increases the risk of in-stent restenosis and stent thrombosis. Lesion preparation before stent implantation is a crucial component in managing calcified coronary lesions in order to facilitate stent delivery and allow optimal stent expansion. Clinical guidelines recommend the use of rotational atherectomy before implantation for severely calcified lesions that cannot be crossed by a balloon catheter or adequately dilated [24]. A randomized controlled trial of patients with complex calcified angiographic lesions was unable to clearly show the clinical advantage of rotational atherectomy before paclitaxel-eluting stent implantation compared with balloon predilation alone; therefore, balloon dilation with provisional rotablation before stenting remains the default strategy for complex calcified lesions in the DES era [25, 26]. The potential benefits of orbital atherectomy or laser angioplasty for severely calcified lesions have been recommended by other studies [26, 27]. Hence it is important to identify and evaluate different morphology of calcified lesions that may need modification before stent implantation. Little data is available on the importance of lesion modification in lesions containing nodular calcification before stent implantation. Our study showed that nodular calcification is associated with stent under-expansion after PCI and may benefit from lesion modification.
The evaluation of calcified plaque by optical coherence tomography
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) has been increasingly used to guide percutaneous coronary intervention procedures and improve the outcome of patient with coronary artery disease after implantation of stent [28,29,30]. OCT can penetrate and assess the three-dimensional extent of calcium, whereas the evaluation of calcium by IVUS is limited because ultrasound is almost entirely reflected from the calcium surface. Additionally, OCT can provide precise evaluation for superficial calcification that might be related to poor stent expansion [31, 32]. Recently, Fujino et al. reported an OCT-based calcium scoring system and the risk of stent underexpansion was increased in lesions with calcium score of 4. Lesions with calcium score of 0 to 3 had excellent stent expansion, whereas the lesions with a score of 4 had poor stent expansion and aggressive lesion modification should be considered when treating them [7]. Our study reveals higher incidence of calcium score of 4 in nodular calcification group; suggesting plaque modification before stent implantation might be helpful. The amount and extent of target lesion calcification has been suggested to be an important contributing factor in stent expansion but the morphology of the calcified lesions was not reported [6]; utilizing OCT, we highlighted the impact of nodular calcium protruding into the lumen causing an inadequate stent expansion which may result in abnormal sheer stress that might be associated with smaller stent area.
Study limitations
First, this was a retrospective, observational study with a modest number of patients. Second, our classification of calcification into nodular and non-nodular based on morphology is novel and therefore has not yet been validated. Third, in patients with TIMI flow grade 0/1, manual thrombectomy was performed to re-establish effective vessel patency, allowing safe and high-quality OCT imaging data collection at the culprit site. However, the potential effect of the thrombus aspiration catheter on superficial plaque integrity and atherothrombotic components assessed by OCT must be given serious consideration. Fourth, the analyzed cross-sections using OCT could be inconsistent between pre-and post-PCI. Finally, this study was conducted with only postprocedural, hence, a large scale with long-term follow up is required.
Conclusion
Calcified plaque adversely impacts stent implantation and remain a challenge for an interventional cardiologist. However, the characteristics of calcification morphology may influence the extent of this impact. When considering morphology features, this study demonstrated that the presence of nodular calcification (protruding mass with an irregular surface covered by an intact fibrous cap) is associated with a smaller minimum stent area, and a higher incidence of stent underexpansion in patients with acute coronary syndrome treated with primary PCI. Lesions with nodular calcification may benefit from plaque modification (specialized balloons and atherectomy devices) before stent implantation.
Availability of data and materials
All relevant data and materials are included in the manuscript. The datasets will be available from the corresponding author on reasonable requests after study completion.
Abbreviations
- ACS:
-
Acute coronary syndrome
- PCI:
-
Percutaneous coronary intervention
- OCT:
-
Optical coherence tomography
- IVUS:
-
Intravascular ultrasound
- TLR:
-
Target lesion revascularization
- DES:
-
Drug-eluting stents
- STEMI:
-
ST-segment elevation myocardial infarction
- NSTE-ACS:
-
Non-ST-segment elevation acute coronary syndrome
- ECG:
-
Electrocardiogram
- UAP:
-
Unstable angina pectoris
- MLD:
-
Minimal lumen diameter
- RLD:
-
Reference lumen diameter
- DS:
-
Diameter stenosis
- TIMI:
-
Initial thrombolysis in myocardial infarction
- FCT:
-
Fibrous cap thickness
- TCFA:
-
Thin-cap fibroatheroma
- MSA:
-
Minimum stent area
- SED:
-
Stent edge dissection
- MACE:
-
Major adverse cardiac events
References
Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63(17):1703–14.
Jinnouchi H, Kuramitsu S, Shinozaki T, Kobayashi Y, Hiromasa T, Morinaga T, Ando K. Two-year clinical outcomes of newer-generation drug-eluting stent implantation following rotational atherectomy for heavily calcified lesions. Circ J. 2015;79(9):1938–43.
di Vito L, Yoon JH, Kato K, Yonetsu T, Vergallo R, Costa M, Bezerra HG, Arbustini E, Narula J, Crea F, Prati F, Jang IK. Comprehensive overview of definitions for optical coherence tomography-based plaque and stent analyses. Coron Artery Dis. 2014;25(2):172–85.
Hao H, Fujii K, Shibuya M, Imanaka T, Kawakami R, Hatakeyama K, Asada Y, Masuyama T, Hirota S. Different findings in a calcified nodule between histology and intravascular imaging such as intravascular ultrasound, optical coherence tomography, and coronary angioscopy. JACC Cardiovasc Intervent. 2014;7(8).
Mori H, Finn AV, Atkinson JB, Lutter C, Narula J, Virmani R. Calcified nodule: an early and late cause of in-stent failure. JACC Cardiovasc Intervent. 2016;9(13):e125-6.
Kobayashi Y, Okura H, Kume T, Yamada R, Kobayashi Y, Fukuhara K, Koyama T, Nezuo S, Neishi Y, Hayashida A, Kawamoto T, Yoshida K. Impact of target lesion coronary calcification on stent expansion. 2014;78(September).
Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, Usui E, Yonetsu T, Haag ES, Shlofmitz RA, Kakuta T, Maehara A. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13(18):e2182–9.
Anderson JL. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Vol. 127, Circulation. 2013.
Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Curtis LH, DeMets D, Fleisher LA, Gidding S, Guyton RA, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Stevenson WG, Wijeysundera DN, Yancy CW. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: A report of the American college of cardiology/American heart association task force on practice guidelines. Vol. 130, Circulation. 2014.
Nakajima A, Araki M, Kurihara O, Minami Y, Soeda T, Yonetsu T, Crea F, Takano M, Higuma T, Kakuta T, Adriaenssens T, Lee H, Nakamura S, Jang IK. Comparison of post-stent optical coherence tomography findings among three subtypes of calcified culprit plaques in patients with acute coronary syndrome. Catheterization and Cardiovascular Interventions. 2020;(March):1–12.
Prati F, Regar E, Mintz GS, Arbustini E, di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PWJ. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31(4):401–15.
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, di Mario C, Dudeck D, Falk E, Feldman MD, Fitzgerald P, Garcia H, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CCS, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Rber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PWJC, Shimada K, Shinke T, Shite J, Siegel E, Sonada S, Suter M, Takarada S, Tanaka A, Terashima M, Troels T, Uemura S, Ughi GJ, van Beusekom HMM, van der Steen AFW, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. Journal of the American College of Cardiology [Internet]. 2012;59(12):1058–72. Available from: http://dx.doi.org/https://doi.org/10.1016/j.jacc.2011.09.079
Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62(19):1748–58.
Torii S, Sato Y, Otsuka F, Kolodgie FD, Jinnouchi H, Sakamoto A, Park J, Yahagi K, Sakakura K, Cornelissen A, Kawakami R, Mori M, Kawai K, Amoa F, Guo L, Kutyna M, Fernandez R, Romero ME, Fowler D, Finn A v., Virmani R. Eruptive calcified nodules as a potential mechanism of acute coronary thrombosis and sudden death. J Am Coll Cardiol. 2021;77(13).
Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E, Kanaji Y, Murai T, Yonetsu T, Kakuta T, Maehara A. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imag. 2017;10(8):883–91.
Ong DS, Lee JS, Soeda T, Higuma T, Minami Y, Wang Z, Lee H, Yokoyama H, Yokota T, Okumura K, Jang I-K. Coronary calcification and plaque vulnerability: an optical coherence tomographic study. Circ Cardiovasc Imaging. 2016;9(1):1–8.
Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra HG, Shite J, Généreux P, Nichols M, Jenkins P, Witzenbichler B, Mintz GS, Stone GW. Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound: The ILUMIEN II study (Observational Study of Optical Coherence Tomography [OCT] in Patients Undergoing Fractional Flow Reserve [FFR] and Percutaneous Cor. JACC Cardiovasc Intervent. 2015;8(13):1704–14
Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de La Torre Hernandez JM, Escaned J, Hill J, Prati F, Colombo A, di Mario C, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G, Serruys PW, Alfonso F, Bhindi R, Ali Z, Carter R. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2018;14(6):656–77.
Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, Popma JJ, Ellis SG, Grube E, Dawkins KD, Weissman NJ, Turco MA, Ormiston JA, Stone GW. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents. An Integrated Intravascular Ultrasound Analysis From the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Intervent 2009;2(12):1269–75. https://doi.org/10.1016/j.jcin.2009.10.005
Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, Kim SJ, Vergallo R, Minami Y, Ong DS, Gao L, Lee H, Zhang S, Yu B, Saito Y, Jang IK. Incidence and clinical significance of poststent optical coherence tomography findings: One-year follow-up study from a multicenter registry. Vol. 132, Circulation. 2015. 1020–1029 p.
Sonoda S, Morino Y, Ako J, Terashima M, Hassan AHM, Bonneau HN, Leon MB, Moses JW, Yock PG, Honda Y, Kuntz RE, Fitzgerald PJ. Impact of final stent dimensions on long-term results following sirolimus-eluting stent implantation: Serial intravascular ultrasound analysis from the SIRIUS trial. J Am Coll Cardiol. 2004;43(11):1959–63.
Mosseri M, Satler LF, Pichard AD, Waksman R. Impact of vessel calcification on outcomes after coronary stenting. Cardiovasc Revasc Med. 2005;6(4):147–53.
Généreux P, Madhavan M v., Mintz GS, Maehara A, Palmerini T, Lasalle L, Xu K, McAndrew T, Kirtane A, Lansky AJ, Brener SJ, Mehran R, Stone GW. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes: Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trials. J Am Coll Cardiol. 2014;63(18).
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, Jacobs AK, Albert N, Creager MA, Ettinger SM, Halperin JL, Hochman JS, Kushner FG, Magnus Ohman E, Stevenson W, Yancy CW. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23).
Abdel-Wahab M, Richardt G, Joachim Büttner H, Toelg R, Geist V, Meinertz T, Schofer J, King L, Neumann FJ, Khattab AA. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: The randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Intervent. 2013;6(1):10–9.https://doi.org/10.1016/j.jcin.2012.07.017
Généreux P, Bettinger N, Redfors B, Lee AC, Kim CY, Lee MS, Shlofmitz RA, Moses JW, Stone GW, Chambers JW. Two-year outcomes after treatment of severely calcified coronary lesions with the orbital atherectomy system and the impact of stent types: Insight from the ORBIT II trial. Catheter Cardiovasc Interv. 2016;88(3):369–77.
Latib A, Takagi K, Chizzola G, Tobis J, Ambrosini V, Niccoli G, Sardella G, DiSalvo ME, Armigliato P, Valgimigli M, Tarsia G, Gabrielli G, Lazar L, Maffeo D, Colombo A. Excimer laser lesion modification to expand non-dilatable stents: the ellement registry. Cardiovasc Revasc Med. 2014;15(1):8–12. https://doi.org/10.1016/j.carrev.2013.10.005.
Mehanna E, Bezerra HG, Prabhu D, Brandt E, Chamié D, Yamamoto H, Attizzani GF, Tahara S, van Ditzhuijzen N, Fujino Y, Kanaya T, Stefano G, Wang W, Gargesha M, Wilson D, Costa MA. Volumetric characterization of human coronary calcification by frequency-domain optical coherence tomography. Circ J. 2013;77(9).
Saleh Y, Al-Abcha A, Abdelkarim O, Abdelfattah OM, Abela GS, Hashim H, Goel SS, Kleiman NS. Meta-analysis investigating the role of optical coherence tomography versus intravascular ultrasound in low-risk percutaneous coronary intervention. Am J Cardiol. 2022;164.
Fan LM, Tong D, Mintz GS, Mamas MA, Javed A. Breaking the deadlock of calcified coronary artery lesions: a contemporary review. Catheterization and Cardiovascular Interventions. 2021;97(1).
Kume T, Okura H, Kawamoto T, Yamada R, Miyamoto Y, Hayashida A, Watanabe N, Neishi Y, Sadahira Y, Akasaka T, Yoshida K. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention. 2011;6(6):768–72.
Afolabi A, Hu S, Wang C, Zhu Y, Mustafina I, Lin L, Zheng G, Zhe C, Jia H, Hou J, Yu B. Role of optical coherence tomography in diagnosis and treatment of patients with acute coronary syndrome. Cardiovasc Innov Appl. 2017;2(2):229–35.
Acknowledgements
The authors sincerely thank all colleagues who participated in this study.
Funding
This work was supported by a research grant from the National Natural Science Foundation of China, Harbin, China (81827806/B.Y., and 81330033/B.Y., 81722025/H.J., 81671763/H.J., 81701804/H.S.) and the National Key R&D Program of China (grant no. 2016YFC1301100 to B.Y.).
Author information
Authors and Affiliations
Contributions
A.D., B.Y. and H.J. conceived the idea of the study. A.D. was a major contributor to data acquisition, manuscript drafting and critical manuscript revision. A.D., S.H., E.K. and M.L. analyzed patients’ data and critical manuscript revision. A.D., Z.W., C.Z. and X.F. prepared figures and critical manuscript revision.1–3. A.D., L.H., Y.X., M.Z. and W.M. performed statistical analysis and critical manuscript revision. A.D., Y.S., B.Y., Z.G., and Y.Q. critically revise the manuscript. B.Y., H.J. and G.M. designed the research and critical manuscript revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was performed in accordance with the relevant guidelines and regulations of the Declaration of Helsinki and was approved the Institutional Review Board (Ethics Committee) of the 2nd Affiliated Hospital of Harbin Medical University (Harbin, China). All patients provided written informed consent to participate.
Consent for publication
All patients provided consent to publication.
Competing interests
The authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Demuyakor, A., Hu, S., Koniaeva, E. et al. Impact of nodular calcification in patients with acute coronary syndrome (ACS) treated with primary percutaneous coronary intervention (PCI). BMC Cardiovasc Disord 22, 103 (2022). https://doi.org/10.1186/s12872-022-02551-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-022-02551-7