Abstract
Background
The cellular adhesion pathway has been suggested as playing an important role in the pathogenesis of atrial fibrillation (AF). However, prior studies that have investigated the role of adhesion pathway proteins in risk of AF have been limited in the number of proteins that were studied and in the ethnic and racial diversity of the study population. Therefore we aimed to study the associations of fifteen adhesion pathway proteins with incident AF in a large, diverse population.
Methods
Multi-Ethnic Study of Atherosclerosis participants from four races/ethnicities (n = 2504) with protein levels measured were followed for incident AF (n = 253). HGF protein was measured on Exam 1 samples (N = 6669; AF n = 851). Cox proportional hazards regression was used to assess the association of AF with 15 adhesion pathway proteins. Bonferroni correction was applied to account for multiple comparisons.
Results
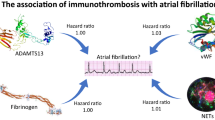
After adjusting for potential confounding variables (age, sex, race/ethnicity, height, body mass index, systolic blood pressure, antihypertension therapy, diabetes status, current smoker, current alcohol use, and total and HDL cholesterol), and accounting for multiple testing (P < 0.05/15 = 0.0033), circulating levels of the following proteins were positively associated with a higher risk of AF: MMP-2 (HR per standard deviation increment, 1.27; 95% CI 1.11‒1.45), TIMP-2 (HR 1.28; 95% CI 1.12‒1.46), VCAM-1 (HR 1.32; 95% CI 1.16‒1.50), and SLPI (HR 1.22; 95% CI 1.07‒1.38). The association between proteins and AF did not differ by race/ethnicity.
Conclusions
Circulating levels of MMP-2, TIMP-2, VCAM-1, and SLPI were positively associated with an increased risk of incident AF in a diverse population. Our findings suggest that adhesion pathway proteins may be important risk predictors of AF.
Similar content being viewed by others
Background
Atrial fibrillation (AF) is the most common chronic cardiac arrhythmia, with an estimated prevalence in 2010 of between 3 and 6 million individuals in the United States [1, 2]. Although widely studied, the pathophysiology of AF is not completely understood. Inflammation has been suggested as an underlying mechanism and a risk factor for AF [3,4,5].
The Framingham Offspring Study assessed the association of 12 circulating inflammatory markers and found that circulating osteoprotegerin was associated with incident AF [6].Furthermore, there is growing evidence that the cellular adhesion pathway, a biological pathway of inflammation, may play a role in AF. For example, in the Women’s Health Study, markers of systemic inflammation, including high sensitivity C-reactive protein (CRP), soluble intercellular adhesion molecule-1 (ICAM-1), and fibrinogen, were significantly associated with AF [7]. A prospective, population-based cohort study that studied thirteen inflammation markers found that the vascular cell adhesion molecule 1 (VCAM-1) was significantly associated with new-onset AF in the general community [8].
However, there are important gaps in our knowledge of the relationship between adhesion pathway proteins and risk of AF. Prior studies included only a limited number of adhesion pathway proteins and have been limited in their ethnic/racial diversity [6,7,8,9,10]. Thus, the aim of this study was to determine the association of fifteen adhesion pathway proteins with risk of AF in the diverse Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study population
The MESA study, as previously described, recruited 6,814 African, Chinese, Hispanic, and non-Hispanic white Americans aged 45 to 84 years (47% male) with no history of cardiovascular disease (CVD) [11]. Hepatocyte growth factor (HGF) was measured at Exam 1 in 6669 participants. A stratified random sample including 720 individuals for each of the 4 races/ethnicities (n = 2880) was used to measure the circulating levels of adhesion pathway proteins at Exam 2. There were 2550 subjects with plasma and/or serum and AF data available. Of these, 30 had a CVD event prior to Exam 2 and were excluded. An additional 16 had an AF event prior to Exam 2 and were excluded, thus resulting in 2504 subjects for analysis (see Additional file 1: Figure S1). All the participants gave written informed consent, and the MESA study protocol and its ancillary studies received approval by the appropriate ethics committees: the University of Minnesota Institutional Review Board (IRB) and the IRBs at all of the other participating centers. The study was done in accordance with the Declaration of Helsinki.
Protein measurements
Blood was obtained from fasting participants at Exam 1 (2000–2002) and Exam 2 (2002–2004) and stored at − 70 °C. Quantitative sandwich enzyme-linked immunosorbent assays were used to measure the adhesion pathway proteins (in years 2010–2012). The 15 circulating proteins were chosen based on biological plausibility of associating with heart disease as determined by published literature or network interaction and had a high-quality commercial assay available. P-selectin, Regulated on Activation, Normal T cell Expressed and Secreted (RANTES), stromal-derived factor 1α (SDF-1α) and transforming growth factor β1 (TGF-β1) were measured in EDTA plasma, while matrix metalloproteinase 1 (MMP-1), matrix metalloproteinase 2 (MMP-2), tissue inhibitor of metalloproteinase 2 (TIMP-2), ICAM-1, VCAM-1, L-selectin, HGF, chemokine ligand 21 (CCL-21), secretory leukocyte protease inhibitor (SLPI), E-cadherin, and interleukin 2 soluble receptor (IL-2 sR) were measured in serum. Details of the assay type and performance are specified in Additional file 1: Table S1.
Covariates
All covariates were measured at Exam 1, which will serve as baseline for the HGF analyses, and Exam 2, which will serve as baseline for all other analyses. Demographics, education, smoking history, current alcohol use, and medication use were collected using self-administered and interviewer-administered questionnaires. In addition to the questionnaire, participants were asked to bring all medications to exams. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Resting blood pressure was measured three times in the seated position using an automated oscillometric sphygmomanometer (Dinamap). The average of the last 2 readings was used in the analyses. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or taking antihypertensive medication. Serum glucose was assayed by a glucose oxidase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostic, Rochester, NY). Diabetes mellitus was defined as use of insulin or other diabetes mellitus medication, self-reported physician diagnosis, or fasting glucose ≥ 126 mg/dL. Glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula [12]. Total cholesterol was measured in EDTA plasma using a cholesterol oxidase method (Roche Diagnostics, Indianapolis, IN) on a Roche COBAS FARA centrifugal analyzer. High-density lipoprotein (HDL) cholesterol was measured in EDTA plasma by the cholesterol oxidase method after precipitation of non-high-density lipoprotein cholesterol with magnesium/dextran (Roche Diagnostics, Indianapolis, IN). Heart failure (HF) was classified as definite or probable. Definite or probable HF both required HF symptoms such as shortness of breath or edema. Definite HF required one or more other criteria, such as pulmonary edema/congestion by chest X-ray; dilated ventricle or poor LV function; or evidence of left ventricular diastolic dysfunction. If definite HF criteria were not available, probable HF was defined as HF diagnosed by a physician and medical treatment for HF. Participants not meeting any criteria, including only a physician diagnosis of HF without any other evidence, were considered as having no HF.
Atrial fibrillation ascertainment
Each participant was contacted at intervals of 9 to 12 months during follow-up to gather information on recent lifestyle changes and new CVD conditions, treatments, hospitalizations and procedures [11]. AF was identified using hospital discharge diagnoses, MESA study electrocardiograms, death certificates, or for those enrolled in fee-for-service Medicare, from inpatient and outpatient Medicare claims data. AF was defined by International Classification of Disease-9 (ICD-9) codes for AF or atrial flutter (ICD-9 codes 427.31 and 427.32, respectively). Incident AF was defined as the first occurrence of an AF diagnosis from baseline through the end of follow-up on December 31, 2014. A first diagnosis of AF made during the same hospitalization as cardiac surgery was not counted as incident AF, but a subsequent AF diagnosis in the same person, not associated with cardiac surgery, was considered their first AF episode. Follow-up for all analyses was calculated as the time elapsed from the baseline examination to whichever came first: first AF event, loss to follow-up, death, or December 31, 2014.
Statistical methods
Bonferroni correction was used to account for multiple comparisons and a P value of < 0.0033 was considered statistically significant (0.05/15 proteins = 0.0033) for all analyses. Baseline characteristics of participants are presented by race/ethnicity and compared using the chi-squared test for categorical variables and the Kruskal–Wallis test for continuous variables. The association of protein levels with incident AF was assessed using Cox proportional hazards regression. For analyses of proteins measured at Exam 2, subjects with incident AF between Exams 1 and 2 were excluded and time was indexed to the Exam 2 visit date. We adjusted for known risk factors for CVD, and for atrial fibrillation specifically. Model 1 was adjusted for age, sex, and race/ethnicity. Model 2 was comprised of Model 1 covariates and height, BMI, systolic blood pressure, antihypertensive use, diabetes mellitus, smoking status, alcohol use, and total and HDL cholesterol. As a sensitivity analysis, heart failure was additionally added to Model 2 as a time-dependent variable as events occurred during follow-up to assess whether it was a plausible mediator in the association between proteins and incident AF. For all analyses, race/ethnicity-specific models were fit in addition to models pooling race/ethnicity that included race/ethnicity as a covariate. Additionally, we tested for multiplicative interactions of proteins with race/ethnicity and age by including cross-product terms in the pooled statistical model. Race/ethnicity was defined as White, Chinese, Black, and Hispanic. Due to the limited missing data at baseline and Exam 2 for the covariates of interest, imputation was not performed.
Results
Table 1 summarizes the characteristics of MESA participants at Exam 2 that had plasma protein measurements (n = 2504; mean age 63; 47% male). The characteristics of the Exam 1 participants are summarized in Additional file 1: Table S2. The distributions of the proteins are summarized in Additional file 1: Figure S2. All of the protein levels varied by race/ethnicity, except for MMP-1 (Table 2).
During the 25,413 person-years of follow up from Exam 2 (average: 10.2 years), 253 incident cases of AF were identified (44 African Americans, 74 Chinese Americans, 57 Hispanic Americans, and 78 non-Hispanic white Americans). After adjustment for age, sex, and race/ethnicity, MMP-2, TIMP-2, VCAM-1, HGF, SLPI, and IL-2 sR were associated with a higher risk of AF with each standard deviation increase in protein level (P < 0.0033; Table 3).
Further adjustment for height, body mass index, systolic blood pressure, antihypertension therapy, diabetes status, current smoker, current alcohol use, and total and HDL cholesterol did not appreciably change point estimates for MMP-2, TIMP-2, VCAM-1, or SLPI. In contrast, full adjustment attenuated the association of HGF with AF (HR 1.10; 95% CI 1.02‒1.18) and IL-2 sR (HR 1.20; 95% CI 1.06‒1.36). Further adjustment for heart failure did not materially change any of the results. The association between proteins and AF did not differ by race/ethnicity (Additional file 1: Table S3) nor age (data not shown).
Discussion
In a large, diverse population of over 2500 individuals (approximately 50% male), higher levels of circulating MMP-2, TIMP-2, VCAM-1, and SLPI were associated with a higher risk of AF, independent of traditional CVD risk factors. While there is growing evidence that adhesion pathway proteins may be associated with AF, prior studies have primarily focused on a limited number of proteins with small sample sizes and limited ethnic and racial diversity [6,7,8,9,10]. Thus, by studying a large number of proteins in a large, ethnic and racially diverse population, we were able to comprehensively evaluate the association between proteins in the cellular adhesion pathway and risk of AF. Our findings have important implications for preventing and improving care of patients at risk for AF.
We found a significant association between MMP-2 and risk of AF. MMP-2 is a gelatinase and in addition to breaking down extracellular matrix, MMP-2 is thought to play a role in proliferation, vascular remodeling, and oxidized LDL induced smooth muscle migration [13,14,15]. Xu et al. found that upregulated MMP-2 expression and downregulated TIMP-2 expression in the atrial tissue of AF patients was associated with the development of sustained AF [16]. Diao and colleagues also demonstrated that persistent AF patients had notably increased MMP-2 expression in left atrial tissue [10]. One prospective study found that increased circulating levels of MMP-2 were independent predictors of myocardial infarction, stroke, peripheral embolization, and death among patients with AF [17]. These associations give further insight on the role of MMP-2 in the mechanism of atrial remodeling and the development and perpetuation of AF.
There was an association between higher levels of TIMP-2 and increased risk of AF. TIMP-2 is a metallopeptidase inhibitor and directly suppresses the proliferation of endothelial cells through MMP-dependent mechanisms. In contrast to our findings, results from a meta-analysis found that lower circulating levels of TIMP-2 were associated with an increased risk of AF, albeit inclusion of different subtypes of AF was postulated as an explanation for the inconsistent findings with prior literature [18]. The higher circulating levels of TIMP-2 found in our study might be explained by its activation upon elevated levels of MMP-2, or by abnormal myocyte response in the remodeled atria [19].
Similar to previous studies, we found a positive association between VCAM-1 and risk of AF [8, 9, 20]. VCAM-1 is a member of the immunoglobulin family of adhesion proteins and in tandem with its receptor, VLA-4, mediates leukocyte-endothelial cell adhesion and signal transduction [21]. Of the two prospective cohort studies assessing the association of VCAM-1 with incident AF, one reported that baseline levels of VCAM-1 predicted post-operative AF after coronary artery bypass surgery and the other found that soluble levels of VCAM-1 had a positive, dose–response relationship with long-term risk of AF [8, 9]. Our study corroborates these findings and expands on the prior work by using a racially/ethnically diverse population.
SLPI was associated with AF risk. SLPI has anti-inflammatory actions regulating TGF-βand IL10 production and suppressing MMP production and activity [22,23,24,25]. Normally found in mucosal tissue, monocytes, and neutrophils, SLPI is expressed in epithelial cells during injury and is thought to prevent the excessive tissue destruction associated with delayed wound healing [26, 27]. To the best of our knowledge, no other studies have assessed the relationship between SLPI and AF.
After full adjustment for confounders, HGF and IL-2 sR were modestly associated with AF. IL-2 sR is the soluble form of the receptor for IL-2, a cytokine well-known for its role in T-lymphocyte function [28, 29]. The soluble receptor of IL-2 is secreted in response to inflammation [28, 29]. Evidence suggests that inflammation seems to trigger AF, although AF might help maintain an inflammatory environment as well [4]. HGF is produced in vascular cells, functions as a potent inducer of LFA-1, and reduces surface expression of L-selectin [30]. Higher circulating levels of HGF have been reported in patients with CVD [31,32,33]. Li et al. found that local HGF levels in samples from the right atrium of AF patients were reduced, confirming dysfunction in the local production of HGF. Systemic HGF levels have been found to increase via the endocrine pathway from sources like the lungs and liver as a result of vascular endothelial injury [34]. Katoh et al. reported higher circulating levels of HGF in patients with non-valvular AF [31]. The association we found between HGF and AF is consistent with this result.
Clinical implications
In this study we found that several proteins in the cellular adhesion pathway, specifically MMP-2, TIMP-2, VCAM-1, and SLPI, are associated with increased risk of AF. These findings have important clinical implications. Our results indicate that strategies aimed at reducing cellular adhesion during the inflammatory response may prevent risk of AF. However, further investigation is needed to determine whether interventions targeting components of the adhesion pathway could eventually lead to new tools for defining AF risk or prevention of AF.
Limitations
The present study had some limitations. First, incident AF is likely underestimated given that asymptotic AF may go undetected. As a result, we could be underestimating the association of proteins and AF due to misclassification or our results could be specific to symptomatic cases. We were underpowered to detect association differences across races/ethnicities. Thus, assessing the heterogeneity by race/ethnicity in larger populations is an area of future research. Finally, proteins were measured in the cohort at a single point in time and AF events could have occurred more than a decade after their measurement. However, circulating levels of these proteins have been shown to be relatively stable over time, indicating that a single measurement is a reasonable biomarker for an individual’s exposure to the proteins [35, 36].
Conclusions
In conclusion, soluble levels of MMP-2, TIMP-2, VCAM-1, and SLPI were associated with an increased risk of AF in a diverse population from the United States. Our findings suggest that adhesion pathway proteins may be important risk predictors of AF.
Availability of data and materials
The datasets analyzed during the current study may be requested from the MESA Coordinating Center.
Abbreviations
- AF:
-
Atrial fibrillation
- BMI:
-
Body mass index
- CCL-21:
-
Chemokine ligand 21
- CRP:
-
C-reactive protein
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- GFR:
-
Glomerular filtration rate
- HDL:
-
High-density lipoprotein
- HF:
-
Heart failure
- HGF:
-
Hepatocyte growth factor
- ICAM:
-
Intercellular adhesion molecule 1
- ICD-9:
-
International Classification of Disease-9
- IL-2 sR:
-
Interleukin 2 soluble receptor
- MMP-1:
-
Matrix metalloproteinase 1
- MMP-2:
-
Matrix metalloproteinase 2
- MESA:
-
Multi-Ethnic Study of Atherosclerosis
- RANTES:
-
Regulated on activation, normal T cell expressed and secreted
- SDF-1α:
-
Stromal-derived factor 1α
- TGF-β1:
-
Transforming growth factor β1
- SLPI:
-
Secretory leukocyte protease inhibitor
- TIMP-2:
-
Tissue inhibitor of metalloproteinase 2
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52.
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–603.
Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50(21):2021–8.
Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–70.
Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27(2):136–49.
Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104(1):92–6.
Conen D, Ridker PM, Everett BM, Tedrow UB, Rose L, Cook NR, et al. A multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. 2010;31(14):1730–6.
Willeit K, Pechlaner R, Willeit P, Skroblin P, Paulweber B, Schernthaner C, et al. Association between vascular cell adhesion molecule 1 and atrial fibrillation. JAMA Cardiol. 2017;2(5):516–23.
Verdejo H, Roldan J, Garcia L, Del Campo A, Becerra E, Chiong M, et al. Systemic vascular cell adhesion molecule-1 predicts the occurrence of post-operative atrial fibrillation. Int J Cardiol. 2011;150(3):270–6.
Diao SL, Xu HP, Zhang B, Ma BX, Liu XL. Associations of MMP-2, BAX, and Bcl-2 mRNA and protein expressions with development of atrial fibrillation. Med Sci Monit. 2016;22:1497–507.
Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92(6):1565–9.
Auge N, Maupas-Schwalm F, Elbaz M, Thiers JC, Waysbort A, Itohara S, et al. Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation. 2004;110(5):571–8.
Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation. 1997;96(10):3555–60.
Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109(3):363–8.
Ehrlich JR, Kaluzny M, Baumann S, Lehmann R, Hohnloser SH. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011;100(11):1029–36.
Liu Y, Xu B, Wu N, Xiang Y, Wu L, Zhang M, et al. Association of MMPs and TIMPs with the occurrence of atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. 2016;32(6):803–13.
Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, Kofune M, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22(9):987–93.
Hammwohner M, Ittenson A, Dierkes J, Bukowska A, Klein HU, Lendeckel U, et al. Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp Biol Med (Maywood). 2007;232(4):581–9.
Hope SA, Meredith IT. Cellular adhesion molecules and cardiovascular disease. Part I. Their expression and role in atherogenesis. Intern Med J. 2003;33(8):380–6.
Bedel A, Negre-Salvayre A, Heeneman S, Grazide MH, Thiers JC, Salvayre R, et al. E-cadherin/beta-catenin/T-cell factor pathway is involved in smooth muscle cell proliferation elicited by oxidized low-density lipoprotein. Circ Res. 2008;103(7):694–701.
Zhang Y, DeWitt DL, McNeely TB, Wahl SM, Wahl LM. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J Clin Invest. 1997;99(5):894–900.
Schneeberger S, Hautz T, Wahl SM, Brandacher G, Sucher R, Steinmassl O, et al. The effect of secretory leukocyte protease inhibitor (SLPI) on ischemia/reperfusion injury in cardiac transplantation. Am J Transplant. 2008;8(4):773–82.
Sano C, Shimizu T, Sato K, Kawauchi H, Tomioka H. Effects of secretory leucocyte protease inhibitor on the production of the anti-inflammatory cytokines, IL-10 and transforming growth factor-beta (TGF-beta), by lipopolysaccharide-stimulated macrophages. Clin Exp Immunol. 2000;121(1):77–85.
Ashcroft GS, Lei K, Jin W, Longenecker G, Kulkarni AB, Greenwell-Wild T, et al. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6(10):1147–53.
Sallenave JM. The role of secretory leukocyte proteinase inhibitor and elafin (elastase-specific inhibitor/skin-derived antileukoprotease) as alarm antiproteinases in inflammatory lung disease. Respir Res. 2000;1(2):87–92.
Rubin LA, Galli F, Greene WC, Nelson DL, Jay G. The molecular basis for the generation of the human soluble interleukin 2 receptor. Cytokine. 1990;2(5):330–6.
Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79.
Mine S, Tanaka Y, Suematu M, Aso M, Fujisaki T, Yamada S, et al. Hepatocyte growth factor is a potent trigger of neutrophil adhesion through rapid activation of lymphocyte function-associated antigen-1. Lab Invest. 1998;78(11):1395–404.
Katoh H, Shimada T, Inoue S, Takahashi N, Shimizu H, Ohta Y, et al. Reduced high serum hepatocyte growth factor levels after successful cardioversion in patients with atrial fibrillation. Clin Exp Pharmacol Physiol. 2004;31(3):145–51.
Bielinski SJ, Berardi C, Decker PA, Larson NB, Bell EJ, Pankow JS, et al. Hepatocyte growth factor demonstrates racial heterogeneity as a biomarker for coronary heart disease. Heart. 2017;103(15):1185–93.
Bell EJ, Larson NB, Decker PA, Pankow JS, Tsai MY, Hanson NQ, et al. Hepatocyte growth factor is positively associated with risk of stroke: the MESA (Multi-Ethnic Study of Atherosclerosis). Stroke. 2016;47(11):2689–94.
Li M, Yi X, Ma L, Zhou Y. Hepatocyte growth factor and basic fibroblast growth factor regulate atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease via the mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2013;6(5):1121–6.
De Bellis A, Di Martino S, Fiordelisi F, Muccitelli VI, Sinisi AA, Abbate GF, et al. Soluble intercellular adhesion molecule-1 (sICAM-1) concentrations in Graves’ disease patients followed up for development of ophthalmopathy. J Clin Endocrinol Metab. 1998;83(4):1222–5.
Giovannoni G, Lai M, Thorpe J, Kidd D, Chamoun V, Thompson AJ, et al. Longitudinal study of soluble adhesion molecules in multiple sclerosis: correlation with gadolinium enhanced magnetic resonance imaging. Neurology. 1997;48(6):1557–65.
Acknowledgements
We thank the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators can be found at http://www.mesa-nhlbi.org.
Funding
This work was supported by the National Institutes of Health Grants N01HC95159, N01 HC95160, N01 HC95161, N01 HC95162, N01 HC95163, N01 HC95164, N01 HC95165, N01 HC95166, N01 HC95167, and N01 HC95168, R01 HL127659, N01 HC95169, R01 HL98077, T32 HL07111-40, UL1 TR000040, and UL1 TR001079). Role of the Funding Source The funding source had no involvement in the study design, collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception or design of the work, or the acquisition, analysis, or interpretation of data for the work: IJM, SMM, EJB, NBL, PAD, MAG, NQH, SRH, JSP, MYT and SJB. Statistical analysis: PAD and NBL. Drafting of the manuscript: IJM, SMM and SJB. Revising the manuscript critically for important intellectual content: IJM, SMM, EJB, NBL, PAD, MAG, NQH, SRH, JSP, MYT and SJB. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the participants gave written informed consent, and the MESA study protocol and its ancillary studies received approval by the appropriate ethics committees: the University of Minnesota Institutional Review Board (IRB # IRB00000438), Wake Forest University IRB (# IRB00008492), Columbia University IRB (# IRB00002973), Johns Hopkins University IRB (# 00001656), Northwestern University IRB (# IRB00005003), University of California Los Angeles IRB (# 00000172), University of Washington IRB (# IRB00005647). The study was done in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Assay characteristics by protein, Multi-Ethnic Study of Atherosclerosis, 2002-2004. Table S2. Exam 1 characteristics by race/ethnicity, Multi-Ethnic Study of Atherosclerosis, 2000-2002. Table S3. Adjusted hazard ratios for incident atrial fibrillation per one standard deviation increase in protein by ethnicity/race, Multi-Ethnic Study of Atherosclerosis, 2002-2014. Figure S1. Flow diagram of Exam 2 study participation. Figure S2. Distribution of proteins levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mendez, I.J., Manemann, S.M., Bell, E.J. et al. Adhesion pathway proteins and risk of atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. BMC Cardiovasc Disord 21, 436 (2021). https://doi.org/10.1186/s12872-021-02241-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-021-02241-w