Abstract
Background
The PEGASUS-TIMI 54 trial inclusion criteria effectively identified high-risk patients with recent myocardial infarction (MI) who would benefit from continuing dual antiplatelet therapy (DAPT) with ticagrelor for more than 12 months. It is unknown how many real-world patients meet these criteria during the acute phase of ST-elevation MI (STEMI), or the extent to which these criteria predict a patient's risk and prognosis. Study objectives were: (1) determine the proportion of PEGASUS-TIMI 54-like patients (PG-l) in a real-world cohort of patients hospitalized with STEMI and to assess their ischemic and hemorrhagic risk; (2) examine their ischemic and hemorrhagic in-hospital events (major adverse cardiovascular and cerebrovascular events [MACCE] and clinically relevant bleeding); (3) evaluate their long-term outcomes and the impact on the long-term prognosis of the type of DAPT prescribed at discharge.
Methods
This observational study was conducted in 1086 patients admitted to hospital with a diagnosis of STEMI between February 2011 and March 2018 and enrolled in the CARDIO-STEMI Sanremo registry. Patients’ demographic and clinical characteristics, procedural variables, and individual ischemic and hemorrhagic risk scores were assessed in-hospital. Four-year survival was also analyzed.
Results
The proportion of PG-I patients was 69.2%. Compared with non-PG-l patients, PG-l patients were older, had more multivessel disease and comorbidities, and experienced more frequent MACCE (8.3% vs. 3.6%, p = 0.005) and clinically significant bleeding events (6.7% vs. 2.7%, p = 0.008), a higher rate of in-hospital death (6.5% vs. 1.5%, p < 0.001), and higher follow-up mortality rate (14.8% vs. 7.7%; p = 0.002). Four-year survival was significantly lower in the PG-l group (83.9% vs. 91.8%; Log-rank = 0.001) and was related to the cumulative number of concurrent risk factors. In the unadjusted analysis, survival was greater in patients discharged on ticagrelor than on another P2Y12 inhibitor (90.2% vs. 76.7%, Log-rank = 0.001), and the difference was particularly evident in PG-l patients.
Conclusions
The risk of MACCE for PG-l patients increased with the number of concurrent PEGASUS-TIMI 54 risk features. Treatment with ticagrelor on discharge was associated with improved survival rates during 4 years of follow-up.
Similar content being viewed by others
Background
In Western Europe, 32.8% of patients hospitalized for acute coronary syndrome (ACS) have ST-segment elevation myocardial infarction (STEMI) [1]. According to current guidelines, STEMI patients should receive dual antiplatelet therapy (DAPT) with aspirin (ASA) and a P2Y12 inhibitor (with ticagrelor or prasugrel preferred over clopidogrel) for at least 12 months, regardless of whether or not patients underwent percutaneous coronary intervention (PCI) [2, 3]. DAPT prevents the recurrence of ischemic events such as myocardial infarction (MI), stroke, or death from cardiovascular (CV) causes unrelated to the treated coronary lesion, which may occur beyond 1 year after coronary stenting [4, 5]. However, the clinical benefit of continued treatment with P2Y12 inhibitors is tempered by a higher risk of bleeding [4, 6], which is correlated with treatment duration [7,8,9,10,11,12,13,14]. The clinical trials ARCTIC-Interruption [15], DAPT [4], DES-LATE [16], OPTIDUAL [17], and meta-analyses [18, 19], showed that the benefit of reduced ischemic events associated with prolonging DAPT beyond 12 months was counterbalanced by an increase in bleeding risk, although study populations were heterogeneous, so no definitive conclusion about the optimal duration of DAPT could be reached. While it has been proposed that this risk could be mitigated in patients with high bleeding risk by a shorter DAPT duration of at least 6 months [3, 20,21,22], evidence from the PEGASUS-TIMI 54 (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin) trial suggested a benefit with prolonged DAPT in patients with high ischemic risk and prior MI [7].
The PEGASUS-TIMI 54 study evaluated the use of DAPT beyond 12 months in 21,162 patients who had had an MI 1 to 3 years prior to enrolment and had a high ischemic risk [23]. The study inclusion criteria also required patients to be ≥ 50 years of age and to have ≥ 1 of the following additional risk factors: age ≥ 65 years, diabetes mellitus, recurrent MI, multivessel coronary artery disease (CAD) or impaired renal function (creatinine clearance < 60 mL/min) [23]. Patients received ASA 75–150 mg/day and add-on ticagrelor 60 or 90 mg twice daily or placebo, and were followed for a median of 33 months [24]. Compared with placebo, ticagrelor was associated with a reduced risk of CV death, MI, or stroke over 3 years and a neutral effect on overall mortality [24]. The two ticagrelor doses were associated with an increased risk of Thrombolysis in Myocardial Infarction (TIMI) major bleeding compared with placebo but the risk of fatal bleeding or intracranial hemorrhage did not differ between ticagrelor and placebo [24]. The benefit of ticagrelor was consistent across the major clinical subgroups of the PEGASUS-TIMI 54 population, 53.4% of whom had experienced a STEMI [24].
Based on the favorable benefit-risk profile, long-term ticagrelor may represent an attractive option, although the benefits seen in the PEGASUS-TIMI 54 trial may not extend to patients at low risk of ischemic events [25, 26] or to patients with a high risk of bleeding, since patients with recent bleeding or requiring oral anticoagulation were excluded from the study [25,26,27]. The 2017 guidelines of the European Society of Cardiology (ESC) recommend that physicians consider extending DAPT with ticagrelor 60 mg twice daily + ASA beyond 1 year in patients who have tolerated DAPT for 12 months without bleeding, and using clopidogrel or prasugrel as an alternative choice if ticagrelor is not feasible or tolerated [2, 3].
The PEGASUS inclusion criteria may help clinicians to promptly identify patients at high ischemic risk [24], because the number of risk factors present in an individual patient is closely related to the risk of a major adverse cardiac event during follow up [28]. Our hypothesis is that the inclusion criteria in the PEGASUS-TIMI 54 trial could be a useful tool for the identification of high-risk patients, defined as PEGASUS-like (PG-l) patients, immediately after STEMI and for prognosis estimation and therapeutic decision-making. The use of such a tool, to the best of our knowledge, has never been tested.
The aim of the study was (1) to evaluate the proportion of STEMI patients enrolled in the Italian CARDIO-STEMI Sanremo registry who met the PEGASUS-TIMI 54 trial inclusion criteria (PG-l patients) during hospitalization; (2) to evaluate the ischemic risk (using the GRACE score [29]) and the hemorrhagic risk (using the PRECISE-DAPT [21] and CRUSADE [30] scores) of these patients and the incidence of hospital events (death, major adverse CV or cerebrovascular event [MACCE], any clinically significant bleeding based on Bleeding Academic Research Consortium (BARC) type ≥ 2); and (3) to evaluate the overall long-term survival in PG-l and non-PG-l patients and the impact of the type of DAPT on the long-term prognosis in these groups.
Methods
This study used data from the CARDIO-STEMI Sanremo registry, a single-center, observational study that was conducted at the Sanremo Hospital (Italy) according to national and international guidelines. The protocol was compliant with ethical standards and privacy rule requirements for research and approved by the local ethics committee (Comitato Etico Regione Liguria based at the IRCCS San Martino of Genoa; resolution number 039REG2016). Each patient in the registry provided written informed consent for participation, including consent to be contacted for follow-up information and for their data to be included in subsequent analyses.
All the consenting patients admitted to the hospital with a diagnosis of STEMI between February 2011 and March 2018 were enrolled; for every patient, demographic and clinical characteristics were recorded and individual ischemic risk factors (i.e. age, diabetes mellitus, a prior spontaneous MI, multivessel CAD, creatinine clearance < 60 mL/min) and hemorrhagic risk scores were assessed during hospitalization; TIMI flow grade (before and after PCI), the rate of in-hospital MACCE, ST segment resolution, bleeding, and survival were also collected. MACCE were defined according the PLATO trial criteria [11]; MI was defined according to the fourth universal definition of MI [31]; bleeding was assessed following the standardized definition provided by Mehran et al. [32]. Hemorrhagic risk factors were assessed using the CRUSADE [30] and PRECISE-DAPT [21] scores; the GRACE risk score was used to assess ischemic risk [29]. The PRECISE DAPT score predicts the risk of bleeding in patients treated with DAPT; a PRECISE DAPT score above the recommended cut-off point (≥ 25) identifies patients who might benefit from DAPT for < 12 months [21]. Patients were treated according to the prevailing guidelines at the time of their enrolment, all of which advised continuation of DAPT for at least 1 year after discharge in patients who had undergone PCI. Follow-up was performed through a telephone interview with the patient or the patient’s family physician, with the support of the local health registry.
Patients were categorized into two cohorts, depending on whether their demographic and clinical characteristics were consistent with the inclusion criteria for the PEGASUS-TIMI 54 study (age ≥ 50 years and ≥ 1 high-risk criterion). Patients who met these criteria were designated PEGASUS-like (PG-l) and all other patients were designated as non-PG-l patients. Comparisons were made between the characteristics treatment patterns, and outcomes in the two groups.
Statistical analyses
The patients’ baseline demographic and clinical characteristics were reported using relevant descriptive statistics (means ± standard deviation [SD], median and interquartile range [IQR], or percentage). Kolmogorov–Smirnov tests were used to assess the normal distribution of continuous variables; those with skewed distribution were compared using the Mann–Whitney U test. Categorical variables and continuous variables that were not normally distributed were compared using the χ2 test and Kruskal–Wallis test, respectively. Medians across groups were compared using a Median test for two independent medians.
A stepwise block Cox multivariate analysis was used to identify predictors of long-term mortality, with age, diabetes, PCI, ≥ 50% ST resolution, renal function, ejection fraction and therapy at discharge included as candidate variables.
For all tests, a p value < 0.05 was considered significant. Survival analyses were generated using the Kaplan–Meier method and compared with the log-rank test. Statistical analyses were undertaken using SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
The demographic and baseline data for the 1086 patients (73.5% males) enrolled in the CARDIO-STEMI Sanremo registry are shown in Table 1. Median age (range) of patients was 66 (56–77) years, with 90.3% of the patients aged ≥ 50 years and 55.3% aged ≥ 65 years. Overall, 17.4% of patients had diabetes, 9.8% had previous MI, 40.5% had multivessel CAD, and 28.3% had impaired renal function. Only 5% of patients had experienced a previous hemorrhage.
The PEGASUS-TIMI 54 inclusion criteria (age ≥ 50 years and ≥ 1 high-risk criterion) were met by 751 (69.2%) patients (PG-l patients). The demographic and baseline data in the PG-l population and the non-PG-l population are shown in Table 2. The PG-l population was older than the non-PG-l group, with 74.2% of PG-l patients being ≥ 65 years old and 41.1% being ≥ 75 years old (p < 0.001). Hypertension was more prevalent among PG-l patients (61.9% vs. 41.8%, p < 0.001), while no significant difference was found between the two groups for dyslipidemia. Non-PG-l patients were twice as likely to be smokers and to have a family history of CAD compared with PG-l patents, who included a higher proportion of patients with impaired renal function (38.2% vs. 6.6%; p < 0.001) and multivessel CAD (52.3% vs. 14.0%; p < 0.001).
The GRACE and CRUSADE scores were significantly higher in the PG-l than the non-PG-l cohort (114 [95–132] vs. 77 [63–90], p < 0.001, and 28.5 [19–39] vs. 16 [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23], p < 0.001, respectively). In our study, the fraction of patients at high bleeding risk (PRECISE DAPT score ≥ 25) was also significantly higher in the PG-l group (41.7% vs. 33.2%, p = 0.018).
No significant between-group differences were seen for the proportion of patients treated with PCI (90.4% vs. 91.9%, p = 0.44) or with ≥ 50% ST resolution, achieved by 81.3% of the PG-l population versus 81.9% of non-PG-l patients (p = 0.86). The frequency of thromboaspiration and the use of radial access were similar between groups (37% vs. 33%, p = 0.40 and 65.8% vs. 60.1, p = 0.12, respectively). Similarly, no significant differences were reported between the PG-l and non-PG-l groups in the proportion of patients presenting with spontaneous reperfusion (initial TIMI flow of ≥ 2 in 41.8% vs. 38.0%, p = 0.29) and achieving reperfusion after angioplasty (a final TIMI flow of ≥ 2 in 94.3% vs. 95.0%, p = 0.76). The left ventricular ejection fraction during hospitalization was lower in PG-l than non-PG-l patients (median [range] 45% [40–55] vs. 50% [45–55], p < 0.001).
In keeping with the high-risk profile of the PG-l cohort (older age, high prevalence of multivessel CAD, higher GRACE and CRUSADE scores, and lower ejection fraction), adverse outcomes during hospitalization were significantly more common in the PG-l group than the non-PG-l group, including BARC ≥ 2 bleeding (6.7% vs. 2.7%, p = 0.008) and MACCE, defined as in-hospital all-cause death, AMI, stent thrombosis or acute ischemic stroke and cerebrovascular events (8.3% vs. 3.6%, p = 0.005, respectively). Similarly, PG-l patients had a longer hospital stay (median [range] 5 [4,5,6,7] days vs. 4 [4, 5] days, p < 0.001), and a nearly fivefold higher rate of in-hospital death (6.5% vs. 1.5%, p < 0.001).
The median (range) long-term follow-up for STEMI patients was 1142 (577–1810) days, and mean ± SD follow-up was 1207 ± 697 days. The Kaplan–Meier estimated 4-year survival rate was significantly lower among PG-l than non-PG-l patients (83.9% vs. 91.8%; Log-rank = 0.001; Fig. 1). The 4-year survival was also dependent on the number of PEGASUS-TIMI 54 high risk criteria that were present (Log-rank = 0.001), with survival decreasing as the number of concomitant risk criteria increased (Fig. 2).
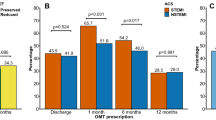
Unless contraindicated, all patients were treated with a P2Y12 inhibitor (prasugrel, ticagrelor, or clopidogrel) on a background of ASA (DAPT). The majority of patients were discharged on ticagrelor (52.7%), followed by clopidogrel (28.9%) and prasugrel (12.3%). The discharge therapy with clopidogrel and prasugrel varied significantly with age (p = 0.001), with clopidogrel prescribed more frequently for older than younger patients, and prasugrel prescribed more often for younger patients. However, the proportion of patients receiving ticagrelor varied little between age groups. No statistically significant differences were noted in sex, age, frequency of in-hospital bleeding (BARC ≥ 2) or the proportion of patients with ≥ 50% ST resolution between those treated with ticagrelor and those who received clopidogrel or prasugrel. However, patients who received ticagrelor versus another P2Y12 inhibitor were more often treated with PCI (97.6% vs. 85.9%; p < 0.001) and had a lower follow-up mortality (unadjusted rate 8.10% vs. 19.0%, p < 0.001).
In the PG-l group, ticagrelor at discharge was associated with significantly lower mortality during follow-up compared with another P2Y12 inhibitor at discharge, with a 4-year survival probability of 90.2% versus 76.7% (Log-rank p = 0.001), as shown in the Kaplan–Meier analysis shown in Fig. 3a. There was no statistically significant difference in mortality between ticagrelor and no ticagrelor at discharge among patients in the non-PG-l group (Fig. 3b; p = 0.158).
In the stepwise Cox multivariate analysis, ticagrelor therapy at discharge was found to be the only independent predictor of follow-up mortality (Relative Risk [RR] 0.62, 95% Confidence Interval [CI]: 0.39–0.98, p = 0.04), and only in the PG-l group, but not in non-PG-l patients (RR 0.70, 95% CI: 0.20–2.40, p = 0.57). Although ticagrelor was associated with reduced mortality in the PG-l group vs. the non-PG-l group, the difference was not statistically significant (p for heterogeneity is 0.86); the lack of a significant effect on mortality in the non-PG-l group may be due to the small number of events/wide confidence intervals.
Discussion
Nearly 70% of the patients admitted to hospital for STEMI met the PEGASUS-TIMI 54 trial inclusion criteria. When compared with non-PG-l patients, PG-l patients were at increased risk of in-hospital and long-term mortality; they were older, with lower ejection fraction, more complex CAD, and in some cases had one or more comorbid conditions. The GRACE score was significantly higher in the PG-l cohort, who experienced a longer hospital stay and an almost five times higher rate of in-hospital death. The 4-year survival of the PG-l population was significantly lower and positively related to the number of associated PEGASUS-TIMI 54 high risk factors: the higher the number of concurrent risk factors, the higher the mortality. This is consistent with previous research by Parodi et al. in similar analyses of patients with ACS and PCI followed for up to 3 years [28]. We can hypothesize that a simple association of risk factors, or their inclusion within more complex scores, could adequately identify patients with a more favorable net clinical benefit from prolonged DAPT. This could translate into a new simple bedside risk-assessment tool based on the PEGASUS-TIMI 54 criteria for the risk stratification of patients with acute MI at index hospitalization.
By adopting the PEGASUS-TIMI 54 criteria we have also been able to identify a population of patients with a high risk of bleeding: the PG-l cohort had a significantly higher CRUSADE score, significantly more patients with a PRECISE-DAPT score > 25, and a significantly greater incidence of in-hospital BARC ≥ 2 bleeding compared with the non-PG-l cohort. While the risk of bleeding was increased in the PG-I cohort, it is worth bearing in mind that interpreting this risk must be balanced against the level of ischemic risk. As shown in the PRECLUDE study [33], prior bleeding is the best predictor of bleeding, so if prior major bleeding is excluded, patients with ischemic risk factors are more likely to have a new ischemic event than bleeding. This suggests that, while our PG-I cohort might have had an increased risk of bleeding, their risk of ischemic occurrence may be greater. The trade-off between ischemic benefit and bleeding risk is of paramount importance for cardiologists who need to assess therapies and manage patients, and the higher bleeding risk associated with DAPT is often outweighed by the benefits of a reduced risk of ischemic events. Considering that bleeding and ischemic risks are often strongly correlated, risk scores enable the stratification of patients and may facilitate tailored decision-making on the type and duration of DAPT [34, 35]. Current ESC recommendations for long-term secondary prevention with DAPT acknowledge this delicate balance of risk, and recommend DAPT for patients at high or moderately increased ischemic risk without high bleeding risk [36].
Among patients discharged on DAPT, prasugrel was preferentially used in younger patients whereas treatment with clopidogrel was more frequent among elderly patients. In contrast, the proportion of patients discharged on ticagrelor did not differ with respect to age, showing that, in the real-life clinical practice of this hospital setting, physicians consider ticagrelor a safe therapeutic option. Indeed, the PLATO study had already shown better follow-up survival for patients treated with ticagrelor versus clopidogrel [11]; a similar benefit in a real-world STEMI population had also been highlighted by our study group [37].
Finally, although results show that ticagrelor was associated with lower mortality rates for the next 12 months compared with other antiplatelet agents, the difference was statistically significant only for PG-l patients (unadjusted analysis). A possible explanation for this is the small number of patients in the non-PG-l group, limiting the power of that analysis. For PG-l patients, the benefit of therapy with ticagrelor 90 mg twice daily persisted for the first 12 months, was sustained over time and was appreciable also at long term follow-up.
From a practical point of view, the better survival among patients receiving ticagrelor could be explained as an extended clinical benefit of ticagrelor in the long term, compared with other antiplatelet agents, which might be due to the decrease in the risk of thrombotic events, without a concomitant increase in the risk of major bleeding, consistent with the findings from the PLATO study and from the sub-study in the STEMI population [11, 38].
Study limitations
This is a real-life study performed on data independently gathered from more than 1000 patients enrolled in a single-center, observational study, with an average 4-year follow-up, and is affected by the inherent limitations of a non-randomized prospective study. No MACCE or bleeding events were recorded after the hospital phase. This limitation may have affected the recording of any favorable effect on the rate of MACCE occurring only after 30 days [24]. However, this is a frequent limitation of hospital registry data. Our study did collect long-term mortality data, which can be considered as a surrogate measure of post-discharge complications including MACCE and bleeding. Another limitation of this study is the lack of data collection on hemorrhagic events during the first 12 months of follow-up; therefore, we could not establish the proportion of patients eligible for prolonged use of DAPT beyond 12 months. Nor did we collect data on use of DAPT beyond 12 months. A history of bleeding was an exclusion criterion for the PEGASUS-TIMI 54 trial. This study lacks the statistical power to show a reduction in mortality for non-PG-l patients over the 4-year follow-up. Finally, the potential for confounding by measured or unmeasured variables cannot be ruled out.
Conclusions
Nearly 70% of the patients enrolled in the CARDIO-STEMI Sanremo registry match the PEGASUS-TIMI 54 inclusion criteria, which successfully identified a population of real-world STEMI patients at a high risk of MACCE or death. When compared with non-PG-l patients, the prognosis for PG-l patients, who are older, and may have one or more comorbid conditions, is worse during both short- and long-term follow-up, and the risk of an adverse outcome progressively increases with the number of concurrent PEGASUS-TIMI 54 high-risk features that are present. Thanks to their ease of use in daily clinical practice, the PEGASUS-TIMI 54 inclusion criteria, along with other scores such as the DAPT score and the PRECISE-DAPT score, should become a more frequently used tool to support clinical decision-making about the duration of DAPT. Notwithstanding the limitations of this real-life registry study, our data also suggest that treatment with ticagrelor on discharge is associated with improved 4-year survival rates in patients who meet the criteria for PEGASUS-TIMI 54 inclusion.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACS:
-
Acute coronary syndrome
- ASA:
-
Aspirin
- BARC:
-
Bleeding Academic Research Consortium
- CV:
-
Cardiovascular
- CI:
-
Confidence interval
- CAD:
-
Coronary artery disease
- DAPT:
-
Dual antiplatelet therapy
- ESC:
-
European Society of Cardiology
- IQR:
-
Interquartile range
- MACCE:
-
Death, major adverse CV or cerebrovascular event
- MI:
-
Myocardial infarction
- PG-l:
-
PEGASUS-like
- PEGASUS-TIMI 54:
-
Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin
- RR:
-
Relative risk
- SD:
-
Standard deviation
- STEMI:
-
Segment elevation myocardial infarction
- TIMI:
-
Thrombolysis in Myocardial Infarction
References
Andre R, Bongard V, Elosua R, et al. International differences in acute coronary syndrome patients’ baseline characteristics, clinical management and outcomes in Western Europe: the EURHOBOP study. Heart. 2014;100(15):1201–7.
Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77.
Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–60.
Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–66.
Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315(16):1735–49.
D’Ascenzo F, Moretti C, Bianco M, et al. Meta-analysis of the duration of dual antiplatelet therapy in patients treated with second-generation drug-eluting stents. Am J Cardiol. 2016;117(11):1714–23.
Gurbel PA, Bliden KP, Butler K, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121(10):1188–99.
Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–33.
Navarese EP, Andreotti F, Schulze V, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618.
Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29(1):21–30.
Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502.
Collet JP, Silvain J, Barthelemy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384(9954):1577–85.
Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129(3):304–12.
Helft G, Steg PG, Le Feuvre C, et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J. 2016;37(4):365–74.
Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65(13):1298–310.
Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385(9985):2371–82.
Costa F, Tijssen JG, Ariotti S, et al. Incremental value of the CRUSADE, ACUITY, and HAS-BLED risk scores for the prediction of hemorrhagic events after coronary stent implantation in patients undergoing long or short duration of dual antiplatelet therapy. J Am Heart Assoc. 2015;4(12).
Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389(10073):1025–34.
Valgimigli M, Ariotti S, Costa F. Duration of dual antiplatelet therapy after drug-eluting stent implantation: will we ever reach a consensus? Eur Heart J. 2015;36(20):1219–22.
Bonaca MP, Bhatt DL, Braunwald E, et al. Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. Am Heart J. 2014;167(4):437–44.
Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–800.
Alexopoulos D. Long-term ticagrelor therapy in patients with prior myocardial infarction significantly reduces ischaemic events, albeit with increased bleeding. Evid Based Med. 2015;20(4):132.
Keaney JF Jr. Balancing the risks and benefits of dual platelet inhibition. N Engl J Med. 2015;372(19):1854–6.
Mearns BM. Antiplatelet therapy. Long-term ticagrelor use in patients with history of MI. Nat Rev Cardiol. 2015;12(5):260.
Parodi G, Bellandi B, Tarantini G, et al. Clinical events beyond one year after an acute coronary syndrome: insights from the RECLOSE 2-ACS study. EuroIntervention. 2017;12(16):2018–24.
Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–33.
Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non-ST-segment-elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119(14):1873–82.
Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618–51.
Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–47.
Lindholm D, Sarno G, Erlinge D, et al. Combined association of key risk factors on ischaemic outcomes and bleeding in patients with myocardial infarction. Heart. 2019;105(15):1175–81.
Matteau A, Yeh RW, Camenzind E, et al. Balancing long-term risks of ischemic and bleeding complications after percutaneous coronary intervention with drug-eluting stents. Am J Cardiol. 2015;116(5):686–93.
van Hattum ES, Algra A, Lawson JA, Eikelboom BC, Moll FL, Tangelder MJ. Bleeding increases the risk of ischemic events in patients with peripheral arterial disease. Circulation. 2009;120(16):1569–76.
Knuuti J, Wijns W, Saraste A, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2019.
Vercellino M, Sanchez FA, Boasi V, et al. Ticagrelor versus clopidogrel in real-world patients with ST elevation myocardial infarction: 1-year results by propensity score analysis. BMC Cardiovasc Disord. 2017;17(1):97.
Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131–41.
Acknowledgements
The authors thank Maurizio Tarzia, an independent medical writer, and Catherine Rees and Tracy Harrison of Springer Healthcare Communications, for providing editorial assistance.
Funding
Cardio-STEMI is an independent study that was funded through departmental resources. Editorial assistance was funded by AstraZeneca. AstraZeneca was not involved in study design, data collection, analysis or interpretation of the data.
Author information
Authors and Affiliations
Contributions
FAS, VB and MV contributed to conception and study design. FAS designed the software to support data collection and acted as data manager. FAS, MV, VB, CT, PC, NP, DP, LG and SC collected data. VB, FAS and MV helped in data analysis and interpretation. VB performed the statistical analysis and acted as corresponding author. VB, FAS and MV were the major contributors in drafting the manuscript. GM revised the manuscript critically for important intellectual content and supervised the development of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by local ethical review boards. The protocol was compliant with ethical standards and privacy rule requirements for research and approved by the local ethics committee (Comitato Etico Regione Liguria based at the IRCCS San Martino of Genoa; resolution number 039REG2016). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sanchez, F., Boasi, V., Vercellino, M. et al. Risk definition and outcomes with the application of the PEGASUS-TIMI 54 trial inclusion criteria to a “real world” STEMI population: results from the Italian “CARDIO-STEMI SANREMO” registry. BMC Cardiovasc Disord 21, 144 (2021). https://doi.org/10.1186/s12872-020-01780-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01780-y