Abstract
Background
Recent studies have demonstrated that complement C1q tumor necrosis factor related proteins (CTRPs) have diverse biological influences on the cardiovascular system. CTRP 3 is a member of the CTRP superfamily, which may play a pivotal role in the pathogenesis of coronary artery disease (CAD). Here, we investigated whether serum levels of CTRP 3 are associated with the prevalence and the severity of CAD.
Methods
In this study, 145 eligible participants were included who underwent coronary angiography. According to the result of the coronary angiography, all participants were divided into two groups: non-CAD group (n = 66) and CAD group (n = 79). The CAD group was further divided into single-vessel (n = 25), double-vessel (n = 30) and triple-vessel (n = 24) disease groups in line with different lesioned vessels of CAD. Plasma CTRP 3 concentration was determined by enzyme-linked immunosorbent assay (ELISA).
Results
Serum levels of CTRP 3 were significantly higher in CAD patients than in non-CAD patients (CAD: 56.68 ± 3.63 ng/ml, non-CAD: 44.10 ± 3.20 ng/ml, p < 0.01). Significant differences of CTRP 3 levels were also found between single-vessel group and triple-vessel group (single-vessel group: 44.80 ± 3.14 ng/ml, triple-vessel group: 75.07 ± 9.41 ng/ml, p < 0.005). Multiple logistic regression analysis revealed that CTRP 3 levels, together with HDL cholesterol and glucose, correlated with CAD.
Conclusions
Elevated serum CTRP 3 levels were closely related to the prevalence and severity of CAD, suggesting that it might be regarded as a novel biomarker for CAD.
Similar content being viewed by others
Background
Given the dramatic changes in lifestyle worldwide, increasing obesity-related disease including type 2 diabetes, dyslipidemia and hypertension have become a serious problem in society. Obesity causes a great deal of metabolic disorders which can culminate in the development of atherosclerotic diseases including coronary artery disease (CAD) [1,2,3,4]. Multiple pro-inflammatory cytokines including tumor necrosis factor-a (TNF-a) and IL-6 are up-regulated in cases of obesity and intensify the prevalence and the progression of CAD [5,6,7].
The complement C1q tumor necrosis factor related protein (CTRP) superfamily is a newly found cluster of adipokines, which share a common structure composed of collagenous and globular C1q-like domains, and 15 members in humans have been identified presently [8, 9]. C1q/tumor necrosis factor related protein-3 (CTRP 3) belongs to the CTRP family, which is abundantly expressed in adipose tissue and chondrocytes [10]. CTRP 3 has been reported for the first time to have comparable potency to APN on vasorelaxation in C57BL mice in 2011 [11]. Circulating CTRP 3 also improves insulin sensitivity and is strongly associated with glucose and lipid metabolism [12]. A recent in vivo study using the CTRP 3 transgenic mice model illustrated that overexpression of CTRP 3 in the obese state can reduce the systemic inflammation [13]. Accumulating evidence has demonstrated that CTRPs have diverse biological influences on the cardiovascular system [14, 15].
CTRP 3, a potent anti-inflammatory adipokine, has attracted increasing attention in its role underlying the pathogenesis of CAD [16]. Recent studies have demonstrated that CTRP 3 exhibits protective properties on cardiac and vascular remodeling in mice [17]. However, there is little research exploring the relevance of CTRP 3 with CAD. Here we conducted a study on serum CTRP 3 and the prevalence or severity of CAD.
Methods
Study subjects
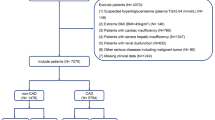
One hundred five CAD patients were enrolled from inpatients that underwent coronary angiography at Ruijin Hospital between 2013 and 2015. The criteria of CAD we used was a 50% or greater organic stenosis of at least one major coronary artery, as confirmed by coronary angiogram. With the help of cardiovascular angiographic system, the lesion can be classified into left main, anterior descending, circumflex and right coronary artery lesion. A stenosis ≥50% was considered as CAD, two stenoses ≥50% was considered as double-vessel disease and more than two stenoses was defined as triple-vessel disease. We excluded patients with acute myocardial infarction, congestive heart failure, valvular heart disease, cerebral infarction, thrombotic diseases, severe hepatic and renal dysfunction, infections, hemodialysis and malignancy. With reference to their coronary angiography results, they were divided into a CAD group (n = 79) and a non-CAD group (n = 66, with normal coronary artery angiography). The CAD group was further grouped into three subgroups by their different coronary lesioned vessels: single-vessel group (n = 25), double-vessel group (n = 30) and triple-vessel group(n = 24). All participants were given written informed consent. This study was approved by the ethics committee of Ruijin Hospital.
Laboratory methods
Blood samples were obtained from CAD patients and non-CAD subjects after overnight fasting. Plasma CTRP 3 levels were measured quantitatively by enzyme-linked immunosorbent assay using the commercially available ELISA kit (Senxiong Biotech Industry Company, Shanghai, China) and we followed the manufacturer’s recommendations (the detail of the ELISA Assay Procedure is presented in Additional file 1). Other serum indexes were determined by standard assays. Blood pressure (BP) was measured with an appropriate arm cuff and a mercury column sphygmomanometer after at least 10 min’ rest in sitting position. Body mass index (BMI) was calculated as the ratio of weight (kg) to squared height (m2).
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software. Values were presented as mean ± standard error (SE) for continuous variables. The measurement data was conducted by the normality test. The difference of continuous variables was compared using the t test and the Mann-Whitney U test between groups. The count data was assessed using chi-squared test. The Kruskal-Wallis H test was used for multiple comparisons. Bonferroni’s correction was used to adjust for multiple comparisons. Association between CAD and all other parameters was first examined by simple logistic correlation analysis, and then evaluated by multiple logistic regression analysis using parameters selected from single analysis. Logistic regression analysis was used to identify the association between CAD and other parameters. All statistical tests used a P value of 0.05 in a two-tail test as statistically significant.
Results
Clinical characteristics
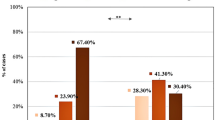
Clinical characteristics in CAD and non-CAD groups are displayed in Table 1. Compared with the non-CAD group, patients in the CAD group showed a more frequent proportion of males, smokers and diabetics. We found that CAD patients had significantly higher BMI, systolic BP, fasting glucose and CTRP 3 (Fig. 1) than non-CAD patients. High density lipoprotein-cholesterol (HDL-c) level was lower in the CAD group than that in the non-CAD group. There were no statistical differences in age, frequency of smokers, diastolic BP, triglyceride (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-c) between the two groups.
The clinical characteristics of the CAD subgroups are listed in Table 2. There were significant differences in the CTRP 3 levels and proportion of males between these groups. However, there were no statistical differences in age, BMI, diastolic BP, diastolic BP, Glucose, triglyceride (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-c) and high density lipoprotein-cholesterol (HDL-c).
Relationship between CTRP 3 and different coronary lesioned vessels of CAD
In order to explore the relationship between serum CTRP 3 and the different coronary lesioned vessels of CAD, CTRP 3 levels in different subgroups were compared. CAD patients were grouped by the different coronary lesioned vessels, the CTRP 3 level in single-vessel, double-vessel and triple-vessel disease group was 44.80 ± 3.14, 51.87 ± 4.02, 75.07 ± 9.41 ng/ml, respectively. Circulating CTRP 3 levels (Fig. 2) were increased with the number of lesioned coronary vessels, and it was significantly different between single-vessel and triple-vessel group (P < 0.005). There was no significant difference between both the single-vessel and double-vessel as well as double-vessel and triple-vessel disease groups.
Association of CTRP 3 levels with CAD
To determine the association between CTRP 3 and CAD, single and multiple logistic regression analyses were performed between CAD and non-CAD groups. In single logistic regression we found that BMI, systolic BP, fasting glucose, HDL-c and CTRP3 were significantly associated with CAD (Table 3). Multiple logistic regression analysis with BMI, systolic BP, fasting glucose, HDL-c and CTRP 3 demonstrated that HDL-c, glucose and CTRP 3 were markedly associated with CAD.
Discussion
CTRP 3 is a newly discovered adipokine with anti-inflammatory properties, which may underpin the pathogenesis of obesity-linked diseases including CAD. Several studies have reported the association of circulating CTRP 3 with obesity and CAD in vitro and in vivo [18,19,20]. Functional knowledge gained from multiple mice models has promoted deep investigations of plasma CTRP 3 levels in humans; however, conflicting results have been shown for its relationship with diabetes and metabolic syndrome [14, 21, 22]. Our study demonstrated that the plasma levels of circulating CTRP 3 are increased in patients with CAD compared to non-CAD subjects, and the CTRP 3 levels positively correlate with the progress of disease. Multiple logistic regression analysis showed that HDL-c, glucose and CTRP 3 level were significantly related with CAD. In contrast, our result disagreed with Reza Fadaei’s study which reported that patients with CAD had markedly decreased circulating CTRP 3 concentration [23]. A defensive response to counteract the metabolic stress or resistance to CTRP3 action might account for our paradoxical increase of serum CTRP 3.
CTRP 3 exerts protective effects on the cardiovascular system by promoting angiogenesis and preventing unbalanced cardiovascular remodeling. Yi first demonstrated that the replenishment of CTRP 3 effectively promotes post-ischemic angiogenesis by elevating cardiomyocyte-endothelial cell communication and attenuates adverse remodeling in C57/BL6 mice with coronary artery occlusion [24]. Recently, Li found the CTRP 3/cartducin gene could be transiently up-regulated in a balloon-injured rat carotid artery tissue during a period characterized by neointimal formation [25]. All these observations proved that CTRP 3 levels were closely related to the pathogenesis of CAD. Consistent with these findings, our data in this study indicated that CTRP 3 levels in CAD group were much higher than non-CAD group, and may contribute to the defensive responses protecting the cardiovascular system. Circulating CTRP 9 had been demonstrated to be associated with the severity of CAD. However, there is little research about the relationship of CTRP 3 levels with CAD [15].
There were several limitations to this study. First, the sample size was small and most of the study subjects were from Shanghai Province where people have similar lifestyles. These results might not be generalizable to populations in other areas. Secondly, cholesterol-lowering statin medications which were reported to play important roles in the development of atherosclerosis were not taken into account in this study [26, 27]. Third, this was a cross-sectional study, which limited our ability to conclude a cause-and-effect relationship between the CTRP3 and CAD. Future studies conducted on a larger population that exclude potential confounding factors such as medication treatment history are needed.
Conclusion
In conclusion, we have demonstrated that serum CTRP 3 levels are elevated in CAD patients than non-CAD patients, and the CTRP 3 levels positively correlate with the progress of the disease. These results indicate that CTRP 3 may play an important role in the pathophysiology of CAD, suggesting that CTRP 3 may represent a novel biomarker for CAD.
Abbreviations
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CTPR 3:
-
Complement C1q tumor necrosis factor related protein-3
- HDL:
-
High density lipoprotein-cholesterol
- LDL:
-
Low density lipoprotein-cholesterol
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7.
Kastorini CM, Georgousopoulou E, Vemmos KN, Nikolaou V, Kantas D, Milionis HJ, et al. Comparative analysis of cardiovascular disease risk factors influencing nonfatal acute coronary syndrome and ischemic stroke. Am J Cardiol. 2013;112(3):349–54.
Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11(5):276–89.
Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015. A systematic analysis for the global burden of disease study 2015. Lancet (London, England). 2016;388(10053):1725–74.
Sun YM, Tian Y, Li X, Liu YY, Wang LF, Li J, et al. Effect of atorvastatin on expression of IL-10 and TNF-alpha mRNA in myocardial ischemia-reperfusion injury in rats. Biochem Biophys Res Commun. 2009;382(2):336–40.
Naya M, Tsukamoto T, Morita K, Katoh C, Furumoto T, Fujii S, et al. Plasma interleukin-6 and tumor necrosis factor-alpha can predict coronary endothelial dysfunction in hypertensive patients. Hypertension research : official journal of the Japanese Society of Hypertension. 2007;30(6):541–8.
Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, Kaartinen M, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101(12):1372–8.
Wong GW, Wang J, Hug C, Tsao TS, Lodish HF. A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci U S A. 2004;101(28):10302–7.
Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. The Biochemical journal. 2008;416(2):161–77.
Hofmann C, Chen N, Obermeier F, Paul G, Buchler C, Kopp A, et al. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis. 2011;17(12):2462–71.
Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, et al. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol. 2011;31(11):2616–23.
Qu H, Deng M, Wang H, Wei H, Liu F, Wu J, et al. Plasma CTRP-3 concentrations in Chinese patients with obesity and type II diabetes negatively correlate with insulin resistance. Journal of clinical lipidology. 2015;9(3):289–94.
Petersen PS, Wolf RM, Lei X, Peterson JM, Wong GW. Immunomodulatory roles of CTRP3 in endotoxemia and metabolic stress. Phys Rep. 2016;4(5):e12735.
Choi KM, Hwang SY, Hong HC, Choi HY, Yoo HJ, Youn BS, et al. Implications of C1q/TNF-related protein-3 (CTRP-3) and progranulin in patients with acute coronary syndrome and stable angina pectoris. Cardiovasc Diabetol. 2014;13:14.
Wang H, Wang R, Du D, Li F, Li Y. Serum levels of C1q/TNF-related protein-1 (CTRP-1) are closely associated with coronary artery disease. BMC Cardiovasc Disord. 2016;16:92.
Kopp A, Bala M, Buechler C, Falk W, Gross P, Neumeier M, et al. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology. 2010;151(11):5267–78.
Lin S, Ma S, Lu P, Cai W, Chen Y, Sheng J. Effect of CTRP3 on activation of adventitial fibroblasts induced by TGF-beta1 from rat aorta in vitro. Int J Clin Exp Pathol. 2014;7(5):2199–208.
Akiyama H, Furukawa S, Wakisaka S, Maeda T. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol Cell Biochem. 2007;304(1–2):243–8.
Maeda T, Wakisaka S. CTRP3/cartducin is induced by transforming growth factor-beta1 and promotes vascular smooth muscle cell proliferation. Cell Biol Int. 2010;34(3):261–6.
Schmid A, Kopp A, Hanses F, Karrasch T, Schaffler A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/−2 phosphorylation in mice in vivo. Biochem Biophys Res Commun. 2014;452(1):8–13.
Wolf RM, Steele KE, Peterson LA, Magnuson TH, Schweitzer MA, Wong GW. Lower circulating C1q/TNF-related protein-3 (CTRP3) levels are associated with obesity: a cross-sectional study. PLoS One. 2015;10(7):e0133955.
Choi KM, Hwang SY, Hong HC, Yang SJ, Choi HY, Yoo HJ, et al. C1q/TNF-related protein-3 (CTRP-3) and pigment epithelium-derived factor (PEDF) concentrations in patients with type 2 diabetes and metabolic syndrome. Diabetes. 2012;61(11):2932–6.
Fadaei R, Moradi N, Baratchian M, Aghajani H, Malek M, Fazaeli AA, et al. Association of C1q/TNF-related protein-3 (CTRP3) and CTRP13 serum levels with coronary artery disease in subjects with and without type 2 diabetes mellitus. PLoS One. 2016;11(12):e0168773.
Yi W, Sun Y, Yuan Y, Lau WB, Zheng Q, Wang X, et al. C1q/tumor necrosis factor-related protein-3, a newly identified adipokine, is a novel antiapoptotic, proangiogenic, and cardioprotective molecule in the ischemic mouse heart. Circulation. 2012;125(25):3159–69.
Li JM, Zhang X, Nelson PR, Odgren PR, Nelson JD, Vasiliu C, et al. Temporal evolution of gene expression in rat carotid artery following balloon angioplasty. J Cell Biochem. 2007;101(2):399–410.
Anderson TJ. New hope for lipid-lowering beyond Statins: effect of IMPROVE-IT on understanding and implementation of atherosclerosis prevention. The Canadian journal of cardiology. 2015;31(5):585–7.
Feig JE, Feig JL, Kini AS. Statins, atherosclerosis regression and HDL: insights from within the plaque. Int J Cardiol. 2015;189:168–71.
Acknowledgements
Authors would like to acknowledge with gratitude all collaborating researchers in collecting information during this study. We would also like to thank the subjects who participated in this study.
Funding
This research was supported by grants from the medicine-engineering cross fund (NO: YG2014MS55).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
LHS and SHW designed the research and participated in data collection, analysis, result interpretation and manuscript writing. YL and WL contributed to data collection, result interpretation and manuscript writing. All authors read and approved the final manuscript and consented to publish this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao-tong University School of Medicine. All of participants were given written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Method S1. ELISA Assay Procedure. (DOCX 135 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, S., Ling, Y., Liang, W. et al. Association of serum C1q/TNF-related protein-3 (CTRP-3) in patients with coronary artery disease. BMC Cardiovasc Disord 17, 210 (2017). https://doi.org/10.1186/s12872-017-0646-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-017-0646-7