Abstract
Background
This study aimed to compare the adverse clinical outcomes associated with a short and a prolonged duration of Dual Anti-Platelet Therapy (DAPT) in patients with Diabetes Mellitus (DM) after undergoing Percutaneous Coronary Intervention (PCI).
Methods
Medline/PubMed, EMBASE and the Cochrane library were searched for studies comparing the short and prolonged DAPT use in patients with DM. Adverse outcomes were considered as the clinical endpoints in this analysis. Odds Ratios (OR) with 95 % Confidence Intervals (CI) were used to express the pooled effect on discontinuous variables and the pooled analyses were performed with RevMan 5.3.
Results
Fifteen studies with a total number of 25,742 patients with DM were included in this current analysis which showed no significant differences in primary endpoints, net clinical outcomes, myocardial infarction and stroke with OR: 1.03, 95 % CI: 0.65–1.64; P = 0.90, OR: 0.96, 95 % CI: 0.69–1.34; P = 0.81, OR: 0.85, 95 % CI: 0.70–1.04; P = 0.12 and OR: 0.94, 95 % CI: 0.65–1.36; P = 0.75 respectively. Revascularization was also similar between these 2 groups of patients with DM. However, even if mortality favored prolonged DAPT use, with OR: 0.87, 95 % CI: 0.76–1.00; P = 0.05, the result only approached significance. Also, stent thrombosis insignificantly favored a prolonged DAPT duration with OR: 0.56, 95 % CI: 0.27–1.17; P = 0.12. Thrombolysis In Myocardial Infarction (TIMI) defined major and minor bleeding were not significantly different in these diabetic patients with OR: 0.91, 95 % CI: 0.60–1.37; P = 0.65 and OR: 1.08, 95 % CI: 0.62–1.91; P = 0.78 respectively. However, bleeding defined by the Bleeding Academic Research Consortium (BARC) classification was significantly higher with a prolonged DAPT use in these diabetic patients with OR: 1.92, 95 % CI: 1.58–2.34; P < 0.00001.
Conclusion
Following PCI, a prolonged DAPT use was associated with similar adverse clinical outcomes but with a significantly increased BARC defined bleeding compared to a short term DAPT use in these patients with DM. However, even if mortality and stent thrombosis favored a prolonged DAPT use, these outcomes only either reached statistical significance or were insignificant respectively, showing that a clear decision about recommending a prolonged duration of DAPT to patients with DM might not be possible at this moment, warranting further research in this particular subgroup.
Similar content being viewed by others
Background
According to guidelines, Dual Anti-Platelet Therapy (DAPT) with aspirin and P2Y12 inhibitors, mainly clopidogrel, is recommended for at least one year following Percutaneous Coronary Intervention (PCI) with Drug Eluting Stents (DES) [1]. However, even in this new era, several Randomized Controlled Trials (RCTs) still could not predict the exact duration of DAPT use and suggested that this issue might possibly be solved only using a larger number of randomized patients following PCI with DES [2]. To be more clear, in a previously published study comparing 6 months with 12 months DAPT use, the authors stated that larger trials would be able to completely solve this issue [3]. Even if several meta-analyses comparing the short and prolonged DAPT use in the general population following PCI showed a longer duration of DAPT to be associated with favorable clinical outcomes [4], other meta-analyses showed no benefits of a prolonged DAPT duration [5] giving rise to controversies. However, whether these results also apply to the subgroup of patients with Diabetes Mellitus (DM) have seldom been studied. Therefore, this study aimed to compare the adverse clinical outcomes associated with a short and prolonged duration of DAPT use in patients with DM following PCI.
Methods
Data sources and search strategy
Medline/PubMed, EMBASE and the Cochrane library were searched for studies comparing the short and prolonged use of DAPT in patients with Acute Coronary Syndrome (ACS) following PCI by typing the words ‘dual anti-platelet therapy, diabetes mellitus and percutaneous coronary intervention’. Another search was performed using the phrase ‘prolonged clopidogrel use, diabetes mellitus and percutaneous coronary intervention’. To widen this search, the abbreviations ‘DAPT, DM, and PCI’ as well as the term ‘coronary angioplasty’ were also used. In addition, reference lists of suitable studies were also reviewed for relevant articles. Only articles published in English were considered in this search process.
Inclusion and exclusion criteria
Studies were included if:
-
(a)
They were randomized trials or observational studies.
-
(b)
They compared short and prolonged DAPT use and included patients with DM.
-
(c)
They reported adverse outcomes as their clinical endpoints.
-
(d)
They were published in English.
Studies were excluded if:
-
(a)
They were meta-analyses, case studies or editorials.
-
(b)
They did not involve patients with DM.
-
(c)
They did not report adverse outcomes as their clinical endpoints.
-
(d)
They did not compare short with prolonged DAPT use, but instead, compared aspirin monotherapy with DAPT following PCI.
-
(e)
They were duplicates or involved the same trial.
Definitions, outcomes and follow up
DM was defined as a state of high blood sugar levels observed at least on two separate occasions, with a fasting blood glucose test or an oral glucose tolerance test, with or without symptoms (asymptomatic) such as polydipsia (frequent thirst), polyuria (frequent urination) and weight loss.
Adverse clinical outcomes which were analyzed in this study included:
-
(a)
Primary endpoint which was a composite endpoint of all-cause death, Myocardial Infarction (MI), stroke, revascularization and stent thrombosis.
-
(b)
MI (any type or any classification of MI) was relevant including the universal definition [6].
-
(c)
Target Lesion Revascularization (TLR).
-
(d)
Target Vessel Revascularization (TVR).
-
(e)
All-cause death (cardiac and non-cardiac).
-
(f)
Stroke.
-
(g)
Net Adverse Clinical and Cerebral Events (NACCE) were defined as a composite of all-cause death, all MI, stroke or major bleeding.
-
(h)
Stent thrombosis which was defined by the Academic Research Consortium (ARC) [7].
-
(i)
Bleeding:
-
(1)
All/Any bleeding.
-
(2)
Major and Minor bleeding defined by Thrombolysis in Myocardial Infarction (TIMI) [8].
-
(3)
Bleeding defined according to the Bleeding Academic Research Consortium (BARC) [9] which was further divided into BARC type 2, BARC type 3 and BARC type 5.
The adverse clinical outcomes reported have been listed in Table 1.
Short and prolonged duration of DAPT
Short and prolonged duration of DAPT use were based on the following criteria:
If the short term DAPT duration period was 3 months, its corresponding prolonged duration period should be more than 3 months (6, 12, 24, or more).
If the short term duration of DAPT was 6 months, its corresponding prolonged duration period should be more than 6 months (12, 18, 24, or more).
If the short term duration of DAPT use was 12 months, its corresponding prolonged duration period should be more than 12 months.
Therefore, a prolonged duration of DAPT was defined as the use of DAPT during a period of time longer than the actual short term duration corresponding to that particular trial. Different trials had different short and prolonged duration of DAPT use. Table 2 further illustrated the short and prolonged duration periods of DAPT use in each of the studies included in this meta-analysis.
Data extraction and review
Two authors (PKB and CMY) independently assessed the studies involved and reviewed the methodological quality of each eligible trial. Information regarding the study/trial names, time period of patients’ enrollment, adverse clinical outcomes reported, the follow up periods, data concerning the total number of patients with DM classified into the short and prolonged DAPT groups respectively, the total number of clinical events reported in each subgroup, as well as information concerning the baseline features of the patients were carefully extracted and cross checked. During the data extraction process, any disagreement which occurred between these two authors was carefully discussed, and if they could not reach a consensus, the disagreement was solved and a final decision was made by the third author (FH). The bias risk among the trials (low risk, moderate risk and high risk) was assessed with the components recommended by the Cochrane Collaboration [10]. The six components of the bias risk were as follow:
-
A.
Sequence generation
-
B.
Allocation sequence concealment
-
C.
Blinding of participants and personnel
-
D.
Blinding of outcome assessment
-
E.
Incomplete outcome data
-
F.
Selective outcome reporting and other potential bias
The trials included in this study were analyzed according to these six components and a bias grade was given accordingly after a careful assessment. A grade ranging from A to E was considered whereby grade A was allocated if an extremely low risk of bias was reported, while a grade E was allocated if a very high risk of bias was observed. Note that these bias grades were just an approximation according to what the authors were able to assess. The bias risk grades allocated to each trial were provided in Table 1. Note that observational studies were ignored during this assessment.
Methodological and statistical analysis
Recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement were followed in this study [11]. Heterogeneity among the subgroups was assessed using the Cochrane Q-statistic test whereby a P value less than 0 · 05 was considered statistically significant whereas P value ≥ 0.05 was considered statistically insignificant. I2-statistic test which also assessed heterogeneity, whereby an I2 with a low percentage (<25 %) represented a low heterogeneity, an I2 with a percentage between 25 and 50 % represented a moderate heterogeneity and an I2 with a high percentage above 50 % denoted an increasing heterogeneity. If I2 was less than 50 %, a fixed effect model was used during this subgroup analysis. However, if I2 was more than 50 %, a random effect model was used. Publication bias was visually estimated by assessing funnel plots. Odds Ratios (OR) with 95 % Confidence Intervals (CIs) were calculated for categorical variables and the pooled analyses were performed with RevMan 5.3 software. Ethical committee or medical institutional board approval was not required since this is a systematic review and meta-analysis of several studies.
Results
Search result
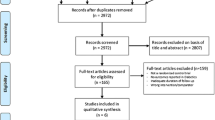
Two thousand two hundred seventy four articles were obtained from PubMed/Medline, EMBASE, the Cochrane Library and from suitable reference lists. After a careful selection and assessment of titles and abstracts, 2168 articles were eliminated since they were not related to the topic of this research. Among the 106 remaining articles, 52 articles were further eliminated since they were duplicates. Fifty-four full-text articles were assessed for eligibility. Ten studies were further eliminated since they were meta-analyses, 11 studies were case studies, 2 studies were protocol of future ongoing trials, 6 articles were letter to editors, and 10 articles were associated with the same trial. Finally, 15 studies (Brar2008 [12], I-LOVE IT 2 [13], ISAR-SAFE [14], Tarantini2016 [15], ARCTIC [16], OPTIMIZE [17], RESET [18], EXCELLENT [3], PEGASUS [19], DAPT [20], Sardella2011 [21], PRODIGY [22], Thukkani2015 [23], ENDEAVOR [24], ITALIC [25]) that satisfied all the inclusion and exclusion criteria of this current analysis, were included. The flow diagram representing the study selection has been illustrated in Fig. 1.
Study Tarantini2016 [15] was a sub-study of the SECURITY trial [26] including patients only with DM and trials DES LATE [27] and REAL-LATE ZEST-LATE [28] were excluded because they compared aspirin monotherapy versus DAPT, instead of prolonged DAPT use versus short term DAPT use.
General features of the studies included
A total number of 25,742 patients with DM (13,350 patients assigned to short term DAPT group whereas 12,392 patients assigned to prolonged DAPT group) were included. Patients were enrolled from the year 2002 to the year 2015. The general features of the studies included have been listed in Table 2.
Baseline features of the studies included
Table 3 summarized the baseline characteristics of the patients included in this meta-analysis.
Mean age was reported in years. Patients who were enrolled in this study had a mean age ranging from 60.0 to 70.0 years. Trials ITALIC [25], ISAR-SAFE [14] and ARCTIC [16] had a majority of males patients. Trial ISAR-SAFE [14] and study Thukkani2015 [23] involved a high number of patients with hypertension. According to the baseline features reported, no significant difference was observed among patients assigned to either a short or prolonged duration of DAPT use.
Main analysis
Results of this analysis have been summarized in Table 4.
This current analysis showed no significant differences in primary endpoints and net clinical outcomes in patients with DM whether with a short or prolonged treatment period with DAPT with OR: 1.03, 95 % CI: 0.65–1.64; P = 0.90 and OR: 0.96, 95 % CI: 0.69–1.34; P = 0.81 respectively. MI was also not significantly different with OR: 0.85, 95 % CI: 0.70–1.04; P = 0.12. However, even if mortality favored prolonged DAPT use, with OR: 0.87, 95 % CI: 0.76–1.00; P = 0.05, the result only approached statistical significance. These results have been illustrated in Fig. 2.
TVR and TLR were also similarly manifested between these 2 groups with OR: 0.85, 95 % CI: 0.58–1.24; P = 0.39 and OR: 0.90, 95 % CI: 0.57–1.41; P = 0.63 respectively. Stroke was also not significantly different with a short term or prolonged DAPT use with these patients with DM, with OR: 0.94, 95 % CI: 0.65–1.36; P = 0.75. However, even if stent thrombosis favored a prolonged DAPT use with OR: 0.56, 95 % CI: 0.27–1.17; P = 0.12, this result was not statistically significant. These results have been illustrated in Fig. 3.
Bleeding events were also analyzed in these patients with DM. Any bleeding was not significantly different between a short and a prolonged DAPT use with OR: 1.22, 95 % CI: 0.72–2.08; P = 0.46. TIMI defined major and minor bleeding were also not significantly different in these diabetic patients with OR: 0.91, 95 % CI: 0.60–1.37; P = 0.65 and OR: 1.08, 95 % CI: 0.62–1.91; P = 0.78 respectively. However, bleeding defined by the BARC classification was significantly higher with a prolonged DAPT use in these diabetic patients with OR: 1.92, 95 % CI: 1.58–2.34; P < 0.00001. When bleeding defined by BARC classification was further subdivided, a significantly higher BARC bleeding types 2 and 3 were observed with a prolonged DAPT use with OR: 1.98, 95 % CI: 1.50–2.61; P < 0.00001 and OR: 1.78, 95 % CI: 1.34–2.37; P < 0.0001 respectively. But even if BARC type 5 also favored a short term DAPT use, with OR: 1.40, 95 % CI: 0.59–3.30; P = 0.44, the result was not statistically significant. Results analyzing bleeding events have been illustrated in Fig. 4.
Because the duration period of DAPT was not similar in all the studies included, that is, a few studies had a short term DAPT duration period of 3 months, 6 months and 12 months respectively, and a long term DAPT duration period of 12 months, 18 months or 24 months respectively, which might have influenced the results of this analysis, another subgroup analysis was conducted only with a short term DAPT duration of 6 months versus a long term duration of 12 months. However, this analysis also showed no significant difference in net clinical outcomes, mortality, MI, TVR, TLR, stent thrombosis and stroke with OR: 0.95, 95 % CI: 0.56–1.60; P = 0.84, OR: 1.14, 95 % CI: 0.44–2.97; P = 0.79, OR: 0.79, 95 % CI: 0.39–1.60; P = 0.51, OR: 0.94, 95 % CI: 0.45–1.96; P = 0.86, OR: 1.01, 95 % CI: 0.45–2.26; P = 0.99, OR: 0.55, 95 % CI: 0.15–2.01; P = 0.36 and OR: 1.02, 95 % CI: 0.33–3.18; P = 0.97 respectively. These results comparing 6 months versus 12 months DAPT use have been illustrated in Fig. 5.
Sensitivity analysis
For all of the above analyses, sensitivity analyses yielded consistent results. Based on a visual inspection of the funnel plots obtained, there has been very low evidence of publication bias for the included studies that assessed all clinical endpoints reported (including the adverse clinical outcomes and the bleeding events analyzed) in these patients with DM. The funnel plots have been illustrated in Figs. 6 and 7.
Discussion
This study aimed to compare the adverse clinical outcomes associated with a short and prolonged DAPT use in patients with DM following PCI. Results of this study showed that a prolonged duration of DAPT use was not associated with any significant difference in adverse clinical outcomes when compared to a short term duration of DAPT use in these patients with DM. The result for mortality which favored a prolonged DAPT use reached near significance but was not statistically significant in this analysis whereas even if stent thrombosis favored a prolonged DAPT use, the result was also not statistically significant. In addition, TIMI defined major and minor bleeding were also not significantly different. However, bleeding defined according to BARC classification was significantly higher with the prolonged DAPT use.
In part similar to the results of this current analysis, the systematic review and meta-analysis comparing the duration of DAPT following DES implantation showed short term DAPT use to be associated with a significantly lower rate of bleeding, and higher rates of stent thrombosis [29]. Note that their study involved more than 30 % of patients with DM. However, their meta-analysis showed all-cause mortality to be insignificantly higher in the long term duration group, which was not the case in our study. In addition, this current study only showed a significantly increased bleeding rate according to the BARC classification, without any significant difference for stent thrombosis. Another meta-analysis published by Navarese et al. showed that compared to a DAPT duration period of 12 months, a short term DAPT use was associated with a significantly lower rate of bleeding events, without any apparent increase in ischemic complications and therefore the authors concluded that a short term DAPT could be considered in most patients following PCI [5].
Furthermore, the meta-analysis published by Yang et al. showed no difference in efficacy outcomes associated with a short or prolonged duration of DAPT use after intracoronary DES implantation [30]. However, a longer duration of DAPT (≥12 months) was associated with increased risk of bleeding complications. The study by Udell et al. also concluded that DAPT use beyond one year was associated with increased bleeding events, without any increase in cardiovascular mortality [4]. In addition, the PRODIGY trial which involved more than 20 % of patients with DM, showed a 24 months of clopidogrel use not to be associated with any increase in adverse clinical outcomes compared to the use of clopidogrel during a short term period of 6 months [31]. This trial compared device specific outcomes relative to different duration of DAPT in 3 different types of DES (everolimus eluting stents, paclitaxel eluting stents, zotarolimus eluting stents) and bare metal stents, suggesting that the optimal duration of DAPT could also possibly be stent specific.
Moreover, patients with DM showed comparable adverse clinical outcomes to that of patients without DM whether during a 6-months treatment with DAPT or a prolonged duration of DAPT following PCI with implanted second generation DES [15].
Nevertheless, the study by Valgimigli et al. showed that along with an increased risk of bleeding associated with a prolonged duration of DAPT use, an increased risk of stroke was also observed [32]. However, our results which involved patients with DM, did not show any significant difference in stroke rate between these two groups. The ITALIC trial also showed no significantly different bleeding or thrombotic events when 6 months DAPT use was compared to 24 months DAPT use after PCI [25]. However, the ITALIC trial involved patients implanted with newer generation DES and also involved patients with good response to aspirin.
The study by Siddiqqi et al. which consisted of more than 50 % of patients with DM, showed a prolonged duration of DAPT not to be associated with any increased risk of bleeding. However, their study involved patients with chronic kidney disease and the exact type of bleeding assessed was not specified [33]. The study by Thukkani et al. also showed results that favored the prolonged use of clopidogrel to be associated with a lower risk of death and MI only in patients with DM implanted with DES [23]. However, that study did not show any benefit of prolonged clopidogrel use in patients without DM or in patients implanted with bare metal stents. Moreover, the DAPT trial also showed a lower rate of mortality to be associated with a prolonged use of clopidogrel after PCI [20]. However, result of this analysis which involved only patients with DM, did not reach statistical significance in the subgroup analyzing mortality.
Even if the subgroup analysis of the OPTIMIZE trial that assessed how short-term DAPT did not show any significantly increase risk for clinical events at 1 year in patients with DM undergoing PCI with a specific 2nd generation DES [34], other studies have shown second generation DES to be associated with higher adverse outcomes in patients with DM compared to the general population [35]. In addition, studies showed increasing adverse clinical outcomes to be associated with insulin-treated DM compared to non-insulin treated DM irrespective of the duration of DAPT [36, 37].
Furthermore, the observational study conducted by Eisenstein et al. examining consecutive patients receiving DES at Duke Heart Center between the year 2000 and 2005, concluded that the extended use of clopidogrel in patients implanted with DES might be associated with a lower risk of death and MI [2]. However, the authors concluded that only larger trials will be able to confirm their results, but unfortunately, even if this current analysis involved a pooling of data from several randomized trials (but only including patients with DM), the result analyzing mortality only nearly reached statistical significance, but was not statistically significant.
Even if the result for stent thrombosis was not significant in patients with DM, other studies have shown a prolonged use of DAPT to be associated with a lower risk of stent thrombosis compared to a shorter duration of DAPT use after PCI. To prove this point, the TYCOON registry showed an extended use of DAPT (2 years) to be associated with a lower rate of stent thrombosis following PCI with DES [38]. However, in contrast, other studies showed clopidogrel use beyond one year not to reduce the risk of stent thrombosis or other adverse clinical outcomes after PCI [39].
Previous studies have already compared the clinical outcomes associated with duration of DAPT in the subset of patients treated for in-stent restenosis [23]. This current analysis showed compared the adverse clinical outcomes between a short and prolonged DAPT use in patients with DM. To be noted, the duration of DAPT use might vary in other subgroups of patients. As it is said, one size shoe approach for DAPT duration is unlikely to fit all the patients, further investigations including other subgroups of patients such as patients with chronic obstructive pulmonary disease who underwent PCI [40, 41], should be conducted.
Novelty
This study is new in several ways. Even if many studies have compared the short and prolonged use of DAPT following PCI, this is among the first meta-analyses comparing the adverse clinical outcomes associated with the short and prolonged use of DAPT in patients with DM. Moreover, this analysis involved several newly published articles which were not included in other recently published meta-analyses representing another novelty.
Limitations
Similar to other studies, this study also has limitations. First of all, due to the small population of patients with DM, this study might not provide robust results. Secondly, different studies reported different duration of DAPT use, as well as different follow up periods. Even if we have tried to compare only 6 months versus 12 months DAPT use in these patients with DM in order to solve this particular issue, we might have only partly succeeded showing that this point should still be considered as a limitation in this study. Moreover, data analyzing several bleeding subgroups were limited, which might have influenced the results.
Conclusion
Following PCI, a prolonged DAPT use was associated with similar adverse clinical outcomes but with a significantly increased BARC defined bleeding compared to a short term DAPT use in these patients with DM. However, even if mortality and stent thrombosis favored a prolonged DAPT use, these outcomes only either reached statistical significance or were insignificant respectively, showing that a clear decision about recommending a prolonged duration of DAPT to patients with DM might not be possible at this moment, warranting further research in this particular subgroup.
Abbreviations
- ACS:
-
Acute coronary syndrome
- BARC:
-
Bleeding academic research consortium
- DAPT:
-
Dual antiplatelet therapy
- DES:
-
Drug eluting stents
- DM:
-
Diabetes mellitus
- PCI:
-
Percutaneous coronary intervention
- TIMI:
-
Thrombolysis in myocardial infarction
References
Task Force members, Windecker S, Kolh P, Alfonso F, et al. ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–619.
Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297(2):159–68.
Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505–13.
Udell JA, Bonaca MP, Collet JP, et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup ofpatients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur Heart J. 2016;37(4):390–9.
Navarese EP, Andreotti F, Schulze V, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. 2015;350:h1618.
Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–53.
Cutlip DE, Windecker S, Mehran R, Academic Research Consortium, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51.
No authors. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med. 1985;312(14):932–6.
Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736–47.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcareinterventions: explanation and elaboration. BMJ. 2009;339:b2700.
Brar SS, Kim J, Brar SK, et al. Long-term outcomes by clopidogrel duration and stent type in a diabetic population with de novo coronary artery lesions. J Am Coll Cardiol. 2008;51(23):2220–7.
Han Y, Xu B, Xu K, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-ElutingStent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv. 2016;9(2):e003145.
Schulz-Schüpke S, Byrne RA, Ten Berg JM, et al. Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy afterdrug-eluting stenting. Eur Heart J. 2015;36(20):1252–63.
Tarantini G, Nai Fovino L, Tellaroli P, et al. Optimal duration of dual antiplatelet therapy after second-generation drug-eluting stent implantation in patientswith diabetes: The SECURITY (Second-Generation Drug-Eluting Stent Implantation Followed By Six- VersusTwelve-Month Dual Antiplatelet Therapy)-diabetes substudy. Int J Cardiol. 2016;207:168–76.
Collet JP, Silvain J, Barthélémy O, ARCTIC investigators, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384(9954):1577–85.
Feres F, Costa RA, Abizaid A, OPTIMIZE TrialInvestigators, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510–22.
Kim BK, Hong MK, Shin DH, RESET Investigators, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60(15):1340–8.
Bonaca MP, Bhatt DL, Steg PG, Storey RF, Cohen M, Im K, Oude Ophuis T, Budaj A, Goto S, López-Sendón J, Diaz R, Dalby A, Van de Werf F, Ardissino D, Montalescot G, Aylward P, Magnani G, Jensen EC, Held P, Braunwald E, Sabatine MS. Ischaemic risk and efficacy of ticagrelor in relation to time from P2Y12 inhibitor withdrawal in patients withprior myocardial infarction: insights from PEGASUS-TIMI 54. Eur Heart J. 2016;37(14):1133–42.
Mauri L, Kereiakes DJ, Yeh RW, DAPT Study Investigators, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–66.
Sardella G, Mancone M, Biondi-Zoccai G, et al. Beneficial impact of prolonged dual antiplatelet therapy after drug-eluting stent implantation. J Interv Cardiol. 2012;25(6):596–603.
Campo G, Tebaldi M, et al. Short- versus long-term duration of dual antiplatelet therapy in patients treated for in-stent restenosis: a PRODIGY trial substudy (Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia). J Am Coll Cardiol. 2014;63(6):506–12.
Thukkani AK, Agrawal K, Prince L, et al. Long-term outcomes in patients with diabetes mellitus related to prolonging clopidogrel more than 12 MonthsAfter coronary stenting. J Am Coll Cardiol. 2015;66(10):1091–101.
Kandzari DE, Barker CS, Leon MB, Mauri L, Wijns W, Fajadet J, Mehran R. Dual antiplatelet therapy duration and clinical outcomes following treatment with zotarolimus-eluting stents. JACC Cardiovasc Interv. 2011;4(10):1119–28.
Gilard M, Barragan P, Noryani AA, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant toaspirin: the randomized, multicenter ITALIC trial. J Am Coll Cardiol. 2015;65(8):777–86.
Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64(20):2086–97.
Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129(3):304–12.
Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374–82.
Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65(13):1298–310.
Yang J, Fan ZX, Yang CJ, Wang HB. A meta-analysis of randomized clinical trials comparing shorter (less or equal than 6 months) and longer (more or equal than 12 months) dual anti-platelet therapy following drug-eluting coronary stents. Iran Red Crescent Med J. 2015;17(7):e26904.
Valgimigli M, Campo G, Monti M, Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015–26.
Valgimigli M, Park SJ, Kim HS, et al. Benefits and risks of long-term duration of dual antiplatelet therapy after drug-eluting stenting: a meta-analysis of randomized trials. Int J Cardiol. 2013;168(3):2579–87.
Siddiqi OK, Smoot K, Dufour AB, et al. Outcomes with prolonged clopidogrel therapy after coronary stenting in patients with chronic kidney disease. Heart. 2015;101(19):1569–76.
Fausto F, Ricardo Costa D, et al. Impact pf short versus long term DAPT in patients with diabetes mellitus undergoing percutaneous intervention with endeavour zotarolimus eluting stents. A subanalysis of the large, prospective, randomized, multicenter OPTIMIZE trial. J Am Coll Cardiol. 2014;63(14):61862–3.
Silber S, Serruys PW, Leon MB, et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc Interv. 2013;6(4):357–68.
Park KW, Lee JM, Kang SH, et al. Everolimus-eluting Xience v/Promus versus zotarolimus-eluting resolute stents in patients with diabetes mellitus. JACC Cardiovasc Interv. 2014;7(5):471–81.
Bundhun PK, Li N, Chen MH. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol. 2015;14:135.
Tanzilli G, Greco C, Pelliccia F, et al. Effectiveness of two-year clopidogrel + aspirin in abolishing the risk of very late thrombosis after drug-eluting stent implantation (from the TYCOON [two-year ClOpidOgrel need] study). Am J Cardiol. 2009;104(10):1357–61.
Park DW, Yun SC, Lee SW, et al. Stent thrombosis, clinical events, and influence of prolonged clopidogrel use after placement of drug-elutingstent data from an observational cohort study of drug-eluting versus bare-metal stents. JACC Cardiovasc Interv. 2008;1(5):494–503.
Campo G, Guastaroba P, Marzocchi A, Santarelli A, Varani E, Vignali L, Sangiorgio P, Tondi S, Serenelli C, De Palma R, Saia F. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest. 2013;144(3):750–7.
Campo G, Pavasini R, Malagù M, Mascetti S, Biscaglia S, Ceconi C, Papi A, Contoli M. Chronic obstructive pulmonary disease and ischemic heart disease comorbidity: overview of mechanisms and clinical management. Cardiovasc Drugs Ther. 2015;29(2):147–57.
Acknowledgement
This research was supported by Youth Science Foundation of Guangxi Medical University (No. GXMUYSF201308), Scientific Project of Guangxi Higher Education (No. KY2015ZD028) and National Natural Science Foundation of China (No. 81560046).
Funding
There was no external source of funding for this research.
Availability of data and materials
All data and materials used in this research are freely available. References have been provided.
Authors’ contributions
PKB, CMY and FH were responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the initial manuscript and revising it critically for important intellectual content. PKB wrote this manuscript. All authors read and approved the final manuscript.
Authors’ information
Dr Pravesh Kumar Bundhun (M.D) is the first author. From the Department of Cardiovascular Diseases, the First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was not applicable for this systematic review and meta-analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bundhun, P.K., Yanamala, C.M. & Huang, F. Should a prolonged duration of dual anti-platelet therapy be recommended to patients with diabetes mellitus following percutaneous coronary intervention? A systematic review and meta-analysis of 15 studies. BMC Cardiovasc Disord 16, 161 (2016). https://doi.org/10.1186/s12872-016-0343-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-016-0343-y