Abstract
Background
Because infiltrative cardiomyopathy and hypertrophic cardiomyopathy (HCM) share clinical and hemodynamic features of left ventricular (LV) hypertrophy and abnormal diastolic function, it is often difficult to distinguish these entities.
Methods
We investigated the potential role of high-sensitivity cardiac troponin T (hs-cTnT) for differentiation of infiltrative cardiomyopathy from HCM.
Results
The study group consisted of 46 consecutive patients with infiltrative cardiomyopathies or HCM in whom sarcomere protein gene mutations were identified at Kochi Medical School Hospital; of these, there were 11 patients with infiltrative cardiomyopathy (cardiac amyloidosis in 8 patients and Fabry disease in 3 patients) and 35 HCM patients. Serum hs-cTnT level was significantly higher in patients who had infiltrative cardiomyopathy than in those who had HCM (0.083 ± 0.057 ng/ml versus 0.027 ± 0.034 ng/ml, p < 0.001), whereas brain natriuretic peptide levels did not differ between the two groups. In two age-matched the 2 cohorts (patients evaluated at > 40 years at age), hs-cTnT level, maximum LV wall thickness, posterior wall thickness, peak early (E) transmitral filling velocity, peak early diastolic (Ea) velocity of tissue Doppler imaging at the lateral corner and E/Ea ratios at both the septal and lateral corners were significantly different between the two groups. As for diagnostic accuracy to differentiate the two groups by using receiver operating characteristic analysis, hs-cTnT was the highest value of area under the curve (0.939) and E/Ea (lateral) was second highest value (0.914).
Conclusions
Serum hs-cTnT is a helpful diagnostic indicator for accurate differentiation between infiltrative cardiomyopathy and HCM.
Similar content being viewed by others
Background
Infiltrative cardiomyopathies such as cardiac amyloidosis and Fabry disease are difficult to differentiate from hypertrophic cardiomyopathy (HCM) because these cardiomyopathies share clinical and hemodynamic features of left ventricular (LV) hypertrophy and abnormal diastolic function [1–13]. Amyloidosis is a systemic and progressive disease and frequently involves more than one organ. Cardiac involvement in amyloidosis is the most important prognostic factor, and when cardiac amyloidosis is the first or main manifestation of the disease, correct diagnosis is sometimes difficult [1–5]. Fabry disease is a relatively prevalent cause of LV hypertrophy and is associated with significant morbidity and early death due to heart failure or ventricular arrhythmias [6–9, 14]. Since disease-specific enzyme replacement therapy is now available for Fabry disease, correct diagnosis is important [15, 16]. Although cardiac amyloidosis and cardiac involvement in Fabry disease show concentric LV hypertrophy, LV hypertrophy is usually asymmetric and predominantly septal in HCM, and there is often a considerable phenotypic overlap in infiltrative and hypertrophic cardiomyopathies (Fig. 1) [17].
Long-axis two-dimensional echocardiograms (diastole phase) of patients with cardiomyopathies. a: a cardiac amyloidosis patient with concentric left ventricular (LV) hypertrophy, b: a cardiac amyloidosis patient with asymmetric septal hypertrophy (ASH) at first glance (but actually concentric hypertrophy rather than ASH if moderator band is removed), c: a cardiac Fabry patient with concentric LV hypertrophy, d: a cardiac Fabry patient with ASH (in a terminal stage patient with Fabry disease, LV systolic dysfunction with localized thinning of the base of the LV posterior wall is seen.), e: a hypertrophic cardiomyopathy (HCM) patient with ASH, f: a HCM patient with concentric LV hypertrophy at first glance
Recently, a new generation of high-sensitivity assays for cardiac troponins has been developed to identify minimal cardiac damage in the setting of acute coronary syndromes [18, 19]. Elevations of cardiac troponin levels in high-sensitivity assays have been reported to be associated with poor outcome in not only ischemic heart disease but also non-ischemic heart failure [20, 21]. Although high-sensitivity troponin levels seem to be different among various cardiomyopathies, there has been no detailed comparison of these biomarkers’ values [22–27]. In the present study, we investigated the potential role of high-sensitivity cardiac troponin T (hs-cTnT) for differentiation of infiltrative cardiomyopathy from HCM.
Methods
Subjects
The study group consisted of 46 consecutive patients with infiltrative cardiomyopathies or HCM in whom sarcomere protein gene mutations were identified at Kochi Medical School Hospital; of these, there were 11 patients with infiltrative cardiomyopathy (cardiac amyloidosis in 8 patients and Fabry disease in 3 patients) and 35 HCM patients. In this study, we excluded patients with evidence of coronary artery disease and patients with renal failure (estimated glomerular filtration rate (eGFR) < 30 ml/min per 1.73 m2). Informed consent was obtained from all patients or their parents in accordance with the guidelines of the Ethics Committee on Medical Research of Kochi Medical School.
Clinical evaluation
The diagnosis of amyloidosis was made by biopsy study of an involved organ that demonstrated typical Congo red birefringence when viewed under polarized light. Of the 8 patients with cardiac amyloidosis, 2 had AL amyloidosis and 6 were considered to have senile amyloidosis with transthyretin. The diagnosis of Fabry disease was based on low plasma alfa-galactosidase A activity, family surveys, and tissue confirmation. The diagnosis of HCM was based on echocardiographic demonstration of a hypertrophied, nondilated LV (maximum LV wall thickness ≥ 15 mm) in the absence of systemic hypertension or other cardiac disease (e.g. aortic stenosis) capable of producing clinically evident hypertrophy at some point of clinical course. In this study, all HCM patients with sarcomere gene mutations in whom serum hs-cTnT was measured were enrolled. Sarcomere gene mutations in our current study were S297X, V593fs, V762D, R945fs and R1138C in cardiac myosin-binding protein C gene, R663C and N562K in cardiac beta-myosin heavy chain gene, D46V in cardiac troponin T gene and R162W in cardiac troponin I gene. These were not found in at least 200 chromosomes from healthy Japanese individuals. There were 7 patients with dilated phase of HCM defined as LV systolic dysfunction of global ejection fraction (EF) < 50 %.
Evaluation of patients included medical history, clinical examination, 12-lead electrocardiography, and conventional and Doppler echocardiography. Maximum LV wall thickness was defined as the greatest thickness in any single segment. Left ventricular end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) were measured from M-mode and 2-D images obtained from parasternal long-axis views. Global EF was determined from apical two- and four-chamber views. Mitral inflow velocities were determined using pulsed-wave Doppler with the sample volume positioned at the tips of the mitral leaflets in the four-chamber view. Peak early (E) and late (A) transmitral filling velocities were measured. Tissue Doppler imaging was performed in the pulse-Doppler mode to allow for a spectral display and recording of mitral annulus velocities at septal and lateral corners. Peak early diastolic (Ea) velocity was measured, and the E/Ea ratio was calculated. LV outflow tract gradient was calculated form continuous-wave Doppler using the simplified Bernoulli equation. LV outflow tract obstruction was defined as the presence of basal LV outflow gradient ≥ 30 mmHg at rest. Right ventricular hypertrophy was defined as thickness of right ventricular free wall > 5 mm.
Peripheral venous blood samples were collected for measurements of serum hs-cTnT and plasma brain natriuretic peptide (BNP) at the same time in clinically stable condition. Blood was taken at a random time mainly in our outpatient clinic. Serum hs-cTnT was measured by Elecsys Troponin T - High Sensitive immunoassay (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The normal range of this troponin marker in an apparently healthy adult population is less than or equal to 0.014 ng/ml (99 percentile). Plasma BNP was measured using an enzyme immunoassay (TOSOH II; TOSOH, Tokyo, Japan).
Statistical analysis
All data are expressed as mean ± SD or frequency (percentage). Differences between clinical variables of the infiltrative cardiomyopathy group and HCM group were examined with univariate analysis. For analysis of continuous variables, a t-test or Wilcoxon rank sum test was used, and a chi-square test or Fisher’s exact test was used for analysis of categorical variables. The diagnostic accuracies of parameters including biomarkers and echocardiographic indices were compared by receiver operating characteristic (ROC) analysis. Age, New York Heart Association functional class, eGFR, E/Ea (lateral), and hs-cTnT were included in a multivariate logistic regression model to identify independent correlations of the differentiation between the two types of cardiomyopathies. Statistical analysis was performed using SPSS (version 14.0 J) statistical software (SPSS Japan Inc., Tokyo).
Results
Patients characteristics
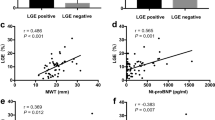
Clinical characteristics of the patients in the present study are summarized in Table 1. Patients who had infiltrative cardiomyopathy were older than patients who had HCM. Patients with infiltrative cardiomyopathy were more symptomatic than patients with HCM. Figure 2 shows the distribution of hs-cTnT and BNP values in all patients. There was less overlap of hs-cTnT values than BNP values between the two groups. Serum hs-cTnT level was significantly higher in patients who had infiltrative cardiomyopathy than in those who had HCM, while BNP levels did not differ between the two groups (Table 1). Table 2 shows the echocardiographic measurements in the two groups. Maximum LV wall thickness was smaller and posterior wall thickness was greater in patients with infiltrative cardiomyopathy than in those with HCM, although interventricular septal wall thickness was not different. There was no significant difference in the LV or left atrial sizes between the two groups. In Doppler echocardiographic measurements, E wave velocity was higher in patients who had infiltrative cardiomyopathy than in patients who had HCM. Ea at the lateral corner was significantly lower and E/Ea ratios at both the septal and lateral corners were significantly higher in patients who had infiltrative cardiomyopathy than in patients who had HCM.
Differentiation of the two groups in patients evaluated at > 40 years of age
In clinical practice, phenotypic expression in infiltrative cardiomyopathies is usually after middle-age. We therefore focused on patients evaluated at > 40 years of age in order to clarify the usefulness of hs-cTnT for differentiation of infiltrative cardiomyopathy from HCM. Table 3 shows the clinical characteristics of the two groups of patients evaluated at > 40 years of age. Hs-cTnT level, maximum LV wall thickness, posterior wall thickness, early filling velocity, Ea (lateral) and E/Ea ratios at both the septal and lateral corners were significantly different between the two groups. Area under the curve values in ROC curves are shown in Table 4. Hs-cTnT was highest and E/Ea (lateral) was second highest to differentiate the two groups. The multivariate logistic regression analysis showed that the independent determinants of the differentiation between the two types of cardiomyopathies were hs-cTnT (p = 0.047) and E/Ea (lateral) (p = 0.028). When the cut-off levels were defined as hs-cTnT of 0.035 ng/ml and E/Ea (lateral) of 11, combined measurements of these parameters resulted in 100 % sensitivity and 95 % specificity for the diagnosis of infiltrative cardiomyopathy (Fig. 3).
Discussion
To the best of knowledge, this is the first report that a new generation of assays, hs-cTnT, is able to differentiate accurately between infiltrative cardiomyopathy, including cardiac amyloidosis and cardiac involvement of Fabry disease, and HCM. Serum hs-cTnT is a helpful diagnostic indicator and should be measured in the evaluation of patients with thickening of the LV wall.
Although the underlying cause is different, infiltrative cardiomyopathy and HCM share clinical and hemodynamic features of LV hypertrophy and abnormal diastolic function [1–13]. It is often difficult to distinguish these entities by routine clinical examinations. Diagnosis of cardiac amyloidosis based on conventional modalities is often only possible once the disease is in a relatively advanced stage [1–5]. In hemodynamic parameters on echocardiography, a restrictive pattern of transmitral flow velocity, which is considered to be characteristic for cardiac amyloidosis, is not always observed in patients with this disease: cardiac amyloidosis can present as an abnormal relaxation pattern, depending on the left atrial or LV end-diastolic pressure. In Fabry disease, cardiac involvement occurs in the majority of patients and is mainly manifested as LV hypertrophy. There have been several reports of Fabry disease sometimes being undiagnosed in HCM cohorts [6–9]. On the other hand, clinical management differs among these cardiomyopathies in terms of prognosis and treatment. Compared with HCM, cardiac amyloidosis and Fabry disease are more progressive and show a poorer prognosis. Furthermore, there are several effective treatments available for these infiltrative cardiomyopathies: high-dose chemotherapy and stem-cell transplantation for AL amyloidosis and enzyme replacement therapy for Fabry disease [15, 16, 28]. To optimize survival in patients with these infiltrative cardiomyopathies, early diagnosis and institution of therapy are essential.
In the present study, we evaluated the value of hs-cTnT to distinguish between infiltrative cardiomyopathies and HCM. Serum hs-cTnT level is a useful marker to differentiate two groups. The mechanisms of myocyte injury and release of cardiac troponins in patients with non-ischemic heart failure or cardiomyopathies remain unresolved. Various reasons have been proposed for high troponin levels, including increased wall stress, myocyte damage from inflammatory cytokines or oxidative stress, altered calcium handling, and coronary microvascular dysfunction [29]. For the hypertrophied myocardium, coronary microvascular dysfunction is considered to be the most plausible mechanism for elevation of cardiac troponins. In fact, microvascular dysfunction has been reported in cardiac amyloidosis, Fabry disease, and HCM [30–32]. Microvascular dysfunction and subsequent ischemia may be important components of the disease progression in patients with cardiac hypertrophy. Although the reason is unclear why hs-cTnT level is higher in patients who had infiltrative cardiomyopathy than in those who had HCM, we speculate that apoptotic or necrotic injury may be induced by the toxic effect of accumulated substances themselves in infiltrative cardiomyopathy.
In the present study, E/Ea at lateral corner (not septal corner) was second highest in area under the curve values in ROC curves to differentiate the two groups. This may result from the findings that amyloid deposition in cardiac amyloidosis is diffuse, whereas LV hypertrophy is usually asymmetric and predominantly septal in HCM.
Limitations
There are several limitations to be acknowledged in the present study. First, the number of subjects was small and some of the statistical analyses might have been affected. We need to have more data on hs-cTnT levels in various clinical severities in each disease entity. A diagnostic challenge remains in patients with infiltrative cardiomyopathies who have relatively mild abnormalities on echocardiography. Second, due to the retrospective design of the study, it is possible that there is a selection bias, although the study population consisted of consecutive patients with cardiomyopathies. Third, we could not distinguish between cardiac amyloidosis and cardiac involvement of Fabry disease within infiltrative cardiomyopathies by using hs-cTnT measurements. This biomarker has not been fully evaluated in Fabry disease.
Conclusions
Measurement of serum hs-cTnT enables accurate discrimination between infiltrative cardiomyopathy and HCM. The combination of this biomarker and conventional echocardiographic parameters helps to differentiate these cardiomyopathies.
References
Falk RH, Comenzo RL, Skinner M. The systemic amyloidosis. N Engl J Med. 1997;337:898–909.
Cueto-Garcia L, Reeder GS, Kyle RA, Wood DL, Seward JB, Naessens J, et al. Echocardiographic findings in systemic amyloidosis: spectrum of cardiac involvement and relation to survival. J Am Coll Cardiol. 1985;6:737–43.
Falk RH, Plehn JF, Deering T, Schick Jr EC, Boinay P, Rubinow A, et al. Sensitivity and specificity of the echocardiographic features of cardiac amyloidosis. Am J Cardiol. 1987;59:418–22.
Palka P, Lange A, Donnelly JE, Scalia G, Burstow DJ, Nihoyannopoulos P. Doppler tissue echocardiographic features of cardiac amyloidosis. J Am Soc Echocardiogr. 2002;15:1353–60.
Oki T, Tanaka H, Yamada H, Tabata T, Oishi Y, Ishimoto T, et al. Diagnosis of cardiac amyloidosis based on the myocardial velocity profile in the hypertrophied left ventricular wall. Am J Cardiol. 2004;93:864–9.
Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, et al. An atypical variant of Fabry’s disease in men with left ventricular hypertrophy. N Engl J Med. 1995;333:288–93.
Sachdev B, Takenaka T, Teraguchi H, Tei C, Lee P, McKenna WJ, et al. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation. 2002;105:1407–11.
Monserrat L, Gimeno-Blanes JR, Marin F, Hermida-Prieto M, Garcia-Honrubia A, Perez I, et al. Prevalence of Fabry disease in a cohort of 508 unrelated patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2007;50:2399–403.
Elliott P, Baker R, Pasquale F, Quarta G, Ebrahim H, Mehta AB. Hughes DA, and ACES study group: Prevalence of Anderson-Fabry disease in patients with hypertrophic cardiomyopathy: the European Anderson-Fabry Disease Survey. Heart. 2011;97:1957–60.
Weidmann F, Strotmann JM. Use of tissue Doppler imaging to identify and manage systemic diseases. Clin Res Cardiol. 2008;97:65–73.
Spirito P, Seidman CE, McKenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N Engl J Med. 1997;336:775–85.
Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42:1687–713.
Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–55.
Takenaka T, Teraguchi H, Yoshida A, Taguchi S, Ninomiya K, Umekita Y, et al. Terminal stage cardiac findings in patients with cardiac Fabry disease: an electrocardiographic, echocardiographic, and autopsy study. J Cardiol. 2008;51:50–9.
Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, et al. Safety and efficacy of recombinant human alpha-galactosidase A: replacement therapy in Fabry’s disease. N Engl J Med. 2001;345:9–16.
Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Stork S, et al. Long-term effects of enzyme replacement therapy on Fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation. 2009;119:524–9.
Ochi Y, Kubo T, Kitaoka H. Repeated heart failure in a 74-year-old man with left ventricular hypertrophy. Heart. 2014;100:710.
Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–67.
Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009;361:868–77.
Sato Y, Fujiwara H, Takatsu Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J Cardiol. 2012;60:160–7.
Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, et al. Nal-HeFT Investigators: Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9.
Kristen AV, Giannitsis E, Lehrke S, Hegenbart U, Konstandin M, Lindenmaier D, et al. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood. 2010;116:2455–61.
Apridonidze T, Steingart RM, Comenzo RL, Hoffman J, Goldsmith Y, Bella JN, et al. Clinical and echocardiographic correlates of elevated troponin in amyloid light-chain cardiac amyloidosis. Am J Cardiol. 2012;110:1180–4.
Dispenzieri A, Gertz MA, Kumar SK, Lacy MQ, Kyle RA, Saenger AK, et al. High sensitivity cardiac troponin T in patients with immunoglobulin light chain amyloidosis. Heart. 2014;100:383–8.
Qian G, Wu C, Zhang Y, Chen YD, Dong W, Ren YH. Prognostic value of high-sensitivity cardiac troponin T in patients with endomyocardial-biopsy proven cardiac amyloidosis. J Geriatr Cardiol. 2014;11:136–40.
Kubo T, Kitaoka H, Yamanaka S, Hirota T, Baba Y, Hayashi K, et al. Significance of high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;62:1252–9.
Jenab Y, Pourjafari M, Darabi F, Boroumand MA, Zoroufian A, Jalali A. Prevalence and determinants of elevated high-sensitivity cardiac troponin T in hypertrophic cardiomyopathy. J Cardiol. 2014;63:140–4.
Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL, et al. High-dose melphalan and autologous stem cell transplantation in patietns with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93.
Takashio S, Yamamuro M, Izumiya Y, Sugiyama S, Kojima S, Yamamoto E, et al. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin T release measured by a highly sensitive assay in patients with nonischemic heart failure. J Am Coll Cardiol. 2013;62:632–40.
Abdelmoneim SS, Bernier M, Bellavia D, Syed IS, Mankad SV, Chandrasekaran K, et al. Myocardial contrast echocardiography in biopsy-proven primary cardiac amyloidosis. Eur J Echocardiogr. 2008;9:338–41.
Elliott PM, Kindler H, Shah JS, Sachdev B, Rimoldi OE, Thaman R, et al. Coronary microvascular dysfunction in male patients with Anderson-Fabry disease and the effect of treatment with alpha galactosidase A. Heart. 2006;92:357–60.
Timmer SA, Germans T, Gotte MJ, Russel IK, Lubberink M, Ten Berg JM, et al. Relation of coronary microvascular dysfunction in hypertrophic cardiomyopathy to contractile dysfunction independent from myocardial injury. Am J Cardiol. 2011;107:1522–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TK, TS, and HK conceived the idea for the study and planned the investigations. TK, YB, TH, KT, NY, TF, and HK undertook clinical investigations of patients. SY measured serum high-sensitivity cardiac troponin T. NK performed statistical analysis and TI supervised statistical analysis for this paper. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kubo, T., Baba, Y., Hirota, T. et al. Differentiation of infiltrative cardiomyopathy from hypertrophic cardiomyopathy using high-sensitivity cardiac troponin T: a case-control study. BMC Cardiovasc Disord 15, 53 (2015). https://doi.org/10.1186/s12872-015-0043-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-015-0043-z