Abstract
Background
Minimally invasive adrenalectomy is the standard of care for small adrenal tumours. Both the transperitoneal lateral approach and posterior retroperitoneal approach are widely used and have been proven to be safe and effective. However, the prevalence of chronic postsurgical pain has not been specifically investigated in previous studies. The primary goal of this study was to identify the prevalence of chronic postsurgical pain after minimally invasive adrenalectomy.
Methods
A cross-sectional study was performed among all consecutive patients who had undergone minimally invasive adrenalectomy in a single university medical centre. The primary outcome was the prevalence of chronic postsurgical pain. Secondary outcomes were the prevalence of localized hypoesthesia, risk factors for the development of chronic postsurgical pain, and the Health-Related Quality of Life. Three questionnaires were used to measure the prevalence and severity of chronic postsurgical pain, hypoesthesia, and Health-Related Quality of Life. Logistic regression analysis was performed to determine risk factors for development of chronic postsurgical pain.
Results
Six hundred two patients underwent minimally invasive adrenalectomy between January 2007 and September 2019, of whom 328 signed informed consent. The prevalence of chronic postsurgical pain was 14.9%. In the group of patients with chronic postsurgical pain, 33% reported hypoesthesia as well. Young age was a significant predictor for developing chronic postsurgical pain. The prevalence of localized hypoesthesia was 15.2%. In patients with chronic postsurgical pain, Health-Related Quality of Life was significantly lower, compared to patients without pain.
Conclusions
The prevalence of chronic postsurgical pain following minimally invasive adrenalectomy is considerable. Furthermore, the presence of chronic postsurgical pain was correlated with a significant and clinically relevant lower Health-Related Quality of Life. These findings should be included in the preoperative counselling of the patient. In the absence of evidence for effective treatment in established chronic pain, prevention should be the key strategy and topic of future research.
Similar content being viewed by others
Background
Since the introduction in 1992, minimally invasive adrenalectomy (MIA) has become the standard of care for the management of small (≤7 cm) benign adrenal tumours and, in selected cases, for the treatment of small (≤6 cm) malignant tumours [1]. The transperitoneal lateral approach (TLA) and the posterior retroperitoneal approach (PRA) have both been proven to be safe and effective when compared with open adrenalectomy, with low morbidity and complication rates, decreased blood loss, less postoperative pain, shorter hospital stay, and improved cosmetic effects [2, 3]. Nevertheless, patients regularly report chronic postsurgical pain (CPSP) [4]. CPSP is defined as chronic pain that develops or increases in intensity after a surgical procedure or tissue injury and persists beyond the healing process, i.e. at least 3 months after surgery [5]. Since MIA is frequently performed worldwide, it is important to report these functional outcomes, because CPSP can have significant impact on quality of life and health care demand [6]. Predisposing factors described in literature for development of CPSP are the presence of pre-existing pain, early postoperative pain, psychological factors, the surgical procedure itself and patient characteristics, such as age and sex [7, 8].
The primary outcome of this study was the prevalence of CPSP. Secondary outcomes were the prevalence of hypoesthesia, risk factors for CPSP and the impact of CPSP on Health-Related Quality of Life (HRQoL).
Methods
Study design and patient population
All adult patients who underwent MIA in our hospital between February 2007 and September 2019 were included in a cross-sectional study. Patients were approached by phone between October 2019 and January 2020. PRA was introduced in our hospital in 2011. Patients with (presumably) benign tumours or pheochromocytomas ≤7 cm, and a body mass index (BMI) < 35 kg/m2 were eligible for PRA. In all other patients TLA was indicated. Two surgeons performed the procedures, both with more than 15 years of laparoscopic experience. Living patients were approached by telephone, subsequently written information was sent after which informed consent was obtained. All patients were asked to complete three questionnaires at the time of study. The study was approved by the research ethics committee of the Radboud University Nijmegen Medical Centre (2019–5500).
Measures
There were four objectives in this study; (1) assessment of CPSP; (2) assessment of localized hypoesthesia; (3) analysis of possible risk factors for CPSP; and (4) the influence of CPSP on HRQoL. The primary outcome of this study was the prevalence of CPSP. The presence of CPSP was scored when patients replied “yes” to the question if they currently had pain which could be related back to their adrenalectomy, i.e. wound pain, pain at site of the scar, flank pain, referred shoulder pain. When patients answered “yes” to the chronic pain question, they were included in the chronic pain group, and they were asked to fill in the follow up questionnaire: the Dutch version of the McGill Pain Questionnaire (MPQ) [9]. When the answer was “no”, they did not fill-out this questionnaire. To exclude other sources for pain symptoms, all existing comorbidities present at time of surgery were reported. The MPQ is a validated multidimensional pain questionnaire designed to measure the quality and intensity of chronic pain [10]. The main section of this questionnaire includes a list of 63 words, divided into three major classes: the sensory class, the affective class and the evaluative class. Pain intensity is measured quantitatively by the Number of Words Chosen (NWC), and qualitatively by the Pain Rating Index (PRI). The questionnaire includes a Visual Analogue Scale (VAS), to determine pain severity at the time of analysis. Lastly, the MPQ includes a section for localization, duration, and course of pain. The ‘onset of pain’ was one of the questions in the MPQ. This was subjectively filled in, and marks the way patients experienced the onset of the pain, i.e. like a sudden start in minutes/hours or a slow start in days/weeks.
Secondary outcomes were the prevalence of localized hypoesthesia, analysis of possible risk factors for CPSP and HRQoL. When patients answered “yes” to the localized hypoesthesia question they were included in the group with localized hypoesthesia and were asked to fill in the subsequent self-designed follow up questionnaire to determine its localization, duration, and course (Appendix 1). The ‘onset of numbness’ was one of the questions. This was subjectively filled in, and marks the way patients experienced the origination of the numbness feeling; i.e. fast (minutes/hours) or slow (days/weeks) onset.
All patients were asked to fill in the questionnaire regarding HRQoL: the RAND Short Form-36 Health Status Inventory (RAND SF-36) [11]. The RAND SF-36 is a validated questionnaire on HRQoL, separated into eight multi-item scales; physical functioning, role limitations due to physical health problems, role limitations due to emotional problems, general mental health, social functioning, energy/fatigue, bodily pain, and general health perceptions [11].
Perioperative patient data were collected from a prospectively maintained database and included: medical history, age at the time of surgery, BMI, ASA-score, medication and indication for surgery, side of adrenalectomy, duration of surgery, operating technique, conversion to open surgery and postoperative complications, according to the Clavien-Dindo classification. The presence of preoperative pain, preoperative anxiety disorders or depression was obtained from electronic patient documents and scored “yes” if it was reported.
Statistical analysis
The data were assessed for normality using the Shapiro-Wilk Test. To compare normally distributed continuous variables Student t-test was used. Chi-square and ANOVA were used for categorical variables. Correlation between variables that were normally distributed was calculated with the Pearson correlation coefficient and with the Spearman rank correlation method for non-normally distributed variables.
To identify possible predictive factors for CPSP binary logistic regression was performed. The Hosmer-Lemeshow goodness of fit test was used to describe the performance of the regression model. Variables with a significance level of p < .05 in the univariate model were included in the multivariate model. Statistical significance was defined as a p-value <.05. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS IBM Statistics 24; Armonk, NY).
Results
Patient enrolment
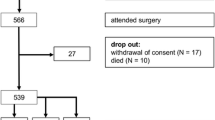
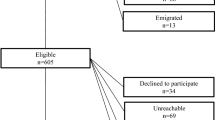
A total of 602 patients underwent MIA between February 2007 and September 2019. Five hundred forty-four patients were approached by phone between October 2019 and January 2020. Fifty-eight patients were lost to follow-up because of death or missing contact information. We received response from 358 (65.8%) patients, of which 328 patients signed informed consent. Of these patients, 172 underwent TLA and 156 PRA. Patient enrolment is depicted in Fig. 1.
Patient characteristics
The mean age of patients at time of adrenalectomy was 54 years and 52% was male. The mean follow-up time was 4.6 years. The most common indications for surgery were primary aldosteronism (PA) (50%), pheochromocytoma (23%), and Cushing’s syndrome (13%). Forty-nine patients (15%) reported pain preoperatively, and 52 (16%) patients used pain medication preoperatively. In the group of patients with CPSP, 25% had reported pain preoperatively and 22% used analgesia before surgery. History or comorbidity of neurological diseases, which included for example cerebral vascular events, hernia nuclei pulposi, and peripheral neuropathy was not significantly different between patients with and without CPSP. Thirty-four patients had any postoperative complication, of which 15 (4.6%) patients had a Clavien-Dindo I complication, 11 (3.3%) patients had a Clavien-Dindo II, and 8 (2.4%) patients had a Clavien-Dindo III complication. Most Clavien-Dindo II complications were postoperative pulmonary infections or urinary tract infections (73%). The Clavien-Dindo III complications included three patients who underwent additional surgery (0.9%), two because of an incisional hernia and one because of an urinoma. Other Clavien-Dindo III complications included pulmonary embolism and postoperative abscess which required percutaneous drainage. Patient characteristics are presented in Table 1.
Prevalence of chronic postsurgical pain
Forty-nine out of 328 patients (14.9%) reported the presence of CPSP, of which 34 received TLA (69.4%) and 15 PRA (30.6%). Onset of CPSP was acute in 18 patients (36.7%) and slow in 28 patients (57.1%). In the group of patients with CPSP, 32.7% reported hypoesthesia as well. In the group of patients with CPSP, most patients (44.9%) experienced continuous pain with fluctuating severity. In this group, the mean general pain intensity measured by VAS was 34 (± 25). The reported localizations were the ipsilateral flank (67.3%), the contralateral flank (16.3%) and other more diverse localizations such as groin, shoulders or arms (16.3%).
The mean Number of Words Chosen (NWC) was 7.9 of 20 words. Sensory descriptors were chosen more frequently than affective or evaluative terms. The most frequently selected words were nagging (n = 21; 42.9%) and stabbing (n = 20; 40.8%) in the sensory class; tiring (n = 25; 51%) in the affective class; and annoying (n = 26; 53.1%) in the evaluative class. The mean pain intensity measured by the total score of the PRI (PRI-Total; PRI-T) was 40.8 (SD 26.7; maximum score 63). Twenty patients (40.8%) were using analgesics for the reported pain, mostly acetaminophen or nonsteroidal anti-inflammatory drugs (34.7%); four patients reported the use of opioids (8.2%). Further characteristics are presented in Table 2.
Prevalence of hypoesthesia
Fifty-two patients (15.8%) reported hypoesthesia, of which 19 patients received TLA (36.5%) and 33 PRA (63.5%). Onset of hypoesthesia was acute in 28 patients (53.8%) and slow in 18 patients (34.6%). Twenty-one patients (40.4%) experienced a continuous feeling of hypoesthesia. The reported localizations were the ipsilateral flank (78.8%), leg or arms (11.5%) or unknown (9.6%). Further characteristics are presented in Table 2.
Risk factors of CPSP
When looking at the univariate binary logistic regression, age, BMI, ASA-score, preexisting pain, surgical complications with Clavien-Dindo score III, TLA, and primary aldosteronism as indication for surgery were significant individual predictors of CPSP (Table 3). When performing multivariate binary logistic regression with these individual predictors, only young age remained a significant predictor for the development of CPSP. There was no collinearity between ASA-score and age. The Hosmer-Lemeshow goodness of fit test was not significant (p = .405).
Health related quality of life
The HRQoL of patients with CPSP after MIA was significantly lower in all subscales compared with patients without pain (Table 4). Major differences were seen in role limitations due to physical health problems between patients without CPSP (mean 76.1 ± SD 38.7) and patients with CPSP (mean 40.6 ± SD 43.3) (p < .001) and on the scale of bodily pain between patients without pain (mean 86.1 ± SD 21.9) and patients with CPSP (mean 59.1 ± SD 20.1) (p < .001). When analyzing pain intensity, a higher mean VAS-score was significantly correlated with a lower score on the following subscales of the RAND-SF36 questionnaire: role limitations due to physical health problems (R2 = 0.13, p = .012), role limitations due to emotional problems (R2 = 0.11, p = .024), general mental health (R2 = 0.18, p = .003) and bodily pain (R2 = 0.30, p = .000). In patients with CPSP the presence of hypoesthesia as well resulted in a significantly lower score of physical functioning (68.2 versus 42.2, p = .001). On the other seven domains there was no significant difference.
Discussion
Although MIA was proven to be safe and effective for a heterogeneous group of patients with adrenal disorders, the prevalence of CPSP has not been reported widely. In this cohort study the prevalence of CPSP following MIA was 14.9%. The presence of CPSP was correlated with a significantly lower HRQoL.
Acosta et al. found a prevalence of 8% chronic back pain in twelve open and 6% in seventeen laparoscopic bilateral adrenalectomies for hypercortisolism [12]. Walz et al. observed an incidence of 8.5% of temporary hypoesthesia and/or relaxation of the abdominal wall after PRA [13]. A study by Bruintjes et al. showed a prevalence of CPSP of 5.7% following laparoscopic donor nephrectomy in relatively healthy live kidney donors. They also showed a significantly lower HRQoL in patients with CPSP on all subscales of the RAND-SF36, except role limitations due to emotional problems [14]. The prevalence of CPSP in our study was higher when compared to the study by Bruintjes et al. There are some possible explanations. First, there is a certain risk of recall bias, which might indicate that patient with pain symptoms are more willing to fill in the questionnaires compared to patients without pain, causing an overestimation of CPSP in this study. Second, we used the visual analogue scale and MPQ to report and classify postoperative pain. Differences in definition, classification systems used or methodology of analysis might impede comparisons among studies [15]. Third, patients with different comorbidities were analyzed in this study, compared to healthy donors in the study by Bruintjes et al. The prevalence of nerve injury-induced neuropathic pain was high in patients with persistent pain after thoracic and breast surgeries, 66 and 68%, respectively. In patients with CPSP after groin hernia repair, the prevalence of neuropathic pain was 31%, and after total hip or knee arthroplasty it was 6% [16]. So, the prevalence of nerve injury-induced neuropathic pain among CPSP cases differs in various types of surgery, probably depending on the likelihood of surgical iatrogenic nerve injury. Although, perioperative nerve injury seems to play an important role in the development of neuropathic pain, nociceptive and inflammatory processes can also be involved [16,17,18]. We found sixteen patients (33%) with a combination of CPSP and symptoms of hypoesthesia. This is in accordance with the study by Johansen et al., who reported a strong association between sensory abnormalities and persistent pain, increasing with higher pain intensities [19]. These findings may indicate that direct neuronal injury is a potential factor for developing CPSP, since nerve damage can result in central sensitization, which is linked to the development of CPSP [20]. Early prevention of central sensitization may provide a mechanism-based approach by blocking nociceptive input, for example by using regional anesthesia or through antihyperalgesic drugs, such as ketamine, subsequently reducing the chance to develop CPSP.
After multivariate regression analysis young age was a significant predictor of CPSP. This predictor has already been described for other surgical procedures than adrenalectomy, such as video-assisted thoracoscopy or thoracotomy [21], breast cancer surgery [22], and hysterectomy [23]. The etiology is not well-understood, but may be the result of a reduction in peripheral nerve functioning that occurs with increased age [24]. Recent publications describe a significant association between major postoperative complications and development of CPSP in much larger cohorts [25, 26]. The presence of a postoperative complications scored Clavien-Dindo III in our population was a significant predictor of CPSP in the univariate regression analysis, but not in the multivariate regression analysis. However, the result could be underpowered, since this group of patients was small.
When looking at pain severity, patients with a higher VAS-score had significantly lower scores on several domains of the RAND-SF36. This means that the presence of more severe pain results in a significantly lower HRQoL. CPSP can lead to functional limitations and psychological distress in patients. Therefore, identifying the risk factors and applying a preventive strategy may help to decrease the incidence of CPSP and the resulting lower HRQoL. Possible preventive strategies include modification of the surgical technique, adequate pain control throughout the perioperative period, and preoperative psychological intervention focusing on psychosocial and cognitive risk factors [27].
The main strength of this study is that we specifically investigated the prevalence of CPSP after MIA as a primary outcome, which was not done before. Furthermore, this study includes a large patient number from an expert centre, with a relatively high response rate compared with other small-scale phone or e-mail surveys [28]. This allowed us to perform multivariate logistic regression analyses to identify independent predictors of CPSP.
We acknowledge a few limitations in our study. First, patients with a variety of indications for surgery, disease-related symptoms and differences in comorbidity were compared. Preoperative patient data revealed that according to the group of patients with CPSP 25% had pre-existent pain symptoms, and 22% used analgesia before surgery. This could have an influence on preoperative and postoperative HRQoL between patients, and may influence their recovery after surgery, with or without the presence of CPSP. Second, because of the delay of inclusion (maximal 12 years from surgery to research contact), loss to follow-up may have influenced the results. We received response from 66% of patients, and 54% patients signed informed consent. Bias from lack of response might cause an incomplete analysis of the data. So, we cannot ensure that the remaining 46% had similar outcomes to those patients we assessed. Third, the questionnaire regarding CPSP was specific to only answer “yes” if the pain could be related back to their adrenalectomy, i.e. in time of onset and specific localization. However, it is still possible that patients with pre-existing pain reported “yes” as well. This could have resulted in an overestimation of the prevalence of CPSP. We tried to reduce this by searching and reporting comorbidities present at time of surgery which could lead to chronic pain symptoms. Finally, no structured preoperative HRQoL data were present in our population.
In conclusion, in this study we have shown a substantial prevalence of CPSP following MIA. The presence of CPSP was significantly correlated with a lower HRQoL. When present, CPSP should be identified in a timely manner, since adequate management by pharmacotherapy, appropriate pain interventions, and/or psychological management, can improve the pain and the physical and social functionality of patients. Furthermore, in the absence of evidence for the most effective treatment in established chronic pain, prevention should be the key strategy. Future trials should focus on etiology and prevention of CPSP after MIA.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author [EvH]. The datasets generated and/or analysed during the current study are not publicly available due to reasons of sensitivity, e.g. human data; containing information that could compromise research participant privacy.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- MIA:
-
Minimally invasive adrenalectomy
- TLA:
-
Transperitoneal lateral approach
- PRA:
-
Posterior retroperitoneal approach
- CPSP:
-
Chronic postsurgical pain
- HRQoL:
-
Health-Related Quality of Life
- BMI:
-
Body mass index
- MPQ:
-
McGill Pain Questionnaire
- RAND SF-36:
-
RAND Short Form-36 Health Status Inventory
- NWC:
-
Number of Words Chosen
- PRI:
-
Pain Rating Index
- VAS:
-
Visual Analogue Scale
References
Gaujoux S, Mihai R. European Society of Endocrine Surgeons (ESES) and European network for the study of adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg. 2017;104(4):358–76.
Conzo G, Tartaglia E, Gambardella C, et al. Minimally invasive approach for adrenal lesions: systematic review of laparoscopic versus retroperitoneoscopic adrenalectomy and assessment of risk factors for complications. Int J Surg. 2016;28(Suppl 1):S118–23.
Wu S, Lai H, Zhao J, et al. Laparoendoscopic single-site adrenalectomy versus conventional laparoscopic adrenalectomy: an updated Meta analysis. Urol J. 2016;13(2):2590–8.
Fletcher D, Stamer UM, Pogatzki-Zahn E, et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32(10):725–34.
Schug SA, Lavand'homme P, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52.
Wu CL, Richman JM. Postoperative pain and quality of recovery. Curr Opin Anaesthesiol. 2004;17(5):455–60.
van Rijckevorsel DC, de Vries M, Schreuder LT, Wilder-Smith OH, van Goor H. Risk factors for chronic postsurgical abdominal and pelvic pain. Pain Manag. 2015;5(2):107–16.
Reddi D, Curran N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med J. 2014;90(1062):222–7 quiz 226.
Vanderiet K, Adriaensen H, Carton H, Vertommen H. The McGill pain questionnaire constructed for the Dutch language (MPQ-DV). Preliminary data concerning reliability and validity. Pain. 1987;30(3):395–408.
Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–99.
Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–27.
Acosta E, Pantoja JP, Gamino R, Rull JA, Herrera MF. Laparoscopic versus open adrenalectomy in Cushing's syndrome and disease. Surgery. 1999;126(6):1111–6.
Walz MK, Alesina PF, Wenger FA, et al. Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery. 2006;140(6):943–8 discussion 948–950.
Bruintjes MHD, van Helden EV, de Vries M, et al. Chronic pain following laparoscopic living-donor nephrectomy: prevalence and impact on quality of life. Am J Transplant. 2019;19(10):2825–32.
van Boekel RLM, Warlé MC, Nielen RGC, et al. Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 2019;269(5):856–65.
Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(1):95–102.
Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–44.
Dualé C, Ouchchane L, Schoeffler P, Dubray C. Neuropathic aspects of persistent postsurgical pain: a French multicenter survey with a 6-month prospective follow-up. J Pain. 2014;15(1):24.e21–0.
Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain. 2012;153(7):1390–6.
van Helmond N, Steegers MA, Filippini-de Moor GP, Vissers KC, Wilder-Smith OH. Hyperalgesia and persistent pain after breast Cancer surgery: a prospective randomized controlled trial with perioperative COX-2 inhibition. PLoS One. 2016;11(12):e0166601.
Steegers MA, Snik DM, Verhagen AF, van der Drift MA, Wilder-Smith OH. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9(10):955–61.
Steegers MA, Wolters B, Evers AW, Strobbe L, Wilder-Smith OH. Effect of axillary lymph node dissection on prevalence and intensity of chronic and phantom pain after breast cancer surgery. J Pain. 2008;9(9):813–22.
Pinto PR, McIntyre T, Nogueira-Silva C, Almeida A, Araújo-Soares V. Risk factors for persistent postsurgical pain in women undergoing hysterectomy due to benign causes: a prospective predictive study. J Pain. 2012;13(11):1045–57.
Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208.
Hanley C, Ladha KS, Clarke HA, Cuthbertson BC, Wijeysundera DN. Association of postoperative complications with persistent post-surgical pain: a multicentre prospective cohort study. Br J Anaesth. 2022;128(2):311–20.
Willingham M, Rangrass G, Curcuru C, et al. Association between postoperative complications and lingering post-surgical pain: an observational cohort study. Br J Anaesth. 2020;124(2):214–21.
Thapa P, Euasobhon P. Chronic postsurgical pain: current evidence for prevention and management. Korean J Pain. 2018;31(3):155–73.
Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50(10):1129–36.
Acknowledgements
No other persons but those named in the list of authors have made substantial contributions to this manuscript.
Funding
The authors declare that they received no funding.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. Conception and design: EvH, AvU, MCW, JFL. Acquisition of data: EvH, AvU, MCW, JFL. Analysis and interpretation of data: EvH, AvU, MCW, JFL, GJS, CK. Drafting of manuscript: EvH, AvU, MCW, JFL. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: EvH, AvU, MCW, JFL. Obtaining funding: no funding applicable. Administrative, technical or material support: EvH, MCW, AvU, JFL. Supervision: MCW, JFL.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the research ethics committee of the Radboud University Nijmegen Medical Centre (2019–5500), on the basis of the Dutch Code of conduct for health research, the Dutch Code of conduct for responsible use, the Dutch Personal Data Protection Act and the Medical Treatment Agreement Act. We confirm that all methods were performed in accordance with the Declaration of Helsinki. All participants provided informed consent for the study, which used an opt-in consent method.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Helden, E.V., van Uitert, A., Albers, K.I. et al. Chronic postsurgical pain after minimally invasive adrenalectomy: prevalence and impact on quality of life. BMC Anesthesiol 22, 153 (2022). https://doi.org/10.1186/s12871-022-01696-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01696-4