Abstract
Background
In recent years, high flow nasal oxygen (HFNO) has been widely used in clinic, especially in perioperative period. Many studies have discussed the role of HFNO in pre- and apneic oxygenation, but their results are controversial. Our study aimed to examine the effectiveness of HFNO in pre- and apneic oxygenation by a meta-analysis of RCTs.
Methods
EMBASE, PUBMED, and COCHRANE LIBRARY databases were searched from inception to July 2021 for relevant randomized controlled trails (RCTs) on the effectiveness of HFNO versus standard facemask ventilation (FMV) in pre- and apenic oxygenation. Studies involving one of the following six indicators: (1) Arterial oxygen partial pressure (PaO2), (2) End expiratory oxygen concentration (EtO2), (3) Safe apnoea time, (4) Minimum pulse oxygen saturation (SpO2min), (5) Oxygenation (O2) desaturation, (6) End expiratory carbon dioxide (EtCO2) or Arterial carbon dioxide partial pressure(PaCO2) were included. Due to the source of clinical heterogeneity in the observed indicators in this study, we adopt random-effects model for analysis, and express it as the mean difference (MD) or risk ratio (RR) with a confidence interval of 95% (95%CI). We conducted a risk assessment of bias for eligible studies and assessed the overall quality of evidence for each outcome.
Results
Fourteen RCTs and 1012 participants were finally included. We found the PaO2 was higher in HFNO group than FMV group with a MD (95% CI) of 57.38 mmHg (25.65 to 89.10; p = 0.0004) after preoxygenation and the safe apnoea time was significantly longer with a MD (95% CI) of 86.93 s (44.35 to 129.51; p < 0.0001) during anesthesia induction. There were no significant statistical difference in the minimum SpO2, CO2 accumulation, EtO2 and O2 desaturation rate during anesthesia induction between the two groups.
Conclusions
This systematic review and meta-analysis suggests that HFNO should be considered as an oxygenation tool for patients during anesthesia induction. Compared with FMV, continuous use of HFNO during anesthesia induction can significantly improve oxygenation and prolong safe apnoea time in surgical patients.
Similar content being viewed by others
Introduction
Oxygenaton is fundamental to safe anaesthetic practice and anaesthesiologists need to be skilled in oxygenation techniques. Hypoxemia during anesthesia induction is one of the leading causes of anesthesia-related morbidity and mortality [1], and anesthesiologists should take it seriously and avoid its occurrence. It has been reported that cardiac arrests can occur in 2–3% of intubation procedure in intensive care unit (ICU), and is strongly related to hypoxemia or absence of preoxygenaion before intubation [2]. Preoxygenation before anesthesia induction can increase alveolar oxygen reserve of patients by denitrogenation, so as to increase safe apnoea time and reduce the incidence of hypoxemia and subsequent complications during endotracheal intubation. Consequently, the Difficult Airway Society guidelines recommended that all patients should be preoxygenated before induction of general anesthesia [3]. The standard method of preoxygenation is performed using a facemask with an adequate seal between the patient and the circuit for 3 min with a fresh gas flow of 10 L·min−1[4].In addition, apneic oxygenation can also prolong safe apnoea time and reduces the incidence of arterial oxygen desaturation during intubation [5]. Preoxygenation and apneic oxygenation are especially important in patients whereby bag-mask ventilation after the induction of anesthesia is to be avoided and in patients at higher risk of hypoxemia [5, 6].
HFNO is composed of an air/oxygen blender, an active humidifier, a single heated circuit and a nasal cannula, which can provide constant inhaled oxygen concentration of 0.21–1.0 and oxygen flow rate of 1–60 L·min−1 or even higher [7]. It has been proposed that the use of HFNO can generate continuous positive airway pressure, reduce anatomical dead space, improve mucociliary clearance and reduce the work of breathing [8,9,10,11]. In 2015, HFNO was first used for preoxygenation and apneic oxygen in patients with predicted difficult airway, and was proposed that HFNO can significantly prolong the safe apnoea time of patients under general anesthesia [6]. Many clinical anesthesiologists has carried out extensive and in-depth research on the application of HFNO in perioperative period, especially in the pre- and apneic oxygenation efficacy of HFNO during anesthesia induction. However, many studies have reached controversial results. There was a systematic review and meta-analysis have indicated the use of HFNO in the intraoperative setting can reduce the risk of O2 desaturation, increase safe apnoea time and SpO2min in patients at higher risk of hypoxemia [12]. However, it was based on small-sampled studies and did not restrict the control group to standard face mask ventilation. In addition, recent published RCTs can be included in our systematic and meta-analysis [13,14,15,16,17,18,19].
Therefore, we conducted a systematic review and meta-analysis to update the existing evidence and gain further insight into the effectiveness of HFNO compared with FMV for pre- and apneic oxygenation during anesthesia induction. We selected 6 indicators to compare the use of HFNO and FMV during anesthesia induction. Among them, PaO2 and EtO2 can represent the efficacy of pre-oxygenation, safe apnoea time, SpO2min and O2 desaturation can represent the efficiency of pre-oxygenation, and EtCO2 or PaCO2 can be used to observe the effect of apneic oxygenation on patient ventilation [4].
Methods
Search strategy
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [20]. The PRISMA Checklist is provided in Additional file 1. English databases including PUBMED, EMBASE, and COCHRANE LIBRARY were searched from inception to December 2021 to find RCTs exploring the effectiveness of HFNO compared with FMV for pre- and apneic oxygenation in adult patients (> 18 years old). According to the PICOS approach, the following terms were selected: “High flow nasal oxygen,””HFNO,” “High flow nasal cannula,” “HFNC,” “Transnasal humidified rapid-insufflation ventilatory exchange,” “THRIVE,” “Facemask,” “Facemask ventilation,” “Preoxygenation,” “Intubation,” “Anesthesia induction,””Randomised controlled trial,” “RCT,” “randomized,” “controlled,”. We also searched Google Scholar and clinical trail registry to identify grey literature and checked the reference list of all included studies to identify additional studies missed from the original electronic search. This study does not contain any conference abstracts.
Inclusion and exclusion criteria
Inclusion criteria were as follows: 1)comparing the effects of HFNO and FMV during anesthesia induction; 2)involving one of the following six indicators: (1) PaO2, (2) EtO2, (3) safe apnoea time, (4) SpO2min, (5) O2 desaturation, (6) EtCO2 or PaCO2, at anesthesia induction period for pre- or apenic oxygenation; 3) randomized controlled trials. We excluded studies if they 1) were intensive care unit and pediatric patients; 2)were non mask controlled experiments, including bite block or nasal cannula ventilation; 3)were not able to extract data; 4) were not available for full text.
Articles selection and data extraction
Titles and abstracts were independently screened by 2 authors (Song, Sun). Following selection of abstracts, full text of articles identified for possible inclusion were obtained and assessed for inclusion independently by the 2 reviewers (Song, Sun). Disagreements were resolved by consensus or by consulting the senior author (Su). Study characteristics were extracted independently by 2 authors (Shi, Liu) using a standard data collection form in an Excel worksheet. The following information was extracted from each study: author, year of publication, type of surgery, number of patients, intervention characteristics and inclusion indicators. The 6 indicators extracted were PaO2, EtO2, safe apnea time, SpO2min, O2 desaturation and EtCO2 or PaCO2. The data were extracted independently by two authors (Shi, Liu) and then reviewed by the senior author (Su). When there is missing data, contact the relevant author to obtain the missing data.
When comparing the safe apnoea time, the included articles have different definitions. Two defined from the cessation of spontaneous breathing until the SpO2decreased to 90% or the apnoea time reached 6 min or 10 min [13, 14], one defined the apnoea time from the onset of cessation of breathing until the SpO2 decreased to 95% or the apnoea time reached 6 min [21] and one defined from the cessation of spontaneous breathing until the SpO2decreased to 92% [18]. And there are also differences in the definition of deoxysaturation, desaturation was defined as SpO2≦90% in two studies [22, 23], SpO2≦93% in two studies [15, 24] and SpO2≦92% in one study [18]. In our analysis, we directly compared this indicator without adopting a unified definition.
Risk of bias assessment
Two reviewers (Song, Sun) independently assessed risk of bias in included studies using the Cochrane Collaboration risk-of-bias tool [25]. Studies were categorized into high, low, or unclear risk of bias according to the following predefined criteria: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential sources of bias. Each study was compared for consistency, with any disagreement resolved by discussion between the two reviewers (Song, Sun) or mediated by a third reviewer (Su).
Statistical analysis
Meta-analysis was performed using Review Manager (RevMan version 5.4.1, The Nordic Cochrane Centre, Copenhagen, Denmark). Categorical and continuous variable summary data from each individual study were entered into Review Manager. The statistical method used for categorical outcome (O2 desaturation) was Mantel–Haenszel and the effect measure was risk ratio (RR). The statistical method used for continuous outcome (PaO2, EtO2, safe apnoea time, SpO2min, EtCO2 or PaCO2) was inverse variance and the effect measure was mean difference. Due to the source of clinical heterogeneity in the observed indicators in this study, we adopt random-effects model for analysis. Subgroup analysis and sensitivity analysis excluding literature one by one were used to explore the causes of high heterogeneity. Forest plots, RR (95% confidence interval [CI]), mean difference (95% CI), and heterogeneity (χ2 and I2) were generated for the 6 outcomes. For studies that showed results in median and range or interquartile range, the methodology of Wan et al. [26] was used to convert them into mean and standard deviation.
Results
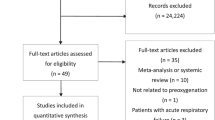
The initial electronic search retrieved 1965 citations, and the grey literature search identified additional 408 studies. This process identified 121 potentially eligible studies for full-text review. After duplicate and ineligible studies were removed, 14 RCTs with a total of 1012 participants were finally included in our systematic review and meta-analysis (Fig. 1) [13,14,15,16,17,18,19, 21,22,23,24, 27,28,29]. The characteristics of included studies are presented in Table 1. The methodological quality of the involved trails is shown in Fig. 2. Two studies were multi-center RCT [15, 17] and the reminder were single-center RCTs. All 14 studies included one or more of the following outcomes: (1) PaO2, (2) EtO2, (3) safe apnoea time, (4) SpO2min, (5) O2 desaturation, (6) EtCO2 or PaCO2, at anesthesia induction period for pre- or apenic oxygenation.
PaO2
Eight RCTs compared the PaO2 after preoxygenation between HFNO and FMV group, including a total of 391 patients. HFNO was administered at flow rates between 30 and 70 L·min−1 while the flow rate of FMV group was 6–15 L·min−1 during preoxygenation. Meta-analysis based on the eight studies showed a statistically significant higher PaO2 after preoxygenation in the HFNO group than FMV group with a MD (95% CI) of 67.82 mmHg (29.25 to 106.40; p = 0.0006). Due to high heterogeneity, we performed the sensitivity analysis by excluding the eight studies one by one, and found that by excluding Yasser MO et al.’s article could significantly reduce heterogeneity. And still statistically significant with a MD (95% CI) of 57.38 mmHg (25.65 to 89.10; p = 0.0004; Fig. 3). The seven RCTs included a total of 291 patients. The source of heterogeneity may come from different patient populations, different pre-oxygenation time and different ways to use HFNO in the included articles. Subgroup analysis showed no significant difference in PaO2 between after preoxygenation and after intubation (p = 0.70; Fig. 3). Funnel plot analysis suggested visually no significant asymmetry, suggesting a low chance of publication bias (Additional file 2 and S1).
Forest plots of PaO2 in HFNO versus FMV after preoxygenation and after intubation. Subgroup analysis shows the PaO2 after preoxygenation versus after intubation. CI indicates confidence interval; df, degrees of freedom; HFNO, a high-flow nasal oxygen; FMV, facemask ventilation; IV, inverse variance, 02 oxygen, standard deviation
EtO2
Five studies compared the EtO2 between HFNO and FMV group. Meta-analysis based on the five studies showed that EtO2 was similar in the HFNO group versus FMV group with a MD (95% CI) of -3.34% (-8.83 to 2.14; p = 0.23; Fig. 4). Due to high heterogeneity, we performed the sensitivity analysis by excluding the five studies one by one, but there was no significant change in heterogeneity. The source of heterogeneity may come from different patient populations, different pre-oxygenation time and different ways to use HFNO in the included articles. Three studies [16, 27, 29] compared the EtO2 after preoxygenation and two studies [15, 19] compared the EtO2 after intubation. Subgroup analysis showed that there was no significant difference in EtO2 between after preoxygenation and intubation (MD -5.82; 95%CI -11.96 to 0.33; p = 0.06 and MD 0.71; 95%CI -16.90 to 18.32; p = 0.94; Fig. 4).
Forest plots of ETO2 in HFNO versus FMV after preoxygenation and intubation. Subgroup analysis shows the ETO2 after preoxygenation versus after intubation. CI indicates confidence interval; df, degrees of freedom; HFNO, hign flow anasal oxygen; FMV, facemask ventilation; IV, inverse variance; o2 oxygen; SD, standard deviation
Safe apnea time
Four RCTs compared safe apnoea time during the peri-intubation period between HFNO and FMV. In all four RCTs, facemask assisted ventilation was not implemented in control groups during apneic oxygenation. Airway patency was carefully maintained using a chin left or jaw thrust in all subjects. From meta-analysis of the four RCTs, safe apnoea time was significantly longer in HFNO compared with FMV group by a MD (95% CI) of 110.36 s (50.56 to 170.16; p = 0.0003). Due to the high heterogeneity, we excluded the literature one by one for sensitivity analysis. We found that when excluding Yasser MO et al.'s research can significantly reduce heterogeneity, and there were still statistical differences with a MD (95% CI) of 86.93 s (44.35 to 129.51; p < 0.0001; Fig. 5A). The source of heterogeneity may come from different patient populations, different pre-oxygenation time, different ways to use HFNO and the different definitions of the safe apnoea time in the included articles.
A Forest plots of safe apnea time in HFNO versus FMV after preoxygenation and intubation. B Forest plots of SpO2min in HFNO versus FMV during intubation. C Forest plots of the rate of desaturation in HFNO versus FMV during intubation. CI indicates confidence interval; MD, mean difference; RR, risk ratio; df, degree of freedom; HFNO, high flow nasal oxygenation; FMV, face mask ventilation; IV, inverse variance; O2, Oxygenation; SD, standard deviation
Minimum O2 saturation(SpO2min)
Three RCTs compared the SpO2min during the peri-intubation period between HFNO and FMV. Meta-analysis showed that the SpO2min was similar in HFNO and FMV subjects with a MD (95% CI) of 3.17% (-1.37 to 7.70; p = 0.17; Fig. 5B). Due to the high heterogeneity, we excluded the studies one by one for sensitivity analysis. After excluding Sjöblom A et al.’s study, the heterogeneity decreased slightly, but there was a significant statistical difference in HFNO verses FMV with a MD (95% CI) of 4.91% (1.49 to 8.32; p = 0.005). The source of heterogeneity may come from different patient populations, different pre-oxygenation time and different ways to use HFNO in the included articles.
O2 desaturation
Five RCTs compared the rate of O2 desaturation during intubation period between HFNO and FMV group. Meta-analysis showed that the rate of peri-intubation O2 desaturation was similar in HFNO group versus FMV group with a RR (95% CI) of 0.66 (0.14 to 3.12; p = 0.60; Fig. 5C). The source of heterogeneity may come from different patient populations, different defination of desaturation, different pre-oxygenation time and different ways to use HFNO in the included articles.
PaCO2 or end-tidal CO2
Nine RCTs compared the EtCO2 or PaCO2 between HFNO group and FMV group during intubation period. Since both EtCO2 and PaCO2 can reflect the accumulation of CO2 in the body, we analyzed EtCO2 and PaCO2 together. Meta-analysis showed that the CO2 accumulation was similar in HFNO group versus FMV group with a MD (95% CI) of 0.56 mmHg (-0.81 to 1.93; p = 0.43; Fig. 6). We also performed subgroup analysis with EtCO2 and PaCO2, and found no significant statistical difference (p = 0.09) between the EtCO2 group (MD -0.18; 95% CI -1.25 to 0.89; p = 0.75) and the PaCO2 group (MD 2.59; 95% CI -0.38 to 5.57; p = 0.09; Fig. 6). The source of heterogeneity may come from the difference between EtCO2 and PaCO2, the different pre-oxygenation time and the different apneic oxygenation time in the included articles. Funnel plot analysis suggested visually no significant asymmetry, suggesting a low chance of publication bias (S2).
Forest plots of EtCO2 or PaCO2 in HFNO versus FMV after intubation. Subgroup analysis shows the EtCO2 versus PaCO2 after intubation. CI indicates confidence interval; df, degrees of freedom; HFNO, high-flow nasal oxygen; FMV, facemask ventilation; IV, inverse variance; O2, oxygen; SD, standard deviation
Discussion
This systematic review and meta-analysis shows that compared with FMV, HFNO can significantly improve oxygenation and prolong safe apnoea time during anesthesia induction, but there is no significant statistical difference in the rate of O2 desaturation, EtO2, minimum SpO2 and CO2 level.
Meta-analysis showed that compared with FMV group, PaO2 in HFNO group was higher during anesthesia induction (p = 0.0004) and subgroup analysis showed that there was no significant difference (p = 0.70) in PaO2 between after preoxygenation and after intubation. This finding shows that compared with FMV, the use of HFNO during anesthesia induction can significantly improve the oxygenation of patients, which has been confirmed by previous studies. The oxygenation efficacy of HFNO in awake fibre-optic intubation in patients with difficult airways has been studied and found that HFNO can significantly improve oxygenation and prolong the safe apnoea time [30]. Previous studies have shown that HFNO can provide a stable inspired oxygen concentration, the distal positive airway pressure generated by high flow gas can increase end-expiratory lung volume, alveolar oxygen partial pressure and reduce intrapulmonary shunt, and the less dead space ventilation than FMV due to the washout effect of THRIVE [9, 19, 23, 28]. This may be a potential mechanism for HFNO to increase the PaO2 compared with FMV during pre-oxygenation.
In addition, we found that safe apnoea time during anesthesia induction was longer in HFNO group than FMV group (p < 0.0001). This finding is in line with the previous research conclusions in both ICU and operating room [5, 31, 32]. It has been reported that HFNO can significantly prolong safe apnoea time when used for preoxygenation and apneic oxygenation during surgery in patients with predictable difficult airway, with a median apnoea time of 14 min and a maximum of 65 min [6]. HFNO can provide continuous supply for patients with apnoea through the effect of apneic oxygenation during intubation period, so as long to prolong safe apnoea time [6, 9]. Taking advantage of the fact that HFNO can significantly prolong the safe apnoea time, many medical institutions have successfully carried out tubeless anesthesia, especially in short operations with shared airway such as subglottic stenosis and upper airway surgeries [33, 34]. However, studies recently published indicates that although the apneic oxygenation of HFNO can ensure the oxygenation of patients and maintain long-term tubeless anesthesia, it is easy to result in CO2 accumulation and respiratory acidosis when the apnoea time is greater than 30 min [35, 36]. This extends previous knowledge and has implications for the safe application of HFNO during prolonged procedures.
However, there are still some differences compared with previous studies. In our findings, there was no significant difference in the rate of O2 desaturation (p = 0.60) and the SpO2min (p = 0.17) between HFNO and FMV subjects during intubation period. These findings are not exactly consistent with the studies on HFNO in the ICU. Previous studies have shown that the use of HFNO during endotracheal intubation can reduce the incidence of hypoxemia and increase the minimum O2 saturation in ICU patients [9, 35, 37]. But, an observational study showed that the use of HFNO during emergency intubation can reduce the incidence of desaturation in patients with high risk hypoxemia [38]. And there were also studies showing no differences [39,40,41]. The reason for this difference may be that, unlike ICU patients, surgical patients have well compensated cardiopulmonary function.
According to our findings, we can recognize that HFNO is an effective oxygenation tool in general anesthesia surgery. Oxygenation is of paramount importance in anesthesia induction period, especially in patients with difficult airways and high-risk hypoxemia patients. Previous studies have demonstrated the effectiveness of HFNO use in these specific populations [6, 9, 37]. However, in this paper, we did not compare the use of HFNO and FMV in these special populations, and therefore cannot suggest that HFNO is superior to FMV when used in these populations.
The strengths of this review include a comprehensive search strategy using major biomedical databases for published data and grey literature, and a focus on clinically relevant outcomes. Secondly, we followed a rigorous methodology. The review of eligibility criteria, data extraction, and outcome methodology assessment were all performed in duplicate with a high degree of inter-rater agreement. Thirdly, this review contained the largest number of RCTs published on this topic, which allowed outcomes to meet the optimal information size and allowed us to make more reliable inferences.
Several potential limitations are also present in this meta-analysis. First, we included 14 RCTs and observed six indicators, and there were relatively few articles included in each index, even though this is the largest number of RCTs that can be searched. Second, in this article, we included different populations into the meta-analysis. Due to the limited number of articles included in each observation index, we did not conduct subgroup analysis for different populations. Third, in this meta-analysis, although we reduced the heterogeneity through sensitivity analysis, each observation index still has heterogeneity. Finally, due to the limited articles included in each indicator, we only evaluated the publication bias of PaO2 and CO2 indicators.
Conclusion
This systematic review and meta-analysis comprehensively evaluated the effectiveness of HFNO verses FMV for pre- and apneic oxygenation during anesthesia induction. According to our findings, compared with FMV, HFNO can improve oxygenation of patients during pre-oxygenation, and its continuous application during induction of anesthesia can significantly prolong the safe apnoea time. We suggest that HFNO should be considered as an oxygenation tool during anesthesia induction in patients undergoing general anesthesia surgery. Further well-powered RCTs should focus on comparing the effectiveness of HFNO verses FMV in special surgical populations, such as patients with hypoxemia, patients with difficult airway and pediatric patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files]. All data are from EMBASE, PubMed and Cochrane library databases (https://www.elsevier.com/solutions/embase-biomedical-research; https://pubmed.ncbi.nlm.nih.gov/advanced/; https://www.cochranelibrary.com/).
Abbreviations
- HFNO:
-
High flow nasal oxygen
- RCTs:
-
Randomized controlled trails
- FMV:
-
Facemask ventilation
- PaO2 :
-
Arterial oxygen partial pressure
- EtO2 :
-
End expiratory oxygen concentration
- SpO2 min :
-
Minimum pulse oxygen saturation
- EtCO2 :
-
End expiratory carbon dioxide
- PaCO2 :
-
Arterial carbon dioxide partial pressure
- MD:
-
Mean difference;
- RR:
-
Risk ratio
- 95%CI:
-
Confidence interval of 95%
- ICU:
-
Intensive care unit
References
Woodall NM, Cook TM. National census of airway management techniques used for anaesthesia in the UK: first phase of the Fourth National Audit Project at the Royal College of Anaesthetists. Br J Anaesth. 2011. https://doi.org/10.1093/bja/aeq339.
De Jong A, Rolle A, Molinari N, Paugam-Burtz C, Constantin JM, Lefrant JY, Asehnoune K, Jung B, Futier E, Chanques G, Azoulay E, Jaber S. Cardiac Arrest and Mortality Related to Intubation Procedure in Critically Ill Adult Patients: A Multicenter Cohort Study. Crit Care Med. 2018. https://doi.org/10.1097/CCM.0000000000002925.
Frerk C, Mitchell VS, McNarry AF, Mendonca C, Bhagrath R, Patel A, O’Sullivan EP, Woodall NM, Ahmad I; Difficult Airway Society intubation guidelines working group. Difficult Airway Society. guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth. 2015;2015. https://doi.org/10.1093/bja/aev371.
Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: Physiologic Basis, Benefits, and Potential Risks. Anesth Analg. 2017. https://doi.org/10.1213/ANE.0000000000001589.
Wong DT, Yee AJ, Leong SM, Chung F. The effectiveness of apneic oxygenation during tracheal intubation in various clinical settings: a narrative review. Can J Anaesth. 2017. https://doi.org/10.1007/s12630-016-0802-z.
Patel A, Nouraei SA. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015. https://doi.org/10.1111/anae.12923.
Nishimura M. High-flow nasal cannula oxygen therapy in adults. J Intensive Care. 2015. https://doi.org/10.1186/s40560-015-0084-5.
Riva T, Meyer J, Theiler L, Obrist D, Bütikofer L, Greif R, Nabecker S. Measurement of airway pressure during high-flow nasal therapy in apnoeic oxygenation: a randomised controlled crossover trial. Anaesthesia. 2021. https://doi.org/10.1111/anae.15224.
Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, Pesenti A. Physiologic Effects of High-Flow Nasal Cannula in Acute Hypoxemic Respiratory Failure. Am J Respir Crit Care Med. 2017. https://doi.org/10.1164/rccm.201605-0916OC.
Hasani A, Chapman TH, McCool D, Smith RE, Dilworth JP, Agnew JE. Domiciliary humidification improves lung mucociliary clearance in patients with bronchiectasis. Chron Respir Dis. 2008. https://doi.org/10.1177/1479972307087190.
Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011. https://doi.org/10.1093/bja/aer265.
Spence EA, Rajaleelan W, Wong J, Chung F, Wong DT. The Effectiveness of High-Flow Nasal Oxygen During the Intraoperative Period: A Systematic Review and Meta-analysis. Anesth Analg. 2020. https://doi.org/10.1213/ANE.0000000000005073.
Osman Y.M., Abd El-Raof R. High flow nasal cannula oxygen preventing deoxygenation during induction of general anaesthesia in caesarean section: A randomized controlled trial. Trends Anae Crit Care. 2021;40:23. https://doi.org/10.1016/j.tacc.
Hua Z, Liu Z, Li Y, Zhang H, Yang M, Zuo M. Transnasal humidified rapid insufflation ventilatory exchange vs. facemask oxygenation in elderly patients undergoing general anaesthesia: a randomized controlled trial. Sci Rep. 2020;10:5745. https://doi.org/10.1038/s41598-020-62716-2.
Sjöblom A, Broms J, Hedberg M, Lodenius Å, Furubacke A, Henningsson R, Wiklund A, Nabecker S, Theiler L, Jonsson Fagerlund M. Pre-oxygenation using high-flow nasal oxygen vs. tight facemask during rapid sequence induction. Anaesthesia. 2021;76:1176. https://doi.org/10.1111/anae.15426.
Rosén J, Frykholm P, Fors D. High-flow nasal cannula versus face mask for preoxygenation in obese patients: A randomised controlled trial. Acta Anaesthesiol Scand. 2021;65:1381. https://doi.org/10.1111/aas.13960 Epub ahead of print.
Tremey B, Squara P, De Labarre H, Ma S, Fischler M, Lawkoune JD, Le Guen M. Hands-free induction of general anesthesia: a randomised pilot study comparing usual care and high-flow nasal oxygen. Minerva Anestesiol. 2020. https://doi.org/10.23736/S0375-9393.20.14456-0.
Lyons C, McElwain J, Coughlan MG, O’Gorman DA, Harte BH, Kinirons B, Laffey JG, Callaghan M. Pre-oxygenation with facemask oxygen vs high-flow nasal oxygen vs high-flow nasal oxygen plus mouthpiece: a randomised controlled trial. Anaesthesia. 2021;77:40. https://doi.org/10.1111/anae.15556 Epub ahead of print.
Zhou S, Zhou Y, Cao X, Ni X, Du W, Xu Z, Liu Z. The efficacy of high flow nasal oxygenation for maintaining maternal oxygenation during rapid sequence induction in pregnancy: A prospective randomised clinical trial. Eur J Anaesthesiol. 2020. https://doi.org/10.1097/EJA.0000000000001395.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009. https://doi.org/10.1371/journal.pmed.1000100.
Wong DT, Dallaire A, Singh KP, Madhusudan P, Jackson T, Singh M, Wong J, Chung F. High-Flow Nasal Oxygen Improves Safe Apnea Time in Morbidly Obese Patients Undergoing General Anesthesia: A Randomized Controlled Trial. Anesth Analg. 2019. https://doi.org/10.1213/ANE.0000000000003966.
Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017. https://doi.org/10.1111/anae.13799.
Ng I, Krieser R, Mezzavia P, Lee K, Tseng C, Douglas N, Segal R. The use of Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) for pre-oxygenation in neurosurgical patients: a randomised controlled trial. Anaesth Intensive Care. 2018. https://doi.org/10.1177/0310057X1804600403.
Lodenius Å, Piehl J, Östlund A, Ullman J, Jonsson Fagerlund M. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. 2018;73:564. https://doi.org/10.1111/anae.14215.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. www.handbook.cochrane.org.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014. https://doi.org/10.1186/1471-2288-14-135.
Hanouz JL, Lhermitte D, Gérard JL, Fischer MO. Comparison of pre-oxygenation using spontaneous breathing through face mask and high-flow nasal oxygen: A randomised controlled crossover study in healthy volunteers. Eur J Anaesthesiol. 2019. https://doi.org/10.1097/EJA.0000000000000954.
Heinrich S, Horbach T, Stubner B, Prottengeier J, Irouschek A, Schmidt J. Benefits of Heated and Humidified High Flow Nasal Oxygen for Preoxygenation in Morbidly Obese Patients Undergoing Bariatric Surgery: A Randomized Controlled Study. J Obes Bariatrics. 2014. https://doi.org/10.13188/2377-9284.1000003.
Shippam W, Preston R, Douglas J, Taylor J, Albert A, Chau A. High-flow nasal oxygen vs. standard flow-rate facemask pre-oxygenation in pregnant patients: a randomised physiological study. Anaesthesia. 2019;74:450. https://doi.org/10.1111/anae.14567.
Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre-optic intubation using a high-flow nasal oxygen-delivery system. Br J Anaesth. 2015. https://doi.org/10.1093/bja/aev262.
Renda T, Corrado A, Iskandar G, Pelaia G, Abdalla K, Navalesi P. High-flow nasal oxygen therapy in intensive care and anaesthesia. Br J Anaesth. 2018. https://doi.org/10.1016/j.bja.2017.11.010.
Helviz Y, Einav S. A Systematic Review of the High-flow Nasal Cannula for Adult Patients. Crit Care. 2018. https://doi.org/10.1186/s13054-018-1990-4.
To K, Harding F, Scott M, Milligan P, Nixon IJ, Adamson R, McNarry AF. The use of Transnasal Humidified Rapid-Insufflation Ventilatory Exchange in 17 cases of subglottic stenosis. Clin Otolaryngol. 2017. https://doi.org/10.1111/coa.12921.
Bharathi MB, Kumar MRA, Prakash BG, Shetty S, Sivapuram K, Madhan S. New Visionary in Upper Airway Surgeries-THRIVE, a Tubeless Ventilation. Indian J Otolaryngol Head Neck Surg. 2021. https://doi.org/10.1007/s12070-021-02491-2.
Piosik ZM, Dirks J, Rasmussen LS, Kristensen CM, Kristensen MS. Exploring the limits of prolonged apnoea with high-flow nasal oxygen: an observational study. Anaesthesia. 2021. https://doi.org/10.1111/anae.15277.
Booth AWG, Vidhani K, Lee PK, Coman SH, Pelecanos AM, Dimeski G, Sturgess DJ. The Effect of High-Flow Nasal Oxygen on Carbon Dioxide Accumulation in Apneic or Spontaneously Breathing Adults During Airway Surgery: A Randomized-Controlled Trial. Anesth Analg. 2021. https://doi.org/10.1213/ANE.0000000000005002.
Guitton C, Ehrmann S, Volteau C, Colin G, Maamar A, Jean-Michel V, Mahe PJ, Landais M, Brule N, Bretonnière C, Zambon O, Vourc’h M. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive Care Med. 2019. https://doi.org/10.1007/s00134-019-05529-w.
Doyle AJ, Stolady D, Mariyaselvam M, Wijewardena G, Gent E, Blunt M, Young P. Preoxygenation and apneic oxygenation using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange for emergency intubation. J Crit Care. 2016. https://doi.org/10.1016/j.jcrc.2016.06.011.
Vourc’h M, Asfar P, Volteau C, Bachoumas K, Clavieras N, Egreteau PY, Asehnoune K, Mercat A, Reignier J, Jaber S, Prat G, Roquilly A, Brule N, Villers D, Bretonniere C, Guitton C. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015. https://doi.org/10.1007/s00134-015-3796-z.
Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S. High-Flow Nasal Cannula Versus Bag-Valve-Mask for Preoxygenation Before Intubation in Subjects With Hypoxemic Respiratory Failure. Respir Care. 2016. https://doi.org/10.4187/respcare.04413.
Chaudhuri D, Granton D, Wang DX, Einav S, Helviz Y, Mauri T, Ricard JD, Mancebo J, Frat JP, Jog S, Hernandez G, Maggiore SM, Hodgson C, Jaber S, Brochard L, Burns KEA, Rochwerg B. Moderate Certainty Evidence Suggests the Use of High-Flow Nasal Cannula Does Not Decrease Hypoxia When Compared With Conventional Oxygen Therapy in the Peri-Intubation Period: Results of a Systematic Review and Meta-Analysis. Crit Care Med. 2020. https://doi.org/10.1097/CCM.0000000000004217.
Acknowledgements
Not applicable.
Funding
This research was funded by the Key R&D Project of Jilin Province Science and Technology Development Program (No. 202020404165YY).
Author information
Authors and Affiliations
Contributions
Jian-li Song made substantial contributions conception and design of the study; Jian-li Song and Yan Sun searched and screened literature; Yu-bo Shi, Xiao-yin Liu and Zhen-bo Su extracted data from the collected literature and analyzed the data; Jian-li Song and Yan Sun wrote the manuscript; Yu-bo Shi, Xiao-yin Liu and Zhen-bo Su revised the manuscript; all the authors approved the final version of manuscript. Jian-li Song and Yan Sun contributed equally to this work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No patients or members of public were involved in the present study. No patients were asked to advise on the interpretation or writing up of results.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA checklist.
Additional
file 2. S1: Funnel plot of PaO2. S2: Funnel plot of CO2 accumulation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, Jl., Sun, Y., Shi, Yb. et al. Comparison of the effectiveness of high-flow nasal oxygen vs. standard facemask oxygenation for pre- and apneic oxygenation during anesthesia induction: a systematic review and meta-analysis. BMC Anesthesiol 22, 100 (2022). https://doi.org/10.1186/s12871-022-01615-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-022-01615-7