Abstract
Background
Perioperative neurocognitive disorders (PND) is a common postoperative complication including postoperative delirium (POD), postoperative cognitive decline (POCD) or delayed neurocognitive recovery. It is still controversial whether the use of intraoperative cerebral function monitoring can decrease the incidence of PND. The purpose of this study was to evaluate the effects of different cerebral function monitoring (electroencephalography (EEG) and regional cerebral oxygen saturation (rSO2) monitoring) on PND based on the data from randomized controlled trials (RCTs).
Methods
The electronic databases of Ovid MEDLINE, PubMed, EMBASE, Cochrane Library database were systematically searched using the indicated keywords from their inception to April 2020. The odds ratio (OR) or mean difference (MD) with 95% confidence interval (CI) were employed to analyze the data. Heterogeneity across analyzed studies was assessed with chi-square test and I2 test.
Results
Twenty two RCTs with 6356 patients were included in the final analysis. Data from 12 studies including 4976 patients were analyzed to assess the association between the EEG-guided anesthesia and PND. The results showed that EEG-guided anesthesia could reduce the incidence of POD in patients undergoing non-cardiac surgery (OR: 0.73; 95% CI: 0.57–0.95; P = 0.02), but had no effect on patients undergoing cardiac surgery (OR: 0.44; 95% CI: 0.05–3.54; P = 0.44). The use of intraoperative EEG monitoring reduced the incidence of POCD up to 3 months after the surgery (OR: 0.69; 95% CI: 0.49–0.96; P = 0.03), but the incidence of early POCD remained unaffected (OR: 0.61; 95% CI: 0.35–1.07; P = 0.09). The remaining 10 studies compared the effect of rSO2 monitoring to routine care in a total of 1380 participants on the incidence of PND. The results indicated that intraoperative monitoring of rSO2 could reduce the incidence of POCD (OR 0.53, 95% CI 0.39–0.73; P < 0.0001), whereas no significant difference was found regarding the incidence of POD (OR: 0.74; 95% CI: 0.48–1.14; P = 0.17).
Conclusions
The findings in the present study indicated that intraoperative use of EEG or/and rSO2 monitor could decrease the risk of PND.
Trial registration
PROSPREO registration number: CRD42019130512.
Similar content being viewed by others
Background
Protecting brain functions is one of the essences of anesthesia practice. There is an increasing concern about the potential effects of anesthetics on perioperative neurocognitive disorders (PND) [1]. PND is a common complication after major surgeries including postoperative delirium (POD), postoperative cognitive decline (POCD) and delayed neurocognitive recovery. Its incidence rate ranges from 10 to 50% in general population. In high-risk patients, the incidence rate could reach as high as 50–70% [2,3,4]. PND is associated with several poor prognosis, such as higher mortality, long-term cognitive decline, dementia, re-admission and prolonged length of hospitalization. It also increases the financial burdens to the public, reaching up to $ 16 billion for US health care cost every year [5,6,7,8,9,10].
It has been revealed by several studies that the risk of PND is increased by either that excessively deep anesthesia or lower level of regional cerebral oxygen saturation during the operation [11,12,13]. These findings provided evidence to support the necessities of maintaining proper depth of anesthesia and enhancing cerebral perfusion, therefore increasing the level of cerebral oxygen saturation. Various monitoring technologies have become available to monitor the cerebral function. For example, electroencephalography (EEG) is a commonly used method to monitor the depth of anesthesia [14]. Regional cerebral oxygen saturation (rSO2) monitoring detected by near infrared reflected spectroscopy (NIRS) can be used to monitor cerebral saturation and to alert cerebral ischemia [15]. Previous meta-analysis indicated that the use of cerebral monitors during surgery correlated with a reduced risk of PND [16, 17]. However, this conclusion appeared to be controversial as some recently published large randomized controlled trials showed that the use of cerebral monitors didn’t benefit the reduction of PND incidence after major surgeries [18, 19].
To better understand the effects of cerebral function monitoring on PND and to provide clearer guidance to clinicians, we conducted this systematic review to investigate the relationship between intraoperative cerebral function monitoring and the adverse clinical outcomes.
Methods
This review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement (PRISMA) guidelines [20]. This systematic review and meta-analysis had been registered in the international prospective register of systematic reviews (CRD42019130512 https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=130512).
Search strategy
Two investigators (DL and DXC) performed a systematic search in the databases of Ovid MEDLINE, PubMed, EMBASE, Cochrane Library database, and other databases updated to April 2020. The searching keywords included “cerebral monitoring”, “electroencephalography”, “cerebral oxygenation”, “postoperative delirium”, “postoperative cognitive decline”, and “randomized controlled trial”. The search terms were modified for each database. Any conflict about search results between the two investigators (DL and DXC) was resolved by discussion and the consensus was reached. The literature search strategy is provided in Additional file 1: Material 1.
Eligibility criteria
Prior to the systematic review and meta-analysis, the inclusion criteria were predetermined by all authors. Inclusion criteria were as the following: (1) the study was randomized controlled trial (RCT), regardless of the language and status; (2) included patients were adults aged 18 years or older who underwent general anesthesia for surgery; (3) the incidence of PND under the EEG or rSO2 monitoring was compared to the PND outcome without the usage of EEG or rSO2 monitoring in the study; (4) the occurrence of PND evaluated by validated scale was reported in the study. The exclusion criteria were: (1) non-randomized studies; (2) non full-text studies; (3) ongoing studies; (4) the outcome data could not be extracted and used to analyze.
Data collection and quality assessment
Data was extracted by two investigators (DL and DXC) independently using a standardized form based on the Population Interventions Comparisons Outcomes (PICO) approach. The extracted information included the first author, year of publication, study design, sample size, outcome variables and assessment scale, summative results and conclusion. The methodological quality of the included studies was with using the Cochrane risk of bias scale, which contains seven specified domains [21]. Risk of bias were classified as high, low or unclear for each item. The methodological quality assessment was conducted by two investigators independently, and the occurred conflicts were resolved by a third investigator (QL) referring to the original article, if any.
Statistical analysis
Data analyses were performed using the Review Manager (version 5.3) software. The inspection level for the pooled data were two-sided, and P < 0.05 was regarded as statistically significant. The odds ratio (OR) and mean difference (MD) with 95% confidence interval (CI) were employed to analyze the categories and continuous data. Heterogeneity across studies was assessed with chi-square test and I2 test, and I2 > 50% or P < 0.10 was considered as significantly heterogenous. The random-effect model was adopted if the heterogeneity existed among the studies, whereas the fixed-effect model was applied if no significant heterogeneity was detected. Sensitivity analysis was conducted to assess the impact of single study to the overall analysis [22,23,24,25]. Publication bias was assessed by using the funnel plot test.
Results
Literature search
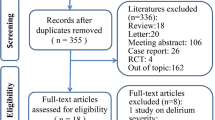
The initial search in PubMed, Ovid, EMBASE, Cochrane library, and other databases identified 3309 reports. Duplicates removal reduced the number of reports to 2630. Then, 2589 studies were further excluded after reviewing the title and abstracts. The full text of the remaining 41 studies were retrieved for evaluation, 19 out of the 41 studies were further excluded due to one or more of the following reasons: not RCT (n = 2); review (n = 6); non-general anesthesia patients (n = 3); or other studies which data could not be extracted or used to analyze (n = 8). Reviewing the reference lists of the retrieved studies did not identify any new eligible study. Finally, 22 RCTs were included in the present this review [18, 19, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. A flow diagram illustrating the literature search and trials screening process was shown in Fig. 1.
Characteristics of included studies
As listed in Table 1, a total number of 6356 patients included in the 22 RCTs were enrolled in this meta-analysis [18, 19, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Among these 22 RCTs, 10 studies used EEG as the guide for anesthesia depth [18, 26,27,28,29,30,31,32,33,34], 10 studies evaluated the effects of rSO2 monitoring on PND [19, 35,36,37,38,39,40,41,42,43] and the last 2 trials deployed both of the two monitoring [44, 45]. Ten out of the 22 trials included patients who underwent cardiac surgery [18, 19, 27, 36, 38,39,40,41, 43, 45], whereas the other 12 studies were conducted in among patients undergoing non-cardiac major surgery, including abdominal surgery, ENT surgery, hip fracture repair surgeries and others [26, 28,29,30,31,32,33,34, 37, 39, 42, 44]. The risk of bias of included studies was assessed and the result was shown in Fig. 2. Two studies were at the high level in terms of risk of bias. One of them was lacking of the methods of allocation and blinding [33], and the other had a high dropout rate [44]. Eleven studies [19, 30, 32, 34,35,36, 38, 40,41,42,43] were at unclear risk of bias due to the unclear blinding of outcome assessments (detection bias) or unclear blinding of participants and study personnel (performance bias). The remaining 9 studies rated as low in terms of the risk of bias.
Cerebral functional monitoring and perioperative neurocognitive disorders (PND)
Electroencephalography (EEG) guided anesthesia
Postoperative delirium (POD)
After pooling and analyzing the data from the 10 studies using EEG to guide the depth of anesthesia (n = 4451, EEG monitoring = 2214, routine care = 2237), it is noticed that in general, the EEG-guided anesthesia group had a reduced risk of POD compared to the group of routine care (OR: 0.75; 95% CI: 0.60–0.93; P = 0.008) (Fig. 3a) with a significant, heterogeneity detected among the included studies (P = 0.004, I2 = 61%). Then we divided the participants into non-cardiac or cardiac subgroups according to the types of surgeries that patients received and re-analyzed the effect of EEG on the risk of POD. The results demonstrated that in the non-cardiac surgery subgroup (8 studies, n = 3600, EEG monitoring = 1793, non-EEG group = 1807), the use of EEG-guided anesthesia correlated with a reduction of POD incidence (OR: 0.73; 95% CI: 0.57–0.95; P = 0.02), whereas in the cardiac surgery subgroup, no correlation was detected between the use of EEG and the reduction of POD incidence (2 studies, n = 541, EEG monitoring = 272, non-EEG group = 269; OR: 0.44; 95% CI: 0.05–3.54; P = 0.44). It is noted that the study conducted by Whitlock et al. was excluded from the subgroup analysis because it included both cardiac surgery and thoracic surgery without detailed information on the number of patients in the each subgroup. The funnel plot demonstrated that no publication bias existed among the included studies (Fig. 3b).
Postoperative cognitive decline (POCD)
Four studies (n = 2435, EEG monitoring = 1200, routine care = 1235) reported the incidence of POCD within 1–7 days after surgery after non-cardiac surgery, and three of them also reported the incidence of POCD 3 months after the surgery (n = 2047, EEG monitoring = 1011, routine care = 1036). It was found that EEG-guided anesthesia did not reduce the incidence of POCD in the early postoperative stage (OR: 0.61; 95% CI: 0.35–1.07; P = 0.09). However, the incidence of POCD 3 months after the surgery was reduced upon the use of intraoperative EEG monitoring (OR: 0.69; 95% CI: 0.49–0.96; P = 0.03). Only one study by Ballard and his colleagues, reported the incidence of POCD at the time of 1 year postoperatively (n = 59, EEG monitoring = 27, routine care = 32), but it did not suggest the advantages of EEG monitoring with respect to the incidence of POCD (OR: 0.27; 95% CI: 0.03–2.57; P = 0.25) (Fig. 4).
Regional cerebral oxygen saturation (rSO2) monitoring
Postoperative delirium (POD)
Four studies including 765 patients undergoing cardiac surgery reported the outcome of incidence of POD between the rSO2 monitoring group (n = 378) and the routine care group (n = 387). All the four studies showed no difference in terms of POD between the two groups. Our meta-analysis also revealed a comparable result about the incidence of POD between the cerebral oxygenation monitoring group and the routine care group (OR: 0.74; 95% CI: 0.48–1.14; P = 0.17) (Fig. 5).
Postoperative cognitive decline (POCD)
Seven studies involving 805 patients analyzed the effect of rSO2 monitoring on the incidence of POCD after major surgeries compared to that of routine care (n = 805, rSO2 monitoring group, n = 411; routine care, n = 394). Among these 7 studies, Slater et al. focused on cardiac surgery [43], while Cox and his colleagues reported the result of their work in non-cardiac surgery [37]. Despite the different types of surgeries studied, both groups reported no difference found between the rSO2 monitoring group and the routine care group in terms of the incidence of POCD. However, the remaining five trials, three of which conducted in patients undertaking cardiac surgeries [36, 39, 40] and two of which performed in patients undertaking non-cardiac surgeries [35, 42], reported that the incidence of POCD in the routine care group was significantly higher than that of rSO2 monitoring group. Our meta-analysis also revealed a significant lower incidence of the POCD in the rSO2 monitoring group (OR: 0.53, 95% CI 0.39–0.73; P < 0.0001) (Fig. 6) compared to that of routine care group without heterogeneity detected (P = 0.19; I2 = 32%).
Discussion
In the present systematic review and meta-analysis, data of 6356 patients from 22 RCTs was analyzed, including 3169 patients who received EEG or/and rSO2 monitoring, and 3187 patients who received routine care. In non-cardiac surgery patients, we found the incidence of POD significantly decreased in EEG-guided anesthesia group compared to that of routine care group. Both EEG-guided anesthesia and rSO2 monitoring were correlated with a significant lower incidence of POCD despite the types of surgery.
EEG-guided anesthesia
Twenty studies including 4976 patients assessed the association between the EEG-guided anesthesia and PND [18, 26,27,28,29,30,31,32,33,34]. Our meta-analysis showed that EEG-guided anesthesia could reduce the incidence of POD in patients undergoing non-cardiac surgeries but not cardiac surgery patients. In addition, deployment of intraoperative EEG monitoring could reduce the incidence of POCD up to 3 months after the surgery, but had no effect on the incidence of early POCD.
Prior to our study, Punjasawadwong et al. [46] and Kristen et al. [16] have performed two meta-analyses separately, each included 3 RCTs (n = 2197) and 5 RCTs (n = 2654) respectively, to evaluate the impact of EEG monitoring on POD and POCD. These two meta-analyses both reported that the EEG-guided anesthesia could reduce the incidence of POD. However, Kristen et al. did not draw a conclusion on whether EEG monitoring affect POCD due to the high heterogeneity among their included RCTs [16]. Both studies pointed out that the quality of the research evidence was moderate, and further studies should be required to clarify whether the appropriate cerebral function monitoring during surgery can reduce the incidence of PND. Recently, several studies have further explore this important issue. It is worth noting that a large-sample RCT (n = 1213) conducted by Wildes and his colleagues proposed that EEG-guided anesthesia cannot reduce the incidence of POD [18], which is inconsistent with the results from previous large-sample studies [29, 30]. In 2013, Chan et al. performed an RCT including 902 patients and revealed that the incidence rate of PND was lower in patients receiving EEG-guided anesthesia than that in patients receiving routine care [29]. In addition, Radtke and his colleagues analyzed data extracted from 1155 patients and concluded that EEG monitoring correlated with a significant reduction of POD incidence and a decreasing tendency on incidence of POCD [30]. The discrepant findings among the above-mentioned studies may attribute to the differences of their methodology and the heterogeneity of the studied population. Compared to the studies by Chan et al. or Radtke et al. [29, 30], the study conducted by Wildes et al. [18] included patients with more severe conditions as more than 30% of the patients in Wildes’ study had ASA ≥ 3, or had a history of falls, or planned cardiothoracic surgery, which might partially eliminate the effect of EEG on POD [47,48,49]. In our study, a similar conclusion that using EEG cannot reduce the incidence of POD was drawn in the subgroup of patients who underwent cardiac surgeries. For these high-risk patients, it is recommended by several clinical practice guidelines that a multi-component strategy is required to prevent the incidence of POD, indicating that a single approach of monitoring has a limited role in preventing the high-risk patients from POD [50, 51]. In our study, heterogeneity among included studies existed throughout the analysis except the subgroup analysis. Further large-scale RCTs should be conducted to confirm the conclusion.
The underlying mechanisms of the POD prevention by EEG monitoring remains unclear. One hypothesis is that the use of EEG monitoring makes it possible to avoid excessively anesthesia, therefore to specifically reduce the incidence and cumulative duration of intraoperative burst suppression. Previous studies have shown that burst suppression is an independent risk factor of POD [52, 53]. In addition, Hesse et al. have demonstrated that every incidence of burst suppression during the anesthesia maintenance was associated with a 75% increase in odds of POD [54]. Furthermore, higher incidence or longer duration of burst suppression are significantly associated with the incidence of POD [55, 56]. On the other hand, the use of EEG monitoring also reduced the dosage of general anesthetics, such as volatile agents and propofol [57, 58]. Previous studies have reported that excessively exposure to potent volatile agents might increase the incidence of POD [59]. Particularly, most of these studies were performed in geriatric patients whose aging brains were more sensitive to anesthetic agents, therefore, were more likely to experience the burst suppression and POD [60, 61].
Regional cerebral oxygenation monitoring
1380 participants from 10 studies comparing the effect of rSO2 monitoring to routine care was included in our study [19, 35,36,37,38,39,40,41,42,43]. The results indicated that intraoperative monitoring of cerebral oxygenation could reduce POCD, but have no effect on POD.
Prior to our research, Yu et al. conducted a meta-analysis to evaluate the impact of cerebral near infrared reflected spectroscopy (NIRS) monitoring on the following clinical outcomes, including cerebral oxygen desaturation events, neurological outcomes, non-neurological outcomes and socioeconomic impact. The results from the study suggested that the effects of rSO2 monitoring on POCD or POD are uncertain due to the low quality of the evidence and high heterogeneity among included studies [17].
Since the total amount of oxygen consumed by the brain is about 20% of body oxygen supply, the cerebral function is extremely vulnerable to hypoxemia. A study found that 50 to 75% of patients undergoing cardiac surgery experienced once or more rSO2 desaturations during cardiopulmonary bypass (CPB) [62], and that prolonged low rSO2 values have been associated with significantly higher risks of POCD [63, 64]. Evidence indicates that perioperative rSO2 levels below a certain level (50–60%) are associated with an increase in neurological complications and an increase in mortality. It also suggested not to interpret rSO2 levels based on absolute values rather than follow the trend analysis instead, by interpreting the relative changes of rSO2 levels with respect to an individual baseline value [65]. Therefore, rSO2 monitoring has been used to mitigate the cerebral oxygen desaturation during surgery.
NIRS is an emerging noninvasive technique of monitoring brain oxygenation and increasingly being used in various clinical settings. This provides an opportunity for early recognition of imbalances of oxygen delivery and consumption and rSO2 desaturation [66]. Clinicians can take more active treatment measures to prevent prolonged rSO2 desaturation, thereby avoiding neurological and other major complications. However, the clinical benefits of this technology have been questioned. In a multicenter prospective randomized study conducted by Deschamps et al. which included 201 patients [38], the authors found that NIRS-guided intervention can prevented the decreases of rSO2 in cardiac surgery but did not reduce the incidence of PND. These findings were consistent with two single-center RCTs conducted by Lei et al. and Slater et al. respectively [41, 43]. Although our meta-analysis suggest that rSO2 monitoring can reduce POCD, the quality of the included studies is not uniform, and the definition and evaluation methods of POCD were also different. Therefore, further and larger multi-center RCTs are needed to confirm our conclusions.
In our meta-analysis, there were two studies evaluated the effects of the combination of combined use of EEG monitoring and regional cerebral saturation monitoring on reducing PND [44, 45]. The RCT conducted by Ballard and colleagues showed that the use of EEG and rSO2 monitor can significantly reduce the incidence of POCD at1, 12 and 52 weeks postoperatively [44]. But another study conducted by Kunst et al. found that in elderly patients undergoing coronary artery bypass graft surgery (CABG), the combined use of EEG and rSO2 monitoring can reduce the incidence of POD rather than POCD [45]. Nevertheless, It was reported in both studies suggested that the combined use of EEG and rSO2 monitoring reduced the incidence of PND. This result suggested that combination of multiple monitoring approaches is better than one.
NIRS guided rSO2 monitoring can provide clinicians with information on the quality of cerebral oxygen delivery. However, NIRS cannot reflect brain function. EEG can provided objective measures assessing brain cortical function according to differently dynamic waveform, and the generation of electrical activity requires adequate cerebral perfusion and cerebral oxygen. Previous studies showed the observed EEG pattern consisted only of voltage suppression at rSO2 level was less than 20%, while the delta background activity was seen at rSO2 level was greater than 40%. The emergence of delta background activity may be a sign of cortical functional recovery [67]. In addition, Horst et al. investigated the relationship between rSO2 and EEG in preterm infants. The authors concluded that there is a significant relationship between electrocortical activity and oxygen consumption as cerebral oxygen metabolism increases with increasing EEG amplitude [68]. So by the combination of EEG data and NIRS data, the clinicians were able to potentially assess the impact of cerebral oxygen delivery on cortical function as determined via EEG patterns [67]. Both the increased brain oxygen consumption and the decreased brain electrical activity reflect the compromised cerebral oxygen delivery or perfusion, and early warning and intervention may prevent the neurological impairment. Although the potential mechanism of rSO2 on EEG under anesthesia has not been clarified, considering the possibility of intraoperative burst suppression caused by excessive anesthesia and cerebral oxygen desaturation caused by insufficient perfusion, the collaborative application of both monitoring may provide benefits for reducing the incidence of PND.
Several limitations of this meta-analysis should be acknowledged: (1) smaller number of included trials and less solidarity of the results due to the absence of adjustment variables such as age, gender and the type of surgery; (2) there were different scales to assess the incidence of POD or POCD in our included studies, such as CAM/CAM-ICU, DSM-IV, MMSE and other scales which had different specificity and sensitivity. However, we carefully read the definitions of POD and POCD in each original text to extract the data with as little heterogeneity as possible; (3) this study is based on the published articles, the publication bias is inevitable; (4) the analysis of this study is based on data at study-level, whereas the original data from individual patients was not available.
Conclusions
In conclusion, the findings of our study indicated that the use of EEG or/and rSO2 monitor correlated with a lower risk of PND. Based on this, we recommend intraoperative EEG or/and rSO2 monitoring during surgery to decrease the risk of PND. In addition, for high-risk patients, multiple monitoring approaches should be combined to optimize the anesthesia management and to prevent the incidence of PND. Further research should be conducted to verify the identified correlation between the use of EEG or/and rSO2 monitoring and PND.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PND:
-
Perioperative Neurocognitive Disorders
- EEG:
-
Electroencephalography
- POD:
-
Postoperative delirium
- rSO2 :
-
Regional cerebral oxygen saturations
- POCD:
-
Postoperative cognitive decline
- NIRS:
-
Near infrared reflected spectroscopy
- PICO:
-
Population Interventions Comparisons Outcomes
- ETAC:
-
End-tidal anesthetic concentration
- ENT:
-
Ear, nose, and throat
- CABG:
-
Coronary artery bypass graft surgery
- CAM:
-
Confusion assessment method
- DSM IV:
-
Diagnostic and Statistical Manual of Mental Disorders
- MMSE:
-
Mini mental state examination
- CPB:
-
Cardiopulmonary bypass
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- RCTs:
-
Randomized controlled trials
- MD:
-
Mean difference
References
Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Nomenclature Consensus Working Group. Recommendations for the Nomenclature of Cognitive Change Associated With Anaesthesia and Surgery-2018. Anesth Analg. 2018;127:1189–95. https://doi.org/10.1097/ALN.0000000000002334..
Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin. 2015;33:505–16. https://doi.org/10.1016/j.anclin.2015.05.007.
Iamaroon A, Wongviriyawong T, Sura-Arunsumrit P, et al. Incidence of and risk factors for postoperative delirium in older adult patients undergoing non-cardiac surgery: a prospective study.[J]. BMC Geriatr. 2020;20:40. https://doi.org/10.1186/s12877-020-1449-8.
Brown CH. Delirium in the cardiac surgical ICU. Curr Opin Anaesthesiol. 2014;27:117–22. https://doi.org/10.1097/ACO.0000000000000061.
Tropea J, LoGiudice D, Liew D, et al. Poorer outcomes and greater healthcare costs for hospitalised older people with dementia and delirium: a retrospective cohort study. Int J Geriatr Psychiatry. 2017;32:539–47. https://doi.org/10.1002/gps.4491.
Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–51. https://doi.org/10.1001/jama.2010.1013.
Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–8. https://doi.org/10.1097/SLA.0b013e31818e4776.
Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–9. https://doi.org/10.1056/NEJMoa1112923.
Tosun Tasar P, Sahin S, Akcam NO, et al. Delirium is associated with increased mortality in the geriatric population. Int J Psychiatry Clin Pract. 2018;22:200–5. https://doi.org/10.1080/13651501.2017.1406955.
Jackson P, Khan A. Delirium in critically ill patients. Crit Care Clin. 2015;31:589–603. https://doi.org/10.1016/j.ccc.2015.03.011.
Lu X, Jin X, Yang S, Xia Y. The correlation of the depth of anesthesia and postoperative cognitive impairment: a meta-analysis based on randomized controlled trials. J Clin Anesth. 2018;45:55–9. https://doi.org/10.1016/j.jclinane.2017.12.002.
Eertmans W, De DC, Genbrugge C, Marcus B, Bouneb S, Beran M. Association between postoperative delirium and postoperative cerebral oxygen desaturation in older patients after cardiac surgery. Br J Anaesth. 2019. https://doi.org/10.1016/j.bja.2019.09.042.
Momeni M, Meyer S, Docquier MA, Lemaire G, Kahn D, Khalifa C, et al. Predicting postoperative delirium and postoperative cognitive decline with combined intraoperative electroencephalogram monitoring and cerebral near-infrared spectroscopy in patients undergoing cardiac interventions. J Clin Monit Comput. 2019;33(6):999–1009. https://doi.org/10.1007/s10877-019-00253-8.
Fahy Brenda G, Chau Destiny F. The Technology of Processed Electroencephalogram Monitoring Devices for Assessment of Depth of Anesthesia [J]. Anesth Analg. 2018;126:111–7. https://doi.org/10.1213/ANE.0000000000002331.
Murkin John M. Cerebral oximetry: monitoring the brain as the index organ.[J]. Anesthesiology. 2011;114:12–3. https://doi.org/10.1097/ALN.0b013e3181fef5d2.
MacKenzie KK, Britt-Spells AM, Sands LP, et al. Processed Electroencephalogram Monitoring and Postoperative Delirium: A Systematic Review and Meta-analysis [J]. Anesthesiology. 2018;129:417–27. https://doi.org/10.1097/ALN.0000000000002323.
Yu Y, Zhang K, Zhang L, Zong H, Meng L, Han R. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst Rev. 2018;1:Cd010947.
Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA. 2019;321:473–83. https://doi.org/10.1001/jama.2018.22005.
Uysal S, Lin HM, Trinh M, Park CH, Reich DL. Optimizing cerebral oxygenation in cardiac surgery: A randomized controlled trial examining neurocognitive and perioperative outcomes. J Thoracic Cardiovasc Surg. 2020;159(3):943–53 e3.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Higgins J, Altman DG. Assessing risk of bias in included studies. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, England: Cochrane Collaboration; 2008. chap 8.
Deeks JJ, Higgins JPT, Altman DG. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford, UK: The Cochrane Collaboration; 2008. chap 9.
Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;47:15–7.
Jildenstål PK, Hallén JL, Rawal N, Gupta A, Berggren L. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. European journal of anaesthesiology. 2011;28(3):213–9.
Whitlock EL, Torres BA, Lin N, et al. Postoperative delirium in a substudy of cardiothoracic surgical patients in the BAG-RECALL clinical trial. Anesth Analg. 2014;118:809–17. https://doi.org/10.1213/ANE.0000000000000028. .
Jildenstal PK. Does Depth of Anesthesia Influence Postoperative Cognitive Dysfunction or Inflammatory Response Following Major ENT Surgery? J Anesth Clin Res 2012;03(06). https://doi.org/10.1097/ANA.0b013e3182712fba.
Chan MT, Cheng BC, Lee TM, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. .
Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110 Suppl 1:i98–105. https://doi.org/10.1093/bja/aet055. .
Zhou Y, Li Y, Wang K. Bispectral Index Monitoring During Anesthesia Promotes Early Postoperative Recovery of Cognitive Function and Reduces Acute Delirium in Elderly Patients with Colon Carcinoma: A Prospective Controlled Study using the Attention Network Test. Med Sci Monit. 2018;24:7785–93. https://doi.org/10.12659/MSM.910124. .
Qiang lulu, Xu Guanghong, Fang Weiping, et al.(Chinese) The impact of bispectral index-guided anesthesia on the incidence of postoperative delirium in elderly patients with chronic anemia. Int J Aneth Resus. 2016;37:415–9.
Li Yongjun, Sun Heliang, Li Xiaoshuang, et al.(Chinese) The effect of BIS monitoring on the incidence of postoperative delirium in elderly patients undergoing abdominal surgery. Jiangsu Med J. 2014;40:977–978.
Tang Christopher J, Zhongnan J, Sands Laura P, et al. ADAPT-2: A Randomized Clinical Trial to Reduce Intraoperative EEG Suppression in Older Surgical Patients Undergoing Major Noncardiac Surgery. [J] Anesth Analg. 2020;131:1228–36. https://doi.org/10.1213/ANE.0000000000004713.
Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Danelli G, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesthesia and analgesia. 2005;101(3):740–7.
Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric-Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2015;47(3):447–54.
Cox RM, Jamgochian GC, Nicholson K, Wong JC, Namdari S, Abboud JA. The use of near-infrared spectroscopy and cognitive function after arthroscopic shoulder surgery in the beach chair position: a randomized prospective blinded study. Journal of shoulder and elbow surgery. 2018;27(4):e138–e9. .
Deschamps A, Hall R, Grocott H, Mazer CD, Choi PT, Turgeon A, et al. Cerebral oximetry monitoring to maintain normal cerebral oxygen saturation during highrisk cardiac surgery: a randomized controlled feasibility trial. Anesthesiology. 2016;124(4):826–36.
Kara I, Erkin A, Sacli H, Demirtas M, Percin B, Diler MS, et al. The effects of near-infrared spectroscopy on the neurocognitive functions in the patients undergoing coronary artery bypass grafting with asymptomatic carotid artery disease: a randomized prospective study. Annals of Thoracic and Cardiovascular Surgery. 2015;21(6):544–50.
Mohandas BS, Jagadeesh AM, Vikram SB. Impact of monitoring cerebral oxygen saturation on the outcome of patients undergoing open heart surgery. Annals of Cardiac Anaesthesia 2013;16(2):102–6.
Lei L, Katznelson R, Fedorko L, Carroll J, Poonawala H, Machina M, et al. Cerebral oximetry and postoperative delirium after cardiac surgery: a randomised, controlled trial. Anaesthesia. 2017;72(12):1456–66.
Murniece S, Soehle M, Vanags I, Mamaja B. Near Infrared Spectroscopy Based Clinical Algorithm Applicability During Spinal Neurosurgery and Postoperative Cognitive Disturbances. Medicina. 2019;55(5):179. https://doi.org/10.3390/medicina55050179.
Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, 3rd, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. The Annals of thoracic surgery. 2009;87(1):36–44.
Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PloS one. 2012;7(6):e37410.
Kunst G, Gauge N, Salaunkey K, Spazzapan M, Amoako D, Ferreira N, et al. Intraoperative Optimization of Both Depth of Anesthesia and Cerebral Oxygenation in Elderly Patients Undergoing Coronary Artery Bypass Graft Surgery-A Randomized Controlled Pilot Trial. [J] Cardiothorac Vasc Anesth. 2020;34:1172–81. https://doi.org/10.1053/j.jvca.2019.10.054.
Punjasawadwong Y, Chau-in W, Laopaiboon M, et al. Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non-cardiac and non-neurosurgical procedures in adults. Cochrane Database Syst Rev. 2018;5:CD011283. https://doi.org/10.1002/14651858.CD011283.pub2.
Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7.
Brouquet A, Cudennec T, Benoist S, et al. Impaired mobility, ASA status and administration of tramadol are risk factors for postoperative delirium in patients aged 75 years or more after major abdominal surgery. Ann Surg. 2010;251:759–65. https://doi.org/10.1097/SLA.0b013e3181c1cfc9.
Raats JW, van Eijsden WA, Crolla RM, et al. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One. 2015;10:e0136071. https://doi.org/10.1371/journal.pone.0136071.
Hughes Christopher G, Boncyk Christina S, Culley Deborah J, et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Delirium Prevention [J]. Anesth Analg. 2020. https://doi.org/10.1213/ANE.0000000000004641.
American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults [J]. Am Geriatr Soc. 2015;63(1):142–50. https://doi.org/10.1111/jgs.13281.
Fritz BA, Kalarickal PL, Maybrier HR, et al. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 2016;122:234–42. https://doi.org/10.1213/ANE.0000000000000989.
Soehle M, Dittmann A, Ellerkmann RK, et al. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study [J], BMC Anesthesiol. 2015;15:61. https://doi.org/10.1186/s12871-015-0051-7.
Hesse S, Kreuzer M, Hight D, et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the post-anaesthesia care unit: an early sign of postoperative complications. Br J Anaesth. 2018. https://doi.org/10.1016/j.bja.2018.09.016.
Besch G, Liu N, Samain E, et al. Occurrence of and risk factors for electroencephalogram burst suppression during propofol-remifentanil anaesthesia [J]. Br J Anaesth. 2011;107:749–56. https://doi.org/10.1093/bja/aer235.
Plummer GS, Ibala R, Hahm E, et al. Electroencephalogram dynamics during general anesthesia predict the later incidence and duration of burst-suppression during cardiopulmonary bypass. [J]. Clin Neurophysiol. 2019;130:55–60. https://doi.org/10.1016/j.clinph.2018.11.003.
Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery [J]. Cochrane Database Syst Rev. 2014:CD003843. https://doi.org/10.1002/14651858.CD003843.pub3.
Quesada N, Júdez D, Martínez UJ, et al. Bispectral index monitoring reduces the dosage of Propofol and adverse events in sedation for Endobronchial ultrasound.[J]. Respiration. 2016;92:166–75. https://doi.org/10.1159/000448433.
Leung JM, Sands LP, Lim E, et al. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium?[J]. Am J Geriatr Psychiatry. 2013;21:946–56. https://doi.org/10.1016/j.jagp.2013.01.069.
Purdon PL, Pavone KJ, Akeju O, et al. The ageing brain: age-dependent changes in the electroencephalogram during propofol and sevoflurane general anaesthesia. [J]. Br J Anaesth. 2015;115:i46–57. https://doi.org/10.1093/bja/aev213.
Martin G, Glass PS, Breslin DS, et al. A study of anesthetic drug utilization in different age groups. J Clin Anesth. 2003;15:194–200. https://doi.org/10.1016/s0952-8180(03)00030-8.
Subramanian B, Nyman C, Fritock M, et al. A multicenter pilot study assessing regional cerebral oxygen desaturation frequency during cardiopulmonary bypass and responsiveness to an intervention algorithm. Anesth Analg. 2016;122:1786–93.
Heringlake M, Garbers C, Kabler JH, et al. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiol. 2011;114:58–69. https://doi.org/10.1097/ALN.0b013e3181fef34e.
Rogers CA, Stoica S, Ellis L, et al. Randomized trial of near-infrared spectroscopy for personalized optimization of cerebral tissue oxygenation during cardiac surgery. Br J Anaes. 2017;119:384–93.
Scheeren TWL, Kuizenga MH, Holger M, Struys MMRF, Matthias H. Electroencephalography and Brain Oxygenation Monitoring in the Perioperative Period. Anesth Analg. 128(2):265–77. https://doi.org/10.1213/ANE.0000000000002812.
Scheeren TWL, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 26(4):279–87. https://doi.org/10.1007/s10877-012-9348-y.
Reagan EM, Nguyen RT, Ravishankar ST, et al. Monitoring the Relationship Between Changes in Cerebral Oxygenation and Electroencephalography Patterns During Cardiopulmonary Resuscitation: A Feasibility Study. Crit Care Med. 46(5):757–63. https://doi.org/10.1097/CCM.0000000000003014.
Ter Horst HJ, Verhagen EA, Paul K, et al. The relationship between electrocerebral activity and cerebral fractional tissue oxygen extraction in preterm infants.[J]. Pediatr Res. 2011;70:384–8. https://doi.org/10.1203/PDR.0b013e3182294735.
Acknowledgements
Not applicable.
Funding
This study is supported by National Supported by National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University Z2018A10. The funders contribute to the modification and polishing of the language in this manuscript.
Author information
Authors and Affiliations
Contributions
DL, DXC, and QL designed the study, DL and DXC conducted database searches and extracted study data, DL performed the data analysis, and was the major contributor to the manuscript writing, QL provided critical review and revision to the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Literature search strategy.
Additional file 2:
PRISMA Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ding, L., Chen, D.X. & Li, Q. Effects of electroencephalography and regional cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a systematic review and meta-analysis. BMC Anesthesiol 20, 254 (2020). https://doi.org/10.1186/s12871-020-01163-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-020-01163-y