Abstract
Background
Postoperative nausea and vomiting (PONV) is one of the most common adverse outcomes after strabismus surgery. The primary outcome of this prospective, randomized, double-blind study was to compare the incidences of nausea or vomiting, and patient satisfaction of ondansetron and ramosetron after strabismus surgery under general anesthesia. The secondary outcome was to investigate whether the number of involved extraocular muscles (EOMs) in strabismus surgery was related to PONV.
Methods
One hundred and five patients (aged 18–60 years) undergoing strabismus surgery were allocated randomly to one of the three groups: placebo, ondansetron, or ramosetron. Patients received 2 ml placebo, 4 mg ondansetron, or 0.3 mg ramosetron at the end of surgery. Each of the three groups was subdivided into two subgroups according to the number of EOMs involved in the surgery: subgroup S, single-muscle correction; subgroup M, multiple-muscle correction. The incidences of nausea or vomiting, and patient satisfaction at 2, 24 and 48 h after surgery were analyzed as primary outcome. With regard to subgroups S and M in the placebo, ondansetron and ramosetron groups, incidences of nausea or vomiting, and patient satisfaction at 2, 24 and 48 h after surgery were analyzed as seconadary outcome.
Results
The incidence of nausea was significantly lower in the ramosetron group at 2 h (9.4 %) than in the placebo (45.2 %) and ondansetron (34.7 %) groups (P < 0.05). The incidence of nausea was also significantly lower in the ramosetron group at 24 h than in the other groups (P < 0.05). Patients in the ramosetron group were more satisfied at 2 h (8.11 ± 0.98) and 24 h (8.50 ± 0.67) after surgery than those in the other groups (P < 0.05). With regard to subgroups S and M in the placebo, ondansetron and ramosetron groups, there were no significant differences in either the incidence of nausea or patient satisfaction.
Conclusion
Ramosetron has superior antiemetic activity to ondansetron in adult strabismus surgery patients. The number of EOMs involved in strabismus surgery was not related to the incidence of PONV.
Trial registration
Clinical Research Information Service (CRiS) Identifier: KCT0000688. Date of registration: 27 February 2013.
Similar content being viewed by others
Background
Postoperative nausea and vomiting (PONV) is one of the most common adverse outcomes after strabismus surgery under general anesthesia, with an incidence of 37–80 % [1–4]. PONV may delay discharge from hospital or cause unexpected hospitalization in severe cases not only that it lowers patient’s satisfaction after surgery.
There are several known methods for PONV prophylaxis. Serotonin (5-HT3) receptor antagonists are recommended as the first-line regimen for PONV prophylaxis [5]. Ondansetron, the first 5-HT3 receptor antagonist used clinically for the prevention and management of PONV, acts less selectively on the 5-HT3 receptor than other 5-HT3 antagonists. Systemic review has revealed that the prophylactic effect of ondansetron on nausea is questionable although it has fine prophylactic effect on vomiting [6, 7]. Ramosetron, a newer 5-HT3 antagonist, has higher affinity to the 5-HT3 receptor and longer duration of action, and has a similar or greater prophylactic effect on PONV compared with older 5-HT3–receptor antagonists such as granisetron and ondansetron [8–10].
Traction of extraocular muscles (EOMs) is one of the factors that trigger PONV after strabismus surgery, which induces the oculoemetic reflex [11]. We hypothesized that more PONV would occur as the number of EOMs involved in strabismus surgery increases, causing more muscle traction. To our knowledge, no previous reports of the relationship between the numbers of involved EOMs and PONV have been published.
Thus, we primarily designed this prospective, randomized, double-blind study to compare the incidences of nausea or vomiting, and patient satisfaction of ondansetron and ramosetron after strabismus surgery under general anesthesia in patients aged > 18 years. The secondary outcome was to investigate whether the number of involved extraocular muscles (EOMs) in strabismus surgery was related to PONV.
Methods
Study population
One hundred twenty patients aged between 18 and 60 years, ASA physical status I and II, and scheduled for strabismus surgery under general anesthesia were enrolled from April 2011 to April 2012 in this prospective, randomized, double-blind clinical study. Patients who took any analgesics, steroids or antiemetics 24 h before surgery, or who had gastrointestinal diseases were excluded.
Recruited patients were allocated randomly to receive one of the following three drugs at the end of surgery: intravenous (IV) placebo (normal saline), ondansetron (4 mg), or ramosetron (0.3 mg) [12, 13]. A computerized randomization list was generated, and identical syringes (2 ml total) containing each drug were prepared by personnel not involved in the study, according to the list.
Study protocol
All patients were allowed to take solid food up to 8 h before surgery and water up to 2 h before surgery. No premedication was administered to the patients. Electrocardiogram, non-invasive blood pressure, heart rate, and pulse oximetry were measured continuously at 5-min intervals, starting from the time of arrival in the operating room. General anesthesia was induced with 2 mg/kg propofol and 0.6 mg/kg rocuronium. After tracheal intubation, anesthesia was maintained with 1.5–2 vol.% sevoflurane, medical air in oxygen [fraction of inspired O2 (FiO2) = 0.5], and continuously infused IV remifentanil 0.05–0.1 μg/kg/min, keeping end-tidal CO2 between 35 and 40 mmHg throughout the surgery. Ringer’s lactate solution was administered at 6–8 ml/kg/h during surgery. The nasopharyngeal temperature was monitored and maintained at 36 ± 1 °C throughout surgery using a warming pad. The types of strabismus surgery included were unilateral rectus muscle recession, unilateral rectus muscle resection, bilateral rectus muscle recession, and bilateral rectus muscle resection. At the end of the surgical procedure, sevoflurane and remifentanil administration were discontinued. IV 0.2 mg/kg pyridostigmine and 0.008 mg/kg glycopyrrolate were administered for reversal of muscle relaxation, along with the assigned study drug. The trachea was extubated when spontaneous respiration of the patient was adequate.
After the operation, the patients were transferred to the postanesthesia care unit. Every episode of nausea or vomiting was recorded during the first 2 h after surgery, and the presence of nausea at 2 h after surgery was asked by a member of the nursing staff who was blinded to the study drug used. She also filled out the data table including the followings: presence of nausea and vomiting, use of rescue antiemetics, patient’s satisfaction, degree of pain, use of rescue analgesics, and presence of adverse effects of the study drugs, such as headache, dizziness, or drowsiness. The patients were asked to grade their satisfaction from 0 to 10 (VRS, verbal rating scale), where 0 represents total dissatisfaction and 10 represents the most satisfactory subjective answer. The degree of pain was recorded using a visual rating scale (VRS) with options from 0 (not painful at all) to 10 (extremely painful).
If VRS score was > 3 for > 5 min, 30 mg IV ketorolac was administered for rescue analgesia. If patients complained of severe pain (VRS > 7), 1 μg/kg fentanyl was administered. When patients complained of nausea or vomitted, 10-mg IV metoclopramide was administered as a rescue antiemetic.
Patients were discharged from the hospital unless they showed severe complications of anesthesia, such as fever or desaturation due to laryngospasm or atelectasis. At 24 and 48 h after surgery, a resident of the Department of Ophthalmology who was also blinded to the study drug used completed the data table via telephone call.
After completion of data collection, patients were subdivided into two groups according to the number of EOMs involved in the surgery: subgroup S, single-muscle correction; and subgroup M, multiple-muscle correction.
Statistical analyses
To estimate the required sample size, a power analysis was used. We aimed for an 80 % probability (β = 0.2) of detecting a 50 % difference with a significance level (α) of 0.05, based on the incidence of PONV being 50 % in the placebo group and 20 % in the ramosetron group from our pilot study; thus 25 patients per group were required. To account for exclusion of some patients, we enrolled 35 patients in each group. Statistical analysis was performed using the SPSS, version 18.0 (SPSS, Chicago, IL, USA). Demographic data were analyzed by Student’s t-test. Differences in the incidence of nausea and vomiting, and use of rescue antiemetics were analyzed by χ 2 test. Patient’s satisfaction and pain were compared using one-way ANOVA with post hoc Bonferroni correction for multiple comparisons; data are presented as mean ± SD. Intention-to-treat (ITT) analysis was conducted using last-observation-carried-forward (LOCF) imputation for missing values. A value of P < 0.05 was considered to indicate statistical significance.
Results
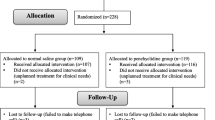
Among 120 patients recruited, 12 patients were excluded because they did not meet inclusion criteria; they showed ASA physical status >2. Another three patients were excluded because they did not agree to participate. Hence, 105 were randomly allocated to three groups. Among 16 follow-up loss patients, three patients and 13 patients did not respond to 24 h and 48 h telephone call, respectively (Fig. 1). Since persistent prophylactic effect on PONV was in concern, all the follow-up loss patients were excluded. There were no significant differences in patients’ characteristics, Apfel’s four risk factors for PONV (female gender, nonsmoking, prior history of motion sickness or PONV, and the use of intraoperative opioids) [14], and the duration of surgery and anesthesia among the groups (Table 1).
The total incidences of nausea were 29.2 % (26/89) at 2 h, 14.6 % (13/89) at 24 h, and 1.1 % (1/89) at 48 h after surgery. The incidence of nausea was significantly lower in the ramosetron group at 2 h (9.4 %) than in the placebo (45.2 %) and ondansetron (34.6 %) groups (P < 0.05). The incidence of nausea was also significantly lower in the ramosetron group at 24 h (3.1 %) than in the placebo (22.6 %) and ondansetron (19.2 %) groups (P < 0.05). There was no significant difference in the incidence of nausea at 48 h after surgery. The ondansetron group, comparing with the placebo group, showed significantly lower incidence of nausea only at 2 h after surgery (Fig. 2a). The ITT analysis using LOCF imputation for missing values revealed similar results (Fig. 2b). No patient showed vomiting after surgery. The incidence of rescue antiemetic use was identical significantly lower in the ramosetron group than in the other groups. No patients required fentanyl as rescue analgesics (Table 2).
Patients in the ramosetron group were more satisfied at 2 h (8.11 ± 0.98) and 24 h (8.50 ± 0.67) after surgery than those in the placebo (6.84 ± 1.34, 7.25 ± 1.29, respectively at 2 h and 24 h) and those in the ondansetron (7.28 ± 1.83, 7.27 ± 1.59, respectively at 2 h and 24 h) groups (P < 0.05). There was no significant difference in satisfaction in the patients at 48 h after surgery among any of the groups. The placebo and ondansetron groups showed no significant difference in patient satisfaction at all times after surgery (Fig. 3). There were no significant differences in the degree of pain and incidence of adverse effects of antiemetics among the groups (Tables 3 and 4).
With regard to subgroups S and M in the placebo, ondansetron and ramosetron groups, there were no significant differences in either the incidence of nausea or patient satisfaction (Figs. 4 and 5). The ITT analysis also showed similar results.
Incidence of nausea regarding the number of extraocular muscles involved Subgroup S, single-muscle correction; subgroup M, multiple-muscle correction. Placebo subgroup S (n = 16), subgroup M (n = 15); Ondansetron subgroup S (n = 12), subgroup M (n = 14); Ramoseron subgroup S (n = 17), subgroup M (n = 15)
Patient satisfaction regarding the number of EOMs involved. Subgroup S, single-muscle correction; subgroup M, multiple-muscle correction. Placebo subgroup S (n = 16), subgroup M (n = 15); Ondansetron subgroup S (n = 12), subgroup M (n = 14); Ramoseron subgroup S (n = 17), subgroup M (n = 15). VRS, verbal rating scale
Discussion
PONV is one of the most common causes of unexpected hospitalization in ambulatory strabismus surgery [15]. Although the mechanism of PONV after strabismus surgery are not still clearly identified, traction of EOMs, besides from agents used for general anesthesia, is a well-known risk factor for PONV [11, 16, 17].
In the present study, the incidence of postoperative nausea without prophylaxis was 45.2 % at 2 h and 22.6 % at 24 h after strabismus surgery. This is slightly higher than the previously reported incidence of PONV after strabismus surgery [18–20]. Meanwhile, the incidence of postoperative vomiting without prophylaxis was 0 % in this study unlike those previous studies. However, analysis in these differences is complicated because of differences in study population and anesthetic techniques.
Drugs used to alleviate PONV act on serotonergic, dopaminergic, histaminergic, or cholinergic receptors in the chemoreceptor trigger zone [4, 21, 22]. Among these, the 5-HT3 receptor antagonists prevents the emetogenic signal from tranmission to the nucleus tractus solitarii, where most vagal afferents terminate, by regulating serotonin release. 5-HT3 receptor antagonists exert their effect by inhibiting the binding of serotonin to the 5-HT3 receptors; they are copious in nucleus tractus solitarii and the chemoreceptor trigger zone which, in turn, project signals to the emetic center located in the brainstem [23, 24].
Among 5-HT3 receptor antagonists, ondansetron was the first agent used clinically for PONV. A dose of 4 mg IV at the end of surgery has been reported to be effective for PONV prophylaxis, with a relatively short plasma half-life (3.5–4.7 h) and duration of action (~12 h) [13]. Tramer et al. [7] reported less pronounced antiemetic effect of ondansetron comparing to its anti-vomiting effect. Ondansetron, comparing with placebo, did not improve the patient’s satisfaction at any time point although it reduced the incidence of nausea only at 2 h after in our study.
Meanwhile, ramosetron, a newer 5-HT3 receptor antagonist, has greater affinity for 5-HT3 receptors and greater potency in consequence, and a longer plasma half life (5.8 ± 1.2 h) and duration of action than other 5-HT3 receptor antagonists [6, 8, 9]. Whether ramosetron has a greater antiemetic effect than other 5-HT3 receptor antagonists is controversial [25–30]. Our data suggest that ramosetron has a greater antiemetic effect than ondansetron within 24 h after strabismus surgery. Strabismus surgery is known to be a particularly emetogenic surgical procedure [1, 3, 31]. Improving patients’ satisfaction and stronger anti-emetic effect within 24 h after surgery comparing to ondansetron suggest that ramosetron should be administered after strabismus surgery to prevent nausea, because most postoperative nausea occurred within 24 h after surgery in our study population. Meanwhile, the antiemetic effect of ramosetron at 48 h after surgery was similar to that of ondansetron, which corresponds to the report of Halm et al. [10].
Among various ocular surgeries under general anesthesia, the strabismus surgery is one of the most common surgeries that would result in PONV. Traction of EOMs induces the ocluoemetic reflex and oculocardiac relex, causing bradycardia and hypotension, which are risk factors for PONV [11]. Therefore, we hypothesized that more PONV would occur as the number of EOMs involved in strabismus surgery increases, causing more muscle traction. However, this was not supported by our data. The effect of traction of EOMs on PONV after strabismus surgery still remains obscure. Further study is needed to reveal the mechanism of PONV after strabismus surgery.
Pain is a well-known risk factor for PONV, and nausea but not vomiting is the predominant symptom [11, 16]. As in the present study, several others have reported that adult patients complain of minimal pain after strabismus surgery with a VAS score < 3 [32, 33]. Moreover, the degree of pain did not differ among any of our groups. Therefore, we suggest that pain has less of an effect on PONV after strabismus surgery.
There are several limitations in our study. First, relatively small number of study population was included. Second, we did not discriminate the patients who had strabismus surgery in both eyes from those who had surgery in only one eye. Although we failed to demonstrate an association between the number of EOMs involved in strabismus surgery and PONV, the binocularity might have been related to PONV. Third, the equivalent doses of ramosetron and ondansetron has not been clearly identified. Various studies have compared the efficacy of 0.3 mg of ramosetron with 4–16 mg of ondansetron [9, 10, 24, 27, 34, 35]. In a previous animal study, ondansetron did not show a linear dose response curve [36], which indicates that it is quite complicated to decide the equivalent doses of ramosetron and ondanseston. Further investigation to verify the equivalent doses of ramosetron and ondansetron is in contemplation.
Conclusions
In conclusion, the incidence of postoperative nausea was high until 24 h after strabismus surgery. Therefore, prevention of postoperative nausea during the 24 h after strabismus surgery is crucial. Ramosetron had an antiemetic efficacy greater than that of ondansetron or placebo during the first 24 h after strabismus surgery in adult patients. The number of EOMs involved in strabismus surgery was not associated with the incidence of PONV.
Abbreviations
EOM, extraocular muscles; PONV, postoperative nausea and vomiting; TIVA, total intravenous anesthesia; VAS, visual analogue scale; VRS, verbal rating scale
References
Abramowitz MD, Oh TH, Epstein BS, Ruttimann UE, Friendly DS. The antiemetic effect of droperidol following outpatient strabismus surgery in children. Anesthesiology. 1983;59:579–83.
Bowhay AR, May HA, Rudnicka AR, Booker PD. A randomized controlled trial of the antiemetic effect of three doses of ondansetron after strabismus surgery in children. Paediatr Anaesth. 2001;11:215–21.
Kuhn I, Scheifler G, Wissing H. Incidence of nausea and vomiting in children after strabismus surgery following desflurane anaesthesia. Paediatr Anaesth. 1999;9:521–6.
Sadhasivam S, Shende D, Madan R. Prophylactic ondansetron in prevention of postoperative nausea and vomiting following pediatric strabismus surgery: a dose–response study. Anesthesiology. 2000;92:1035–42.
Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71.
Gan TJ. Selective serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting: are they all the same? CNS Drugs. 2005;19:225–38.
Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ. Efficacy, dose–response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology. 1997;87:1277–89.
Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc). 2002;38:75–89.
Choi YS, Shim JK, Yoon DH, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976). 2008;33:E602–6.
Hahm TS, Ko JS, Choi SJ, Gwak MS. Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. Anaesthesia. 2010;65:500–4.
Kenny GN. Risk factors for postoperative nausea and vomiting. Anaesthesia. 1994;49(Suppl):6–10.
Ayuhara H, Takayanagi R, Okuyama K, Yoshimoto K, Ozeki T, Yokoyama H, et al. Receptor occupancy theory-based analysis of interindividual differences in antiemetic effect of 5-HT3 receptor anatagonists. Int J Clin Oncol. 2009;14:518–24.
Sun R, Klein KW, White PF. The effect of timing of ondansetron administration in outpatients undergoing otolaryngologic surgery. Anesth Analg. 1997;84:133–6.
Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700.
Gayer S, Lubarsky DA. Cost-effective Antiemesis. Int Anesthesiol Clin. 2003;41:145–64.
Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth. 1992;69:24S–32S.
Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–84.
Ebrahim Soltani AR, Mohammadinasab H, Goudarzi M, Arbabi S, Mohammadinasab A, Mohammadinasab F, et al. Comparing the efficacy of prophylactic p6 acupressure, ondansetron, metoclopramide and placebo in the prevention of vomiting and nausea after strabismus surgery. Acta Med Iran. 2011;49:208–12.
Treschan TA, Zimmer C, Nass C, Stegen B, Esser J, Peters J. Inspired oxygen fraction of 0.8 does not attenuate postoperative nausea and vomiting after strabismus surgery. Anesthesiology. 2005;103:6–10.
Tramèr MR, Fuchs-Buder T, Sansonetti A, Rifat K. Low incidence of the oculocardiac reflex and postoperative nausea and vomiting in adults undergoing strabismus surgery. Can J Anaesth. 1997;44:830–5.
Klockgether-Radke A, Feldmann M, Braun U, Mühlendyck H. Droperidol versus metoclopramide. Prevention of emesis following strabismus surgery in children. Anaesthesist. 1992;41:254–9.
Schlager A, Mitterschiffthaler G, Pühringer F. Rectally administered dimenhydrinate reduces postoperative vomiting in children after strabismus surgery. Br J Anaesth. 2000;84:405–6.
Broomhead CJ. Physiology of postoperative nausea and vomiting. Br J Hosp Med. 1995;53:327–30.
Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43.
Ansari MM, Siddiqui OA, Haleem S, Varshney R, Akhtar S, Khan FA. Comparison of ramosetron and ondansetron for control of post-operative nausea and vomiting following laparoscopic cholecystectomy. Indian J Med Sci. 2010;64:272–80.
Lee JW, Park HJ, Choi J, Park SJ, Kang H, Kim EG. Comparison of ramosetron’s and ondansetron’s preventive anti-emetic effects in highly susceptible patients undergoing abdominal hysterectomy. Korean J Anesthesiol. 2011;61:488–92.
Ryu J, So YM, Hwang J, Do SH. Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Surg Endosc. 2010;24:812–7.
Banerjee D, Das A, Majumdar S, Mandal RD, Dutta S, Mukherjee A, et al. PONV in Ambulatory surgery: a comparison between Ramosetron and Ondansetron: a prospective, double-blinded, and randomized controlled study. Saudi J Anaesth. 2014;8:25–9.
Roh GU, Yang SY, Shim JK, Kwak YL. Efficacy of palonosetron versus ramosetron on preventing opioid-based analgesia-related nausea and vomiting after lumbar spinal surgery: a prospective, randomized, and double-blind trial. Spine (Phila Pa 1976). 2014;39:E543–9.
Ryu JH, Lee JE, Lim YJ, Hong DM, Park HP, Han JI, et al. A prospective, randomized, double-blind, and multicenter trial of prophylactic effects of ramosetronon postoperative nausea and vomiting (PONV) after craniotomy: comparison with ondansetron. BMC Anesthesiol. 2014;14:63.
Oh AY, Kim JH, Hwang JW, Do SH, Jeon YT. Incidence of postoperative nausea and vomiting after paediatric strabismus surgery with sevoflurane or remifentanil-sevoflurane. Br J Anaesth. 2010;104:756–60.
Dagtekin O, Wiese P, Wolter K, Hermann MM, Pietruck C, Kampe S. Haloperidol versus haloperidol plus ondansetron for the prophylaxis of postoperative nausea and vomiting after ophthalmologic surgery. Pharmacology. 2009;83:205–10.
Kachko L, Katz J, Axer-Siegel R, Friling R, Goldenberg-Cohen N, Simhi E, et al. Sub-Tenon’s ropivacaine block for pain relief after primary strabismus surgery. Curr Eye Res. 2010;35:529–35.
Shi Y, He X, Yang S, Ai B, Zhang C, Huang D, et al. Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: a prospective randomized controlled study. Chemotherapy. 2007;53:44–50.
Kim SI, Kim SC, Baek YH, Ok SY, Kim SH. Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynecological surgery. Br J Anaesth. 2009;103:549–53.
Andrews PL, Bhandari P, Davey PT, Bingham S, Marr HE, Blower PR. Are all 5-HT3 receptor antagonists the same? Eur J Cancer. 1992;28A(Suppl):S2–6.
Acknowledgements
We express our gratitude to Professor Yong-gyu Park (Department of statistics, College of Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea) for his support with statistical analysis.
Availability of data and materials
The data will not be made available in order to protect the participants identity.
Author’s contributions
JJ, SHP, HJP and SYS participated in the design of the study. JJ and SYS were responsible for sample collections and logistics. JJ and SYS drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by Ethical Committee of Seoul St. Mary’s Hospital, Catholic University of Korea, ID: KIRB-00332_18-001, and was registered at cris.nih.go.kr, ID: KCT0000688. Oral and written consent was obtained from all of the patients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Joo, J., Park, S., Park, H.J. et al. Ramosetron versus ondansetron for postoperative nausea and vomiting in strabismus surgery patients. BMC Anesthesiol 16, 41 (2015). https://doi.org/10.1186/s12871-016-0210-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-016-0210-5