Abstract
Trehalose serves as a crucial osmolyte and plays a significant role in stress tolerance. The influence of exogenously added trehalose (1 and 5 mM) in alleviating the chromium (Cr; 0.5 mM) stress-induced decline in growth, photosynthesis, mineral uptake, antioxidant system and nitrate reductase activity in Vigna radiata was studied. Chromium (Cr) significantly declined shoot height (39.33%), shoot fresh weight (35.54%), shoot dry weight (36.79%), total chlorophylls (50.70%), carotenoids (29.96%), photosynthesis (33.97%), net intercellular CO2 (26.86%), transpiration rate (36.77%), the content of N (35.04%), P (35.77%), K (31.33%), S (23.91%), Mg (32.74%), and Ca (29.67%). However, the application of trehalose considerably alleviated the decline. Application of trehalose at both concentrations significantly reduced hydrogen peroxide accumulation, lipid peroxidation and electrolyte leakage, which were increased due to Cr stress. Application of trehalose significantly mitigated the Cr-induced oxidative damage by up-regulating the activity of reactive oxygen species (ROS) scavenging enzymes, including superoxide dismutase (182.03%), catalase (125.40%), ascorbate peroxidase (72.86%), and glutathione reductase (68.39%). Besides this, applied trehalose proved effective in enhancing ascorbate (24.29%) and reducing glutathione content (34.40%). In addition, also alleviated the decline in ascorbate by Cr stress to significant levels. The activity of nitrate reductase enhanced significantly (28.52%) due to trehalose activity and declined due to Cr stress (34.15%). Exogenous application of trehalose significantly improved the content of osmolytes, including proline, glycine betaine, sugars and total phenols under normal and Cr stress conditions. Furthermore, Trehalose significantly increased the content of key mineral elements and alleviated the decline induced by Cr to considerable levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Heavy metal contamination has become a serious problem worldwide and has resulted in a significant threat to sustainable food production. The accumulation of heavy metals and metalloids in soils significantly affects plant growth, development, and productivity by influencing cellular division, photosynthesis, enzyme activity, and tolerance mechanisms [1, 2]. Chromium (Cr) is a toxic metal that potentially affects crop growth and development worldwide [3, 4]. It has been reported that Cr drastically reduces growth and development by affecting photosynthesis, mineral assimilation, and redox homeostasis reflecting an insignificant decline in [5]. Among the key symptoms of Cr toxicity are included: delayed seed germination, reduced biomass production, leaf chlorosis and photosynthetic arrest, altered nutrient uptake and assimilation, restricted water uptake and tissue proliferation and enzyme inhibition ultimately affecting the yield potential of the plant [6,7,8,9]. Wheat plants treated with Cr exhibit significant alteration in photosynthetic attributes like the number of active PSII reaction centers, electron transport and activity of PSII heterogeneity [10]. Cr exists as Cr(III) and Cr(VI) valence states in which Cr(VI) is more toxic and persistent in soil, and has higher solubility and mobility in water [11, 12]. Plants take Cr from the soil through phosphate and sulphate transporters [12]. Among the natural sources of Cr accumulation mineral leaching is the prime contributor while the anthropogenic sources contributing 70% of the total Cr accumulation in soil include the effluent from paper and pulp mills, leather tanning industries, non-ferrous base metal smelters, refineries, thermal generating stations and the urban stormwater runoff [12,13,14,15].
Once taken up by plants Cr triggers toxic damaging effects by inducing the formation of reactive oxygen species thereby hampering normal growth and metabolism in plants by interfering with pigment synthesis, photosynthesis, root growth and osmolyte metabolism [16]. Plants have naturally occurring tolerance mechanisms to mitigate the negative effects of stresses to safeguard the metabolism and other machinery for better growth and yield performance [17, 18]. The prime mechanisms and pathways contributing to stress tolerance in plants include antioxidant system, osmolyte accumulation and efficient exclusion and sequestration of toxic ions [18,19,20,21]. Plants exhibiting significant up-regulation of the tolerance mechanisms mitigate the damaging effects of stresses efficiently [22,23,24,25]. Several strategies including molecular interventions, the use of novel management strategies, exogenous supplementation of minerals, phytohormones and metabolites, etc. have been exploited to improve the tolerance mechanisms to strengthen the potentiality of plants to withstand adverse environmental conditions. For example, the exogenous application of nitric oxide [26] and acetylcholine [27] improved tolerance to mercury and salinity treatments through the strengthening of tolerance mechanisms. In wheat, Adrees et al. (2015) have demonstrated that the application of mannitol improves the functioning of the antioxidant system thereby reducing oxidative damage and the Cr uptake [28].
In addition to proline, glycine betaine, etc., trehalose is considered a key sugar osmolyte having a substantial role in imparting stress tolerance. It plays a role in the regulation of plant growth, development, and metabolism in response to extreme environments such as high temperature, salinity, drought, and cold stress [29]. Trehalose 6-phosphate (T6P) was considered to be a signaling metabolite communicating the carbohydrate state of plants to other pathways involved in growth, development and responses to the environment [30]. Therefore, the various metabolic functions of trehalose may be caused by its role in sugar signal transduction. It restricts the growth of pathogens and also elicits the tolerance to withstand stress conditions [31]. As a protectant under stressful conditions, trehalose alleviates stress-induced physiological and biochemical disorders, delays leaf abscission and stimulates flowering [32]. In rice treatment of trehalose has been reported to alleviate the damaging effects of cadmium [33] and salinity [34].
Vigna radiata is an important legume crop also known as green gram or mung bean and is grown worldwide for its edible seeds that are rich in carbohydrates, proteins and minerals. The nitrogen-fixing ability of green gram benefits to improve the soil fertility and hence contributes to betterment of plant growth. The excess accumulation of metals including Cr can be toxic to growth of Vigna radiata and hence can result in a significant decline in its yield. In this backdrop it was hypothesized that the exogenous application of trehalose can mitigate the negative effects of Cr on growth, photosynthesis and mineral uptake by up-regulating the antioxidant functioning and osmolyte accumulation.
Materials and methods
Plant Material
Seeds of Vigna radiata were surface sterilized and sown in earthen pots filled with sand and soil in a ratio of 3:1. At the time of sowing all pots were irrigated with 250 mL full-strength Hoagland nutrient solution. In this study, all chemicals used were of analytical grade. Specifically, trehalose was procured from Sigma-Aldrich, Germany. Five days after germination two healthy and uniformly growing seedlings were maintained in each pot and were irrigated with full-strength Hoagland solution on every alternate day for another five days. Ten days after normal seedling growth pots were divided into two groups and were irrigated with normal Hoagland and modified Hoagland solution containing 0.5 mM Cr (in the form of K2Cr2O7) to induce Cr stress. Treatment of trehalose (1 and 5 mM) was given foliarly using 0.1% tween-20 as surfactant and during the treatment of trehalose, pots were covered with polythene to avoid the penetration of trehalose into roots. Trehalose was given twice a week (15 mL per pot). Pots were arranged in a complete randomized block design with five replicates for each treatment and were kept in a greenhouse under natural conditions with day/ night temperatures (oC) of 35/26 ± 5 and relative humidity (percent) of 75/55 ± 8. Twenty days after Cr and trehalose treatment plant were uprooted carefully and analyzed for different parameters as described below.
Estimation of total chlorophylls and carotenoids, and gas exchange parameters
The content of total chlorophylls and carotenoids was estimated according to [35] and optical density was taken at 480, 645 and 663 nm. LI-6400 photosynthesis system (Li-Cor, USA) was used for the estimation of net photosynthesis, intercellular CO2 and transpiration rate, and the observations were recorded in a fully expanded leaf between 09:00–12:00 h.
Measurement of osmolytes and RWC
The extraction of soluble sugar was in ethanol and estimations were carried out according to the anthrone method [36]. For estimating proline content method of [37] was adopted and extraction was 3% sulphosalicylic acid. Optical density was taken at 520 nm. Glycine betaine was estimated according to [38] and absorbance was recorded at 365 nm. For relative water content (RWC) determination method of [39] was used. Leaf discs were punched from fresh leaf tissue and their fresh weight (FW) was taken and turgid weight (TW) of same leaf discs was taken after allowing them to gain turgidity in distilled water for 1 h. Dry weight (DW) of discs was recorded after oven drying the discs for 24 h at 80 °C. Calculation was carried using formula:
Lipid peroxidation, hydrogen peroxide and electrolyte leakage
For lipid peroxidation [40], method was followed and 100 mg fresh tissue was extracted in 1% TCA. The formation of malonaldehyde (MDA) content was recorded at 532 and 600 nm. Hydrogen peroxide was estimated by crushing 500 mg fresh tissue in 0.1% TCA and supernatant was mixed with potassium phosphate buffer (pH 7.0) and 1 mL potassium iodide solution. Absorbance was taken at 390 nm [41]. Electrolyte leakage was measured following [42].
Assay of nitrate reductase
Nitrate reductase (E.C. 1.6.6.1.) activity was assayed following [43] and absorbance was recorded at 540 nm.
Determination of antioxidant enzyme activities
Extraction of antioxidant enzymes was carried out by homogenizing 500 mg fresh tissue in sodium phosphate buffer (50 mM, pH 7.0) containing 1% PVP, 0.5 mM EDTA and 0.1 mM PMSF. After centrifuging at 15,000 g for 20 min at 4 °C, supernatant was used for enzyme assay. Thereafter the activity of superoxide dismutase [44], catalase [45], ascorbate peroxidase [46] and glutathione reductase [47] was measured. Protein determination method of [48] was used.
Measurement of ascorbate and reduced glutathione
Ascorbate (AsA) was extracted in 6% TCA and supernatant was mixed with 2% dinitrophenylhydrazine and thiourea, and absorbance was taken at 530 nm [49]. Reduced glutathione was extracted by homogenizing fresh tissue in phosphate buffer (pH 8.0) and supernatant was mixed with 5, 5-dithiobis-2-nitrobenzoic acid and absorbance was taken at 412 nm [50].
Estimation of total phenols
Total phenols were extracted in methanol and after centrifuging at 10,000 g for 10 min supernatant was reacted with Folin–Ciocalteu reagent. Optical density was measured at 765 nm [51].
Estimation of nutrients and chromium
For the estimation of nitrogen method of [52] was adopted. The content of K, Ca and Mg were determined using atomic absorption Spectrophotometer (Shimadzu AA-6300, Japan) [53]. The spectrophotometric method described by [54] was used for the estimation of phosphorous. Sulphur was estimated using the turbidimetric method [55].
Estimation of cr content in shoot & root is missing
Cr content in shoots and roots was quantified at 50 days after seeding? following the method of McGrath & Cunliffe [56] and Ouzounidou et al. [57]. Root and shoot samples (0.5 g each) were digested in 15 ml conc. HNO3:HClO4 (3:1, v/v) on a hot plate, by gradually increasing the temperature up to 275 °C till yellow fumes began to emerge from the flask. H2O2 was added once the density of fumes decreased. After cooling, distilled water was added to the samples to achieve a total volume of 25 ml. Cr content was determined by obtaining absorbance values using flame atomic absorption spectrophotometry.
Statistical analysis
The obtained data were analyzed using one-way analysis of variance (ANOVA), using Minitab® Statistical Software version 20, and the results were described as a mean of three replicates ± standard error [58]. The least significant was calculated using Duncan’s Multiple Range Test at p < 0.05.
Results
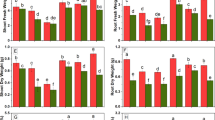
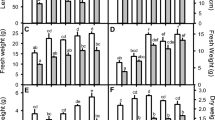
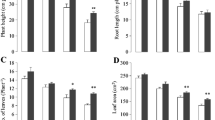
Cr stress significantly reduced shoot height, fresh weight, and dry weight of the shoot. However, trehalose application not only increased these parameters but also alleviated the decline induced by Cr. Relative to control, treatment of Cr declined shoot height by 39.33%, shoot fresh weight by 35.54% and shoot dry weight by 36.79% (Table 1). Application of trehalose at both concentrations alleviated the decline to significant levels (Table 1). Treatment of Cr declined the content of N, P, K, S, Mg and Ca by 35.04%, 35.77%, 31.33%, 23.91%, 32.74% and 29.67% respectively as compared to control. Application of trehalose mitigated to decline to considerable levels with maximal mitigation due to 5 mM trehalose. Percent decline in Cr + 5 mM trehalose-treated plants was 1.02%, 2.11%, 2.96%, 1.49%, 2.29% and 7.64% for N, P, K, S, Mg and Ca respectively over control (Table 1). Under normal conditions, both concentrations of trehalose enhanced N, P, K, S, Mg and Ca maximally by 26.27%, 31.69%, 44.52%, 60.89%, 33.58% and 42.90% respectively due to 5 mM trehalose (Table 1). Relative to control, Cr content in the shoot decreased by 17.41% and 60.78% due to the application of 1- and 5-mM trehalose over the Cr-stressed counterparts (Table 1). A more or less similar impact was observed in the root (Table 1).
Supplementation of trehalose significantly increased total chlorophylls, carotenoids, photosynthesis, net intercellular CO2 and transpiration rate with maximal increases of 49.76%, 18.39%, 56.68%, 42.06% and 43.94% observed due to 5 mM concentration of trehalose. Treatment of Cr resulted in a decline of 50.70% in total chlorophylls, 29.96% in carotenoids, 33.97% in photosynthesis, 26.86% in net intercellular CO2 and 36.77% in transpiration rate over the control. Application of trehalose to Cr-stressed plants considerably mitigated Cr-induced decline in total chlorophylls, carotenoids, photosynthesis, net intercellular CO2 and transpiration rate with maximal alleviation observed due to 5 mM trehalose (Figs. 1 and 2).
Effect of chromium stress on (A) Net photosynthesis, (B) intercellular CO2 concentration and (C) stomatal conductance in Vigna radiata plants grown with and without exogenous application of trehalose (Tre; 1 and 5 mM). Data is mean (± SE) of three replicates and means followed by same letters are not significantly different at p < 0.05
Treatment of Cr increased the accumulation of sugars, proline and glycine betaine. Application of trehalose at 1 mM concentrations increased the content of sugars proline and glycine betaine by 26.45%, 20.97% and 10.40% respectively while as 5% trehalose caused an increase of 96.30%, 85.34% and 31.79% respectively. Application of trehalose to Cr-treated plants enhanced the sugars, proline and glycine betaine. Sugars, proline and glycine betaine maximally increased by 200.13%, 203.42% and 63.58% respectively in Cr + 5 mM trehalose-treated plants (Fig. 3A-C). Relative to control, RWC was decreased by 32.00% due to Cr stress and increased by 8.62% due to 5 mM trehalose treatment. The decline in RWC was alleviated by the supplementation of trehalose (Fig. 3D).
Effect of chromium stress on (A) proline, (B) glycine betaine, (C) sugar and (D) relative water content (RWC) in Vigna radiata plants grown with and without exogenous application of trehalose (Tre; 1 and 5 mM). Data is mean (± SE) of three replicates and means followed by same letters are not significantly different at p < 0.05
Cr stress increased the generation of H2O2, lipid peroxidation and electrolyte leakage significantly over control, however, the application of trehalose reduced H2O2, lipid peroxidation and electrolyte leakage under normal as well as Cr treatments. Relative to control, H2O2, lipid peroxidation and electrolyte leakage increased by 131.09%, 149.85% and 139.23% respectively due to Cr stress. Treatment of 5 mM trehalose reduced the H2O2, lipid peroxidation and electrolyte leakage by 44.96%, 47.43% and 48.08% respectively as compared to control. Application of trehalose to Cr-stressed plants alleviated the H2O2, lipid peroxidation and electrolyte leakage with maximal alleviation observed in Cr + 5 mM trehalose-treated plants (Fig. 4A-C).
Effect of chromium stress on (A) hydrogen peroxide, (B) Lipid peroxidation and (C) electrolyte leakage in Vigna radiata plants grown with and without exogenous application of trehalose (Tre; 1 and 5 mM). Data is mean (± SE) of three replicates and means followed by same letters are not significantly different at p < 0.05
The activity of SOD, CAT, APX and GR increased by 60.42%, 44.20%, 33.78% and 28.56% respectively due to the application of 5 mM trehalose. In Cr-stressed plants activity of SOD, CAT, APX and GR increased by 108.98%, 69.15%, 46.79% and 38.64% respectively, however, plants treated with Cr + 5mM trehalose resulted in an increase of 182.03%, 125.40%, 72.86% and 68.39% in SOD, CAT, APX and GR over the control (Figs. 5A and B and 6A and B). The content of AsA decreased by 27.25% and GSH increased by 44.35% due to Cr stress compared to control. Under normal growth conditions, the application of trehalose maximally increased AsA and GSH by 24.29% and 34.40% at 5 mM concentrations. Treatment of trehalose to Cr-stressed plants alleviated the decline in AsA while causing a further increase in GSH content (Fig. 6C and D).
Effect of chromium stress on the activity of (A) superoxide dismutase and (B) catalase in Vigna radiata plants grown with and without exogenous application of trehalose (Tre; 1 and 5 mM). Data is mean (± SE) of three replicates and means followed by same letters are not significantly different at p < 0.05
Effect of chromium stress on the activity of (A) ascorbate peroxidase, (B) glutathione reductase and content of (C) ascorbate and (D) reduced glutathione in Vigna radiata plants grown with and without exogenous application of trehalose (Tre; 1 and 5 mM). Data is mean (± SE) of three replicates and means followed by same letters are not significantly different at p < 0.05
The content of total phenols increased due to the application of trehalose by 19.00% and 64.00% due to 1- and 5-mM concentrations. Seedlings treated with Cr exhibited an increase of 88.00% in total phenols which was further increased due to the application of trehalose. Maximum accumulation of total phenols was observed in plants treated with Cr and 5 mM trehalose attaining an increase of 133.00% over control (Fig. 7).
Cr stress significantly reduced the nitrate reductase activity and the application of trehalose not only increased its activity but also alleviated the decline induced by Cr. Percent decline in activity due to Cr was 34.15% while as trehalose increased the activity by 14.08% and 28.52% at 1 and 5 mM respectively compared to control. The decline in Cr + 5 mM trehalose-treated plants was 2.11% over control (Fig. 8).
Discussion
Rapid industrialization has a significant contribution to increasing metal pollution all around the globe. This has led to a considerable decline in the arable productive land thereby significantly reducing the yield. To protect growth and yield production from the adverse effects of toxic metals different management strategies have been devised and implemented from time to time. The present study aimed to evaluate the potentiality of applied trehalose in the mitigation of Cr toxicity-induced growth and metabolic alterations in Vigna radiata. Treatment of trehalose at 1 and 5 mM proved beneficial in alleviating the Cr-mediated growth reduction to some extent. Earlier it was been reported that Cr stress reduced growth, fresh and dry weight significantly in different plants [5, 9]. Cr potentially reduces the germination, and growth of coleoptile, hinders root elongation and distorts the xylem and phloem [59]. In Vigna radiata, treatment of Cr significantly reduces growth and yield by triggering oxidative damage [4]. Adverse effects of cadmium in rice [33], salinity in wheat [60] and drought in sweet basil [61] on the growth, fresh and dry biomass were mitigated by trehalose. Trehalose application potentially enhances ABA to induce salt tolerance in tomato [62]. Transgenic rice exhibiting up-regulated trehalose biosynthesis depicts better growth, increased photosynthesis, Na/K and relative water content under drought, salinity and sodic stress [63]. Increased growth in trehalose-treated plants may be attributed to significant enhancement in essential mineral ions, increased chlorophyll synthesis and osmolyte accumulation in addition to up-regulated antioxidant system. Trehalose application resulted in significant improvement in the content of key elements including N, P, K, S, Mg and Ca thereby assisting the plants to maintain metabolic functioning and growth under Cr stress conditions. Optimal availability of N [64, 65], P [19, 20], K [66, 67], S [68], Mg [69] and Ca [18] enhance growth and improve stress tolerance by regulating key functions involving enzyme activity, chlorophyll synthesis, mineral uptake and assimilation, protein synthesis, osmolyte accumulation and antioxidant functioning [70]. have also demonstrated a significant decline in the content of K, Mg, Fe and Zn in Cr-stressed lettuce. In the present study trehalose application resulted in a significant enhancement in the uptake of essential mineral elements and reduced the accumulation of Cr thereby contributing to growth improvement and Cr tolerance. Magnesium is a key central component of chlorophyll and trehalose-mediated enhancement in Mg may have contributed significantly to chlorophyll synthesis thereby contributing to increased growth and photoassimilate production. Cr impairs the nutrient balance by reducing their assimilation [71] and a significant reduction in key mineral ions has been reported in paddy under Cr stress [72]. Besides, Cr imparted an obvious decline in the activity of nitrate reductase reflecting in its negative influence on the assimilation of nutrients as well. Earlier Cr-induced reduction in nitrate reductase activity has been reported which severely influences the photosynthetic efficiency [5]. Decline nitrate reductase activity due to Cr stress is mainly due to impaired substrate utilization as has been reported by [73]. Trehalose application may have contributed to improved nutrient uptake by regulating the activity of mineral transport proteins and preventing the inhibitory effects of Cr on the assimilatory pathways. Besides this increased root growth and root penetration as well as improved translocation of nutrients due to trehalose application may have served as a key factor for increased mineral uptake.
Cr stress reduced the chlorophyll and carotenoid content as well as photosynthesis, CO2 concentration and transpiration rate. On the contrary, plants treated with trehalose exhibited a significant increase in these parameters and trehalose was effective in mitigating the Cr-induced decline. Earlier [5], reported a significant decline in chlorophyll, net photosynthesis, stomatal conductance, intercellular CO2 concentration, electron transport rate, PSII functioning and Rubisco activity in Cr-stressed Brassica juncea plants. Cr affects chloroplast morphology and volume besides obvious influence on pigment synthesis and photosynthetic gas exchange parameters in pea [74]. Reduced chlorophyll synthesis, photosynthesis, Fv/Fm and electron transport rate due to Cr stress is attributed to increased generation of toxic free radicals like superoxide, hydrogen peroxide and hydroxyl reflecting in declined growth and biomass production [75]. Exogenous trehalose supplementation has been reported to increase chlorophyll [34] and carotenoid [76] content in Oryza sativa and Raphanus sativus and alleviate the decline induced due to salinity and water deficit stresses respectively. Trehalose supplementation improves photosynthetic carbon assimilation under heat stress in wheat and maize [77] [61]. also demonstrated increased net photosynthesis, intercellular CO2, transpiration rate and stomatal conductance concomitant with significant alleviation of drought-induced decline due to the application of trehalose. Stresses induce ROS production thereby hampering the structural and functional integrity of chloroplast and also up-regulate the functioning of chlorophyll degrading enzymes with a significant decline in the activity of those involved in biosynthesis [27]. Trehalose-treated plants exhibited lesser accumulation of ROS concomitant with increased water content and mineral (N and Mg) content which may have contributed to improved chlorophyll synthesis and photosynthesis in them. Carotenoids contribute to ROS scavenging besides their key role in photoprotection [78] and trehalose-induced increase in carotenoids resulted in improved growth and stress tolerance by improving photosynthesis and photoprotection, phytohormone synthesis and signaling. Plants exhibiting increased synthesis of carotenoids have improved potential to withstand stress [79].
Reduced growth, chlorophyll synthesis and photosynthesis in Cr-stressed plants were correlated with significant enhancement in the oxidative damage measured in terms of H2O2, lipid peroxidation and electrolyte leakage. Increased ROS accumulation and membrane damage in terms of lipid peroxidation and electrolyte leakage are in corroboration with [5, 80, 81] and [4]. Stress conditions trigger the peroxidation of membrane lipids and up-regulate lipoxygenase activity resulting in a significant decline in the integrity of membranes [22, 64, 82] hence functioning of key cellular compartments is impeded. In Cr-stressed Cicer arietinum L [9], has also demonstrated increased H2O2 content, lipid peroxidation and electrolyte leakage resulting in significant growth and yield restriction. Oxidative damage triggered by Cr was alleviated by the application of trehalose at both concentrations with 5 mM to be much more effective. The application of trehalose has been reported to alleviate the damaging effects of salinity stress on growth and photosynthesis by reducing the accumulation of H2O2, lipid peroxidation and lipoxygenase activity in wheat [60]. Excess accumulation of ROS damages cellular structures, hampers photosynthesis and weakens the tolerance mechanisms however exogenous trehalose application prevents the adverse effects to significant levels [61]. The trehalose-mediated decline in ROS, lipid peroxidation, electrolyte leakage and lipoxygenase is reflected in significant protection of proteins in thylakoid membranes and photosynthesis [83]. Trehalose application resulted in a significant enhancement in membrane stability (obvious as reduced oxidative damage) reflecting in increased growth and photosynthesis.
Reduced oxidative damage in trehalose-treated plants can be ascribed to improved functioning of tolerance mechanisms including antioxidant system, and accumulation of osmolyte and secondary metabolite. The antioxidant system is comprised of enzymatic and non-enzymatic components which have a significant role in the prevention of stress-induced oxidative damage to key cellular macromolecules thereby impeding their functioning [17]. SOD neutralizes superoxide while H2O2 is eliminated by CAT or APX and GR [84]. Earlier increased activity of antioxidant enzymes due to exogenous treatment of trehalose has been reported in rice [85], maize [86] and sweet basil [61], thereby alleviating the oxidative damage to growth and photosynthesis. Recently, in mung bean [87] observed that trehalose application increases the functioning of antioxidants resulting in a decline in ROS accumulation, lipid peroxidation and electrolyte leakage concomitant with improved photosynthesis and yield under cadmium stress. Enzymes including APX and GR as well as AsA and GSH are among the main contributors to the ascorbate-glutathione cycle which detoxifies excess H2O2 from key cellular components including chloroplast and mitochondria thereby preventing any damage to their functioning [26, 88, 89]. Glutathione reductase maintains NADP/NADPH ratio so that electron transport does not get so much influenced [89]. Transgenic plants exhibiting up-regulated activity of antioxidant enzymes display increased growth, photosynthetic potential and photoprotection mechanisms for PSII concomitant with increased membrane stability and reduced electrolyte leakage [90]. The decline in AsA content due to Cr stress has been reported by [73] in Ocimum tenuiflorum. Glutathione and AsA form key non-antioxidant components regulating several aspects of plant development and metabolism in addition of their obvious role as redox components [91,92,93]. Glutathione protects thiol groups of proteins thereby preventing their denaturation under stress and acts as a key component for the optimal functioning of ascorbate-glutathione pathway and glyoxalase cycle [94]. Therefore, trehalose-mediated enhancement in the AsA and GSH content prevented the ill effects of Cr on key metabolic pathways and future studies may help to know exact mechanisms.
Tolerance mechanisms in trehalose-treated plants were further improved by the significant enhancement in the accumulation of osmolytes including sugars, proline and glycine betaine. Osmolytes are multifunctional molecules serving as osmoprotectants, ROS scavengers, metal chelators, stress sensors and signaling molecules [95,96,97,98]. Cr stress enhanced proline, glycine betaine and sugars however, trehalose application caused further enhancement thereby inducing better tolerance against Cr. Cr-mediated increase in osmolytes has been reported by others [4, 5, 73, 99]. Cr lead accumulation of proline was not sufficient to counter the photodestructive effects on PSII [100]. Trehalose-induced increase in osmolyte accumulation may have benefited the metabolism and growth by tissue water content maintenance, scavenging ROS and protecting enzyme functioning. Earlier trehalose mediated an increase in the accumulation of proline [101], glycine betaine [61] and sugars [101] [60]. demonstrated a significant improvement in the accumulation of glucose, sucrose, trehalose, total soluble sugars, free amino acids and proline in wheat due to the application of trehalose reflecting a significant alleviation of the damaging effects of salinity.
Moreover, total phenols increased due to the treatment of trehalose in control and Cr stress. Earlier Cr-mediated enhancement in total phenol content has been reported in Brassica juncea [102] and Plantago ovata [103]. In wheat [104], quinoa [105] and sunflower [106] have demonstrated a significant enhancement in the content of phenols due to the exogenous application of trehalose reflecting in improved stress tolerance. heavy metal treatment induces the accumulation of phenolic compounds and trehalose further increases the contents resulting in strengthening the mechanisms against the oxidative effects triggered by heavy metals. Phenolic compounds neutralize ROS to protect the cells [107] [108]. have suggested the role of phenolic compounds in the scavenging of ROS including hydrogen peroxide and superoxide ion.
Conclusions and future prospects
In conclusion, exogenous trehalose significantly alleviated the negative effects of Cr on growth, photosynthesis, and enzyme activity. Oxidative effects of Cr were significantly alleviated by the exogenous trehalose application evident as reduced oxidative damage. Moreover, trehalose application triggered osmolyte accumulation, activated antioxidant mechanism and phenol accumulation to ameliorate the negative influence of Cr. Photosynthesis, mineral content and nitrate reductase activity were increased thereby justifying the beneficial role of trehalose in improving Cr tolerance in Vigna radiata. Although this study could provide a comprehensive understanding of the mechanisms through which trehalose affects Vigna radiata plants under Cr-stressful conditions, the molecular mechanisms remain unknown. Thus, further studies on the mechanisms of trehalose can be worthwhile to unravel the exact mechanisms involved.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ahanger MA, Aziz U, Alsahli A, Alyemeni MN, Ahmad P. Combined Kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by Up-Regulating antioxidant and Nitrogen Metabolism under Cadmium stress. Biomolecules. 2020;10:147.
Soliman M, Alhaithloul HA, Hakeem KR, Alharbi BM, El-Esawi M, Elkelish A. Exogenous nitric oxide mitigates Nickel-Induced oxidative damage in Eggplant by Upregulating antioxidants, Osmolyte Metabolism, and Glyoxalase systems. Plants. 2019;8:562.
Patnaik AR, Achary VMM, Panda BB. Chromium (VI)-induced hormesis and genotoxicity are mediated through oxidative stress in root cells of Allium cepa L. Plant Growth Regul. 2013;71:157–70.
Singh D, Sharma NL, Singh CK, Yerramilli V, Narayan R, Sarkar SK et al. Chromium (VI)-Induced alterations in Physio-Chemical parameters, yield, and yield characteristics in two cultivars of Mungbean (Vigna radiata L). Front Plant Sci. 2021;12.
Asgher M, Per TS, Verma S, Pandith SA, Masood A, Khan NA. Ethylene Supplementation increases PSII Efficiency and alleviates chromium-inhibited photosynthesis through increased Nitrogen and Sulfur Assimilation in Mustard. J Plant Growth Regul. 2018;37:1300–17.
Chen Q, Zhang X, Liu Y, Wei J, Shen W, Shen Z, et al. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 2017;81:253–64.
Ding H, Wang G, Lou L, Lv J. Physiological responses and tolerance of kenaf (Hibiscus cannabinus L.) exposed to chromium. Ecotoxicol Environ Saf. 2016;133:509–18.
Ertani A, Mietto A, Borin M, Nardi S. Chromium in agricultural soils and crops: a review. Water Air Soil Pollut. 2017;228:190.
Singh D, Sharma NL, Singh CK, Sarkar SK, Singh I, Dotaniya ML. Effect of chromium (VI) toxicity on morpho-physiological characteristics, yield, and yield components of two chickpea (Cicer arietinum L.) varieties. PLoS ONE. 2020;15:e0243032.
Mathur S, Kalaji HM, Jajoo A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica. 2016;54:185–92.
Bhalerao SA, Sharma AS, Chromium. As an environmental pollutant. Int J Curr Microbiol App Sci. 2015;4:732–46.
Srivastava D, Tiwari M, Dutta P, Singh P, Chawda K, Kumari M, et al. Chromium stress in plants: toxicity, tolerance and phytoremediation. Sustainability. 2021;13:4629.
Aravindhan R, Madhan B, Rao JR, Nair BU, Ramasami T. Bioaccumulation of Chromium from Tannery Wastewater: an Approach for Chrome Recovery and Reuse. Environ Sci Technol. 2004;38:300–6.
Cassano A, Della Pietra L, Drioli E. Integrated membrane process for the recovery of Chromium salts from Tannery effluents. Ind Eng Chem Res. 2007;46:6825–30.
WHO. Chromium in Drinking-water. Background document for development of WHO Guidelines for drinking-water quality. Geneva: World Health Organization; 2020.
Ali S, Bharwana SA, Rizwan M, Farid M, Kanwal S, Ali Q, et al. Fulvic acid mediates chromium (cr) tolerance in wheat (Triticum aestivum L.) through lowering of cr uptake and improved antioxidant defense system. Environ Sci Pollut Res. 2015;22:10601–9.
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants. 2017;23:731–44.
Elkelish AA, Alnusaire TS, Soliman MH, Gowayed S, Senousy HH, Fahad S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J Appl Bot Food Qual. 2019;92:258–66.
Begum N, Ahanger MA, Zhang L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ Exp Bot. 2020;176:104088.
Begum N, Akhtar K, Ahanger MA, Iqbal M, Wang P, Mustafa NS, et al. Arbuscular mycorrhizal fungi improve growth, essential oil, secondary metabolism, and yield of tobacco (Nicotiana tabacum L.) under drought stress conditions. Environ Sci Pollut Res. 2021;28:45276–95.
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem. 2019;137:144–53.
Ahanger MA, Qi M, Huang Z, Xu X, Begum N, Qin C, et al. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol Environ Saf. 2021;216:112195.
Chen W, Miao Y, Ayyaz A, Hannan F, Huang Q, Ulhassan Z, et al. Purple stem Brassica napus exhibits higher photosynthetic efficiency, antioxidant potential and anthocyanin biosynthesis related genes expression against drought stress. Front Plant Sci. 2022;13:936696.
Qin C, Ahanger MA, Lin B, Huang Z, Zhou J, Ahmed N, et al. Comparative transcriptome analysis reveals the regulatory effects of acetylcholine on salt tolerance of Nicotiana Benthamiana. Phytochemistry. 2021;181:112582.
Qin C, Shen J, Ahanger MA. Supplementation of nitric oxide and spermidine alleviates the nickel stress-induced damage to growth, chlorophyll metabolism, and photosynthesis by upregulating ascorbate–glutathione and glyoxalase cycle functioning in tomato. Front Plant Sci. 2022;13.
Ahmad P, Alam P, Balawi TH, Altalayan FH, Ahanger MA, Ashraf M. Sodium nitroprusside (SNP) improves tolerance to arsenic (as) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere. 2020;244:125480.
Qin C, Ahanger MA, Zhou J, Ahmed N, Wei C, Yuan S, et al. Beneficial role of acetylcholine in chlorophyll metabolism and photosynthetic gas exchange in Nicotiana benthamiana seedlings under salinity stress. Plant Biol. 2020;22:357–65.
Adrees M, Ali S, Iqbal M, Aslam Bharwana S, Siddiqi Z, Farid M, et al. Mannitol alleviates chromium toxicity in wheat plants in relation to growth, yield, stimulation of anti-oxidative enzymes, oxidative stress and cr uptake in sand and soil media. Ecotoxicol Environ Saf. 2015;122:1–8.
Fichtner F, Lunn JE. The role of Trehalose 6-Phosphate (Tre6P) in Plant Metabolism and Development. Annu Rev Plant Biol. 2021;72:737–60.
Fichtner F, Barbier FF, Annunziata MG, Feil R, Olas JJ, Mueller-Roeber B, et al. Regulation of shoot branching in arabidopsis by trehalose 6‐phosphate. New Phytol. 2021;229:2135–51.
Fernandez O, Béthencourt L, Quero A, Sangwan RS, Clément C. Trehalose and plant stress responses: friend or foe? Trends Plant Sci. 2010;15:409–17.
Kosar F, Akram NA, Sadiq M, Al-Qurainy F, Ashraf M. Trehalose: a key Organic Osmolyte effectively involved in plant abiotic stress tolerance. J Plant Growth Regul. 2019;38:606–18.
Wang K, Li F, Gao M, Huang Y, Song Z. Mechanisms of trehalose-mediated mitigation of cd toxicity in rice seedlings. J Clean Prod. 2020;267:121982.
Mostofa MG, Hossain MA, Fujita M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma. 2015;252:461–75.
Arnon DI. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949;24:1–15.
Robert S, Burnett William. Determination of protein-bound carbohydrate in serum by modified Anthrone Method. Anal Chem. 1960;32:885–6.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–7.
Grieve CM, Grattan SR. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–7.
Smart RE, Bingham GE. Rapid Estimates of Relative Water Content. Plant Physiol. 1974;53:258–60.
Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98.
Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66.
Sairam RK, Deshmukh PS, Shukla DS. Tolerance of Drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178:171–8.
Jaworski EG. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 1971;43:1274–9.
Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–66.
Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2nd edition. New York: Academic Press Inc.; 1974. pp. 673–84.
Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–80.
Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–5.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Mukherjee SP, Choudhuri MA. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant. 1983;58:166–70.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7.
Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58.
Subbaiah BV, Asija GL. A rapid procedure for estimation of available nitrogen in soil. Curr Sci. 1956;25:259–60.
Sagner S, Kneer R, Wanner G, Cosson J-P, Deus-Neumann B, Zenk MH. Hyperaccumulation, complexation and distribution of nickel in Sebertia Acuminata. Phytochemistry. 1998;47:339–47.
Olsen SR, Cole CV, Watanabe FS, Dean LA. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington DC.: US Department of Agriculture; 1954.
Chesnin L, Yien CH. Turbidimetric determination of available sulfates. Soil Sci Soc Am J. 1951;15 C:149–51.
McGrath SP, Cunliffe CH. A simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. J Sci Food Agric. 1985;36:794–8.
Ouzounidou G, Eleftheriou EP, Karataglis S. Ecophysical and ultrastructural effects of copper in Thlaspi Ochroleucum (Cruciferae). Can J Bot. 1992;70:947–57.
Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2nd ed. New York Chichester Brisbane [etc.]: J. Wiley & sons; 1984.
Medda S, Mondal NK. Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Annals Agrarian Sci. 2017;15:396–401.
Sadak MS. Physiological role of trehalose on enhancing salinity tolerance of wheat plant. Bull Natl Res Cent. 2019;43:53.
Zulfiqar F, Chen J, Finnegan PM, Younis A, Nafees M, Zorrig W, et al. Application of Trehalose and Salicylic Acid mitigates Drought stress in Sweet Basil and improves Plant Growth. Plants. 2021;10:1078.
Feng Y, Chen X, He Y, Kou X, Xue Z. Effects of Exogenous Trehalose on the metabolism of Sugar and Abscisic Acid in Tomato seedlings under Salt stress. Trans Tianjin Univ. 2019;25:451–71.
Joshi R, Sahoo KK, Singh AK, Anwar K, Pundir P, Gautam RK, et al. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. J Exp Bot. 2020;71:653–68.
Ahanger MA, Qin C, Begum N, Maodong Q, Dong XX, El-Esawi M, et al. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019;19:479.
Iqbal N, Umar S, Khan NA. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol. 2015;178:84–91.
Ahanger MA, Agarwal RM. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol Biochem. 2017;115:449–60.
Ahanger MA, Agarwal RM. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma. 2017;254:1471–86.
Khan MIR, Nazir F, Asgher Mohd, Per TS, Khan NA. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol. 2015;173:9–18.
Mengutay M, Ceylan Y, Kutman UB, Cakmak I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil. 2013;368:57–72.
Dias MC, Moutinho-Pereira J, Correia C, Monteiro C, Araújo M, Brüggemann W, et al. Physiological mechanisms to cope with cr(VI) toxicity in lettuce: can lettuce be used in Cr phytoremediation? Environ Sci Pollut Res. 2016;23:15627–37.
Dube BK, Tewari K, Chatterjee J, Chatterjee C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere. 2003;53:1147–53.
Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L. Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol. 2010;333:597–607.
Rai V, Vajpayee P, Singh SN, Mehrotra S. Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci. 2004;167:1159–69.
Rodriguez E, Santos C, Azevedo R, Moutinho-Pereira J, Correia C, Dias MC. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol Biochem. 2012;53:94–100.
Pandey V, Dixit V, Shyam R. Chromium effect on ROS generation and detoxification in pea (Pisum sativum) leaf chloroplasts. Protoplasma. 2009;236:85–95.
Shafiq S, Akram NA, Ashraf M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci Hort. 2015;185:68–75.
Zhang Z, Sun M, Gao Y, Luo Y. Exogenous trehalose differently improves photosynthetic carbon assimilation capacities in maize and wheat under heat stress. J Plant Interact. 2022;17:361–70.
Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2020;74:1–16.
Li C, Ji J, Wang G, Li Z, Wang Y, Fan Y. Over-expression of LcPDS, LcZDS, and LcCRTISO, genes from Wolfberry for Carotenoid Biosynthesis, enhanced Carotenoid Accumulation, and Salt Tolerance in Tobacco. Front Plant Sci. 2020;11.
Choudhary SP, Kanwar M, Bhardwaj R, Yu J-Q, Tran L-SP. Chromium stress mitigation by polyamine-brassinosteroid application involves Phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE. 2012;7:e33210.
Tripathi A, Tripathi DK, Chauhan DK, Kumar N. Chromium (VI)-induced phytotoxicity in river catchment agriculture: evidence from physiological, biochemical and anatomical alterations in Cucumis sativus (L.) used as model species. Chem Ecol. 2016;32:12–33.
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M. Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf. 2016;126:245–55.
Luo Y, Li F, Wang GP, Yang XH, Wang W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol Plant. 2010;54:495–501.
Soliman M, Elkelish A, Souad T, Alhaithloul H, Farooq M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants. 2020;26:501–11.
Shahbaz M, Abid A, Masood A, Waraich EA. Foliar-applied trehalose modulates growth, mineral nutrition, photosynthetic ability, and oxidative defense system of rice (Oryza sativa L.) under saline stress. J Plant Nutr. 2017;40:584–99.
Rohman MM, Islam MR, Monsur MB, Amiruzzaman M, Fujita M, Hasanuzzaman M. Trehalose protects Maize plants from salt stress and Phosphorus Deficiency. Plants. 2019;8:568.
Rehman S, Chattha MU, Khan I, Mahmood A, Hassan MU, Al-Huqail AA, et al. Exogenously Applied Trehalose augments cadmium stress tolerance and yield of Mung Bean (Vigna radiata L.) grown in soil and Hydroponic systems through reducing cd uptake and enhancing photosynthetic efficiency and antioxidant Defense systems. Plants. 2022;11:822.
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma. 2018;255:79–93.
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma. 2018;255:459–69.
Wang B, Li Z, Ran Q, Li P, Peng Z, Zhang J. ZmNF-YB16 overexpression improves Drought Resistance and yield by enhancing photosynthesis and the antioxidant capacity of Maize plants. Front Plant Sci. 2018;9.
El-Mogy MM, Atia MAM, Dhawi F, Fouad AS, Bendary ESA, Khojah E, et al. Towards Better Grafting: SCoT and CDDP analyses for prediction of the Tomato rootstocks Performance under Drought stress. Agronomy. 2022;12:153.
Jahan MS, Hasan MM, Alotaibi FS, Alabdallah NM, Alharbi BM, Ramadan KMA, et al. Exogenous putrescine increases Heat Tolerance in Tomato seedlings by regulating Chlorophyll Metabolism and enhancing antioxidant defense efficiency. Plants. 2022;11:1038.
Abd Elbar OH, Elkelish A, Niedbała G, Farag R, Wojciechowski T, Mukherjee S et al. Protective effect of γ-Aminobutyric acid against chilling stress during Reproductive Stage in Tomato plants through modulation of Sugar Metabolism, Chloroplast Integrity, and Antioxidative Defense systems. Front Plant Sci. 2021;12.
Hasanuzzaman M, Nahar K, Bhuiyan TF, Anee TI, Inafuku M, Oku H, et al. Salicylic acid: an All-Rounder in regulating Abiotic stress responses in plants. In: El-Esawi M, editor. Phytohormones - Signaling mechanisms and Crosstalk in Plant Development and stress responses. InTech; 2017.
Ahanger MA, Ahmad P. Role of Mineral nutrients in Abiotic stress tolerance: revisiting the Associated Signaling mechanisms. In: Khan MIR, Reddy PS, Ferrante A, Khan NA, editors. Plant Signaling molecules. Woodhead Publishing; 2019. pp. 269–85.
Giri J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav. 2011;6:1746–51.
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A. Role of proline under changing environments. Plant Signal Behav. 2012;7:1456–66.
Jeandet P, Formela-Luboińska M, Labudda M, Morkunas I. The role of sugars in plant responses to stress and their Regulatory function during development. Int J Mol Sci. 2022;23:5161.
Del Bubba M, Ancillotti C, Checchini L, Ciofi L, Fibbi D, Gonnelli C, et al. Chromium accumulation and changes in plant growth, selected phenolics and sugars of wild type and genetically modified Nicotiana langsdorffii. J Hazard Mater. 2013;262:394–403.
Unal D, Isik NO, Sukatar A. Effects of chromium VI stress on photosynthesis, chlorophyll integrity, cell viability, and proline accumulation in lichen Ramalina farinacea. Russ J Plant Physiol. 2010;57:664–9.
Abdallah MM-S, El Sebai TN, Ramadan AAE-M, El-Bassiouny HMS. Physiological and biochemical role of proline, trehalose, and compost on enhancing salinity tolerance of quinoa plant. Bull Natl Res Cent. 2020;44:96.
Handa N, Kohli SK, Thukral AK, Bhardwaj R, Alyemeni MN, Wijaya L, et al. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3 Biotech. 2018;8:66.
Kundu D, Dey S, Raychaudhuri SS. Chromium (VI) – induced stress response in the plant Plantago ovata Forsk in vitro. Genes Environ. 2018;40:21.
Ibrahim HA, Abdellatif YMR. Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Annals Agricultural Sci. 2016;61:267–74.
Sadak MS, El-Bassiouny HMS, Dawood MG. Role of trehalose on antioxidant defense system and some osmolytes of quinoa plants under water deficit. Bull Natl Res Cent. 2019;43:5.
Kosar F, Akram NA, Ashraf M, Ahmad A, Alyemeni MN, Ahmad P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol Plant. 2021;172:317–33.
Świętek M, Lu Y-C, Konefał R, Ferreira LP, Cruz MM, Ma Y-H, et al. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J Nanotechnol. 2019;10:1073–88.
Czerniewicz P, Sytykiewicz H, Durak R, Borowiak-Sobkowiak B, Chrzanowski G. Role of phenolic compounds during antioxidative responses of winter triticale to aphid and beetle attack. Plant Physiol Biochem. 2017;118:529–40.
Acknowledgements
We also extend our thanks to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23066).
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RG23066).
Author information
Authors and Affiliations
Contributions
The Conceptualization, A.E, A.A, A.H, A.B, A.S; methodology, A.E, A.A, A.H, F.H, B.A, M.E, A.R, N.A, A.B, A.S ; validation, A.E, A.A, A.H, F.H, B.A, M.E, A.R, A.B, A.S ; formal analysis, A.E, A.A, A.H, F.H, B.A, M.E, N.A, A.R, A.B, A.S .; investigation, A.E, A.A, A.B, A.S data curation, A.E, A.A, A.H, A.B, A.S.; writing—original draft preparation, A.E, A.AN.A, N.A, A.B, A.S; writing—review and editing, A.E, A.A, A.H, F.H, B.A, M.E, A.R, A.B, N.A, A.S; visualization, A.E, A.A, A.B, A.S . All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Elkelish, A., Alhudhaibi, A.M., Hossain, A.S. et al. Alleviating chromium-induced oxidative stress in Vigna radiata through exogenous trehalose application: insights into growth, photosynthetic efficiency, mineral nutrient uptake, and reactive oxygen species scavenging enzyme activity enhancement. BMC Plant Biol 24, 460 (2024). https://doi.org/10.1186/s12870-024-05152-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05152-y