Abstract
Background
The era of high throughput sequencing offers new paths to identifying species boundaries that are complementary to traditional morphology-based delimitations. De novo species delimitation using traditional or DNA super-barcodes serve as efficient approaches to recognizing putative species (molecular operational taxonomic units, MOTUs). Tea plants (Camellia sect. Thea) form a group of morphologically similar species with significant economic value, providing the raw material for tea, which is the most popular nonalcoholic caffeine-containing beverage in the world. Taxonomic challenges have arisen from vague species boundaries in this group.
Results
Based on the most comprehensive sampling of C. sect. Thea by far (165 individuals of 39 morphospecies), we applied three de novo species delimitation methods (ASAP, PTP, and mPTP) using plastome data to provide an independent evaluation of morphology-based species boundaries in tea plants. Comparing MOTU partitions with morphospecies, we particularly tested the congruence of MOTUs resulting from different methods. We recognized 28 consensus MOTUs within C. sect. Thea, while tentatively suggesting that 11 morphospecies be discarded. Ten of the 28 consensus MOTUs were uncovered as morphospecies complexes in need of further study integrating other evidence. Our results also showed a strong imbalance among the analyzed MOTUs in terms of the number of molecular diagnostic characters.
Conclusion
This study serves as a solid step forward for recognizing the underlying species boundaries of tea plants, providing a needed evidence-based framework for the utilization and conservation of this economically important plant group.
Similar content being viewed by others
Introduction
While DNA sequences and other kinds of integrative data are increasingly included in the assessment of species boundaries, species delimitation in plants generally remains defined on the basis of morphological characters [1,2,3]. Morphology-based species delimitation is contentious for two reasons. First, intra- and inter-specific variation among closely related taxa often overlaps, commonly including morphological traits that have been used to distinguish taxa [4]. Second, different taxonomists may disagree with each other on the taxonomic significance of the same morphological trait [5]. It is quite common that the same specimens, especially of taxonomically difficult taxa, are assigned different names by different taxonomists, or even by the same taxonomist at different times. Thus, the application of molecular sequence data in delimiting species is a useful adjunct for resolving groups in which morphological data are indecisive, as well as for identifying currently unrecognized species-level diversity [6, 7], which in both cases may reciprocally enhance the application of morphological data [8]. Finally, accelerating the pace of taxonomic work is urgently needed to meet the challenge of the contemporary biodiversity crisis in light of climate change and anthropogenic alteration. For these and other reasons, DNA-based species delimitation and identification has been proposed for and attracted substantial interest as a complement to morphology-based taxonomy [9,10,11]. For this purpose, DNA barcoding techniques espouse the use of homologous DNA fragments applicable across relatively wide phylogenetic scales to identify or delimit taxa [12,13,14,15].
Currently, many empirical studies have investigated the robustness of DNA barcodes in identifying species under the prior knowledge of species identification based on morphology [16,17,18,19,20]. However, for highly morphologically similar taxa, taxonomic discordances between these and morphological identifications are quite common [5]. Operational factors involved in discordance include species over-splitting and lumping, which can significantly affect evaluations of the efficacy of DNA barcodes [20, 21]. In the light of this, de novo molecular species delimitation using DNA barcodes was therefore advocated without prior biological assignments [10, 11]. Originally, DNA barcodes were short DNA fragments selected from organellar and nuclear genomes, such as COI, rbcL, matK, trnH–psbA and nrITS. In the last decade, super-barcode data from the entire plastome have become widely used in identifying species [14, 22, 23], where their greater signal has found use in several taxa. For example, molecular delimitation in Orychophragmus (Brassicaceae) highlighted the application of plastomes to jointly examining species boundaries and establishing phylogenetic relationships [24]. Another case based on Polygonatum kingianum demonstrated the ability to delimit species on the basis of the plastid genome [25].
Tea plants generally refer to plants of Camellia sect. Thea (Theaceae). Almost all of the species from this section can be prepared as a nonalcoholic caffeine-containing beverage [26, 27]. The most commonly and commercially grown tea plants are C. sinensis var. sinensis and C. sinensis var. assamica [26], but other closely related species are potential beverage resources that need further investigation. Further work on potential uses of tea plants is hampered by a complex and controversial taxonomic history. There are three important taxonomic systems of C. sect. Thea. Sealy [28] first systematically studied genus Camellia in modern times and he proposed classification system of it, where only five species were included in C. sect. Thea. However, with more Camellia species being discovered, Sealy’s classification system was gradually replaced by the other two classification systems which are widely used now. One is Chang’s taxonomic system; Chang [29] recognized thirty-two species in four series of C. sect. Thea. The other is Ming’s taxonomic system; Ming [30] recognized twelve species without establishing any series in C. sect. Thea. The dramatic variation between these two taxonomic treatments for C. sect. Thea is due primarily to emphasizing different morphological characters. The existence of these significant differences, leading to conflicting morphology-based classifications, has made traditional taxonomic work and investigation of wild tea relatives in C. sect. Thea intractable. Additionally, new species have continued to be found since these treatments [31,32,33,34,35]. Since the publication of the first classification system for C. sect. Thea (1958), the recognized species diversity of tea plants has increased significantly (up to 54 new taxa published), mainly through in-depth field investigation and analysis of morphological characters [27]. However, this progress leads to doubts on the true level of species diversity of tea plants and confusion on the protection and efficient utilization of tea plants. Significantly, all species of C. sect. Thea are listed as protected in the updated List of National Key Protected Wild Plants of China in 2021 and are ranked as category II, while the numbers of species are not specified [36]. Therefore, understanding species diversity and properly delimiting species boundaries are of realistic value in both conserving and utilizing tea plants.
Based on our careful examination of specimens and wide-range field survey, most tea plants are highly morphologically similar, with small differences in ovary, sepal, and the size of flowers and fruits being the primary morphological variation (Fig. 1). Notably, in some of the diagnostic morphological characters variation often overlaps, making species identification and delimitation in tea plants extremely challenging and also leading to a misestimate of the species diversity of tea plants. Despite the disagreements on the classification of tea plants, previous phylogenetic studies have primarily tested the monophyly of C. sect. Thea [37,38,39] without further detailed study on species delimitation within the section. Molecular species delimitation using DNA barcodes has not yet been applied in C. sect. Thea, with few studies only focusing specifically on C. sinensis that did not further discuss the species delimitation of the section due to limited sampling [34, 40].
Currently, plastomes can be rapidly and inexpensively obtained due to the improvement of next-generation sequencing (NGS) techniques [41,42,43]. Here, we collected 165 samples of major extant species of C. sect. Thea and DNA barcoded by sequencing plastomes. Based on multiple molecular species delimitation approaches, we evaluated the effectivity of the plastome as a super-barcode in delimiting tea plants. We aim to reassess the validity of morphology-based species boundaries of tea plants using plastomes, and to explore the underlying species-level diversity of tea plants.
Materials and methods
Sample collection and sequencing
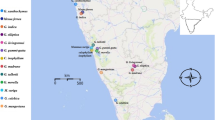
The collected samples were assigned to morphospecies by reference to recent taxonomic literature. Both the Chang and Ming’s classification systems were integrated in this study [29, 30]. Herbarium material (including types) for each species was also investigated, and most samples were collected from type localities. Dr. Shixiong Yang undertook the formal identification of the plant material used in our study. All samples were collected in the field (Fig. 2) with fresh leaf tissue dried in silica gel. In total, plastomes of 165 samples representing 39 morphospecies of C. sect. Thea were sequenced (Table S1), of which 34 morphospecies were represented by more than one individual (2–10) and five morphospecies were singletons (population-level sampling in Table S1). Camellia mairei (GenBank accession: KY406767) and C. reticulata (GenBank accession: KY406793) from C. sect. Camellia were selected as outgroups, whose plastome data were obtained from our previous study [44]. The vouchers were deposited in the Herbarium of the Kunming Institute of Botany (KUN), Chinese Academy of Sciences, Yunnan, China.
Map of geographical distribution of samples of C. sect. Thea for this study. The map was constructed using the mapbox (https://studio.mapbox.com/)
Dried samples were then subjected to total genomic DNA extraction following a modified cetrimonium bromide (CTAB) protocol [45], followed by two alternative sequencing methods. For 115 samples, the plastomes were amplified in overlapping fragments using the long-range PCR method [46], and PCR fragments were then pooled together in roughly equal concentrations for subsequent sequencing. Paired-end sequencing of 250 bp was conducted on the Illumina MiSeq platform at the Molecular Biology Experiment Center, Germplasm Bank of Wild Species in Southwest China. About 200 Mb − 2Gb sequencing data were generated for each sample. For the genome skimming of the remaining 50 samples, paired-end sequencing libraries were generated from total DNA following the manufacturer’s protocol (Illumina) with an insertion size of 350 bp and were sequenced on the Illumina NovaSeq 6000 platform with the 150-bp paired-end sequencing model. Approximately 2 Gb sequencing data were generated for each sample.
Genome assembly, annotation, and alignment
Quality control of raw sequence reads was performed using fastp v0.20.1 [47] with default parameters. Plastomes were first de novo assembled using GetOrangelle pipeline v1.7.5.2 [48] based on the reads from both long-range PCR and genome skimming sequencing. Successfully completed assemblies were annotated using PGA [49], then the intron/exon boundaries were manually checked and adjusted. Most reads generated by long-range PCR method failed to be assembled as a circle plastome using GetOrganelle pipeline v1.7.5.2 [48] and only contigs were obtained. Therefore, for these samples, we used a reference sequence (Camellia remotiserrata; GenBank accession number KY406759) as a mapping reference to complete the assembly. Contigs from long-range PCR reads were mapped to the reference using Bowtie 2 [50] with default parameters. The obtained mapping files were exported in SAM (Sequence Alignment/Map) format. Next, using Geneious v8.02 [51], consensus sequences were extracted from these SAM files and then aligned using MAFFT plugin with default parameters, then manually checked and adjusted. The consensus sequences were annotated according to the reference. Including two outgroups, plastome sequences (excluding one IR region) from 165 individuals were aligned using MAFFT v7.471 [52] with default settings. Poorly aligned regions were refined by trimAl v1.4.rev15 [53] using "-automated1" command, then manually checked and adjusted.
Genetic diversity analysis
Nucleotide diversity across 165 samples was estimated using DnaSP v6.12.03 [54] with a sliding window of 2,000 bp and step size of 200 bp. The maximum intraspecific genetic distances among 39 morphospecies were calculated using MEGA X [55]. Analyses were conducted using the Kimura 2-parameter model [56].
Phylogenetic analysis
Maximum likelihood (ML) analysis was performed using RAxML v8.2.12 [57] based on the General Time Reversible + gamma model (GTR-GAMMA model) with 1000 rapid bootstraps replications. Bayesian inference (BI) analysis was performed using MrBayes v3.2 [58]. Two independent Markov chain Monte Carlo (MCMC) runs were executed. Four chains were run for two million generations with random initial trees and sampling every 100 generations. The first 25% of the trees were discarded as burn-in. Moreover, we carried out Quartet Sampling (QS) analysis based on plastome dataset and the ML tree with 1,000 replicates to dissect phylogenetic discordance within plastome, which were recently found in other organisms [59,60,61,62]. The QS method was designed to evaluate the consistency of information (Quartet Concordance score, QC), the presence of secondary evolutionary histories (Quartet Differential score, QD), the amount of information (Quartet Informativeness score, QI), and the reliability of individual taxa in the tree (Quartet Fidelity score, QF). The resultant trees were visualized and edited in FigTree v1.4.4 [63].
Molecular species delimitation analysis
Two methods (tree-based and distance-based methods) were used for de novo species delimitation of C. sect Thea based on plastome sequences. The above two approaches were performed using the PTP (Poisson Tree Processes) model [64], and ASAP (Assemble Species by Automatic Partitioning) [65], respectively. The PTP model is a tree-based method that models the expected number of substitutions for intra- vs. interspecific gene tree branch lengths, identifying transition points in the tree as delimited species [64]. The PTP modeling was performed with PTP web server (https://species.h-its.org/) with 500,000 replicates using the maximum likelihood implementation (PTP-ML), as well as the Bayesian implementation (PTP-BI). In addition, multirate PTP (mPTP) was performed to further assess the confidence of the previous PTP analyses by accounting for differences between species in sampling and genetic structure [66]. The ML tree was used as input for all PTP analyses.
In contrast, distance-based methods do not utilize the tree topology, but instead rely on genetic distances, such as the ASAP algorithm [65]. ASAP analysis was conducted on the webserver (https://bioinfo.mnhn.fr/abi/public/asap/) based on p distance model using the plastome alignment. The ASAP algorithm partitions sequence into “group” by ascending hierarchical clustering based on sequence similarity [65]. The best two MOTUs partition predicted by ASAP were chosen to compare with other methods.
Comparison of morphospecies and MOTU
The congruence between MOTUs and morphospecies assignment was evaluated by the match ratio (morphology) [67]. The match ratio (morphology) is equal to 2 × Nmorph/(NMOTU + NMORPH), where Nmorph is the number of matches of morphospecies (all samples) with MOTUs, NMOTU is the number of MOTUs, and NMORPH is the number of morphospecies. Since nonmonophyletic species are unlikely to be correctly delimited by any method based on molecular data [68,69,70], for operational purposes, we recognized monophyletic morphospecies based on the ML tree. The congruence among different molecular species delimitation methods were then assessed by the match ratio (monophyly). The match ratio (monophyly) is equal to Nmono/NMONO, where Nmono is the number of MOTUs that were consistent with monophyletic morphospecies, and NMONO is the number of total monophyletic morphospecies.
Finally, to account for uncertainty and limitations in the implementation of individual methods [71], consensus MOTUs (c-MOTUs) were determined following several criteria [70, 72]: (i) MOTUs that were delimited identically by two of three methods were accepted; (ii) the c-MOTUs were monophyletic; (iii) sympatric MOTUs were accepted while allopatric MOTUs were rejected. We thereafter calculated the molecular diagnostic characters (MDC) for each c-MOTU using FASTACHAR v. 0.2.5 software [73].
Results
Sampling, characteristics of sequencing data and datasets
For the newly sequenced 165 samples, the number of cleaned Illumina sequencing reads ranged from 251,240 to 30,000,000. The de novo assembly generated 165 complete or near-complete plastomes, ranged from 130,314 to 133,043 bp when excluding one IR region (Table S1). The aligned dataset of plastome sequences was 131,047 bp after manual adjustment. It included 2,183 variable sites (1.67%), among which 1,771 sites were parsimony-informative (1.35%). Based on the DNA polymorphism analysis, we found that the plastome variability was very low with an overall nucleotide diversity (0.00146) ≤ 0.01 (Table 1).
Phylogenetic reconstruction
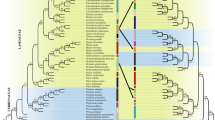
Phylogenetic reconstructions based on RAxML and MrBayes analyses obtained identical topology (Fig. S1), and five clades were identified (Fig. 3). Seventeen morphospecies (43.59%) were recovered as monophyletic, including Camellia atrothea, C. crassicolumna var. shangbaensis, C. danzaiensis, C. dishiensis, C. fangchengensis, C. formosensis, C. glaberrima, C. grandibracteata, C. gymnogyna, C. kwangtungenesis, C. makuanica, C. nanchuanica, C. parvisepala, C. polyneura, C. pubescens, C. quinquelocularis, and C. sinensis var. sinensis (Fig. 3).
Maximum likelihood phylogeny of tea plants with information of morphospecies assignments, information of collection sites, and delimited MOTUs. The branch colors represent the QS scores (QC/QD/QI). Red represents full or strong support (QC ≥ 0.5), purple represents moderate support (0.2 ≤ QC < 0.5), blue represents weak support (0 ≤ QC < 0.2), and green represents counter support (QC < 0), according to Pease et al. (2018). The geographical distribution of collection sites is marked on the voucher number (sample name) with different colors. Taxa names in red indicate monophyly and taxa names in black indicate non-monophyly. Red solid boxes besides taxa names indicate agreement between molecular species delimitation method and morphospecies assignment, while blue solid boxes indicate disagreement. Hollow boxes indicate the final MOTUs
Clade I consisted of three individuals of C. leptophylla while other three individuals formed a subclade clustered within Clade IV, although all of them were collected from the type locality (Longzhou, Guangxi, China). Clade II contained 11 morphospecies, all samples of C. danzaiensis, C. pubescens, C. gymnogyna and C. fangchengensis clustered as monophyletic groups, respectively. Most morphospecies in this clade were characterized by having 3-locule ovary except for C. tachangensis and C. tetracarpa.
Clade III exclusively contained C. remotiserrata (2906, 5298, 5048, 5049, 5071, 5072) and C. nanchuanica (6792), as well as cultivated individuals of C. arborescens (2909) and C. sinensis var. pubilimba (5062), both of which were introduced by wild C. remotiserrata germplasm. Species in this clade exhibited a geographic cluster pattern. Sample 2906 of C. remotiserrata, 2909 of C. arborescens (from Yunnan, Weixin), 5048, 5049, 5071, 5072 of C. remotiserrata (from Sichuan, Yibin), 5298 of C. remotiserrata (from Guizhou Chishui) and 6792 of C. nanchuanica (from Chongqing Nanchuan) overlapped in distribution.
Clade IV included 10 morphospecies, of which six were resolved as monophyletic. Most samples of C. sinensis (C. sinensis var. sinensis, C. sinensis var. assamica, C. sinensis var. dehungensis and C. sinensis var. publimba) were nested in this clade, along with their relatives (C. dishiensis, C. formosensis, C. parvisepaloides, C. parvisepala, and C. grandibracteata). Among the five relatives, except C. grandibracteata, four species have been synonymized under the name of C. sinensis. Samples of C. kwangtungensis, which has been put in C. sect. Glaberrima by Chang & Ren (1998), formed a monophyletic group. Clade V was the largest clade mainly composed of morphospecies with 5-locule ovary, five morphospecies were exceptions. Camellia glaberrima, C. polyneura, and C. sinensis var. kucha were characterized by having 3-locule ovary. Camellia tetracarpa had 4-locule ovary, and C. longlingensis varied from 3-locule to 5-locule ovary.
In the QS analysis, we considered a QC score of ≥ 0.5 to be strong support. In general, strong-supported nodes had long internal branches while low QC values were coupled with short branch lengths (Fig. 3). The mean QC score for the internodes was 0.65. Weak support (QC = 0.039) was found at the branch separating Clade II from the rest of samples (Fig. S2). All the monophyletic morphospecies were recovered with strong support (QC = 1). Moreover, branches with negative QC scores with high QD scores were observed in both 5-loculed and trilocular taxa, suggestive of putative incomplete lineage sorting (ILS) (Fig. S2). The average QI score for nodes was 0.86, and the majority (67%) were above the average, indicating that sequence variation was not a limiting issue. The QF scores for all samples were above 0.5, and the mean QF score was 0.77, indicating that rogue taxa were not an issue affecting phylogenetic inference (Table S2).
Molecular species delimitation
The MOTUs produced by different molecular species delimitation methods conflicted with each other, as well as with the morphology-based species assignments (Table 2). PTP analysis resulted in two MOTU partitioning schemes. One was the maximum likelihood solution (PTP-ML), distinguishing 45 MOTUs with six matches and the match ratios were 0.14 for morphology and 0.35 for monophyly. The other was the Bayesian solution (PTP-BI); PTP-BI produced 100 MOTUs with five matches with the morphology (0.07) and monophyly (0.29). However, mPTP only produced six MOTUs and none of the MOTUs defined by mPTP were congruent with morphospecies. The best two ASAP results produced 54 or 61 MOTUs, thus the resultant match ratios with morphology were 0.26 and 0.21, respectively. Twelve monophyletic morphospecies (0.71) were both recovered in the two ASAP results. In total, 22 non-monophyletic morphospecies were inconsistent with MOTUs delimited by all methods. Additionally, not all monophyletic morphospecies agreed with MOTUs.
A final count of 28 c-MOTUs was therefore suggested. Among these, 16 c-MOTUs were detected by multiple conspecific samples, including c-MOTU-1, 3, 6, 10–13, 15–17, 19, 22, 24–27. Two c-MOTUs were consisted of singletons (c-MOTU-2 and -23). The other 10 c-MOTUs comprised morphospecies complexes, which appeared to be indistinguishable either by morphology or by plastomes. In total, we detected from 11 to 1,252 plastid MDCs among these 28 c-MOTUs. The lowest number of MDCs was found between c-MOTU-11 and c-MOTU-18. The highest number of MDCs was found between c-MOTU-4 and c-MOTU-19 (Table S3).
Discussion
Comparing the performance of molecular species delimitation methods
This study describes a protocol for rapidly obtaining a primary species delimitation scheme. In some cases, plastome data contributes to discovering cryptic diversity or sorting out problematic taxonomic treatments [24, 74, 75], and have formed the primary basis of formal taxonomic revisions [25, 76]. In other cases, an integrative strategy including plastome data has been applied for delimiting species. For example, species boundaries in the Maddenia group of Prunus were clarified based on not only plastomes but also nuclear data and morphology [77]. Compared with the varying steps needed for different types of data, our protocol has the advantage of using highly accessible single-locus data with defined criteria to produce a testable primary taxonomic framework.
The high incongruences between MOTUs and morphospecies in Camellia sect. Thea, together with the observed conflicts within different molecular species delimitation methods indicate that plastomes are unable to completely clarify morphology-based species boundaries of tea plants. The incongruence across delimitation methods is inevitable and attributable to the inherent limitations of methods [65, 78]. Successful applications of molecular species delimitation methods require intellectual and methodological consensus [71]. Different statistical approaches and a priori criteria for delimiting species might result in conflicting delimitations of species boundaries [79]. On the one hand, distance-based methods (e.g. ASAP) are based on a similarity criterion, while tree-based methods (e.g. PTP and mPTP) are based on a phylogenetic topology criterion. In addition, although PTP and ASAP are based on different criteria, they both agree on the premise that all species are expected to be reciprocally monophyletic, which accounts for the increased match rate when only considering monophyletic morphospecies in the study. However, many studies have found that non-monophyly is quite extensive among plant species [80,81,82]. Therefore, these criteria can be practically problematic to apply in delimiting species boundaries given the complex reticulation process in plants, such as introgression and hybridization [83, 84]. This is particularly true for tea plants, where the evolutionary history is further complicated by human intervention, such as describing new taxa from cultivation, and hybridization among tea plants when they are planted together [27, 85, 86].
On the other hand, sampling may also affect the output of different delimitation methods [67, 87, 88], as is the case of tea plants studied here. The discordance between monophyletic morphospecies and MOTUs indicated limited sampling could lead to monophyly ascertainment biases [67, 70]. In addition, oversampling of closely related individuals within one species might risk causing grouping some of individuals within other relatively distinct species, resulting in over-splitting the former species using molecular delimitation methods [89]. In two cases (C. tetracarpa and C. tachangensis), despite being sampled from the same locality, some of their samples nested with morphological distinct species, such as trilocular C. costata and C. yungkiangensis, while the other nested together. As expected, all the methods over-split them, hence violating the prior morphological assignments. In addition, geographic sampling bias may exacerbate intraspecific variation, which has been shown to decrease the efficacy of species delimitation using molecular data [64, 66]. In such cases, mPTP may be more accurate as it can account for divergent intraspecific variation among species [66, 78]. However, the estimations yielded by mPTP tend to be too conservative in empirical studies [90]. As exemplified for Clades II to V, mPTP collapsed many morphologically distinct taxa, some of which are uncontroversial, into one. However, the other methods likewise perhaps over-split compared with the mPTP result. This was observed in groups of monophyletic C. formosensis, which were delimited as several discrete MOTUs by PTP-ML, PTP-BI, and ASAP (Fig. 3). Therefore, considering that the species estimations yielded by these methods are likely the result of their inherent limitations, operational tendency to over-split or lump, and the general difficulty of species identification in Camellia sect. Thea, we propose using multiple methods to cross-validate with each other.
Uncovering the species delimitation of tea plants
Our study sampled 39 morphospecies of tea plants, accounting for 75% of 52 legal names in C. sect. Thea under the Botanical Code [27, 91]. This therefore represents by far the most comprehensive sampling across C. sect. Thea attempted to date. The examined 39 morphospecies of tea plants resulted in 28 c-MOTUs considering the results among methods employed, which reflect those lineages likely to be distinct species. The final c-MOTUs were more than twice as numerous as those recognized in Ming’s taxonomic system while less numerous than Chang’s taxonomic system for C. sect. Thea. The introduction of molecular data, not always agreeing with morphological data, has therefore largely complicated the taxonomy of C. sect. Thea. In general, two interpretations can be drawn from the cases of incongruence between MOTU and morphospecies. One is that misleading morphological variation results in incorrect species delimitation. In these cases, incongruent MOTUs might instead reflect true species boundaries. The other is that molecular species delimitation methods are not powerful enough to solve complex biological factors underlying the speciation process because of limitations in algorithms or data.
We found conditions in tea plants were even more complex as the two interpretations appear to be interleaved. Ten morphospecies were merged with their sister morphospecies in a single c-MOTU partition (c-MOTU-4, 5, 7, 8, 9, 14, 18, 20, 21, and 28). In one case, C. yungkiangensis clustered with C. gymnogyna within one MOTU. However, the merge of C. yungkiangensis and C. gymnogyna was not supported by morphology. Similarly, Ming (1992) treated C. yungkiangensis as a synonym of C. costata [92], but C. yungkiangensis and C. costata nested in different subclades in Clade II in this analysis. Two morphospecies (C. grandibracteata and C. haaniensis) showed similar patterns: C. grandibracteata was merged with C. sinensis var. assamica and C. haaniensis was merged with C. crispula (Fig. 3). C. grandibracteata might be a hybrid of C. sinensis var. assamica and C. taliensis, which was also supported by morphological evidence [86]. In addition, Camellia haaniensis was synonymized under C. crispula [93]. In another case, C. remotiserrata and C. nanchuanica were merged in Clade III. First, Ming (1992) synonymized C. remotiserrata and C. nanchuanica under C. gymnogyna var. remotiserrata [92]. Later, Ming (1999) made a new combination—C. tachangensis var. remotiserrata, and therefore C. remotiserrata and C. nanchuanica became synonyms of C. tachangensis var. remotiserrata [94]. Our results supported that C. remotiserrata and C. nanchuanica should be the same species. However, the relationship between C. remotiserrata and C. tachangensis needs further investigation.
Considering the contrasting case where molecular data recognize more taxa, up to 10 morphospecies of C. sect. Thea were split into different MOTUs, including C. leptophylla, C. arborescens, C. tachangensis, C. tetracarpa, C. sinensis var. pubilimba, C. kwangsiensis, C. kwangnanica, C. crispula, C. sinensis var. kucha, and C. multiplex. The splits of 10 morphospecies were primarily associated with geographical clustering of individuals (in the same place or nearby), except for C. leptophylla and C. sinensis var. pubilimba. Therefore, these cases of splits might reflect the possibility of cryptic species.
Finally, 10 c-MOTUs agreed with morphology assignments: Camellia atrothea, C. danzaiensis, C. dishiensis, C. formosensis, C. kwangtungensis, C. glaberrima, C. makuanica, C. parvisepala, C. quinquelocularis, and C. sinensis var. sinensis. They might be distinct species but still need further validation. For example, Camellia danzaiensis, C. glaberrima and C. kwangtungensis belong to C. sect. Glaberrima, which is morphologically distinct with C. sect. Thea in Chang’s classification system [29]. However, Ming merged C. sect. Glaberrima into C. sect. Thea [92]. In addition, none of the three species were accepted as distinct species in Ming’s classification, in which Camellia danzaiensis and C. kwangtungensis were synonymized under C. costata and C. glaberrima was synonymized under C. gymnogyna [92, 94]. Our phylogenetic results indicated that three species of sect. Glaberrima had a close relationship with species of C. sect. Thea. In addition, all three species were resolved as monophyletic groups and therefore recovered as three c-MOTUs, respectively.
According to botanical codes of nomenclature, scientific names are based on type specimens, which are often not available for DNA analysis, or in some cases are even lost. Therefore, in assemblages of taxonomically difficult morphospecies, species names associated with molecular diagnostic characters tend to have greater taxonomic utility in many contexts than those solely based on morphology.
Conclusion
Our study integrated multiple species delimitation approaches based on plastome data to evaluate the validity of 39 morphospecies in C. sect. Thea. We proposed 28 c-MOTUs, fewer than species assignments based on morphology. Although, the number of molecular diagnostic characters varied irregularly among 28 c-MOTUs. The fact that molecular species delimitation of tea plants conflicts with morphology highlights the incompatibility of extant taxonomic systems of C. sect. Thea. Even without accurate prior biological knowledge, an estimate of species richness and delimitation can be obtained through the simple and fast algorithmic processing of molecular data. While taxonomic decisions based on analyses of plastome data do pose risks, they are useful if the results of molecular species delimitation are viewed as drafts for taxonomy rather than as the sole criterion for species description. Therefore, our research provides taxonomists with a starting point for taxonomic revision of C. sect. Thea.
Availability of data and materials
All DNA sequences generated are available from the GenBank database. GenBank accession numbers of all samples included in this study are provided in Table S1. Alignment matrixes and phylogenetic trees are available from Figshare (https://figshare.com/s/f38c8ab9de141d677973).
References
Cicero C, Mason NA, Jiménez RA, Wait DR, Wang-Claypool CY, Bowie RCK. Integrative taxonomy and geographic sampling underlie successful species delimitation. Ornithology. 2021;138:ukab009.
Hörandl E. Novel approaches for species concepts and delimitation in polyploids and hybrids. Plants. 2022;11:204.
Liu J-Q. The integrative species concept and species on the speciation way. Biodivers Sci. 2016;24:1004–8.
Duminil J, Di Michele M. Plant species delimitation: a comparison of morphological and molecular markers. Plant Biosyst. 2009;143:528–42.
Spooner DM. DNA barcoding will frequently fail in complicated groups: an example in wild potatoes. Am J Bot. 2009;96:1177–89.
Durand J-D, Shen K-N, Chen W-J, Jamandre BW, Blel H, Diop K, et al. Systematics of the grey mullets (Teleostei: Mugiliformes: Mugilidae): molecular phylogenetic evidence challenges two centuries of morphology-based taxonomy. Mol Phylogenet Evol. 2012;64:73–92.
Hubert N, Hanner R. DNA Barcoding, species delineation and taxonomy: a historical perspective. DNA Barcodes. 2015;3:44–58.
Folk RA, Freudenstein JV. “Sky islands” in the eastern U.S.A.? — strong phylogenetic structure in the Heuchera parviflora group (Saxifragaceae). Taxon. 2015;64:254–71.
Engel MS, Ceríaco LMP, Daniel GM, Dellapé PM, Löbl I, Marinov M, et al. The taxonomic impediment: a shortage of taxonomists, not the lack of technical approaches. Zool J Linn Soc. 2021;193:381–7.
Tautz D, Arctander P, Minelli A, Thomas RH, Vogler AP. DNA points the way ahead in taxonomy. Nature. 2002;418:479.
Tautz D, Arctander P, Minelli A, Thomas RH, Vogler AP. A plea for DNA taxonomy. Trends Ecol Evol. 2003;18:70–4.
Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci. 2003;270:313–21.
Hollingsworth PM, Graham SW, Little DP. Choosing and using a plant DNA barcode. PLoS One. 2011;6:e19254.
Hollingsworth PM, Li D-Z, van der Bank M, Twyford AD. Telling plant species apart with DNA: from barcodes to genomes. Philos Trans R Soc B Biol Sci. 2016;371:20150338.
Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci. 2005;102:8369–74.
Fu C-N, Mo Z-Q, Yang J-B, Cai J, Ye L-J, Zou J-Y, et al. Testing genome skimming for species discrimination in the large and taxonomically difficult genus Rhododendron. Mol Ecol Resour. 2022;22:404–14.
Ji Y-H, Yang J, Landis JB, Wang S-Y, Yang Z-Y, Zhang Y-H. Deciphering the taxonomic delimitation of Ottelia acuminata (Hydrocharitaceae) using complete plastomes as super-barcodes. Front Plant Sci. 2021;12:681270.
Liu J, Milne RI, Möller M, Zhu G-F, Ye L-J, Luo Y-H, et al. Integrating a comprehensive DNA barcode reference library with a global map of yews (Taxus L.) for forensic identification. Mol Ecol Resour. 2018;18:1115–31.
Pang X-B, Liu H-S, Wu S-R, Yuan Y-C, Li H-J, Dong JS, et al. Species identification of oaks (Quercus L., Fagaceae) from gene to genome. Int J Mol Sci. 2019;20:5940.
Yu X-Q, Jiang Y-Z, Folk RA, Zhao J-L, Fu C-N, Fang L, et al. Species discrimination in Schima (Theaceae): next-generation super-barcodes meet evolutionary complexity. Mol Ecol Resour. 2022;22:3161–75.
Funk DJ, Omland KE. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst. 2003;34:397–423.
Coissac E, Hollingsworth PM, Lavergne S, Taberlet P. From barcodes to genomes: extending the concept of DNA barcoding. Mol Ecol. 2016;25:1423–8.
Li X-W, Yang Y, Henry RJ, Rossetto M, Wang Y-T, Chen S-L. Plant DNA barcoding: from gene to genome. Biol Rev. 2015;90:157–66.
Hu H, Hu Q-J, Al-Shehbaz IA, Luo X, Zeng T-T, Guo X-Y, et al. Species delimitation and interspecific relationships of the genus orychophragmus (Brassicaceae) inferred from whole chloroplast genomes. Front Plant Sci. 2016;7:1826.
Guo X-R, Shi N-X, Xie P-X, Zhang G-F, Liu H-Y, Ji Y-H. Plastome sequencing for accurate and effective authentication of Polygonatum kingianum (Asparagaceae). Ind Crops Prod. 2022;184:115056.
Mondal TK, Bhattacharya A, Laxmikumaran M, Singh AP. Recent advances of tea (Camellia sinensis) biotechnology. Plant Cell Tissue Organ Cult. 2004;76:195–254.
Yang S-X. Thinking on the taxonomy of Camellia sect. Thea.. J Tea Sci. 2021;41:439–53.
Sealy JR. A revision of the genus Camellia. London: The Royal Horticultural Society; 1958.
Chang H-T, Ren S-X. Theaceae. In: Flora Reipublicae Popularis Sinicae. Beijing: Science Press; 1998. p. 115–37.
Ming T-L, Bartholomew B. Theaceae. In: Flora of China. Beijing & St. Louis: Science Press &Missouri Botanical Garden Press; 2007. p. 367–412.
Le Q-U, Nguyen D-L, Lay L-H. Camellia sinensis var. dulcamara (Camellia, Theaceae), a new var. and subvar. recorded for sect. Thea from Northern Vietnam. J New Biol Rep. 2020;9:44–9.
Le VS, Curry AS, Truong QC, Luong VD, Nguyen TL. Camellia flosculora: a new species of Camellia section Thea series Sinenses (Theaceae) from Vietnam. Brittonia. 2021;73:220–8.
Orel G, Curry AS. In pursuit of hidden Camellias: 32 new Camellia species from Vietnam and China. Sydney: Theaceae Exploration Associates; 2015.
Su M, Hsieh C, Tsou C. The confirmation of Camellia formosensis (Theaceae) as an independent species based on DNA sequence analyses. Bot Stud. 2009;50:477–85.
Wang J-H, Chen Q-H, Lin C-H. A new variety of Camellia (Theaceae) in Guizhou-mist Hyson. Seed. 2011;30:65–6.
Lu Z-L, Qin H-N, Jin X-H, Zhang Z-X, Yang Q-W, Hong D-Y, et al. On the necessity, principle, and process of updating the List of National Key Protected Wild Plants. Biodivers Sci. 2021;29:1577–82.
Wu Q, Tong W, Zhao H-J, Ge R-H, Li R-P, Huang J, et al. Comparative transcriptomic analysis unveils the deep phylogeny and secondary metabolite evolution of 116 Camellia plants. Plant J. 2022;111:406–21.
Zhang Q, Zhao L, Folk RA, Zhao J-L, Zamora NA, Yang S-X, et al. Phylotranscriptomics of Theaceae: generic-level relationships, reticulation and whole-genome duplication. Ann Bot. 2022;129:457–71.
Zhao D-W, Hodkinson TR, Parnell JAN. Phylogenetics of global Camellia (Theaceae) based on three nuclear regions and its implications for systematics and evolutionary history. J Syst Evol. 2022;61:356–68.
De Castro O, Comparone M, Di Maio A, Del Guacchio E, Menale B, Troisi J, et al. What is in your cup of tea? DNA verity test to characterize black and green commercial teas. PLoS One. 2017;12:e0178262.
Hu T, Chitnis N, Monos D, Dinh A. Next-generation sequencing technologies: an overview. Hum Immunol. 2021;82:801–11.
Soltis DE, Gitzendanner MA, Stull G, Chester M, Chanderbali A, Chamala S, et al. The potential of genomics in plant systematics. Taxon. 2013;62:886–98.
Twyford AD, Ness RW. Strategies for complete plastid genome sequencing. Mol Ecol Resour. 2017;17:858–68.
Yu X-Q, Gao L-M, Soltis DE, Soltis PS, Yang J-B, Fang L, et al. Insights into the historical assembly of East Asian subtropical evergreen broadleaved forests revealed by the temporal history of the tea family. New Phytol. 2017;215:1235–48.
Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987;19:11–5.
Yang J-B, Li DZ, Li H-T. Highly effective sequencing whole chloroplast genomes of angiosperms by nine novel universal primer pairs. Mol Ecol Resour. 2014;14:1024–31.
Chen S-F, Zhou Y-Q, Chen Y-R, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90.
Jin J-J, Yu W-B, Yang J-B, Song Y, dePamphilis CW, Yi T-S, et al. GetOrganelle: a fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21:241.
Qu X-J, Moore MJ, Li D-Z, Yi T-S. PGA: a software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods. 2019;15:50.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80.
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–3.
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–302.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–9.
Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–20.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–3.
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42.
Pease JB, Brown JW, Walker JF, Hinchliff CE, Smith SA. Quartet Sampling distinguishes lack of support from conflicting support in the green plant tree of life. Am J Bot. 2018;105:385–403.
Yang Y-Y, Qu X-J, Zhang R, Stull GW, Yi T-S. Plastid phylogenomic analyses of Fagales reveal signatures of conflict and ancient chloroplast capture. Mol Phylogenet Evol. 2021;163:107232.
Zhang R, Wang Y-H, Jin J-J, Stull GW, Bruneau A, Cardoso D, et al. Exploration of plastid phylogenomic conflict yields new insights into the deep relationships of Leguminosae. Syst Biol. 2020;69:613–22.
Zhang X, Sun Y-X, Landis JB, Lv Z-Y, Shen J, Zhang H-J, et al. Plastome phylogenomic study of Gentianeae (Gentianaceae): widespread gene tree discordance and its association with evolutionary rate heterogeneity of plastid genes. BMC Plant Biol. 2020;20:340.
Rambaut A. FigTree. 2018. Available from https://github.com/rambaut/figtree/releases.
Zhang J-J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013;29:2869–76.
Puillandre N, Brouillet S, Achaz G. ASAP: assemble species by automatic partitioning. Mol Ecol Resour. 2021;21:609–20.
Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, et al. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics. 2017;33:1630–8.
Ahrens D, Fujisawa T, Krammer H-J, Eberle J, Fabrizi S, Vogler A. Rarity and incomplete sampling in DNA-based species delimitation. Syst Biol. 2016;65:478–94.
Kekkonen M, Mutanen M, Kaila L, Nieminen M, Hebert PDN. Delineating species with DNA barcodes: a case of taxon dependent method performance in moths. PLoS One. 2015;10:e0122481.
Mutanen M, Kivelä SM, Vos RA, Doorenweerd C, Ratnasingham S, Hausmann A, et al. Species-level para- and polyphyly in DNA barcode gene trees: strong operational bias in European Lepidoptera. Syst Biol. 2016;65:1024–40.
Pentinsaari M, Vos R, Mutanen M. Algorithmic single-locus species delimitation: effects of sampling effort, variation and nonmonophyly in four methods and 1870 species of beetles. Mol Ecol Resour. 2017;17:393–404.
Carstens BC, Pelletier TA, Reid NM, Satler JD. How to fail at species delimitation. Mol Ecol. 2013;22:4369–83.
Kekkonen M, Hebert PDN. DNA barcode-based delineation of putative species: efficient start for taxonomic workflows. Mol Ecol Resour. 2014;14:706–15.
Merckelbach LM, Borges LMS. Make every species count: fastachar software for rapid determination of molecular diagnostic characters to describe species. Mol Ecol Resour. 2020;20:1761–8.
Firetti F, Zuntini AR, Gaiarsa JW, Oliveira RS, Lohmann LG, Van Sluys M. Complete chloroplast genome sequences contribute to plant species delimitation: a case study of the Anemopaegma species complex. Am J Bot. 2017;104:1493–509.
Rivas-Chamorro M, Cadenillas R, Ge X, Jin L, Millán B, Roncal J. Testing species relationships and delimitation in the Amazonian hyperdominant Astrocaryum section Huicungo (Arecaceae) using chloroplast data from genome skimming. Taxon. 2023;72:501–14.
Ji Y, Yang J, Landis JB, Wang S, Jin L, Xie P, et al. Genome skimming contributes to clarifying species limits in Paris section Axiparis (Melanthiaceae). Front Plant Sci. 2022;13:832034.
Su N, Liu B, Wang J, Tong R, Ren C, Chang Z, et al. On the species delimitation of the Maddenia group of Prunus (Rosaceae): evidence from plastome and nuclear sequences and morphology. Front Plant Sci. 2021;12:743643.
Blair C, Bryson RW. Cryptic diversity and discordance in single-locus species delimitation methods within horned lizards (Phrynosomatidae: Phrynosoma). Mol Ecol Resour. 2017;17:1168–82.
Leavitt SD, Divakar PK, Crespo A, Lumbsch HT. A matter of time — understanding the limits of the power of molecular data for delimiting species boundaries. Herzogia. 2016;29:479–92.
Fazekas AJ, Kesanakurti PR, Burgess KS, Percy DM, Graham SW, Barrett SCH, et al. Are plant species inherently harder to discriminate than animal species using DNA barcoding markers? Mol Ecol Resour. 2009;9(Suppl. 1):130–9.
Freudenstein JV, Broe MB, Folk RA, Sinn BT. Biodiversity and the species concept—lineages are not enough. Syst Biol. 2017;66:644–56.
Rieseberg LH, Brouillet L. Are many plant species paraphyletic? Taxon. 1994;43:21–32.
Bangs MR, Douglas MR, Chafin TK, Douglas ME. Gene flow and species delimitation in fishes of Western North America: Flannelmouth (Catostomus latipinnis) and Bluehead sucker (C. Pantosteus discobolus). Ecol Evol. 2020;10:6477–93.
Cooney CR, Thomas GH. Heterogeneous relationships between rates of speciation and body size evolution across vertebrate clades. Nat Ecol Evol. 2021;5:101–10.
Meegahakumbura MK, Wambulwa MC, Thapa KK, Li MM, Möller M, Xu JC, et al. Indications for three independent domestication events for the tea plant (Camellia sinensis (L.) O. Kuntze) and new insights into the origin of tea germplasm in China and India revealed by nuclear microsatellites. PLoS One. 2016;11:e0155369.
Zhao D-W, Yang S-X. Rediscovery of Camellia grandibracteata (Theaceae) with emendate description. J Trop Subtrop Bot. 2012;20:399–402.
Lukic D, Eberle J, Thormann J, Holzschuh C, Ahrens D. Excluding spatial sampling bias does not eliminate oversplitting in DNA-based species delimitation analyses. Ecol Evol. 2021;11:10327–37.
Mason NA, Fletcher NK, Gill BA, Funk WC, Zamudio KR. Coalescent-based species delimitation is sensitive to geographic sampling and isolation by distance. Syst Biodivers. 2020;18:269–80.
Williams PH, Altanchimeg D, Byvaltsev A, Jonghe RD, Jaffar S, Japoshvili G, et al. Widespread polytypic species or complexes of local species? revising bumblebees of the subgenus Melanobombus world-wide (Hymenoptera, Apidae, Bombus). Eur J Taxon. 2020;719:1–120.
Becchimanzi A, Zimowska B, Nicoletti R. Cryptic diversity in Cladosporium cladosporioides resulting from sequence-based species delimitation analyses. Pathogens. 2021;10: 1167.
Thanh NT, Dung LV, Hai Ninh LN, Truong QC, Yang S. Taxonomic notes of three tea-plants (Camellia sect. Thea) recently described in Vietnam. Beverage Plant Res. 2022;0:1–3.
Ming T-L. The revision of Camellia sect. Thea. Acta Bot Yunnanica. 1992;14:115–32.
Jiang Y-Z, Yu X-Q, Zhang T, Peng H, Yang S-X. Taxonomic notes on Camellia crassicolumna and its related species (Theaceae). Phytotaxa. 2023;595:109–14.
Ming T-L. A systematic synopsis of the genus Camellia. Acta Bot Yunnanica. 1999;21:149–59.
Acknowledgements
We thank Prof. Dezhu Li (Kunming Institute of Botany, CAS) for his advice and great help for this study. We thank Dr. Jie Cai and Ende Liu for their help with sample collection, the staff from Molecular Biology Experiment Center in Germplasm Bank of Wild Species for assisting with laboratory work, the staff at KUN for providing access to study specimens of Camellia sect. Thea, and the iFlora High Performance Computing Center of the Germplasm Bank of Wild Species (iFlora HPC Center of GBOWS, KIB, CAS) for computing.
Funding
This work was supported by National Natural Science Foundation of China (No. 32070369), Science and Technology Basic Resources Investigation Program of China "Wild germplasm collection and preservation in Great Gaoligong Mountain" (No. 2021FY100200), the Youth Innovation Promotion Association CAS (No. 2021393), the Yunnan Revitalization Talent Support Program "Young Talent" Project, and the Applied Fundamental Research Foundation of Yunnan Province (202301AT070308).
Author information
Authors and Affiliations
Contributions
XY, CX and SY designed the study. JY and ZH collected the data. YJ and ZH analysed the data. YJ wrote the manuscript. XY, RAF, JY, CX, JZ, HP, JL and SY revised the manuscript; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The current study complies with relevant institutional, national, and international guidelines and legislation for experimental research and field studies on plants (either cultivated or wild), including the collection of plant material. The plant materials involved in this study have been licensed for collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Y., Yang, J., Folk, R.A. et al. Species delimitation of tea plants (Camellia sect. Thea) based on super-barcodes. BMC Plant Biol 24, 181 (2024). https://doi.org/10.1186/s12870-024-04882-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-04882-3