Abstract
Background
Our previous studies revealed that CgbHLH001 transcription factor (TF) played an important role in abiotic stress tolerance, suggesting that its promoter was a potential target in response to stress signals. In addition, the regulatory mechanism of CgbHLH001 TF is still limited.
Results
In the present study, a 1512 bp of 5’-flanking sequence of CgbHLH001 gene was identified, and the sequence carried quite a few of cis-acting elements. The gene promoter displayed strong activity and was induced by multiple abiotic stress. A series of 5’-deletions of the promoter sequence resulted in a gradual decrease in its activity, especially, the 5’ untranslated region (UTR) was necessary to drive promoter activity. Further, CgbHLH001 promoter drove its own gene overexpression ectopically at the transcriptional and translational levels, which in turn conferred the stress tolerance to transgenic Arabidopsis. Transcriptome analysis showed that salt stress induced a large number of genes involved in multiple biological regulatory processes. Differentially expressed genes (DEGs) that mediate phytohormone signal transduction and mitogen-activated protein kinase (MAPK) signaling pathway were widely induced and mostly upregulated under salt stress, and the transcription levels in PbHLH::bHLH-overexpressing transgenic lines were higher than that of 35S::bHLH overexpression.
Conclusions
The CgbHLH001 promoter exhibited a positive response to abiotic stress and its 5’ UTR sequence enhanced the regulation of gene expression to stress. A few important pathways and putative key genes involved in salt tolerance were identified, which can be used to elucidate the mechanism of salt tolerance and decipher the regulatory mechanism of promoters to develop an adaptation strategy for desert halophytes.
Similar content being viewed by others
Background
Gene expression is generally regulated at transcriptional and post-translational levels [1], in which the promoter plays an important role at the transcriptional level [2]. Various cis-acting elements are distributed at a high frequency throughout the upstream sequence around 1–3 kb region from the start codon ATG of a gene [3], including elements responsive to light, abiotic stress, hormone, and other stimuli, which provide the sites for transcription factors (TFs) [4]. Among promoter sequences, the 5’ UTR is vital for the control of gene expression. It may act as a translational enhancer or repressor during the initiation process [5, 6]. For example, the 5’ UTR of the soybean cytosolic glutamine synthetase β1 gene (GS1) enhances GUS expression for 20-fold at translational level [7]. Further studies indicate that the secondary structure, upstream start codon (uAUG), and upstream open reading frames (uORF) apply the vital function in the regulation of translational efficiency [8, 9]. Generally, the function of cis-acting elements can be dissected via truncations of the promoter sequence at the 5’ end. In plants, the recombinant Promoter::GUS (β-glucuronidase gene) construct and the Agrobacterium-mediated transient expression system, as well as the generation of transgenic plants, are commonly used to analyze promoter activity [10]. For example, the tomato ELIP (early light-inducible protein) gene promoter is identified with different positive or negative regulatory elements using seven 5’-deletion variants [11]. The understanding of promoter activity and the role of key elements is essential for utilizing new transgenic technologies in plant molecular breeding.
RNA-seq technology has been widely used to comparatively analyze changes in gene profile induced by stress and further explore the stress-responsive mechanisms. Currently, several plant species have been successfully investigated of the gene expression and function via transcriptomic analyses, such as Arabidopsis thaliana [12, 13], rice [14, 15], tobacco [16], maize [17], and cotton [18]. Plant species (e.g., some halophytes) exhibited poor genetic transformation efficiency need to employ the transgenic Arabidopsis to explore gene functions. Overexpression of Halostachys capsica HcSCL13 gene in transgenic Arabidopsis enhanced plant growth and salt tolerance, transcriptomic analysis revealed that various signaling pathways are involved in controlling plant development in HcSCL13 transgenic Arabidopsis under salt stress [19]. Therefore, transcriptome analysis has become a powerful tool to decipher the molecular basis of plant in response to abiotic stress.
Chenopodium glaucum, as a pioneer halophyte distributed in semi-arid areas of the Xinjiang Uygur Autonomous Region in China, exhibits outstanding characteristics in response to abiotic stress [20]. Halophytes, such as Salsola ferganica and Suaeda aralocaspica, have generally evolved specified morphology or accessory structures (such as succulent leaves, trichomes, and heteromorphic seeds) to adapt to saline conditions, while C. glaucum exhibits normal plant morphology and leaf structures, but still can confer positive environmental impact in improving soil salinity [21, 22]. Therefore, elucidating the mechanism of stress tolerance in C. glaucum has a great significance. In our previous work, a bHLH transcription factor (TF) gene from C. glaucum (named as CgbHLH001) was characterized, which is important in stress response, especially drought tolerance [23], however, the regulatory mechanism of CgbHLH001 TF is still not clear. Based on its promoter sequence (with a potential target in response to stress signals), as well as the downstream gene network regulated by CgbHLH001 TF, in the present study, we further investigated the promoter activity and the mechanism of CgbHLH001 gene driven by self-promoter in response to abiotic stress, and the regulatory networks and key genes responded to salt stress in CgbHLH001 overexpressing Arabidopsis were also analyzed. Figuring out these details, we may gain further insight into the regulatory mechanism of bHLH TF in response to stress.
Results
Analysis of CgbHLH001 promoter activity and 5’ UTR function
Analysis of the promoter sequence and expression pattern of CgbHLH001 gene
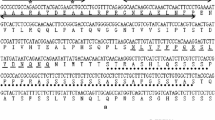
By genomic walking technique, a 1512 bp of 5’ upstream sequence of CgbHLH001 gene (GeneBank accession No. MW544164) was obtained, the transcription start site (TSS) was localized by TSSP software and assigned as ‘ + 1’ (Fig. 1A). A possible promoter sequence was predicted upstream between -1 bp and -1148 bp region. There were many putative cis-acting elements within this region, besides TATA box and CAAT box (core elements), elements on stress responsiveness, plant hormone regulation, light responsiveness, and TF-binding sites were found throughout the predicted promoter sequence, the detailed information was present in Table S1 of the Additional file 8. Notably, within the 5’ UTR region (a 364 bp sequence between the TSS and the ATG start codon), the typical Myb recognition and binding sites, and a Py-rich stretch (highly efficient transcription element) were found (Fig. 1A). The secondary structures of the 5’ UTR suggest a multiple stem-loop structure with -15.85 kcal mol−1 of the folding free energy (∆G) value in DNA (Fig. 1B) and -81.13 kcal mol−1 of ∆G value in RNA (Fig. 1C), these data imply that 5’ UTR sequence of CgbHLH001 may play an important role in gene expression.
Analysis of the promoter and expression patterns of CgbHLH001 gene. A Schematic diagram of the distribution of predicted cis-regulatory elements on CgbHLH001 promoter. B-C Predicted secondary structures of DNA and RNA of the 5’ UTR. D-I Expression patterns of CgbHLH001 in response to different treatments in C. glaucum. The wavelength of the different color light is: white (380–750 nm), red (621–750 nm), yellow (570–750 nm), green (491–570 nm), blue (450–490 nm). Different lowercase letters indicate the significant differences (P < 0.05) existing among different concentrations in D-H; in I, different standard lowercase letters indicate the significant differences among different treatment day(s) with the same light quality; different italic lowercase letters indicate the significant differences among different light qualities at the same treatment time. Values are means ± SD of three biological replicates with two technical replicates of each
The transcriptional expression pattern of CgbHLH001 gene was detected in C. glaucum before its promoter activity could be further explored. Our results showed that low salinity (50 and 100 mM NaCl) stimulated the accumulation of CgbHLH001 transcripts (Fig. 1D); PEG increased gene expression level gradually and significantly within the test concentrations (0 to 20%) (Fig. 1E). For hormone treatments, ABA and MeJA enhanced the expression level at lower concentrations (the former at 2 μM, the latter at 5 and 10 μM), while GA3 had no effect on CgbHLH001 expression at the test concentrations (Fig. 1F, G, H). When treated with different light qualities (including white, red, yellow, green, blue, and darkness), the expression level of CgbHLH001 increased with the time extending, especially under red light and the darkness (Fig. 1I). Our results suggest that cis-elements in CgbHLH001 promoter can respond to different stimuli and consequently activate the transcriptional expression of CgbHLH001 gene.
Effect of 5’-deletions on the activity of CgbHLH001 promoter
Transgenic Arabidopsis harboring PbHLH::GUS was generated to analyze the promoter activity. Histochemical staining showed that stronger blue color was present in leaves compared with that of roots, stems or flowers; shoot apical meristem was also observed apparently blue color, the pseudoseptum and pericarps of the mature silique presented less blue color, and the seed displayed no visible GUS staining (Fig. 2A). Correspondingly, the transcript accumulation of GUS gene in different tissues showed the similar tendency, the leaves exhibited the highest while flowers had the lowest expression level (Fig. 2B). A series of truncates of the CgbHLH001 promoter were generated and fused to GUS reporter gene to define the regions of importance on promoter activity (Fig. 2C), then analyzed by transient expression system in C. glaucum seedlings. With the 5’-deletion increasing, the GUS expression decreased significantly in both mRNA (relative expression) and protein (enzyme activity) levels (Fig. 2D, E). Interestingly, most of the promoter activity was lost after the 5’ UTR-deletion (PbHLH::GUS 1148), suggesting that the 5’ UTR sequence plays an important effect on promoter activity of CgbHLH001.
Analysis of CgbHLH001 promoter activity by 5’-deletions. A Histochemical staining of Arabidopsis seedlings overexpressing PbHLH::GUS FL (full length of the CgbHLH001 promoter). (1) seedling; (2) stem; (3) flowers and early siliques; (4) mature silique, red rectangle on the silique is corresponding to the enlarged inset of seed (right) or pseudoseptum and pericarp (left). Scale bar = 0.5 cm. B Transcriptional expression of GUS gene in different tissues of Arabidopsis overexpressing PbHLH::GUS FL. C Schematic diagram of 5’-deletions of the promoter and constructs. Different truncated fragments of the promoter were constructed into plant expression vector pCAMBIA1300. (1) pCAMBIA1300: no promoter driving GUS gene; (2) PbHLH::GUS 1148, (3) PbHLH::GUS 364, (4) PbHLH::GUS 521, (5) PbHLH::GUS 960: different truncated fragments of CgbHLH001 promoter driving GUS gene; (6) PbHLH::GUS FL: full length of the CgbHLH001 promoter driving GUS gene; (7) pCAMBIA1304: CaMV35S promoter driving GUS gene. HygR: hygromycin resistant gene; E9-ter: terminator signal of the pea rbcS-E9 gene. D GUS histochemical staining in C. glaucum seedlings transiently transformed with different truncations of the CgbHLH001 promoter. E Transcriptional expression of GUS gene in accordance with treatment in D. Different lowercase letters indicate significant differences (P < 0.05) existing among different promoter truncations. In D, E, the number order is corresponding to the constructs in C
Effects of abiotic stress on the activity of CgbHLH001 promoter in transgenic Arabidopsis
To further characterize the activity of CgbHLH001 promoter in response to the abiotic stress, GUS expression and fluorometric GUS activity were analyzed in transgenic Arabidopsis overexpressing CaMV35S::GUS, PbHLH FL::GUS, PbHLH 364::GUS, and PbHLH 1148::GUS. Under normal condition, transgenic seedlings with CaMV35S promoter exhibited the highest GUS expression level, the full-length promoter ranked second, which also had relatively higher activity; the 5’ UTR sequence could drive GUS expression at certain level, however, the promoter without 5’ UTR sequence lost most of the activity (Fig. 3A). When subjected to abiotic stress or phytohormonal treatments, GUS gene driven by PbHLH FL was significantly promoted under mannitol and ABA treatments, those driven by PbHLH 364 or PbHLH 1148 were also significantly enhanced compared to control, except for MeJA treatment (Fig. 3A). Similarly, stronger GUS staining and higher GUS enzyme activity of PbHLH FL transgenic lines were observed under stress treatments, except for 200 mM NaCl treatment (which may partly be the leaf damage under stress); seedlings harboring with 5’ UTR of CgbHLH001 promoter was activated by mannitol and ABA treatments, while CaMV35S promoter showed no significant difference in promotion of GUS activity under abiotic stress compared with that of normal condition (Fig. 3B, C). All these results indicate that CgbHLH001 promoter can respond to abiotic stress and the 5’ UTR is necessary for promoter activity.
Analysis of CgbHLH001 promoter activity under abiotic stress. A Relative expression of GUS gene driven by PbHLHFL, PbHLH364, and PbHLH1148. CaMV35S promoter (P35S) was used as the positive control; Col was used as the negative control. PbHLHFL: full-length CgbHLH001 promoter driving GUS gene; PbHLH364: 5’ UTR sequence of CgbHLH001 promoter driving GUS gene; PbHLH1148: 5’ UTR deletion of full-length CgbHLH001 promoter driving GUS gene. B GUS staining of transgenic Arabidopsis seedlings. C Detection of GUS enzyme activity. The GUS activity was expressed as nmol 4-methylumbelliferone per minute per mg protein. Bars represent means ± SD of three biological replicates. Different lowercase letters indicate significant differences (P < 0.05) existing among different treatments in A and C
Responses of CgbHLH001 gene to abiotic stress driven by self-promoter
The transcripts and protein of CgbHLH001 in C. glaucum were significantly accumulated when subjected to different abiotic stress compared to that of normal condition (Fig. 4A, B), suggesting the important role of CgbHLH001 gene in stress response. To characterize the function of CgbHLH001 gene and the effect of its promoter in response to abiotic stress, T3 generation transgenic Arabidopsis lines overexpressing PbHLH::bHLH or 35S::bHLH were identified by genomic PCR, semi-quantitative RT-PCR, quantitative real-time PCR (qRT-PCR) and Western blot (Fig. 4C-F). The expression of CgbHLH001 gene driven by CaMV35S promoter was significantly higher than that of driven by self-promoter in both mRNA and protein levels (Fig. 4E, F).
Analysis of CgbHLH001 expression in C. glaucum and identification of transgenic Arabidopsis overexpressing CgbHLH001. A-B Detection of CgbHLH001 expression in C. glaucum under different abiotic stress at mRNA and protein levels. C-F Identification of CgbHLH001 in transgenic Arabidopsis by PCR, RT-PCR, qRT-PCR and Western blot. In A, different lowercase letters above the columns indicate significant differences (P < 0.05) between different treatments; in C, + : positive control; -: negative control; M: DL2000 DNA marker; in D, -: negative control; Atactin served as the internal reference; in E, different lowercase letters above the columns indicate significant differences (P < 0.05) between different Arabidopsis lines; in F, Tubulin served as the internal reference. Original images of gels and blots were present in Supporting Fig. 1 in the Additional file 14
To further verify the combined effect of the promoter activity on CgbHLH001 gene function, the phenotypic performances and gene expressions at transcriptional and translational levels were investigated by employing transgenic Arabidopsis lines overexpressing PbHLH::bHLH and 35S::bHLH. The expression of CgbHLH001 (mRNA and protein levels) was activated when exposed to salt stress, although PbHLH promoter had less effect on gene expression compared with the CaMV35S promoter, and changes of protein level were not much more compared with transcripts (Fig. 5A, B). Moreover, WT suffered more than transgenic lines when subjected to 200 mM NaCl treatment for 15 d, in which transgenic Arabidopsis overexpressing 35S::bHLH performed better than that of overexpressing PbHLH::bHLH (Fig. 5C, D). Further analyses of CgbHLH001 in response to drought and cold treatments revealed that increased gene expression and alleviated damage were observed in transgenic lines, and the 35S::bHLH-overexpressing lines had better performance (Fig. S1; Fig. S2 in the Additional files 1, 2). These results indicate that CgbHLH001 promoter is stress-inducible and can drive downstream genes (including CgbHLH001) positively in response to abiotic stress.
Phenotypic performance and gene expression of transgenic Arabidopsis lines (overexpressing 35S::bHLH and PbHLH::bHLH) in response to 200 mM NaCl treatment. A Transcriptional expression of CgbHLH001 gene. B Translational expression of CgbHLH001 protein. C-D Phenotypic observation and survival percentage of transgenic Arabidopsis. OE35S1, 2: 35S::bHLH-overexpressing transgenic Arabidopsis line 1, 2; OEPb1, 2: PbHLH::bHLH-overexpressing transgenic Arabidopsis line 1, 2. Different lowercase letters in A, D indicate significant differences (P < 0.05) existing between different transgenic lines. Original images of blots were present in Supporting Fig. 1 in the Additional file 14
Analysis of transcriptomic data
Overview of the RNA-seq data
Based on the good performance of transgenic Arabidopsis overexpressing CgbHLH001 in response to salt stress, we expect to further elucidate the regulatory mechanism of the gene. Transcriptomic data between WT and transgenic lines (35S::bHLH or PbHLH::bHLH) under normal condition or salt treatment for 1 h were analyzed. In total, 18 libraries were constructed and sequenced. For each sample, 92.39% to 95.71% reads were mapped to the Arabidopsis reference genome after filtering low-quality reads (Table S2 in the Additional file 9). The pairwise Pearson’s correlation coefficients indicated a high consistency among biological replicates (Fig. S3A in the Additional file 3). The principal component analysis (PCA) showed that the transgenic lines were distal to the WT, especially PbHLH::bHLH-overexpressing line under salt treatment (Fig. S3B in the Additional file 3), suggesting that transgenic Arabidopsis can obviously respond to stress. These results indicated that the RNA-seq data were reliable and could be used for further analysis.
Identification of DEGs
Pairwise comparisons were conducted among WT (A), 35S::bHLH (B) and PbHLH::bHLH (C) under normal condition (C) or salt treatment (S) [A(C) vs B(C), A(S) vs B(S), A(C) vs C(C), A(S) vs C(S), B(C) vs C(C), and B(S) vs C(S)] to identify DEGs in response to abiotic stress. Of those, 35S::bHLH and PbHLH::bHLH under salt condition [B(S) vs C(S)] exhibited the least differences with 354 upregulated and 83 downregulated DEGs, indicating that the two transgenic Arabidopsis exhibited similar response to salt stress. In contrast, WT and PbHLH::bHLH under normal condition [A(C) vs C(C)] exhibited the largest differences with 3062 DEGs (1415 upregulated and 1647 downregulated), implying significant difference at transcriptional level in PbHLH::bHLH transgenic Arabidopsis, which were much higher than that in 35S::bHLH transgenic lines (Fig. 6A). KEGG analyses revealed that the most enriched pathways were related to “MAPK signaling pathway” “plant hormone signal transduction” “phenylpropanoid biosynthesis” “starch and sucrose metabolism” and “plant-pathogen interaction” (Fig. 6B).
Numbers of DEGs identified in various comparisons. A Bar graph presents the number of up- and down-regulated DEGs in various comparisons. B KEGG pathway enrichment analysis on the data listed in A. C Upset graphs displaying shared and unique DEGs identified in various comparisons. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment
The upregulated and downregulated DEGs among six comparisons were sorted out to analyze the transcriptional change under salt stress (Fig. 6C). The results revealed 542 DEGs exclusively upregulated and 494 DEGs exclusively downregulated in [A(C) vs C(C)], the former were enriched in photosynthesis (including photosystem I and II), while the latter were in response to hormones (such as cytokinin and gibberellin) (Fig. S4 A, B in the Additional file 4). However, 88 DEGs were exclusively upregulated in [A(C) vs B(C)] and enriched in plant developmental process; whereas 207 DEGs were exclusively downregulated in response to wounding and stress (Fig. S4 C, D in the Additional file 4). With the maximum number of overlapping DEGs between [A(C) vs C(C) and A(C) vs B(C)], 182 genes were commonly upregulated and 415 genes were downregulated. The most enriched GO terms were related to cellular components and plant growth and metabolism (Fig. S4 E, F in the Additional file 4).
Characterization of the candidate genes and signaling pathways regulated by CgbHLH001 gene and its promoter
Comparing between 35S::bHLH and P bHLH ::bHLH transgenic Arabidopsis to characterize the function of CgbHLH001 promoter
Overexpressing 35S::bHLH and PbHLH::bHLH transgenic Arabidopsis under salt stress [B(S) vs C(S)] were selected for analysis. GO annotation in biological process showed that DEGs were involved in plant development (auxin, chitin, root development, and photosynthetic acclimation) and stress response (defense, osmotic, salt, cold, wounding, and hyperosmotic salinity) (Fig. 7A). Furthermore, terms related to salt and osmotic stress were selected to narrow down the candidate genes. All of the 13 DEGs identified were upregulated: 4 genes were commonly upregulated in [B(C) vs C(C) and B(S) vs C(S)], the rest were exclusively upregulated in [B(S) vs C(S)] (Fig. 7B, C). Interestingly, 6 DEGs were identified belonging to ZAT protein family (C2H2 zinc fingers superfamily), which regulate plant development and respond to diverse stress [24]. In addition, the top 50 DEGs with the highest fold change (FC) were selected for functional annotation. The most enriched GO terms were involved in defense response, photosynthetic acclimation, stress response and ROS metabolic process (Fig. S5A in the Additional file 5). All the results showed that CgbHLH001 promoter could activate the downstream stress-responsive genes which consequently mediate the stress tolerance.
Characterization of salt stress-related genes induced by CgbHLH001 gene and its promoter. A-C Comparison of B(S) vs C(S); D-F Comparison of A(S) vs B(S); G-I Comparison of A(S) vs C(S). A, D, G GO annotation of DEGs in biological process. B, C, E, F, H, I Heatmaps of selected DEGs related to salt stress based on FPKM values (B, E, H) or log2FC values (C, F, I). In (A, D, G), gene ratio represents the percentage of selected genes, the circle size represents gene numbers, the bigger the circle, the more the gene numbers. The color of circle represents the p value, the darker the color, the smaller the p value with higher significant difference. In (B, E, H), the FPKM values of DEGs were standardized by log2 (FPKM + 1). The darker the color, the higher the gene expression level. The left in (B, E, H) was clustered by gene expression level. The right in (C, F, I) represent gene name, which is consistent with that in (B, E, H). The darker the color, the larger the gene fold change. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment
The regulation of CgbHLH001 gene overexpression in Arabidopsis
Our results showed that overexpressing CgbHLH001 gene enhanced the abiotic stress tolerance of transgenic Arabidopsis. Here, the WT and 35S::bHLH transgenic lines under salt stress [A(S) vs B(S)] were compared for further analyses. GO annotation associated with biological process revealed that salt/osmotic stress-related DEGs were enriched, a total of 13 DEGs (5 downregulated, 8 upregulated) were screened, which included an upregulated gene SAG21 (senescence-associated gene 21) with higher expression profile (Fig. 7D, E). Three genes were commonly expressed in both [A(C) vs B(C) and A(S) vs B(S)], two of which were downregulated, while the expression was improved under salt treatment (Fig. 7F). Similarly, the most enriched GO terms of the top 50 DEGs with the highest FC were related to kinase activity, signal transduction, secondary metabolic process and response to oxidative stress, indicating that the overexpressing CgbHLH001 gene may mediate signal transduction and hormone-related pathway in response to external stress (Fig. S5B in the Additional file 5).
The response of CgbHLH001 gene overexpression induced by its promoter to salt stress
The phenotypic observation revealed an improved performance of transgenic Arabidopsis than WT in plant growth and development following salt treatments. DEGs of transgenic Arabidopsis before and after salt stress [B(C) vs B(S) and C(C) vs C(S)] were identified to elucidate the mechanism of CgbHLH001 overexpression in response to salt stress. The number of DEGs in PbHLH::bHLH transgenic lines [C(C) vs C(S)] was the highest (Fig. S6A in the Additional file 6). The Venn diagrams were generated to illustrate the similarities and differences between upregulated and downregulated genes (Fig. S6B, C in the Additional file 6). Furthermore, analysis of GO enrichment showed that the upregulated DEGs were positively correlated with multiple abiotic stress responses (Fig. S6D, E in the Additional file 6). Therefore, all the upregulated genes were selected to elucidate the salt-responsive mechanism. A total of 2098 upregulated DEGs were divided into three groups, including DEGs exclusively upregulated in [B(C) vs B(S)] (292), [C(C) vs C(S)] (862) or DEGs commonly upregulated in [B(C) vs B(S) and C(C) vs C(S)] (944) (Fig. 8A). GO analyses in biological process showed that all the three groups were involved in abiotic stress responses, especially the commonly upregulated DEGs, which covered more stress-related genes and presented higher expression levels after salt treatment, such as ERD7 (early-responsive to dehydration 7), EDL3 (EID1-like F-box protein 3), LTI65 (low-temperature-induced 65 kDa protein), and RD29A (desiccation-responsive protein 29A) (Fig. 8B, C). The top 30 DEGs identified by FC values in [B(C) vs B(S) and C(C) vs C(S)] included a proportion of TFs and stress-related genes (Table S3 in the Additional file 10). In this study, a number of differentially expressed (DE) TFs were identified to understand the contribution of TFs in response to salt stress, including AP2/ERF-ERF, bHLH, MYB, WRKY, NAC, and bZIP (Table S4 in the Additional file 11), which plays important roles during stress responses in plants. Among these, C2C2-GATA, TCP and B3 TFs were exclusively downregulated, while C3H was exclusively upregulated. The number of upregulated genes in AP2/ERF-ERF, MYB, NAC, WRKY was significantly higher than the downregulated genes. The better performance of transgenic Arabidopsis under abiotic stress should be attributed to the significant expression of all the stress-related genes, especially the commonly upregulated DEGs.
Characterization of upregulated DEGs in transgenic Arabidopsis under salt stress. A The heatmaps of DEGs between B(C) vs B(S) and C(C) vs C(S). B Top 10 GO functional annotations in biological process corresponding to A. C Heatmaps of DEGs related to salt stress based on log2FC values. In A, the heatmap was generated based on the FPKM value (Z-score method). In B, the red font indicated GO terms related to abiotic stress. B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment
Furthermore, we compared WT and PbHLH::bHLH transgenic Arabidopsis under salt treatment [A(S) vs C(S)] to explore the regulation of CgbHLH001 gene driven by self-promoter. GO enrichment in biological process showed that stress-related terms were widely enriched, including response to abiotic stimuli (cold, salt, temperature, and osmotic stress) and biotic stimuli (defense, wounding, fungus and bacterium) (Fig. 7G). Accordingly, 27 DEGs related to salt and osmotic stress were identified, among them, 21 genes were upregulated and 6 genes were downregulated (Fig. 7H). Twelve DEGs were commonly expressed in both [A(C) vs C(C)] and [A(S) vs C(S)], especially the NHX3 (Na+/H+ antiporter), which was downregulated under normal condition while upregulated after salt treatment. The remained 15 genes were exclusively expressed in [A(S) vs C(S)], all of them were upregulated (Fig. 7I). In addition, the top 50 DEGs of the most enriched GO terms identified by FC values were widely related to responses of abiotic stress (mannitol, salt) and biotic stimuli (Fig. S5C in the Additional file 5). All the results showed that overexpressing CgbHLH001 gene driven by self-promoter presented more positive response to abiotic stress.
Gene expression patterns and functional enrichment of DEGs
To further elucidate the relationship between different Arabidopsis lines, DEGs under salt treatment were categorized into six groups by K-means analysis (Fig. 9A), implying changes in gene expression after overexpressing 35S::bHLH or PbHLH::bHLH. According to the gene expression tendency, six groups were further divided into increasing (C1, C3, C6) and decreasing subgroups (C2, C4, C5). To determine the functional significance of the transcriptional changes of each group, GO classification and KEGG pathways were performed in analysis (Fig. 9B, C). DEGs in group 1 and 3 were enriched in stress responses (such as salt, cold, osmotic and external stimuli), immune response, defense response, and other biotic stimuli, which were mediated by the MAPK signaling pathway, plant hormone signal transduction pathway and plant-pathogen interaction; while group 4 and 5 were mostly involved in compound biosynthesis and metabolism (such as anthocyanin, flavonoid, phenylpropanoid, and isoflavonoid). The expressions of DEGs within group 1 were further analyzed, in which most of the abiotic stress-related genes were transcription factors and protein kinases (Fig. 9D). These results suggest that the function of upregulated DEGs, especially TFs, may be enhanced in salt response.
The dynamic profiles of DEGs in Arabidopsis in response to salt stress. A K-means clustering of the expression profile of DEGs. B Analysis of functional category enrichment among the six clusters. C Analysis of pathways enrichment among the six clusters. D The heatmap of DEGs related to abiotic stress response based on FPKM value (Z-score method). A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (S): salt treatment
Gene co-expression analysis after salt stress by WGCNA (weighted gene co-expression network analysis)
To better understand the regulatory networks of Arabidopsis overexpressing 35S::bHLH and PbHLH::bHLH in response to salt stress, 6 samples with three replicates and their expression data sets were used in WGCNA. A total of 3 co-expression modules were identified (threshold of similarity > 0.25, threshold of gene expression > 1), each module was marked in different color (Fig. 10A). The correlations between modules and samples were analyzed (Fig. 10B), the blue module positively correlated with the PbHLH::bHLH transgenic lines under salt treatment (r = 0.87, p = 0.03), implying a role in salt stress response. Notably, GO annotation of DEGs in blue module revealed that the top 20 of most enriched GO terms were involved in responses to salt stress, oxidative stress, wounding, defense, leaf senescence and other more (Fig. S5D in the Additional file 5). Scatterplots of gene significance (GS) and module membership (MM) in the blue module showed that both were highly correlated, indicating that the candidates were most significantly associated with the stress response of PbHLH::bHLH transgenic Arabidopsis (Fig. 10C). Furthermore, a hub gene MPK11 in this module was focused, which was directly related to plant-pathogen interaction and MAPK signaling pathway (Fig. 10D).
WGCNA of the transcripts in WT, 35S::bHLH and PbHLH::bHLH Arabidopsis lines under salt treatment. A Gene dendrograms of the whole-transcriptome profiles were constructed using average linkage hierarchical clustering, each line represents one gene. The module color underneath the cluster tree shows the results of module assignment by the dynamic tree cut. B Correlations between modules eigengenes and different lines. The color of each module is the same as that in (A). The gene number of each module is shown in the bottom of the module name. The correlation coefficient and p-value are shown in each cell. C Scatterplots of gene significance versus module membership for the blue module. D Co-expression network of members in blue module. The largest inner circle represents the hub gene, and the relationships of all the genes are connected by lines. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment
Hormone signal transduction and MAPK signaling under salt stress
Our analyses showed that DEGs were significantly enriched in plant hormone signal transduction (ko04075) and MAPK signaling pathway (ko04016), which means that the main plant hormone-related genes must be activated. The results confirmed that three ABA receptor genes (PYR/PYL) were downregulated when subjected to salt stress, while the other components of the ABA signaling pathway (PP2Cs, SnRKs, and ABF TFs) were mostly upregulated in transgenic lines. BAK1/BRI1 gene that encodes BR receptor was mostly upregulated in transgenic lines or under salt treatment. In SA signaling pathway, a total of 12 DEGs were identified, of which, 3 NPR1 (nonexpressor of pathogenesis-related) genes were upregulated in transgenic lines under salt treatment, while multiple PR-1 genes were downregulated in transgenic lines, especially in PbHLH::bHLH line. AUX/IAA, GH3 (Gretchen Hagen 3), and SAUR (small auxin-up RNA) are three different types of early auxin-responsive gene families. A number of DEGs related to auxin signaling were identified, and most of the auxin-responsive genes were downregulated in transgenic plants (Fig. 11A). In addition, GA receptor gene GID1 (gibberellin insensitive dwarf1) was upregulated in transgenic lines under salt stress, meanwhile, a lot of DEGs participated in the GA signaling pathway. In the jasmonic acid (JA) signal transduction pathway, JAR1 gene (jasmonic acid-amido synthetase) was downregulated in transgenic lines while upregulated under salt treatment, COI1 gene was downregulated in transgenic lines or under salt treatment, JAZ and MYC genes were upregulated in salt-treated transgenic lines. Protein kinases (PKs) in the MAPK signaling cascade, such as MEKK1, MKK1, MAPKKK17/18, MPK3/6, and MAPK7/14, showed significantly increased expression in transgenic lines under salt treatment, and a better performance was observed in PbHLH::bHLH transgenic Arabidopsis; while MKK4/5 decreased when suffered from salt stress (Fig. 11B). The above data and analyses indicate that multiple phytohormones and the MAPK signaling pathway may have important contribution to the regulatory mechanisms associated with salt stress tolerance in transgenic Arabidopsis.
Transcriptional changes of DEGs involved in plant hormone signal transduction (A) and MAPK signaling pathway (B) to regulate plant in salt tolerance [25]. Blue circle with a ‘P’ inside indicates phosphorylation. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment
Validation of the key genes in transgenic Arabidopsis by qRT-PCR
The expression profiles of 28 genes randomly selected from the identified stress-related DEGs were analyzed by quantitative RT-PCR to compare with their transcriptomic data, including TFs, PKs, ion transporters, hormone- or stress-related genes (Table S5 in the Additional file 12). Among them, TFs and PKs in transgenic lines or under salt treatment were all upregulated. The expression levels of hormone-related genes significantly fluctuated, BRI1 and GH3 were upregulated in transgenic lines or under salt treatment, while IAA3 was downregulated. For the ion transporters, the expression levels of CLC-B (chloride channel protein) and HKT1 (high-affinity K+ transporter) were lower in the transgenic plants than in the WT, while increased under salt stress. NHX3 transcripts were significantly accumulated after salt treatment. All test stress-responsive genes were upregulated under salt treatment. The expression patterns of these DEGs were highly consistent with the FPKM values of RNA-seq data (Fig. 12), indicating that the analyses of RNA-seq-based transcriptional profiles are reliable and all these key genes are involved in salt stress tolerance.
Changes in relative expression level of DEGs analyzed by qRT-PCR and RNA-seq. The left vertical axis indicates the relative expression level determined by qRT-PCR (bar chart), the right vertical axis indicates the FPKM value determined by RNA-seq (line chart). LTI65, low temperature induced 65 kD protein; PXG3, peroxygenase 3; ABF1, ABRE binding factor; ZAT8, zinc finger of Arabidopsis thaliana 8; GSTF6, glutathione S-transferase 6; RLP23, receptor like protein 23; CPK27, calcium-dependent protein kinase 27; MPK3/11, mitogen-activated protein kinase 3/11; SnRK2, serine/threonine-protein kinase 2; MEK1, MAP kinase/ ERK kinase 1; CAMTA3, calmodulin-binding transcription activator 3; PYL4, PYR1-like 4; IAA3, auxin-responsive protein IAA3; BRI1, brassinosteroid insensitive 1; GH3, auxin-responsive GH3 family protein 3; NHX3, Na+/H+ antiporter; CLC-B, chloride channel protein CLC-B; HKT1, high-affinity K+ transporter 1; SAG21, senescence-associated gene 21; ERD7, early responsive to dehydration 7; LEA2, late embryogenesis abundant protein; P5CSB, delta 1-pyrroline-5-carboxylate synthase 2; RD29A, desiccation-responsive protein 29A; SOS1, 2, 3, salt overly sensitive 1, 2, 3; NAC035, NAC domain containing protein 35
Discussion
CgbHLH001 promoter and functional region can respond to abiotic stress
As the key controller of gene expression, the promoter consists of diverse cis-acting elements, motifs, and other regulatory sequences [26]. Many functional cis-elements distributed in the 5’ regulatory region of genes may involve in the transcriptional regulation to control different biological processes and respond to different stimuli [27,28,29]. Therefore, characterization of the functional elements and promoter activity is necessary for understanding of gene expression regulation and application in genetic engineering. In the present study, a 1512 bp promoter sequence of CgbHLH001 gene was identified, which displayed a strong driving activity and was inducible under abiotic stress; the 5’ UTR sequence was necessary to drive the promoter activity.
Cis-regulatory elements can largely control the precise sensitivity and specificity of transcriptional responses [30]. ABRE is known as ABA-responsive element, which is also a cis-acting element on dehydration, high salinity and low temperature responsiveness [4]. ABRE motif in GmRD26 promoter in Glycine max applies a strong induction under drought condition to trigger its downstream gene expression in response to stress [31]. This element in FeDREB1 (dehydration-responsive element binding protein) promoter from Fagopyrum esculentum can activate cold- and drought-responsive gene expression [32]. In the present study, a number of cis-acting elements were predicted in CgbHLH001 promoter, and many associated with phytohormone, light and abiotic stress responsiveness, such as ABRE, G-box, W-box, MYB recognition site, and MYB binding site, which might activate CgbHLH001 when subjected to NaCl, PEG, ABA, MeJA and light treatment in C. glaucum. In accordance with the element distribution in CgbHLH001 promoter, the full length (FL) promoter (1512 bp) presented quite high activity, which was gradually reduced with 5’ stepwise deletion increasing. Our results suggest that cis-elements distributed in CgbHLH001 promoter apply apparent effects on promoter activity.
It has been reported that 5’ UTR plays important roles in regulation of gene expression at transcriptional or/and post-transcriptional level(s) [33]. The changes of 5’ UTR structure in Arabidopsis may result in an over 200-fold variation in mRNA stability and translational efficiency [34,35,36]. 5’ UTR of RBCL (rubisco large subunit) gene in tobacco can compensate for the low-rate transcription by enhancing the mRNA stability in the dark [37]. And also, some 5’ UTRs from higher plants can act as translational enhancer [38,39,40]. The 5’ UTR of AtCOR47 (cold-regulated 47) gene acted as an effective translational enhancer to ensure the stable and high expression of gene under different conditions [6]. In the present study, the absence of 5’ UTR from the PbHLH FL promoter resulted in approximate eightfold reduction of gene expression, suggesting that the 5’ UTR (364 bp) applies a positive effect on the promoter activity. Various regulatory elements, the secondary structure of mRNA and trans-factor accessibility in 5’ UTR all impact on the expression of downstream open reading frame [41]. The 5’UTR RNA sequence of PtDrl02 gene forms a stable secondary structure with -45.1 kcal mol−1 of the folding free energy (∆G), resulting in decreases of GUS mRNA expression and GUS activity [42]. In the present study, we found that 5’ UTR of CgbHLH001 presented much complicated secondary RNA structure with a ∆G value of -81.13 kcal mol−1, which resulted in significant increases at the transcription level and translation efficiency of GUS. Further analysis revealed a Py-rich stretch sequence in this region, which is known to promote gene transcription at high level [43]. Taken together, our results suggest that the 5’ UTR sequence combined with its structure may confer a positive regulatory effect on CgbHLH001 gene expression at transcriptional and translational levels.
Overexpressing P bHLH ::bHLH enhances tolerance to abiotic stress
An increasing number of bHLH TFs have been reported to play an essential role in response to abiotic stress [44,45,46]. Ectopic expression of Pyrus ussuriensis PubHLH1 results in a higher survival rate of transgenic tobacco than WT under chilling treatment [47]. We previously identified a bHLH gene (CgbHLH001) from a halophyte C. glaucum [23], which conferred drought tolerance to transgenic tobacco and maize [48], moreover, enhanced photosynthetic capacity was also achieved in CgbHLH001-overexpressing maize. In the present study, the transgenic Arabidopsis overexpressing PbHLH::bHLH presented a better performance than the WT in relevant gene expression at transcriptional and translational levels when suffered from salt, drought or lower temperature stress. Our results suggest that CgbHLH001 promoter is inducible and can drive CgbHLH001 overexpression ectopically, which in turn enhances stress tolerance in transgenic Arabidopsis. Although CgbHLH001 gene was expressed at a higher transcriptional level in transgenic Arabidopsis, while much lower translational level was present, regardless the CaMV35S or CgbHLH001 promoter was used. Accumulating evidence suggests a mismatch between transcriptional and translational levels, and the obvious difference between mRNA level and protein expression may be attributed to post-transcriptional regulation and/or post-translation modifications [49]. Besides, the apparent difference of the CgbHLH001 protein level was also found between the ectopic expression in Arabidopsis (much lower level) and ontologic expression in C. glaucum (relatively higher level; Fig. 4B) under the same condition. It may be the cytosol environment in Arabidopsis (glycophyte) unfavorable for CgbHLH001 protein (originated from halophyte) accumulation, together with the reason that the protein expression efficiency is determined by multiple factors, including translation rates [50], translation modulation [51], protein’s half-life [52], protein synthesis delay and protein transport [53].
Signaling networks regulated by CgbHLH001 TF responds to abiotic stress
In plant stress responses, receptors or sensors recognize stress signals, which are in turn transmitted via secondary messengers (Ca2+, hormone, and ROS). Various physiological responses are further triggered by signal transduction, in which PKs (CDPKs, MAPKs) control downstream TFs and then regulate the expression of target genes that may ultimately influence the stress tolerance of plants [54]. Based on our analysis, the hormone signal transduction and MAPK signaling pathway were involved in regulating downstream gene network of TF-mediated stress responses, such as CgbHLH001 TF. In the process, multiple PK, TFs and stress-related genes were significantly induced, which may participate in multiple signaling pathways and respond to external stress.
Plant hormone signal transduction is an essential component of plant stress-response signaling pathways, as the most important endogenous substances, phytohormones play critical roles in stress response and growth promotion [55]. The transcriptome analysis of Podocarpus macrophyllus indicate that the transcription of genes involved in biosynthesis and phytohormone signaling pathways have been altered significantly, and genes related to auxin transport and responsiveness are downregulated in response to salt stress [56]. In Chenopodium quinoa, salt stress results in a decrease in signaling components of growth-related phytohormones (auxin, BR) and an increase of components in ABA signaling pathway (PP2Cs), while some other important components of ABA pathway (PYLs, SnRK) are downregulated under long-term salt stress [57]. As an important stress-responsive hormone, ABA plays indispensable roles in regulation of the balance of osmosis, ions, and ROS under salt stress [58]. In the present study, the ABA receptor genes PYR/PYL were significantly upregulated in two types of transgenic Arabidopsis compared to WT. Protein phosphatase 2C (PP2C) is another ABA signaling pathway related gene known to participate in the stress response [59], our results showed a significant increase of PP2C in transgenic Arabidopsis when suffered from salt stress, which finally activated the expression of its substrate ABFs. SA also plays an important role in plant stress tolerance by participating in the accumulation of osmoprotectants and induction of antioxidant enzymes under salt stress [60]. Increase in SA level results in the induction of PR (PATHOGENESIS RELATED) genes, and the NPR1 (NON-EXPRESSOR OF PR GENE 1) functions as the key regulatory elements in SA-dependent activation of PR genes [61]. Our results revealed that NPR1 was induced in two types of transgenic Arabidopsis under salt stress, while the PR-1 was mostly downregulated (Fig. 11). Further exploration of the phytohormone signal transduction and the crosstalk between different gene signals at the physiological levels may partly explain the mechanisms in stress tolerance.
Genes encoding PKs, such as receptor like kinase-Pelle (RLK-Pelle), CDPK, and MAPK, were largely induced among multiple comparisons (Fig. S7 in the Additional file 7). Our DEGs data suggest that MAPK signaling pathway was highly enriched with upregulated genes in transgenic Arabidopsis under salt treatment (Figs. 6B and 11B). RLK-Pelle is the largest gene family in Arabidopsis and rice, which are responsible for multiple abiotic and biotic stresses [62]. In the present study, the RLK-Pelle kinases accounted for much high proportion, especially in [A(C) vs C(C)] and [A(C) vs B(C)], indicating that PKs can be one of the important factors associated with salt tolerance after overexpressing CgbHLH001 gene (Fig. S7 in the Additional file 7).
TFs are activated after perceiving stress signals, the downstream related genes are subsequently regulated to respond to external stress [63, 64]. Numerous upregulated TFs have exclusively been detected in salt-tolerant genotype of barley, including AP2/ERF, bZIP, MYB-related, WRKY, Trihelix, and bHLH, which suggests the crucial roles of these TFs in regulating the downstream genes responsible for salt stress tolerance [65]. Transcriptome analysis between drought-tolerant (Otis) and drought-sensitive (Baronesse) barley genotypes showed that DEGs specifically induced or greatly upregulated under drought stress in Otis but not in Baronesse were important for drought tolerance [66]. In the present study, transcriptome analysis revealed dramatic changes in amount of TFs and AP2/ERF-ERF, and the next ones were NAC, bHLH, WRKY, MYB, C2H2, bZIP and other more, which may alleviate salt-induced damage (Fig. S7 in the Additional file 7). AP2/ERF-ERF genes are plant-specific TFs involved in growth/development and stress responses [67,68,69,70]. Here, abundant DEGs of AP2/ERF-ERF TF were identified in transgenic Arabidopsis compared to WT, less differences were found between two types of transgenic Arabidopsis [B(C) vs C(C)]. The proportion of each TF family in different comparisons varied, indicating that the CgbHLH001 (promoter and gene) in transgenic Arabidopsis may play important role in activating related TFs and functional genes in response to salt stress, and the participation of various PKs and TFs demonstrated that the kinase-mediated signal cascades were involved in salt stress response, which is consistent with our GO and KEGG enrichment analyses (Figs. 6 and 9). Furthermore, the downstream functional genes in signal transduction pathway were significantly upregulated under salt treatment (Fig. 12). However, the relationship between CgbHLH001 and its downstream functional genes still needs to be elucidated.
Conclusion
Based on the previous characterization of the function of CgbHLH001 TF in halophyte C. glaucum [23], we further investigated the promoter activity and biological function of CgbHLH001 gene associated with abiotic stress. In the present study, CgbHLH001 promoter exhibited substantially higher activity and positive response to various abiotic stress, with the 5’ UTR acting as an enhancer of gene expression. Furthermore, CgbHLH001 promoter induced ectopic overexpression of its own gene at transcriptional and translational levels, which results in stress tolerance to transgenic Arabidopsis. The transcriptome data suggest that the stress response of overexpressing CgbHLH001 was involved in multiple biological processes. Here, a large number of DEGs were identified in transgenic Arabidopsis under salt stress, some of which were involved in plant hormone signal transduction and MAPK signaling pathway. Collectively, our findings provide a new insight into the regulatory function of CgbHLH001 promoter and the TF in response to abiotic stress, and suggest a number of candidate genes that can potentially be used in developing stress tolerance crops in the future.
Methods
Plant materials, growth conditions and treatments
Mature seeds of C. glaucum were collected from natural plants growing at the edge of the Gurbantunggut desert at Wujiaqu 103 regiment (44°37'N, 87°26'E; 423 mH) in October 2014, in the Xinjiang Uygur Autonomous Region, China. After collection, the plant was identified by Yongman Lu (a plant taxonomist in Xinjiang University), and voucher specimens (No. CG201410) was deposited at the herbarium (College of Life Science and Technology, Xinjiang University). Seeds were air-dried indoor and cleaned and then stored at 4ºC in sealed brown-paper bags. Wild type (WT, Columbia-0) and transgenic lines of Arabidopsis thaliana, and Chenopodium glaucum were cultivated in pots containing a mixed soil (peat soil: vermiculite = 3:1, v/v) under the greenhouse condition of Xinjiang University, China: at 22ºC (A. thaliana) or 25ºC (C. glaucum), 20–30% relative humidity (RH), 16 h light/8 h dark photoperiod, 100 μmol m−2 s−1 light intensity. Seedlings (plants) were well-watered and applied with half-strength Hoagland solution [71] at an interval of two weeks.
For phenotypic analysis, 3-week-old seedlings of WT and T3 transgenic Arabidopsis lines harboring 35S::bHLH or PbHLH::bHLH were subjected to abiotic stress with 200 mM NaCl treatment for 15 d, natural drought treatment for 20 d or 4ºC treatment for 14 d. Four replicates of each line with 5 plants of each replicate were applied.
For immunoblotting analysis, 4-week-old seedlings of transgenic Arabidopsis or C. glaucum were treated under normal condition (applied with Hoagland solution) or abiotic stress [200 mM NaCl, 300 mM mannitol, 10% PEG treatments (prepared with Hoagland solution), and 4ºC treatment] for 24 h, and sampled for protein extraction.
For transient expression analysis, 2-week-old seedlings of C. glaucum were used in Agrobacterium infiltration.
For qRT-PCR analysis of CgbHLH001 in C. glaucum, (1) NaCl or PEG 6000 stress: mature seeds were sown on two layers of filter paper in a 9 cm Petri dish, which were saturated with 5 mL of different concentrations of solution—NaCl (0, 50, 100, 300 mM), or PEG 6000 (0, 5, 10, 15, 20%), and sealed with cling film in an incubator (25ºC, 30–40% RH, 16 h light/8 h dark, 100 μmol·m−2·s−1 light intensity), 2-week-old seedlings were sampled. (2) Phytohormone treatment: 2-week-old seedlings grown on MS medium (Coolaber, Beijing, China) were carefully transferred into MS solution in addition with ABA (0, 1, 2, 5, 10 μM), GA3 (1, 5, 10, 20 mg·L−1), or MeJA (1, 5, 10, 20 μM), and shaken at 30–50 rpm, 25ºC for 2 h (ABA), or 5 h (GA3 and MeJA), and then sampled. (3) Different light quality treatment: mature seeds were sown on two layers of filter paper (saturated with 5 mL ddH2O) in a 9 cm Petri dish, which was then sealed in a colored transparent box [blue (450–490 nm), green (491–570 nm), yellow (570–750 nm), red (621–750 nm)], the foil wrapped Petri dish was used as darkness treatment. All Petri dishes were placed under normal light (380–750 nm wavelength) in a plant incubator (25ºC, 30–40% RH, 24 h constant light, 100 μmol·m−2·s−1 light intensity). Samples were collected at 0, 1, 3, 5, 7 days after seeds were sown. Three biological replicates for each treatment were applied to all treatments. All samples were frozen immediately at –80ºC upon harvesting for further use.
Cloning and sequence analysis of CgbHLH001 promoter
Genomic DNA of C. glaucum was isolated using DNAsecure Plant Kit (Cat. DP320; Tiangen Biotech., Beijing, China). CgbHLH001 promoter fragment was achieved by using Genome Walking Kit (Cat. 6108; TaKaRa, Dalian, China). Gene specific primers were shown in Table S6 in the Additional file 13. After sequencing, the cis-acting elements were predicted by PlantCARE (http://sphinx.rug.ac.be:8080/PlantCARE/). The transcription start site (TSS) of the promoter was predicted by TSSP-Prediction of PLANT Promoters (http://linux1.softberry.com/berry.phtml?topic=tssp&group=programs&subgroup=promoter). Secondary structures of 5’ UTR were predicted by Mfold RNA/DNA folding software (http://www.bioinfo.rpi.edu/applications/mfold/).
Total RNA extraction and quantitative RT-PCR analysis
Total RNA was isolated from seedlings or plant tissues using Plant RNA Extraction Kit (Omega, USA) according to the manufacturer’s instructions. The reverse transcription reaction was performed with 1.0 μg of total RNA in a volume of 20 μL using the reverse transcriptase M-MLV (TaKaRa, Dalian, China). qRT-PCR was performed using the PerfectStart Green qPCR SuperMix (Cat. AQ601; TransGen, Beijing, China) in a LightCycler 96 Real-Time System (Roche, United States). The reactions were conducted in a 20 μL volume of mixture (containing 10 μL of 2 × SuperMix, 0.4 μL of 10 mM of each primer, 8.2 μL of ddH2O, and 1 μL of cDNA) at conditions: 94ºC 30 s; 40 cycles of 94ºC 5 s, 60ºC 30 s. CgGAPDH (for C. glaucum) or Atactin (for Arabidopsis) was used as an internal reference to normalize the expression level. Primers used for qRT-PCR were shown in Table S6 in the Additional file 13. Three biological replicates with two technical replicates of each were applied. The relative expression level of each gene was calculated by 2−ΔΔCT method [72].
Transient expression assay mediated by Agrobacterium
Single colonies of A. tumefaciens GV3101 harboring pCAMBIA1304-35S::GUS and pCAMBIA1300-PbHLHs::GUS [PbHLHs represent: full length and truncated segments (960 bp, 521 bp, and 364 bp upstream of the ATG codon) of the promoter; 1148 bp upstream of the TSS] were cultured in YEB liquid medium (50 mg·L−1 kanamycin, 40 mg·L−1 gentamicin and 50 mg·L−1 rifampicin) at 28ºC with shaking for overnight. Cultures (200 μL) were transferred to 20 mL fresh YEB medium and incubated at 28ºC with shaking till OD600 = 0.8, then harvested by centrifugation at 8000 rpm for 10 min, after being resuspended, the Agrobacterium slurry was adjusted to OD600 of 0.8 with 1/2 MS solution [containing 120 μM acetosyringone, 2.5% (w/v) sucrose, and 0.01% (w/v) Tween 20, pH 5.8] and employed for the transient transformation, pCAMBIA1300-GUS was used as control. Two-week-old seedlings of C. glaucum were pre-treated for hyperosmosis in 1/2 MS solution [containing 25% (w/v) sucrose, pH 5.8] for 2 h, and then submerged in transformation solution and shaken at 100 rpm at 28ºC for 3 h. Treated seedlings were washed with ddH2O for five times, and then vertically inserted in 1/2 MS solid medium [containing 120 μM acetosyringone, 2.5% (w/v) sucrose, pH 5.8] for co-cultivation. Three days later, seedlings were sampled for GUS staining and qRT-PCR analysis.
Genetic transformation and generation of transgenic lines of A. thaliana
T0 seeds of A. thaliana transformed by the floral dip method [73] were harvested and screened on solid MS medium containing 30 mg·L−1 hygromycin, T3 generation transgenic lines of pCAMBIA1304-35S::GUS, PbHLH::GUS FL, PbHLH::GUS 1148 and PbHLH::GUS 364 were generated and used for GUS analysis (including gene expression, histochemical staining and enzyme activity) under various treatments. Two-week-old seedlings were transferred to 1/2 MS medium containing 200 mM NaCl, 200 mM mannitol, 2 μM ABA, or 10 μM MeJA for 2 d, 2 d, 2 h, or 5 h, respectively. Seedlings grown on 1/2 MS only were used as control. Photographs were captured by stereomicroscope (Nikon SMZ25, Japan). T3 generation transgenic A. thaliana lines harboring 35S::bHLH or PbHLH::bHLH were generated by similar way and used for phenotypic analysis, gene expression and protein extraction.
Analysis of GUS histochemical staining and GUS enzyme activity
For GUS staining, 2-week-old seedlings were immersed in staining solution [containing 1 mM X-Gluc, 100 mM sodium phosphate, pH 7.0; 1 mM potassium ferricyanide (K3Fe(CN)6), 1 mM potassium ferrocyanide (K4Fe(CN)6), 10 mM EDTA, pH 8.0; 0.1% Triton X-100] (prepared just before use and stored in the dark). Samples were treated in the dark in a shaker at 100 rpm at 37ºC overnight. After washed in ddH2O for 2–3 times, seedlings were boiled in de-staining solution (containing glacial acetic acid: anhydrous ethanol = 3:1) for 10 min till became completely transparent, then photographed. Fluorometric GUS activity was detected using 4-methyl-umbelliferyl-β-D-glucuronide (4-MUG) as the substrate. Samples (0.1 g) were harvested and homogenized in extraction buffer (Cat. SL7161, Coolaber, Beijing), after being centrifuged at 12,000 rpm for 10 min, aliquots of supernatant were incubated for 10, 20 min at 37ºC in extraction buffer containing 1 mM 4-MUG. The reaction was terminated by addition of 0.2 M Na2CO3. Fluorescence was then measured on a fluorescent spectrophotometer (F97pro, Shanghai) with 4-methylumbelliferone (4-MU) as standard. Protein concentration was measured using Bradford Kit (Cat. SK1060, Coolaber, Beijing) with bovine serum albumin (BSA) as standard.
Protein extraction and detection in C. glaucum and transgenic Arabidopsis
The total protein of C. glaucum or transgenic Arabidopsis (with 35S::bHLH or PbHLH::bHLH) was extracted using Protein Extraction Kit (Cat PTE001, Coolaber, Beijing). Plant tissues were ground in liquid nitrogen and then transferred into extraction buffer, followed by centrifugation at 12,000 rpm, 4°C for 15 min. The supernatant was used as crude protein solution, the protein concentration was determined by BCA (bicinchoninic acid) Protein Assay Kit (BCAP-2-W, Comin, Jiangsu). The crude protein was mixed with 4 × loading buffer and boiled for 10 min, then used for SDS-PAGE after centrifugation at 12,000 rpm for 10 min. Protein samples (60 μg of each) were resolved by 12% polyacrylamide gel and then transferred to a polyvinylidene fluoride membrane for immunoblotting analysis. The monoclonal mouse antibody against CgbHLH001 protein was developed (Abmart, Shanghai) and used at 1:500 dilution. β-tubulin (1:5000 diluted; M30109F, Abmart, Shanghai) served as internal control. The secondary antibody [Goat Anti-Mouse IgG (H + L) conjugated horseradish peroxidase, TransGen, Beijing] diluted at 1:10,000 for detection. The target protein was visualized by enhanced chemiluminescence (EasySee Western Blot Kit, TransGen, Beijing), and images were acquired by luminescent image analyzer (Amersham Imager 600, GE, USA).
RNA-Sequencing analysis
Three-week-old plants of WT and transgenic Arabidopsis overexpressing 35S::bHLH or PbHLH::bHLH (treated with either normal water or 200 mM NaCl for 1 h) were used for RNA sequencing. High-quality RNA was used (RNA integrity number (RIN) ≥ 8.5, OD260/280 ≥ 1.9 and OD260/230 ≥ 1.5). Sequencing was performed on an Illumina HiSeq2000 Platform (BioMarker technologies Co., Ltd, Beijing, China). Three independent biological replicates were sequenced. For the raw data, fastp v0.21.0 was used to filter adapter, low-quality base and low-complexity reads [74]. Parameters were set as follows: -q (qualified_quality_phred) = 10; -u (unqualified_percent_limt) = 50; -g (trim_poly_g) = 10; -Y (complexity_threshold) = 10; -e (average_qual) = 20; -l (legth_required) = 100; -b (max_len1) = 150; -B (max_len2) = 150. After filtering the raw data, all clean reads were aligned to Arabidopsis reference genome (ftp://ftp.ensemblgenomes.org/pub/plants/release-45/fasta/arabidopsis_thaliana) using Hisat2 v2.0.4 software (JHU, Baltimore, USA) (http://ccb.jhu.edu/software/hisat2/index.shtml). The mapped reads were assembled and merged using StringTie v1.3.4d software (JHU, Baltimore, MD, USA) (https://ccb.jhu.edu/software/stringtie/index.shtml). DEGs were identified using DEseq2 software (false discovery rate < 0.05 and FC ≥ 1.5). Gene Ontology (GO) enrichment analysis of DEGs was implemented via GOseq R packages v3.10.1 based on Wallenius non-central hyper-geometric distribution. Gene function was annotated using six primary databases: National Center for Biotechnology Information (NCBI), non-redundant protein sequences (NR), Kyoto Encyclopedia of Genes and Genomes (KEGG), manually annotated and commented protein sequence (Swiss-Prot), protein family (Pfam) and Gene Ontology (GO). Protein–protein interactions were analyzed using STRING software. Cytoscape software was used to visualize co-expression networks. Weighted gene co-expression network analysis (WGCNA) was used to describe correlation patterns among genes across multiple plants on the platform BMKCloud (www.biocloud.net). The heatmaps were drawn using the TBtools [75]. qRT-PCR of 28 DEGs that were critical to salt stress response was performed to validate the RNA-seq data. AtActin as the reference gene, all the primers used are listed in Table S6 in the Additional file 13.
Statistical analysis
All data were expressed as means ± SD. One-way ANOVA was used to test the significance of main effects, and Tukey’s test was performed for multiple comparisons to determine significant differences between samples at 0.05, 0.01 or 0.001 significance level.
Availability of data and materials
The sequence information of CgbHLH001 promoter is available in the NCBI GenBank (https://www.ncbi.nih.gov/genbank/) under accession number of MW544164. All the raw data from the RNA-seq are available in the Sequencing Read Archive (SRA) of NCBI (PRJNA856615).
References
Palavecino CE, Carrasco-Veliz N, Quest AFG, Garrido MP, Valenzuela-Valderrama M. The 5’ untranslated region of the anti-apoptotic protein Survivin contains an inhibitory upstream AUG codon. Biochem Biophys Res Commun. 2020;526(4):898–905.
Potenza C, Aleman L, Sengupta-Gopalan C. Targeting transgene expression in research, agricultural, and environmental applications: Promoters used in plant transformation. In Vitro Cell Dev-Pl. 2004;40(1):1–22.
Rombauts S, Florquin K, Lescot M, Marchal K, Rouze’ P. Computational approaches to identify promoters and cis-regulatory elements in plant genomes. Plant Physiol. 2003;132:1162–76.
Misra S, Ganesan M. The impact of inducible promoters in transgenic plant production and crop improvement. Plant Gene. 2021;27:100300.
Jiménez-Garza O, Linares-Segovia B, Ruiz-García L, Monroy-Torres R, Hernández-Luna MA. 5’UTR methylation in different genes from workers exposed to volatile organic compounds: a new insight for considering an epigenetic mark as a functional correlate. Toxicol Lett. 2020;330:59–64.
Yamasaki S, Sanada Y, Imase R, Matsuura H, Ueno D, Demura T, et al. Arabidopsis thaliana cold-regulated 47 gene 5’-untranslated region enables stable high-level expression of transgenes. J Biosci Bioeng. 2018;125(1):124–30.
Ortega JL, Wilson OL, Sengupta-Gopalan C. The 5’ untranslated region of the soybean cytosolic glutamine synthetase β1 gene contains prokaryotic translation initiation signals and acts as a translational enhancer in plants. Mol Genet Genomics. 2012;287(11–12):881–93.
Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266(30):19867–70.
Kawaguchi R. Regulation of translational initiation in plants. Curr Opin Plant Biol. 2002;5(5):460–5.
Takeshi M, Hideyuki M, Kazutoshi S, Eiji T, Satoko K, Shinya T, et al. High level expression of transgenes by use of 5’-untranslated region of the Arabidopsis thaliana arabinogalactan-protein 21 gene in dicotyledons. Plant Biotechnol. 2012;29:319–22.
Timerbaev V, Dolgov S. Functional characterization of a strong promoter of the early light-inducible protein gene from tomato. Planta. 2019;250(4):1307–23.
Anna VK, Artem SK, Evgeny SG, Maria DL, Aleksey AP. A high-resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-Seq profiling. Plant J. 2016;88(6):1058–70.
Daniel S, BK W, Janika W, Gerd J. Transcriptomic profiling of the Arabidopsis embryonic epidermis using FANS in combination with RNA-Seq. Methods Mol Biol. 2020;2122:151–64.
Gao Y, Xu H, Shen Y, Wang J. Transcriptomic analysis of rice (Oryza sativa) endosperm using the RNA-Seq technique. Plant Mol Biol. 2013;81(4–5):363–78.
Kumar G, Dasgupta I. Comprehensive molecular insights into the stress response dynamics of rice (Oryza sativa L.) during rice tungro disease by RNA-Seq-based comparative whole transcriptome analysis. J Biosciences. 2020;45(1):27–49.
Li W, Guo YF. RNA-Seq analysis of the transcriptome of leaf senescence in Tobacco. In: Guo Y, editor. Plant Senescence. Methods in Molecular Biology. New York: Humana Press; 2018. p. 331–7.
Liu H, Wu H, Wang Y, Wang H, Chen S, Yin Z. Comparative transcriptome profiling and co-expression network analysis uncover the key genes associated with early-stage resistance to Aspergillus flavus in maize. BMC Plant Biol. 2021;21:216.
Long L, Yang WW, Liao P, Guo YW, Kumar A, Gao W. Transcriptome analysis reveals differentially expressed ERF transcription factors associated with salt response in cotton. Plant Sci. 2019;281:72–81.
Zhang S, Li X, Fan S, Zhou L, Wang Y. Overexpression of HcSCL13, a Halostachys caspica GRAS transcription factor, enhances plant growth and salt stress tolerance in transgenic Arabidopsis. Plant Physiol Bioch. 2020;151:243–54.
Institute of Botany, Academia Sinica. Iconographia Cormophytorum Sinicorum (Supplementum I). Beijing: Science Press; 1983.
Hong SY, Cheon KS, Yoo KO, Lee HO, Cho KS, Suh JT, et al. Complete chloroplast genome sequences and comparative analysis of Chenopodium quinoa and C. album. Front Plant Sci. 2017;8:1696.
Yao Y, Li XT, Wu XY, Fan SJ, Zhang XJ, Qu XJ. Characterization of the complete chloroplast genome of an annual halophyte, Chenopodium glaucum (Amaranthaceae). Mitochondrial DNA B Resour. 2019;4(2):3898–9.
Zhou Z, Wang J, Zhang S, Yu Q, Lan H. Investigation of the nature of CgCDPK and CgbHLH001 interaction and the function of bHLH transcription factor in stress tolerance in Chenopodium glaucum. Front Plant Sci. 2021;11:603298.
Zhang S, Liu J, Zhong G, Wang B. Genome-wide identification and expression patterns of the C2H2-zinc finger gene family related to stress responses and catechins accumulation in Camellia sinensis [L.] O. Kuntze. Int J Mol Sci. 2021;22:4197.
Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Pandey B, Prakash P, Chandra Verma P, Srivastava R. Regulated gene expression by synthetic modulation of the promoter architecture in plants. In: Current Developments in Biotechnology and Bioengineering. 2019;235–255.
Srivastava VK, Raikwar S, Tuteja N. Cloning and functional characterization of the promoter of PsSEOF1 gene from Pisum sativum under different stress conditions using Agrobacterium-mediated transient assay. Plant Signal Behav. 2014;9(9):e29626.
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low- temperature- responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–406.
Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–40.
Mochida K, Yoshida T, Sakurai T, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. In silico analysis of transcription factor repertoire and prediction of stress responsive transcription factors in soybean. DNA Res. 2009;16(6):353–69.
Freitas EO, Melo BP, Loureno-Tessutti IT, Arraes F, Amorim RM, Lisei-De-Sá M, et al. Identification and characterization of the GmRD26 soybean promoter in response to abiotic stresses: potential tool for biotechnological application. BMC Biotechnol. 2019;19:79.
Fang ZW, Xu XY, Gao JF, Wang PK, Liu ZX, Feng BL, et al. Characterization of FeDREB1 promoter involved in cold- and drought-inducible expression from common buckwheat (Fagopyrum esculentum). Genet Mol Res. 2015;14(3):7990–8000.
Cadar AG, Zhong L, Lin A, Valenzuela MO, Lim CC. Upstream open reading frame in 5’-untranslated region reduces titin mRNA translational efficiency. Biochem Biophys Res Commun. 2014;453(1):185–91.
Higgs DC, Shapiro RS, Kindle KL, Stern DB. Small cis-acting sequences that specify secondary structures in a chloroplast mRNA are essential for RNA stability and translation. Mol Cell Biol. 1999;19(12):8479–91.
Vaistij FE, Goldschmidt-Clermont M, Wostrikoff K, Rochaix JD. Stability determinants in the chloroplast psbB/T/H mRNAs of Chlamydomonas reinhardtii. Plant J. 2000;21(5):469–82.
Kim Y, Lee G, Jeon E, Eunju S, Yongjik L, Kang H, et al. The immediate upstream region of the 5’-UTR from the AUG start codon has a pronounced effect on the translational efficiency in Arabidopsis thaliana. Nucleic Acids Res. 2014;1:485–98.
Shiina T, Allison L, Maliga P. rbcL transcript levels in tobacco plastids are independent of light reduced dark transcription rate is compensated by increased mRNA stability. Plant Cell. 1998;10(10):1713–22.
Yamamoto YY, Tsuji H, Obokata J. 5’-leader of a photosystem I gene in Nicotiana sylvestris, psaDb, contains a translational enhancer. J Biol Chem. 1995;270(21):12466–70.
Dansako T, Kato K, Satoh J. 5’ untranslated region of the HSP 18.2 gene contributes to efficient translation in plant cells. J Biosci Bioeng. 2003;95(1):52–8.
Satoh J, Kato K, Shinmyo A. The 5’-untranslated region of the tobacco alcohol dehydrogenase gene functions as an effective translational enhancer in plant. J Biosci Bioeng. 2004;98(1):1–8.
Bradnam KR, Korf I. Longer first introns are a general property of eukaryotic gene structure. PLoS ONE. 2008;3(8):e3093.
Zheng H, Lin S, Zhang Q, Lei Y, Zhang Z. Functional analysis of 5’ untranslated region of a TIR-NBS-encoding gene from triploid white poplar. Mol Genet Genomics. 2009;282(4):381–94.
Karkute SG, Gujjar RS, Rai A, Akhtar M, Singh M, Singh B. Genome wide expression analysis of WRKY genes in tomato (Solanum lycopersicum) under drought stress. Plant Gene. 2018;13:8–17.
Luo P, Li Z, Chen W, Xing W, Yang J, Cui Y. Overexpression of RmICE1, a bHLH transcription factor from Rosa multiflora, enhances cold tolerance via modulating ROS levels and activating the expression of stress-responsive genes. Environ Exp Bot. 2020;178:104160.
Yao P, Sun Z, Li C, Zhao X, Li M, Deng R, et al. Overexpression of Fagopyrum tataricum FtbHLH2 enhances tolerance to cold stress in transgenic Arabidopsis. Plant Physiol Biochem. 2018;125:85–94.
Ren YR, Yang YY, Zhao Q, Zhang TE, Wang CK, Hao YJ, et al. MdCIB1, an apple bHLH transcription factor, plays a positive regulator in response to drought stress. Environ Exp Bot. 2021;188:104523.
Jin C, Huang XS, Li KQ, Yin H, Li LT, Yao ZH, Zhang SL. Overexpression of a bHLH1 transcription factor of Pyrus ussuriensis confers enhanced cold tolerance and increases expression of stress-responsive genes. Front Plant Sci. 2016;7(121):441.
Zhao H, Abulaizi A, Wang C, Lan H. Overexpression of CgbHLH001, a positive regulator to adversity, enhances the photosynthetic capacity of maize seedlings under drought stress. Agronomy. 2022;12:1149.
Wang M, Yang D, Ma F, Zhu M, Shi Z, Miao X. OsHLH61-OsbHLH96 influences rice defense to brown planthopper through regulating the pathogen-related genes. Rice. 2019;12(9):1–12.
Wethmar K, Smink JJ, Leutz A. Upstream open reading frames: Molecular switches in (patho)physiology. BioEssays. 2010;32(10):885–93.
Barrett LW, Fletcher S, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell Mol Life Sci. 2012;69:3613–34.
Tang YC, Amon A. Gene copy-number alterations: A cost-benefit analysis. Cell. 2013;152:394–405.
Liu Y, Beyer A, Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165(3):535–50.
Zhao C, Zhang H, Song C, Zhu JK, Shabala S. Mechanisms of plant responses and adaptation to soil salinity. Annu Rev Plant Biol. 2008;59:651–81.
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, et al. Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul. 2015;75(2):391–404.
Zou L, Li T, Li B, He J, Liao C, Wang L, et al. De novo transcriptome analysis provides insights into the salt tolerance of Podocarpus macrophyllus under salinity stress. BMC Plant Biol. 2021;21:489.
Kiani-Pouya A, Li L, Rasouli F, Zhang Z, Chen J, Yu M, et al. Transcriptome analyses of quinoa leaves revealed critical function of epidermal bladder cells in salt stress acclimation. Plant Stress. 2022;3:100061.
Yu Z, Duan X, Luo L, Dai S, Ding Z, Xia G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020;25(11):1117–30.
Sheen J. Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc Natl Acad Sci USA. 1998;95:975–80.
Ahanger MA, Aziz U, Alsahli AA, Alyemeni MN, Ahmad P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate glutathione cycle in salt stressed Vigna angularis. Biomolecules. 2020;10:42.
van Loon LC, Rep M, Pieterse CM. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–62.
Lehti-Shiu M, Zou C, Hanada K, Shiu SH. Evolutionary history and stress regulation of plant receptor-like kinase/pelle genes. Plant Physiol. 2009;150:12–26.
Hoang XLT, Nhi DNH, Thu NBA, Thao NP, Tran LP. Transcription factors and their roles in signal transduction in plants under abiotic stresses. Curr Genomics. 2017;18:483–97.
Wang H, Wang H, Shao H, Tang X. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front Plant Sci. 2016;7:67.
Gharaghanipor N, Arzani A, Rahimmalek M, Ravash R. Physiological and transcriptome indicators of salt tolerance in wild and cultivated barley. Front Plant Sci. 2022;13:819282.
Harb A, Simpson C, Guo W, Govindan G, Kakani VG, Sunkar R. The effect of drought on transcriptome and hormonal profiles in barley genotypes with contrasting drought tolerance. Front Plant Sci. 2020;11:618491.
Duran-Medina Y, Serwatowska J, Reyes-Olalde JI, Folter SD, Marsch-Martinez N. The AP2/ERF transcription factor DRNL modulates gynoecium development and affects its response to cytokinin. Front Plant Sci. 2017;8:01841.
Li A, Yu X, Cao BB, Peng LX, Gao Y, Feng T, et al. LkAP2L2, an AP2/ERF transcription factor gene of Larix kaempferi, with pleiotropic roles in plant branch and seed development. Russ J Genet. 2017;53(12):1335–42.
Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li XC, et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol. 2007;65(6):719–32.
Quan RD, Hu SJ, Zhang ZL, Zhang HW, Zhang ZJ, Huang RF. Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol J. 2010;8(4):476–88.
Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn. 1950;347:32.
Shi R, Chiang VL. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques. 2005;39(4):519–25.
Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:884–90.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202.
Acknowledgements
We thank the reviewers and all of the editors for their helpful comments and suggestions on this paper.
Funding
This work was supported by the National Natural Science Foundation of China (31660068, 32160057), Open Fund of Xinjiang Uygur Autonomous Region (2016D03015).
Author information
Authors and Affiliations
Contributions
Haiyan Lan, Zixin Zhou and Qinghui Yu designed the experiments. Zixin Zhou and Juan Wang performed all experiments and analyzed the data. Zixin Zhou and Haiyan Lan wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental studies on plants were complied with relevant institutional, national, and international guidelines and legislation. The plant materials used in this manuscript came from the collection of college of life science and technology, Xinjiang University, Urumqi, China. Chenopodium glaucum was identified by Yongman Lu (a plant taxonomist in Xinjiang University), and voucher specimens (No. CG201410) was deposited at the herbarium (College of Life Science and Technology, Xinjiang University).
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Phenotype performance and gene expression of transgenic Arabidopsis lines overexpressing 35S::bHLH and PbHLH::bHLH in response to drought stress. A Transcriptional expression of CgbHLH001 gene. B Translational expression of CgbHLH001 gene. C-D Phenotypic observation and survival percentage of transgenic Arabidopsis. OE35S1, 2: 35S::bHLH-overexpressing transgenic line 1, 2; OEPb1, 2: PbHLH::bHLH-overexpressing transgenic line 1, 2. Different lowercase letters in a indicate significant difference existing between different transgenic lines.
Additional file 2: Fig. S2.
Phenotype performance and gene expression of transgenic Arabidopsis lines overexpressing 35S::bHLH and PbHLH::bHLH in response to 4°C treatment. A Transcriptional expression of CgbHLH001 gene. B Translational expression of CgbHLH001 gene. C-D Phenotypic observation and survival percentage of transgenic Arabidopsis. OE35S1, 2: 35S::bHLH-overexpressing transgenic line 1, 2; OEPb1, 2: PbHLH::bHLH-overexpressing transgenic line 1, 2. Different lowercase letters in A, D indicate significant difference existing between different transgenic lines.
Additional file 3: Fig. S3.
The heatmap of correlation analysis (A) and principal component analysis (B) among replicates in the same group and between different groups. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment.
Additional file 4: Fig. S4.
The most enriched GO terms in different comparisons. A-B 542 upregulated and 494 downregulated DEGs in A(C) vs C(C); C-D 88 upregulated and 207 downregulated DEGs in A(C) vs B(C); E-F 182 upregulated and 415 downregulated DEGs in the overlap between A(C) vs C(C) and A(C) vs B(C). A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition.
Additional file 5: Fig. S5.
The most enriched GO terms in various comparisons. A The most enriched GO terms in the top 50 DEGs with the highest fold change in comparison B(S) vs C(S). B The most enriched GO terms in the top 50 DEGs with the highest fold change in comparison A(S) vs B(S). C The most enriched GO terms in the top 50 DEGs with the highest fold change in comparison A(S) vs C(S). D The most enriched GO terms of DEGs in blue module. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (S): salt treatment.
Additional file 6: Fig. S6.
Analyses of DEGs in CgbHLH001 transgenic plants under salt stress. A Numbers of DEGs in different comparisons. B, C Venn analysis of upregulated or downregulated genes in transgenic plants under salt stress. D, E Go enrichment of upregulated or downregulated genes in transgenic plants under salt stress. In C, gene ratio represents the percentage of selected genes, the circle size represents gene numbers, the larger the circle, the more the gene numbers. The color of circle represents the p value, the darker the color, the smaller the p value, with higher significant difference. The left red font represents abiotic stress related GO terms in biological process.
Additional file 7: Fig. S7.
Statistical analysis of DE TFs and PKs in different comparisons. A: wild type (Col-0); B: 35S::bHLH-overexpressing transgenic Arabidopsis; C: PbHLH::bHLH-overexpressing transgenic Arabidopsis; (C): normal condition; (S): salt treatment.
Additional file 8: Table S1.
Analysis on cis-acting regulatory elements of the promoter of CgbHLH001 gene.
Additional file 9: Table S2.
Overview of RNA-seq data.
Additional file 10: Table S3.
List of top 30 DEGs with the highest fold change of expression level under salt stress.
Additional file 11: Table S4.
List of DE TFs after salt stress treatment.
Additional file 12: Table S5.
Selection of putative salt stress-associated DEGs in transgenic Arabidopsis.
Additional file 13: Table S6.
Primers used in the present study.
Additional file 14: Supporting Fig. 1.
Original images of gels and blots. A: PCR identification of 35S::bHLH-overexpressing transgenic Arabidopsis. B: PCR identification of PbHLH::bHLH-overexpressing transgenic Arabidopsis. C: RT-PCR identification of transgenic Arabidopsis lines. D: Detection of CgbHLH001 expression in C. glaucum under different stress treatments. E-H: Detection of CgbHLH001 expression in transgenic Arabidopsis under normal condition (E), 200 mM NaCl (F), 300 mM Mannitol (G) and 4°C (H) treatments. Tubulin acted as the internal reference.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Z., Wang, J., Yu, Q. et al. Promoter activity and transcriptome analyses decipher functions of CgbHLH001 gene (Chenopodium glaucum L.) in response to abiotic stress. BMC Plant Biol 23, 116 (2023). https://doi.org/10.1186/s12870-023-04128-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-023-04128-8