Abstract
Background
GRAS genes formed one of the important transcription factor gene families in plants, had been identified in several plant species. The family genes were involved in plant growth, development, and stress resistance. However, the comparative analysis of GRAS genes in Rosaceae species was insufficient.
Results
In this study, a total of 333 GRAS genes were identified in six Rosaceae species, including 51 in strawberry (Fragaria vesca), 78 in apple (Malus domestica), 41 in black raspberry (Rubus occidentalis), 59 in European pear (Pyrus communis), 56 in Chinese rose (Rosa chinensis), and 48 in peach (Prunus persica). Motif analysis showed the VHIID domain, SAW motif, LR I region, and PFYRE motif were considerably conserved in the six Rosaceae species. All GRAS genes were divided into 10 subgroups according to phylogenetic analysis. A total of 15 species-specific duplicated clades and 3 lineage-specific duplicated clades were identified in six Rosaceae species. Chromosomal localization presented the uneven distribution of GRAS genes in six Rosaceae species. Duplication events contributed to the expression of the GRAS genes, and Ka/Ks analysis suggested the purification selection as a major force during the evolution process in six Rosaceae species. Cis-acting elements and GO analysis revealed that most of the GRAS genes were associated with various environmental stress in six Rosaceae species. Coexpression network analysis showed the mutual regulatory relationship between GRAS and bZIP genes, suggesting the ability of the GRAS gene to regulate abiotic stress in woodland strawberry. The expression pattern elucidated the transcriptional levels of FvGRAS genes in various tissues and the drought and salt stress in woodland strawberry, which were verified by RT-qPCR analysis.
Conclusions
The evolution and functional analysis of GRAS genes provided insights into the further understanding of GRAS genes on the abiotic stress of Rosaceae species.
Similar content being viewed by others
Background
Transcription factors (TFs) as regulatory proteins, act with promoter cis-acting elements or interact with functional regions of other transcription factors to regulate gene transcription and expression [1]. The main TF family in the plant was first identified in Arabidopsis [2]. In the following years, transcription factors in other plants were identified gradually with the development of the genome era [3]. Several studies had shown that transcription factors play an important role in various regulation processes of plants [4, 5].
As a transcription factor, GRAS genes participated in plant growth and development [6, 7], stress resistance [8, 9], and other life activities [10, 11]. GRAS originated from the three earliest members which were functionally researched, namely, gibberellic acid insensitive (GAI), scarecrow (SCR), and repressor of ga1-3 (RGA) [12,13,14]. In general, GRAS proteins consist of a variable N-terminal domain and a relatively conserved C-terminal domain. The C-terminal contains five extremely conserved fragments: LRI (Leucine-rich regions I), VHIID, LRII (Leucine-rich regions II), PFYRE, and SAW [15,16,17]. The VHIID region between LRI and LRII was a conserved domain, which was present in almost all GRAS proteins [18]. Related studies had shown that the LRI-VHIID-LRII pattern may be involved in the binding of proteins to nucleic acids or the binding of other proteins [19, 20]. In addition, the motifs of PFYRE and SAW were identified as related to GRAS protein structural integrity [21].
At present, the function of GRAS genes had been identified in several plant species. The number of 32 GRAS were found in Arabidopsis [22], 57 in rice [23], 54 in tomato [24], 48 in Chinese cabbage [25], 48 in physic nut [26], 62 in barely [27], and 117 in soybean [28]. Further phylogenetic tree analyses of multi-species divided GRAS proteins into 10 subfamilies, namely, DELLA, AtLAS, AtSHR, AtPAT1, AtSCL3, SCL4/7, HAM, LISCL, AtSCR, and DLT [29]. These GRAS genes from diverse subfamilies had been proven to be involved in various physiological processes in plants. For instance, OsMOC1, SlyLs, and AtLAS were reported to be mainly related to the axillary formation [30,31,32]. Two Arabidopsis GRAS proteins, AtSCR and AtSHR, positively regulated the formation of root and shoot radial patterns [33, 34]. AtSCL21 and AtPAT1 were mainly involved in the signal transduction of etiolated to photomorphogenic in Arabidopsis [35, 36]. Furthermore, several previous studies had shown that GRAS genes participated in plant abiotic stress processes by interacting with bZIP genes [37]. DELLA proteins had been proven to be involved in ABA signaling and enhanced plant tolerance to drought by interacting with the abscisic acid-responsive transcription factor ABF2 [38]. AtSCL14 was shown to interact with the bZIP family gene TGA to activate the broad-spectrum detoxification network [39].
Rosaceae, one of the most economically valuable families, was classified into four subfamilies: Spiraeoideae, Maloideae, Rosoideae, and Amygdaloideae [40]. Rosaceae plants include economical ornamental flowers, numerous fruit species, herbs, nuts, and woody plants [41]. Although the GRAS family has been studied in fruit species, such as apple [42] and strawberry [43], the comparison between GRAS family species in Rosaceae had not been reported. In this study, we selected the representative plants from the four subfamilies of Rosaceae, including woodland strawberry, black raspberry, Chinese rosa, European pear, peach, and apple. The members of the GRAS family were identified in six Rosaceae species, comprehensive phylogenetic analysis, motif analysis, gene duplication, Ka/Ks analysis, and cis-acting element analysis were executed. Combining transcriptome analysis and RT-qPCR analysis, we determined the responses of GRAS family members to drought and salt stress in woodland strawberry. This study may serve as the basis for revealing the evolutionary relationship of the GRAS family in plant growth and development.
Materials and methods
Identification and classification of the GRAS family
To identify the GRAS genes of six Rosaceae species, all protein-coding sequences of Malus domestica (v1.0.a1), Rubus occidentalis (v3.0), Pyrus communis (v2.0), Fragaria vesca (v1.1.a2), Prunus persica (v2.0.a1), and Rosa chinensis (v1.0) were downloaded from the Genome Database for Rosaceae (GDR) site (https://www.rosaceae.org/). The hidden Markov models (HMMs) of the GRAS (PF03514) were downloaded from Pfam 35.0 (http://pfam.xfam.org/), and used to investigate putative GRASs with an e-value cutoff < 1.0 using HMMER v3.1 [44]. To validate the accuracy of the possible GRASs, all candidate GRAS genes were examined and analyzed by Pfam v35.0 and SMART v9.0 (http://smart.embl-heidelberg.de/) [45].
Multiple amino acid alignments of GRAS proteins were aligned and the phylogenetic tree was constructed with the maximum likelihood method and 1000 bootstrap replicates using IQ-TREE v2.1.3 in six Rosaceae species [46]. The Evolview tool was used to improve graphical presentations of the trees [47]. In the clade with bootstrap values larger than 50, the lineage-specific duplications were defined as the GRAS genes appeared in two or more Rosaceae species, and the species-specific duplications occurred in one Rosaceae species [48]. The protein sequences of the GRAS gene in six Rosaceae species and Arabidopsis were aligned by the MUSCLE program in MEGA X and the maximum-likelihood tree was built using IQ-TREE v2.1.3.
Conserved motif analysis of the GRAS genes
The putative motifs in six Rosaceae species GRAS family were predicted by the MEME website (https://meme-suite.org/meme/). The parameter was set according to described previously [49].
Chromosomal location and gene duplication
The chromosomal locations and lengths of the GRASs were obtained from the genome database. Then, the GRAS genes were represented graphically with MapChart. All GRAS protein sequences were searched against themselves by NCBI-BLAST 2.13.0 + [50], and duplication events were investigated using DupGen_finder [51].
Calculation of Ks, Kax/Ks values
To calculate the selection pressure of GRAS genes in six Rosaceae species, the Ks (synonymous substitutions) and Ka/Ks (nonsynonymous to synonymous substitution) ratios of gene pairs were calculated using MEGA X software and Perl script. Ka/Ks > 1 indicated positive selection, Ka/Ks = 1 suggested neutral selection, and Ka/Ks < 1 implied purification selection [52].
Cis-acting elements in the GRAS promoter regions
The 1500 bp upstream sequences from the promoters of GRAS genes in six Rosaceae species were extracted from the assembly file using Perl script. The cis-acting elements in the putative promoter regions were analyzed by PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [53].
GO functional analysis of GRAS genes
To report the predicted functions of GRAS proteins, the GO terms for GRASs in six Rosaceae species were collected from the Gene Ontology Consortium (http://geneontology.org) [54].
Transcriptome analyses of woodland strawberry GRAS genes
To identify tissue-specific expression patterns of GRAS genes in woodland strawberry, RNA-Seq data were collected from Strawberry Genome Resources (http://bioinformatics.towson.edu/strawberry) [55]. The datasets of PRJNA733854 and PRJNA472896, which involved strawberry gene expression upon drought and salt stress, were downloaded from the NCBI website (https://www.ncbi.nlm.nih.gov) [56, 57]. The FPKM (fragments per kilobase million) values were calculated by R v4.0.2. The ‘scale’ parameter was used to normalize transcriptome data. The expression levels of GRAS genes were visualized using the ComplexHeatmap package (v2.4.3) in the R (v4.0.2).
STEM analysis of GRAS genes in woodland strawberry
The DEGs were classified into different clusters according to time sequence profile analysis by Short Times-series Expression Miner (STEM) software [58].
Co-expression network of GRAS with bZIP genes in woodland strawberry
The FPKM values of GRAS and bZIP genes were extracted from the drought and salt stress transcriptomes. The Pearson correlation coefficients (PCCs) between GRAS and bZIP genes were calculated and constructed in a co-expression network using Cytoscape_v3.7.2 (PCCs ≥ 0.6, P < 0.05).
Plant material stress treatment, RNA extraction and RT-qPCR analysis
Woodland strawberry plants (Fragaria vesca, Hawaii4) were grown in greenhouse at Nanjing Agricultural University (Jiangsu, China), and maintained at room temperature under a photoperiod of 16/8 h. Two-month-old plants were used for drought and salt treatment. Tender (the stage before fully expanded mature leaves) and old (the stage of fully expanded mature leaves) leaf samples were collected at 0, 3, 5, and 7 days after water was withheld. In addition, strawberry leaves were used as salt stress treatment and samples were collected at 0, 3, 5, and 7 days after treatment.
Total RNA was extracted using the Plant Total RNA Isolation Kit Plus (Foregene, Chengdu, China). First strand cDNA was synthesized using PrimerScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China). RT-qPCR analysis was performed by Light Cycler 480 II (Roche, Switzerland) with TB Green® Premix Ex Taq™ (TaKaRa, Dalian, China). 18sRNA was used as an internal reference gene to normalize the expression of GRAS genes using the 2−△△Ct method [59]. Each set of RT-qPCR data was calculated with three replicates. The primer sequences used for RT-qPCR were listed in Table S5.
Results
Identification and characterization of the GRAS family in six Rosaceae species
After excluding the redundant sequences, a total of 333 GRAS genes were identified. For the six species, strawberry, apple, black raspberry, pear, Chinese rosa and peach, 51, 78, 41, 59, 56 and 48 GRAS genes were detected, respectively (Table 1). The apple (78) had the maximum number and highest proportion of GRAS genes compared with the other five species. The number of GRAS genes in the other five species was similar.
Phylogenetic analysis of GRAS protein
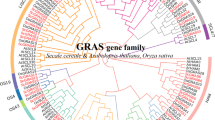
To analyze the phylogenetic relationships of GRAS proteins in six species. The GRAS sequences were used to construct a maximum likelihood tree by IQ-TREE. Arabidopsis was used as an outgroup, and the GRAS genes of six Rosaceae species were divided into ten subfamilies, including PAT1, SHR, SCL, HAM, DELLA, SCL3, LAS, SCL4/7, DLT, SCR (Fig. 1 and Fig. S2). In addition, the clades were shaped by bootstrap values larger than 50 in six Rosaceae species. The 301 GRAS genes (appearing in one Rosaceae species) were identified as 15 species-specific duplicated clades, and the 3 lineage-specific duplicated clades, including 12 GRAS genes appeared in two or more Rosaceae species (Fig. S1).
Conserved motif analysis of GRAS protein
The unique motif sequences provided insight into protein function, in order to identify motif constructions of the GRAS proteins, we submitted 333 GRAS predicted amino acid sequences to the MEME website. A total of 20 motifs were used as the number of queries for the MEME program. Figure 2 showed the four conserved sites in six Rosaceae species, including the VHIID domain, SAW motif, LRI region, and PFYRE motif. This result suggested the highly conserved GRAS gene family.
Chromosomal distribution and duplication events of the GRAS genes
The GRAS genes were distributed unevenly across the chromosomes in six Rosaceae species (Figs. S3, S4, S5, S6, S7 and S8). There was even no GRAS gene distribution on the chromosomes of some species, for instance, no GRAS gene was found on chromosome 16 in apple (Fig. S4), chromosomes 8 and 14 in pear (Fig. S6), and chromosome 4 in woodland strawberry (Fig. S3). In addition, chromosome 5 had the largest number (26) and highest proportion (47%) of the GRAS gene detected in Rosa (Fig. S7).
Gene duplication, as one of the main ways of multigene family expansion, was ubiquitous in plant species [60]. In this study, we identified the number of tandem (TD), whole-genome (WGD), proximal (PD), transposed (TRD) and dispersed (DSD) duplication genes in the GRAS gene family of six Rosaceae species (Additional file 11: Table S3). WGD and TRD events were found in all six Rosaceae species, and 72.88% (30/59) of GRAS genes were identified in pear higher than others in WGD events. TD events occurred in apple, pear, peach, rosa, and strawberry. The largest proportion was 19.23% (15/78) in apple compared to the other five Rosaceae species. The 198 DSD events were identified with 130 GRAS genes in apple, peach, rosa, black raspberry, and strawberry. PD events only appeared in rosa, apple, and strawberry.
GRAS gene evolutionary in six Rosaceae species
The synonymous (Ks) represented the amino acid sequence remaining unchanged after the mutation [61], and the values of Ks in each gene pair were calculated among six Rosaceae species using Perl script. The value of Ks was mainly distributed at 1.2 to 3.8 in apple, strawberry, pear, black raspberry, and peach (Fig. 3). Compared with the other five species, the main distribution of Ks values in rose was 1.0 to 4.4 (Fig. 3E). In apple, the Ks value peaked in the 1.4–1.6 region. The other three Rosaceae species, woodland strawberry, pear, peach with the peak of Ks values between 2.0–2.2 and showed a similar pattern (Fig. 3A, C and D). These results suggested the relatively recent duplication of GRAS genes in apple.
The nonsynonymous substitutions (Ka) and synonymous (Ks) ratio of two protein-coding genes were determined whether the selective pressure acted on the gene [62]. In this study, the Ka/Ks value of 97.7% (5422/5547) of gene pairs was less than 1, indicating that the gene pairs were under purifying selection. The 2.1% (117/5547) of gene pairs had a ratio greater than 1, which implied a positive selection of the gene pairs (Fig. 4 and Table S4).
Functional prediction of GRAS genes in six species
GO annotations of 333 GRAS genes were assigned and classified into three categories: molecular function, biological process, and cellular component (Fig. 5). The results of the biological process showed that the terms asymmetric cell division (GO:0008356), leaf development (GO:0048366), radial pattern formation (GO:0009956), and response to gibberellin (GO:0009739) were enriched. The top GO term in the cellular component was nucleus (GO:0005634). According to the molecular function results, most GRAS genes were associated with transcription factor activity (GO:0003700 and GO:0000989). These results suggested that GRAS genes were mainly expressed in the nucleus as transcription factors and functioned to regulate plant development and respond to hormone-related stress. In addition, 5609 cis-acting elements for all the GRAS gene promoter regions were predicted in six Rosaceae species (Fig. S9). These elements were divided into light responsive, hormone response, metabolism regulation, defense and stress response according to the functions. Elements related to stress occupied a high proportion, including the elements involved in the low-temperature response, drought, defense, and stress response. Many hormone-related responses were also reflected in cis-acting elements, such as auxin, salicylic acid, jasmonic acid, abscisic acid, and gibberellin responses.
Expression patterns of woodland strawberry GRAS genes
We collected the expression patterns of the strawberry GRAS gene family in different tissues, including pollen, microspores, perianth, flowered, receptacle, carpel, anther, leaf, seeding, embryo, ghost, cortex, pitch, style, ovule, and wall. The heatmap showed that at least two-thirds of the genes were highly expressed in almost all tissues (Fig. 6). Among them, the GRAS genes in woodland strawberry were expressed at a low level in pollen. Additionally, the GRAS genes of strawberry had similar expression patterns in the carpel, anther, leaf, seedling, embryo, ghost, cortex, pitch, style, ovule, and wall. These results implied that GRAS genes have participated in the growth and development of woodland strawberry.
Transcriptome and RT-qPCR expression analysis of FvGRASs under abiotic stress
To verify the role of FvGRAS under drought stress, we analyzed the expression pattern of woodland strawberry under drought treatment. After drought treatment, the old leaves and young leaves were clustered at 0d, 3d, 5d, and 7d (Fig. 7A). Heatmap showed that at least half of the GRAS genes were significantly increased in expression upon drought stress. The GRAS genes were divided by STEM analysis, in which two and three profiles had P < 0.01 in old (Fig. 7C) and young leaves (Fig. 7D), respectively. The expression levels of FvGRASs were gradually increasing under drought stress. In strawberry fruit, we found that at least one-third of the GRASs responds to drought and salt stress. Interestingly, the genes differed between salt and drought stress (Fig. 7B), such as mrna30170.1 was highly expressed in drought stress but lower upon salt stress, which indicated that the GRAS family was related to the resistance of strawberry to drought stress.
The values of PCC were calculated to predict the interaction of GRAS-bZIP in woodland strawberry. 91 GRAS-bZIP gene pairs were significantly positively correlated with expression under drought and salt stress (Fig. 8). 255 GRAS-bZIP gene pairs showed negative correlations. These significantly correlated gene pairs may be involved in the activities of response to abiotic stress in strawberry.
Subsequently, according to the classification of different subfamilies, we used RT-qPCR to detect the expression levels of genes in subfamilies (Fig. 9). In the tender leaf tissues under drought stress, the gene expression levels of almost all subfamilies showed a decline and then ascending trend. On the fifth day after treatment, the expression of FvGRAS genes was the lowest in young leaves. Interestingly, the expression levels of mrna14780.1 (SCL4/7 subfamily) and mrna24339.1 (SCR subfamily) were low in tender leaves at different treatment stages. We speculated that these subfamilies may not be involved in the response to drought stress in the young strawberry leaves. In addition, in the old leaves, with the extension of the treatment time, the gene expression trend was slower compared to the tender leaves. For instance, the relative expression levels of LAS (mrna01184.1), PAT (mrna03285.1), SHR (mrna05720.1), SCL3 (mrna18499.1), and LISCL (mrna30167.1) subfamily genes were lower than tender leaves at 7d in old leaves. These results suggested that FvGRAS families respond to drought stress and that the responses in diverse areas seemed to be different.
To further verify the role of the FvGRAS gene family in other abiotic stresses, we detected the relative expression level of the FvGRAS gene after salt treatment (Fig. 10). These results implied that the expression levels of the FvGRAS genes under salt stress were obviously higher than drought stress. The expression of PAT (mrna01015.1), LAS (mrna01184.1), HAM (mrna29558.1), and SCR (mrna24339.1) subfamily genes was more obvious. Interestingly, we observed that genes of different subfamilies were highly expressed at different treatment periods, for instance, mrna01015.1 (PAT) and mrna01184.1 (LAS) were mainly highly expressed on days 3 and 5 after treatment, while mrna29558.1 (HAM), mrna24339.1 (SCR), and mrna30958.1 (DELLA) were mainly highly expressed on day 7 after treatment. In addition, SCR (mrna24339.1) and DELLA (mrna30958.1) subfamily genes increased with the treatment time, while the expression of PAT (mrna01015.1), LAS (mrna01184.1), SCL3 (mrna14438.1) subfamily genes decreased with the treatment time. These results indicated that FvGRAS genes perform unique functions and jointly regulate the resistance of plants to adversity during abiotic stress.
Discussion
Members of the GRAS family played an important regulatory role in multiple biological processes [63, 64]. In rice, OsGRAS23 was demonstrated to be involved in the drought stress response by binding to the promoters of stress-responsive genes [65]. VaPAT1, an inducible GRAS gene, has been identified to increase cold, drought, and salt tolerance in transgenic Arabidopsis [66]. In tomato, overexpression of SlGRAS40 was characterized to enhance tolerance to abiotic stress [67]. In this study, 333 GRAS genes were characterized in six Rosaceae species. The GRAS genes could be divided into ten families: PAT, SHR, SCL4/7, HAM, LISCL, DLT, SCR, SCL3, DELLA, and LAS.
Different duplication patterns drive the expansion of GRAS genes in six Rosaceae
Gene duplication played an important role in the formation of new functional genetic material and the creation of new species [68,69,70]. As a common phenomenon, there were multiple pathways for gene duplication events [71,72,73,74], and the accumulation of beneficial mutations was preserved through selective evolution [75]. As a transcription factor family, the duplication events of GRAS genes had been widely reported [76]. Gene duplication serves as a source of evolutionary novelty for the GRAS gene family in both monocotyledonous and dicotyledonous lineages [77, 78]. Studies had shown that gene duplication events facilitate the expansion of the GRAS gene family in rice and Arabidopsis [23]. In this study, five duplication events were identified in six Rosaceae species. We found a large number of tandem duplications (19.23%), PD events (100%), and DSD events (75.64%) in apple, WGD events (72.88%) in pear, and TRD events (68.29%) in black raspberry, which implied that duplication events have contributed to the expansion of the GRAS gene family, especially in apple.
Species-specific duplications and lineage-specific duplications measured the occurrence of duplication events in a species or species [79]. (301/333) 90.39% species-specific duplicate genes were observed, (12/333) 3.6% lineage-specific duplicate genes were observed. This result suggested that the evolution of GRAS genes involved the participation of lineage-specific duplications and species-specific duplications. Meanwhile, species-specific duplication promoted the expansion of the GRAS genes more than lineage-specific duplication in six species.
GRAS genes evolved differently among the six Rosaceae species
The pairwise Ka/Ks ratio was an important criterion to measure the type of gene selection pressure [80]. Previous studies had demonstrated that HrGRAS genes involved in duplication in sea buckthorn undergo strong purifying selection pressure according to the Ka/Ks ratios [81]. Ka/Ks analysis showed that 88.2% of GRAS genes proceed a purifying selection in barley [27]. Similarly, in this study, as shown in Fig. 4 and Table S4, by calculating the Ka/Ks values of six species, we found that most of the GRAS genes were subjected to purification selection. The Ka/Ks values of all genes in peach were less than 1, indicating that all genes in peach were in a purified state, which may be beneficial to maintaining gene function and eliminating harmful mutations during evolution [82]. In addition, almost all genes in woodland strawberry and European pear had purifying selection. By further analysis of the Ka/Ks ratios, a higher proportion of GRAS genes were subject to positive selection in apple (3.86%) and black raspberry (4.29%). This result showed that apple and black raspberry had a faster evolutionary process than the other four Rosaceae species, revealing that these genes help plants resist various stresses [83].
Different expression patterns of FvGRASs in response to drought and salt stress in woodland strawberry
Previous studies had shown that GRAS genes were involved in plant root system, axillary meristem development, shoot meristem maintenance, and gibberellin signal transduction [7, 11]. For instance, as a key factor in rice tillering, OsMoC1 was involved in the initiation of lateral meristems and the formation and growth of tiller buds [30]. AtSCL28 has been reported to regulate the balance of cell size and number in Arabidopsis [84]. The heatmap showed the expression patterns of GRAS genes in different tissues and developmental stages of woodland strawberry, and it was found that at least two-thirds of GRAS genes were highly expressed in various tissues (Fig. 5), which indicated that GRAS genes were involved in plant growth and development stage in woodland strawberry. However, most of the GRAS genes were expressed at lower levels in pollen, and we inferred that GRAS genes contribute less to the reproductive process in woodland strawberry.
The GRAS members were reported widely involved in regulating stress responses [37, 85]. The analysis of cis-elements in the promoter regions of GmGRAS genes revealed the potential regulation during saline and dehydration stresses in soybean [86]. In the present research, stress-related cis-elements were found in six Rosaceae species, including those for MBS, ABRE, and TC-rich repeats, indicating that GRAS genes may regulate drought and salt stress responses in six Rosaceae species. GRAS genes also participate in stress response by interacting with the bZIP genes in plants. In Arabidopsis, the GRAS regulatory protein member, SCL14 interacts with bZIP subfamily TGA proteins to enhance the plant defense system [39]. In this study, by analyzing previous transcriptome data, we revealed the expression patterns of FvGRASs under drought and salt stress conditions. The results showed that the expression levels of most GRAS genes were significantly changed. In addition, we calculated the correlation coefficient between GRAS and bZIP genes, and the results showed that most GRAS genes have significant positive or negative correlations with bZIP genes, indicating that GRAS genes may respond to abiotic stress by regulating bZIP genes.
To verify whether FvGRASs were involved in the response to abiotic stress, we designed drought and salt stress treatments. The expression levels of GRAS genes in tender leaves were upregulated at 3 d, and decreased at 5 d, and then were upregulated at 7 d under drought conditions. In contrast, the expression levels of mrna24339.1 (SCR) and mrna30958.1 (DELLA) were always up-regulated under salt stress. Interestingly, the HAM subfamily and PAT subfamily had higher expression levels under drought and salt stress that the other subgroups. These data indicated that the FvGRAS family was linked to the responses to drought and salt stresses in woodland strawberry, and the genes had various pathways to participate in various abiotic stresses.
Conclusion
In this study, 51, 78, 41, 59, 56, and 48 GRAS genes were identified in pear, apple, black raspberry, Chinese rosa, strawberry and peach, respectively. Analysis of duplication events in six Rosaceae species revealed that the duplication modes of these six Rosaceae species were diverse, and species-specific duplications contributed more to the expansion of GRAS genes in the six Rosaceae species. In the process of evolution, most of the genes in the six Rosaceae species were in a state of purifying selection. GO and cis-acting element analysis revealed that GRAS genes were involved in environmental stress and hormone signal transduction pathways. In addition, we focused on the analysis of the expression pattern of GRAS genes and the interaction between GRAS and bZIP genes in woodland strawberry. The results showed that FvGRAS genes may had participated in the growth and development of woodland strawberry and played a key role in the response to drought and salt stress. This work laid a foundation for further research on the relationship between abiotic stress and FvGRAS genes in woodland strawberry.
Availability of data and materials
The datasets supporting the conclusions of this article are included in the article and its Additional files.
Abbreviations
- GAI:
-
Gibberellic acid insensitive
- SCR:
-
Scarecrow
- RGA:
-
Repressor of ga1-3
- STEM:
-
Short Times-series Expression Miner software
- FPKM:
-
Fragments per kilobase per million of reads mapped
- RT-qPCR:
-
Quantitative real-time PCR
- Ka:
-
Nonsynonymous substitution
- Ks:
-
Synonymous substitution
- PCC:
-
Pearson correlation coefficient
- TD:
-
Tandem
- WGD:
-
Whole-genome
- PD:
-
Proximal
- TRD:
-
Transposed
- DSD:
-
Dispersed
References
Riechmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000;3(5):423–34.
Guo A, He K, Liu D, Bai S, Gu X, Wei L, et al. DATF: a database of Arabidopsis transcription factors. Bioinformatics. 2005;21(10):2568–9.
Jin JP, Tian F, Yang DC, Meng YQ, Kong L, Luo JC, et al. PlantTFDB 4.0: toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45(D1):D1040–5.
Liu L, White MJ, MacRae TH. Transcription factors and their genes in higher plants functional domains, evolution and regulation. Eur J Biochem. 1999;262(2):247–57.
Amorim LLB, Santos R, Neto JPB, Guida-Santos M, Crovella S, Benko-Iseppon AM. Transcription factors involved in plant resistance to pathogens. Curr Protein Pept Sci. 2017;18(4):335–51.
Morohashi K, Minami M, Takase H, Hotta Y, Hiratsuka K. Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. J Biol Chem. 2003;278(23):20865–73.
Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218(5):683–92.
Mayrose M, Ekengren SK, Melech-Bonfil S, Martin GB, Sessa G. A novel link between tomato GRAS genes, plant disease resistance and mechanical stress response. Mol Plant Pathol. 2006;7(6):593–604.
Bolle C. Functional aspects of GRAS family proteins - ScienceDirect. In: Plant transcription factors. 2016. p. 295–311.
Sun XL, Xue B, Jones WT, Rikkerink E, Dunker AK, Uversky VN. A functionally required unfoldome from the plant kingdom: intrinsically disordered N-terminal domains of GRAS proteins are involved in molecular recognition during plant development. Plant Mol Biol. 2011;77(3):205–23.
Hirsch S, Oldroyd GED. GRAS-domain transcription factors that regulate plant development. Plant Signal Behav. 2014;4(8):698–700.
Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphy GP, et al. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11(23):3194–205.
Silverstone AL, Ciampaglio CN, Sun T. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell. 1998;10(2):155–69.
Laurenzio LD, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86(3):423–33.
Hofmann NR. A structure for plant-specific transcription factors: the GRAS domain revealed. Plant Cell. 2016;28(5):993–4.
Li SP, Zhao YH, Zhao Z, Wu XL, Sun LF, Liu QS, et al. Crystal structure of the GRAS domain of SCARECROW-LIKE 7 in Oryza sativa. Plant Cell. 2016;28(5):1025–34.
Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell. 2002;14(1):57–70.
Liu XY, Widmer A. Genome-wide comparative analysis of the GRAS gene family in Populus, Arabidopsis and rice. Plant Mol Biol Rep. 2014;32(6):1129–45.
Hirsch S, Kim J, Munoz A, Heckmann AB, Downie JA, Oldroyd GED. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in medicago truncatula. Plant Cell. 2009;21(2):545–57.
Lucas MD, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451(7177):480–4.
Lu XH, Liu WQ, Xiang CG, Li XJ, Wang Q, Wang T, et al. Genome-wide characterization of GRAS family and their potential roles in cold tolerance of cucumber (Cucumis sativus L.). Int J Mol Sci. 2020;21(11):3857.
Lee MH, Kim B, Song SK, Heo JO, Yu NI, Lee SA, et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Mol Biol. 2008;67(6):659–70.
Tian CG, Wan P, Sun SH, Li JY, Chen MS. Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Mol Biol. 2004;54(4):519–32.
Niu YL, Zhao TT, Xu XY, Li JF. Genome-wide identification and characterization of GRAS transcription factors in tomato (Solanum lycopersicum). PeerJ. 2017;5(11):e3955.
Song XM, Liu TK, Duan WK, Ma QH, Ren J, Wang Z, et al. Genome-wide analysis of the GRAS gene family in Chinese cabbage (Brassica rapa ssp. Pekinensis). Genomics. 2013;103(1):135–46.
Wu ZY, Wu PZ, Chen YP, Li MR, Wu GJ, Jiang HW. Genome-wide analysis of the GRAS gene family in physic nut (Jatropha curcas L.). Genet Mol Res. 2015;14(4):19211–24.
To VT, Shi Q, Zhang YY, Shi J, Cai WG. Genome-wide analysis of the GRAS gene family in Barley (Hordeum vulgare L.). Genes (Basel). 2020;11(5):553.
Wang TT, Yu TF, Fu JD, Su HG, Chen J, Zhou YB, et al. Genome-wide analysis of the GRAS gene family and functional identification of GmGRAS37 in drought and salt tolerance. Front Plant Sci. 2020;11:604690.
Sun XL, Jones WT, Rikkerink EHA. GRAS proteins: the versatile roles of intrinsically disordered proteins in plant signalling. Biochem J. 2012;442(1):1–12.
Li XY, Qian Q, Fu ZM, Wang YH, Xiong GS, Zeng DL, et al. Control of tillering in rice. Nature. 2003;422(6932):618–21.
Schumacher K, Schmitt T, Rossberg M, Schmitz G, Theres K. The lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc Natl Acad Sci U S A. 1999;96(1):290–5.
Greb T, Clarenz O, Schafer E, Muller D, Herrero R, Schmitz G, et al. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes Dev. 2003;17(9):1175–87.
Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17(3):354–8.
Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101(5):555–67.
Torres-Galea P, Hirtreiter B, Bolle C. Two GRAS proteins, SCARECROW-LIKE21 and PHYTOCHROME A SIGNAL TRANSDUCTION1, function cooperatively in phytochrome a signal transduction. Plant Physiol. 2013;161(1):291–304.
Bolle C, Koncz C, Chua NH. PAT1, a new member of the GRAS family, is involved in phytochrome a signal transduction. Genes Dev. 2000;14(10):1269–78.
Khan Y, Xiong Z, Zhang H, Liu S, Yaseen T, Hui T. Expression and roles of GRAS gene family in plant growth, signal transduction, biotic and abiotic stress resistance and symbiosis formation-a review. Plant Biol (Stuttg). 2022;24(3):404–16.
Wang Z, Liu L, Cheng C, Ren Z, Xu S, Li X. GAI functions in the plant response to dehydration stress in Arabidopsis thaliana. Int J Mol Sci. 2020;21(3):819.
Fode B, Siemsen T, Thurow C, Weigel R, Gatz C. The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell. 2008;20(11):3122–35.
Shulaev V, Korban SS, Sosinski B, Abbott AG, Aldwinckle HS, Folta KM, et al. Multiple models for Rosaceae genomics. Plant Physiol. 2008;147(3):985–1003.
Longhi S, Giongo L, Buti M, Surbanovski N, Viola R, Velasco R, et al. Molecular genetics and genomics of the Rosoideae: state of the art and future perspectives. Hortic Res. 2014;1(2–3):381–92.
Fan S, Zhang D, Gao C, Zhao M, Wu HQ, Li YM, et al. Identification, classification, and expression analysis of GRAS gene family in Malus domestica. Front Physiol. 2017;8:253.
Chen H, Li HH, Lu XQ, Chen LZ, Liu J, Wu H. Identification and expression analysis of GRAS transcription factors to elucidate candidate genes related to stolons, fruit ripening and abiotic stresses in woodland strawberry (Fragaria vesca). Int J Mol Sci. 2019;20(18):4593.
Eddy SR. Profile hidden Markov models. Bioinformatics. 1998;14(9):755–63.
Letunic I, Doerks T, Bork O. SMART: recent updates, new developments and status in 2015. Nucleic Acids Res. 2015;43(D1):257–60.
Nguyen L, Schmidt HA, Haeseler AV, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74.
Subramanian B, Gao SH, Lercher MJ, Hu SN, Chen WH. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019;47(W1):W270–5.
Zhong Y, Zhang XH, Cheng Z-M. Lineage-specific duplications of NBS-LRR genes occurring before the divergence of six Fragaria species. BMC Genomics. 2018;19(1):128.
Liu H, Xiong JS, Jiang YT, Wang L, Cheng Z-M. Evolution of the R2R3-MYB gene family in six Rosaceae species and expression in woodland strawberry. J Integr Agric. 2019;18(12):2753–70.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–402.
Qiao X, Li QH, Yin H, Qi KJ, Li LT, Wang RZ, et al. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019;20(1):38.
Zhang Z, Li J, Zhao XQ, Wang J, Wong GKS, Yu J. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genomics Proteomics Bioinformatics. 2006;4(4):259–63.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Peer YVD, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019;47(D1):D419–26.
Hollender CA, Kang CY, Darwish O, Geretz A, Matthews BF, Slovin J, et al. Floral transcriptomes in woodland strawberry uncover developing receptacle and anther gene networks. Plant Physiol. 2014;165(3):1062–75.
Dong C, Xi Y, Chen XL, Cheng Z-M. Genome-wide identification of AP2/EREBP in Fragaria vesca and expression pattern analysis of the FvDREB subfamily under drought stress. BMC Plant Biol. 2020;21(1):295.
Crizel RL, Perin EC, Vighi IL, Woloski R, Seixas A, Pinto LDS, et al. Genome-wide identification, and characterization of the CDPK gene family reveal their involvement in abiotic stress response in Fragaria x ananassa. Sci Rep. 2020;10(1):11040.
Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC Bioinformatics. 2006;7:191.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Kondrashov FA. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc Biol Sci. 2012;279(1749):5048–57.
Walsh IM, Bowman MA, Soto Santarriaga IF, Rodriguez A, Clark PL. Synonymous codon substitutions perturb cotranslational protein folding in vivo and impair cell fitness. Proc Natl Acad Sci U S A. 2020;117(7):3528–34.
Hurst LD. The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet. 2002;18(9):486.
Chen JW, Yan Q, Li JW, Lei F, Zhang Y, Xu J, et al. The GRAS gene family and its roles in seed development in litchi (Litchi chinensis Sonn). BMC Plant Biol. 2021;21(1):423.
Tong HN, Jin Y, Liu WB, Li F, Fang J, Yin YH, et al. DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 2009;58(5):803–16.
Xu K, Chen SJ, Li TF, Ma XS, Liang XH, Ding XF, et al. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant Biol. 2015;15(1):141.
Yuan YY, Fang LC, Karungo SK, Zhang LL, Gao YY, Li SH, et al. Overexpression of VaPAT1, a GRAS transcription factor from Vitis amurensis, confers abiotic stress tolerance in Arabidopsis. Plant Cell Rep. 2016;35(3):655–66.
Liu YD, Wei H, Xian ZQ, Hu N, Lin DB, Ren H, et al. Overexpression of SlGRAS40 in tomato enhances tolerance to abiotic stresses and influences auxin and gibberellin signaling. Front Plant Sci. 2017;8:1659.
Taylor JS, Peer YVD, Meyer A. Genome duplication, divergent resolution and speciation. Trends Genet. 2001;17(6):299–301.
Xiong AS, Peng RH, Zhuang J, Gao F, Zhu B, Fu XY, et al. Gene duplication and transfer events in plant mitochondria genome. Biochem Biophys Res Commun. 2008;376(1):1–4.
Meyer A. Molecular evolution: duplication, duplication. Nature. 2003;421(6918):31–2.
Shimeld SM. Gene function, gene networks and the fate of duplicated genes. Semin Cell Dev Biol. 1999;10(5):549–53.
Taylor JS, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet. 2004;38(1):615–43.
Wolfe KH, Li WH. Molecular evolution meets the genomics revolution. Nat Genet. 2003;33(3):255–65.
Hurley I, Hale ME, Prince VE. Duplication events and the evolution of segmental identity. Evol Dev. 2005;7(6):556–67.
Panchy N, Lehti-Shiu M, Shiu SH. Evolution of gene duplication in plants. Plant Physiol. 2016;171(4):2294–316.
Wu NN, Zhu Y, Song WL, Li YX, Yan YM, Hu YK. Unusual tandem expansion and positive selection in subgroups of the plant GRAS transcription factor superfamily. BMC Plant Biol. 2014;14:373.
Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci U S A. 2002;99(17):11519–24.
Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, et al. Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol. 2003;131(3):1313–26.
Zhong Y, Yin H, Sargent DJ, Malnoy M, Cheng Z-M. Species-specific duplications driving the recent expansion of NBS-LRR genes in five Rosaceae species. BMC Genomics. 2015;16(1):77.
Hu T, Banzhaf W. Nonsynonymous to synonymous substitution ratio ka/ks: measurement for rate of evolution in evolutionary computation. In: Rudolph G, Jansen T, Beume N, Lucas S, Poplin C, editors. Parallel Problem Solving from Nature – PPSN X. PPSN. Cham: Springer International Publishing; 2008. p. 448–57.
Yu LY, Zhang GY, Lyu ZR, He CY, Zhang JG. Genome-wide analysis of the GRAS gene family exhibited expansion model and functional differentiation in sea buckthorn (Hippophae rhamnoides L.). Plant Biotechnol Rep. 2021;15(4):513–25.
Soares P, Ermini L, Thomson N, Mormina M, Rito T, Röhl A, et al. Correcting for purifying selection: an improved human mitochondrial molecular clock. Am J Hum Genet. 2009;84(6):740–59.
Zhong Y, Zhang XH, Shi Q, Cheng Z-M. Adaptive evolution driving the young duplications in six Rosaceae species. BMC Genomics. 2021;22(1):112.
Nomoto Y, Takatsuka H, Yamada K, Suzuki T, Suzuki T, Huang Y, et al. A hierarchical transcriptional network activates specific CDK inhibitors that regulate G2 to control cell size and number in Arabidopsis. Nat Commun. 2022;13(1):1660.
Zhang B, Liu J, Yang ZE, Chen EY, Zhang CJ, Zhang XY, et al. Genome-wide analysis of GRAS transcription factor gene family in Gossypium hirsutum L. BMC Genomics. 2018;19(1):348.
Wang L, Ding X, Gao Y, Yang S. Genome-wide identification and characterization of GRAS genes in soybean (Glycine max). BMC Plant Biol. 2020;20(1):415.
Acknowledgements
Not applicable.
Funding
This study was supported in part by the funds of Jiangsu Agricultural Science and Technology Innovation Fund [CX(21)3025], Natural Science Foundation of Jiangsu (BK20221009) and China Postdoctoral Science Foundation (2021M701742).
Author information
Authors and Affiliations
Contributions
HL and ZMC conceived this research. YBB, HL, and KZ analyzed the data. YBB wrote the manuscript. HL modified the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental research and method on six Rosaceae species comply with relevant institutional, national, and international guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Phylogenetic tree of GRAS genes among six Rosaceae species. Red oval means species-specific duplication and red square indicate lineage-specific duplication events.
Additional file 2: Fig. S2.
Phylogenetic tree of GRAS genes in the six Rosaceae species and Arabidopsis.
Additional file 3: Fig. S3.
Mapchart shown the distribution of GRAS genes on 7 chromosomes in woodland strawberry.
Additional file 4: Fig. S4.
Mapchart shown the distribution of GRAS genes on 17 chromosomes in apple.
Additional file 5: Fig. S5.
Mapchart shown the distribution of GRAS genes on 8 chromosomes in peach.
Additional file 6: Fig. S6.
Mapchart shown the distribution of GRAS genes on 17 chromosomes in European pear.
Additional file 7: Fig. S7.
Mapchart shown the distribution of GRAS genes on 7 chromosomes in Chinese rosa.
Additional file 8: Fig. S8.
Mapchart shown the distribution of GRAS genes on 7 chromosomes in black raspberry.
Additional file 9: Fig. S9.
Conserved domain analysis of the GRAS gene family among six Rosaceae species.
Additional file 10: Fig. S10.
Heatmap shown the expression of bZIP genes under drought (A) and salt stress (B) in woodland strawberry.
Additional file 11: Table S1.
Total of 333 GRAS genes were identified in six Rosaceae species. Table S2. Ks values of six Rosaceae species. Table S3. Duplication events of six Rosaceae species. Table S4. ka/Ks values of six Rosaceae species. Table S5. Primers used to detect drought stress and salt stress gene expression.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bai, Y., Liu, H., Zhu, K. et al. Evolution and functional analysis of the GRAS family genes in six Rosaceae species. BMC Plant Biol 22, 569 (2022). https://doi.org/10.1186/s12870-022-03925-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03925-x