Abstract
Background
Dendrobium officinale Kimura et Migo, which contains rich polysaccharides, flavonoids and alkaloids, is a Traditional Chinese Medicine (TCM) with important economic benefits, while various pathogens have brought huge losses to its industrialization. NBS gene family is the largest class of plant disease resistance (R) genes, proteins of which are widely distributed in the upstream and downstream of the plant immune systems and are responsible for receiving infection signals and regulating gene expression respectively. It is of great significance for the subsequent disease resistance breeding of D. officinale to identify NBS genes by using the newly published high-quality chromosome-level D. officinale genome.
Results
In this study, a total of 655 NBS genes were uncovered from the genomes of D. officinale, D. nobile, D. chrysotoxum, V. planifolia, A. shenzhenica, P. equestris and A. thaliana. The phylogenetic results of CNL-type protein sequences showed that orchid NBS-LRR genes have significantly degenerated on branches a and b. The Dendrobium NBS gene homology analysis showed that the Dendrobium NBS genes have two obvious characteristics: type changing and NB-ARC domain degeneration. Because the NBS-LRR genes have both NB-ARC and LRR domains, 22 D. officinale NBS-LRR genes were used for subsequent analyses, such as gene structures, conserved motifs, cis-elements and functional annotation analyses. All these results suggested that D. officinale NBS-LRR genes take part in the ETI system, plant hormone signal transduction pathway and Ras signaling pathway. Finally, there were 1,677 DEGs identified from the salicylic acid (SA) treatment transcriptome data of D. officinale. Among them, six NBS-LRR genes (Dof013264, Dof020566, Dof019188, Dof019191, Dof020138 and Dof020707) were significantly up-regulated. However, only Dof020138 was closely related to other pathways from the results of WGCNA, such as pathogen identification pathways, MAPK signaling pathways, plant hormone signal transduction pathways, biosynthetic pathways and energy metabolism pathways.
Conclusion
Our results revealed that the NBS gene degenerations are common in the genus Dendrobium, which is the main reason for the diversity of NBS genes, and the NBS-LRR genes generally take part in D. officinale ETI system and signal transduction pathways. In addition, the D. officinale NBS-LRR gene Dof020138, which may have an important breeding value, is indirectly activated by SA in the ETI system.
Similar content being viewed by others
Background
Dendrobium, one of the largest genera in Orchidaceae, is widely distributed in tropical Asia, Australasia, Australia and New Zealand [1, 2]. There are about 120 Dendrobium species in China, which are epiphytic on rocks and tree trunks and distributed at high elevations up to 1,200 m [2]. Dendrobium orchids, which have accumulated high content of medicinal ingredients [2], are important commercial crops in China because of their horticultural and medicinal values [3]. For example, Dendrobium officinale Kimura et Migo, one of the most valuable Traditional Chinese Medicines (TCMs), is rich in polysaccharides, flavonoids and alkaloids [4, 5]. Because of the great demand and the lack of wild resources, industrial cultivation of D. officinale has been actively promoted in Anhui, Zhejiang, Jiangsu and Guizhou provinces. However, the invasions of pathogens, such as orchid fleck virus, Dendrobium vein necrosis closterovirus, Fusarium oxysporum and Fusarium kyushuense, have led to production reduction, which resulted in great losses for enterprises [6,7,8,9]. Therefore, it is important to identify disease resistance (R) genes and explore the metabolic pathways of resistance to biotic stress based on the D. officinale genome.
The plants have evolved pathogen-associated molecular patterns triggered immunity (PTI) and effector-triggered immunity (ETI) systems to defend against the infections of pathogens [10,11,12]. Among the two systems, PTI will be triggered when the pathogens break through the plant epidermis [13]. With some pathogens bypassing the PTI system, the plants gradually evolved the ETI system, which can recognize specific pathogen effectors, to counter pathogen infection [13, 14]. Plant R genes, of which approximately 80% belong to the NBS gene family, are the major component of the ETI system [15,16,17,18]. NBS proteins are composed of two main domains: (1) nucleotide binding sites (NB-ARCs) domain, which can bind ATP/GTP molecular; (2) C-terminal leucine-rich repeats (LRRs) domain, which recognizes pathogen effectors [19]. NBS genes that retained both the NB-ARC domain and the LRR domain were named NBS-LRR genes because part of NBS genes lacked the variable LRR domain. Based on the different types of domains in the N terminus, the NBS-LRR genes are divided into three subfamilies: TIR-NBS-LRR (TNL), CC-NBS-LRR (CNL) and RPW8-NBS-LRR (RNL) in angiosperm [19]. For example, the rice CNL-type NBS proteins RGA5 and RGA4 can directly bind to Magnaporthe oryzae effectors Avr-Pia and Avr1-Co39 [14, 20]. Shao et al. used 22 angiosperm genomes to identify different types of NBS-LRR genes, the results of which showed that the number of CNL-type NBS-LRR genes (CNL genes) was greater than TNL-type and RNL-type, and no TNL-type NBS-LRR gene was identified in monocots [18]. In grass species, the distribution of NBS-LRR genes in chromosomes shows high aggregation and duplication due to local duplications [17]. In conclusion, NBS genes, as important components of the plant immune systems, are abundant and widely distributed on different chromosomes.
Recently, with the rapid development of third-generation sequencing technology (PacBio and Nanopore), three chromosomal-level genomes were published in the genus Dendrobium, including D. officinale, D. chrysotoxum and D. nobile [2, 21, 22]. The high-quality D. officinale genome was 1.23 Gb, with a contig N50 value of 1.44 Mb, and 93.53% of contig sequences were anchored to 19 pseudochromosomes [2]. These high-quality Dendrobium genomes make it possible for researchers to explore the evolution of Dendrobium NBS genes and uncover molecular pathways of the D. officinale immune systems.
In this study, the NBS genes were identified in six orchids and Arabidopsis thaliana, and the homologous genes of all Dendrobium NBS genes were speculated based on the chromosomal-level genomes. The structure features and cis-elements of the NBS-LRR genes in D. officinale were analyzed. Finally, based on the D. officinale genome, transcriptome analysis was performed on the coding genes by the salicylic acid (SA) treatment. The aims of this study were: (1) to investigate the evolutionary patterns of NBS genes in the genus Dendrobium; (2) to explore the molecular pathways involved in D. officinale immune systems; (3) to reveal the response process of D. officinale immune systems by the SA treatment. We believed that this study will provide us with a comprehensive understanding of the NBS gene evolution in Dendrobium and the molecular pathways of D. officinale immune systems.
Results
Classification of NBS genes in orchidaceae
From the results of Conserved Domain, Pfam and SMART websites, there were 655 putative NBS genes identified in six orchids and A. thaliana (74 genes in D. officinale, 169 genes in D. nobile, 118 genes in D. chrysotoxum, 57 genes in P. equestris, 12 genes in V. planifolia, 15 genes in A. shenzhenica, and 210 genes in A. thaliana) (Table 1, Table S5). The 655 NBS genes were classified into two subclasses: the NBS-LRR subclass (NBS-LRR genes), the proteins of which contain both NB-ARC and LRR domains, and the non-NBS-LRR subclass, the proteins of which lose the LRR domain. Among the NBS-LRR genes, the most abundant genes were the CNL-type (10 genes in D. officinale, 18 genes in D. nobile, 14 genes in D. chrysotoxum, 7 genes in P. equestris, 2 genes in V. planifolia, 4 genes in A. shenzhenica, and 40 genes in A. thaliana), followed by the NL-type. Notably, there were fewer NBS-LRR genes in the orchids than in the A. thaliana, which was consistent with previous studies [23]. In addition, no TNL-type genes were identified in six orchids, which proved that the TIR domain degeneration is a common phenomenon in monocots, and the TNL loss may be potentially driven by NRG1/SAG101 pathway deficiency [18, 24, 25].
Phylogenetic analysis
The 52 chloroplast (cp) genes and ITS sequences, which can well describe the phylogenetic relationships of plants [26, 27], were used to reconstruct the phylogenetic relationships of orchids (Fig. S11). Most nodes were highly supported with ML/BI bootstrap values > = 73%/98%, except for the tree node of genus Goodyera, which is consistent with the fact that the cp genome variation rate was slow [26, 27]. The results showed that the phylogenetic relationships between Vanilla and Dendrobium were not close, which was by the fact that their chromosome numbers were different. In the genus Dendrobium, D. nobile was more closely related to D. officinale, followed by D. chrysotoxum, which was consistent with the previous study [1].
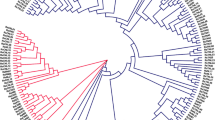
To investigate the phylogenetic relationships of CNL-type NBS-LRR genes (CNL genes) in orchids, the ML phylogenetic trees were reconstructed using the protein sequences of 6 D. officinale genes, 17 D. nobile genes, 12 D. chrysotoxum genes, 7 P. equestris genes, 2 V. planifolia genes, 4 A. shenzhenica genes and 40 A. thaliana genes (Fig. 1). The results showed that the CNL genes were mainly divided into three branches (a, b and c) in orchids. The phylogenetic results of CNL genes in each branch were basically consistent with the orchid species tree (Fig. 1, Fig. S1). However, except for A. thaliana genes, there were only D. nobile and V. planifolia genes in branch a, while there were only D. nobile, D. officinale and D. chrysotoxum genes in branch b, which indicated that the orchid CNL genes have significantly degenerated on branches a and b. At the same time, the orchid CNL genes accounted for 97.4% (37/38) in branch c, which suggested that the orchid CNL genes have undergone significant expansions in branch c.
Syntenic gene analysis
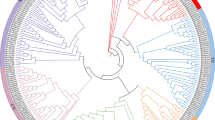
Synteny analysis was conducted on the NBS genes to investigate the gene duplication events using BLASTP and MCScanX. The results revealed that the 19 chromosomes of D. chrysotoxum, D. officinale and D. nobile were highly homologous (Fig. 2). The chromosome collinearity results of the three Dendrobium species showed the corresponding relationships among 19 chromosomes. However, due to the distant phylogenetic relationships, complex collinear relationships were found between the chromosomes of Vanilla and Dendrobium. Meanwhile, the highlighted red lines represented the presence of many homologous NBS gene pairs between the three Dendrobium species, which indicated orthologs may be the main way of NBS gene origins in Dendrobium species.
NBS gene homology analysis
To explore the origin of the Dendrobium NBS genes, the results of BLASTP and MCScanX were used to sort the homologous NBS gene pairs between Dendrobium species. Based on the results of phylogenetic analysis (as shown in Fig. S1), it was assumed that there were orthologs between the D. chrysotoxum and D. officinale NBS genes, and the homologous genes pairs between D. officinale and D. nobile can be explored from the synteny analysis results. The homologies of the NBS genes were classified into three types (ortholog, homochromosomal duplication and heterochromosomal duplication) (Table S6). The results showed that there were 76 orthologous genes, 94 homochromosomal duplication genes and 39 heterochromosomal duplication genes in three Dendrobium species. The gene number differences indicated that the NBS gene number expansions are common events and might before the divergence of families [18, 28, 29].
After arranging the different homology types of NBS genes, the results showed that there were at least 66 orthologous lineages, which were widely distributed in 13 chromosome lineages (Fig. 3). The blue lines represented that there were orthologous relationships between two same type NBS genes, while the red lines represented that the types of NBS genes were different. Remarkably, most of the orthologous lineages were variable, except for four lineages (KAH0465674.1-Dof002599-KAI0519223.1, KAH0448405.1-Dof007745-KAI0487837.1, KAH0456733.1-Dof020566-KAI0499586.1, KAH0458460.1-Dof019452-KAI0501528.1), which indicated that the types of most NBS genes had changed after originating from ortholog events.
The NBS gene homology analysis results among D. officinale, D. nobile, D. chrysotoxum and V. planifolia. The straight lines represent the orthologous relationships between gene pairs, while curves suggest that the homology type of NBS genes was homochromosomal duplication or heterochromosomal duplication. Blue represents that the NBS gene types between gene pairs were the same, and red represents that the types between gene pairs were different. * The only gene, which was non-NBS gene, was explored from the NBS gene homology analysis
Remarkably, the 12 extant V. planifolia NBS genes were all not the orthologous genes of the D. chrysotoxum NBS genes, except for KAG0484437.1, which has lost the NB-ARC domain (Fig. 3). In addition, homochromosomal duplication relationships were used to explore the D. officinale NBS gene origin. It was found that there were 40 homologous lineages incomplete, which suggested that the NBS gene degenerations were common phenomena in Dendrobium.
Gene structure and conserved motif analyses of NBS-LRR genes
The subsequent analyses were focused on the D. officinale NBS-LRR genes, which contained the reserved NB-ARC and LRR domains. The comparison analyses of exon number, gene length and conserved motif were further performed to outline the structure features of D. officinale NBS-LRR genes. The results uncovered a significant positive correlation between exon number and gene length (Pearson's r = 0.9566, P < 0.05) (Fig. 4 and Table S7), which was consistent with previous studies [29]. For example, the ten genes (Dof002831, Dof002838, Dof008501, Dof013257, Dof013264, Dof013262, Dof018917, Dof020138, Dof020707 and Dof024492) had only one exon with the lengths ranging from 1,896 bp to 5,070 bp, while Dof013259 had 11 exons with the length of 85,500 bp.
MEME results revealed that the conserved motif orders of NL-type and CNL-type NBS-LRR genes were conserved (motif 6—motif 5—motif 3—motif 1—motif 7—motif 2—motif 8—motif 4), while other types (CNLCN, NLNL and NLNNL) were highly variable (Fig. 4), suggesting that NL-type and CNL-type NBS-LRR genes possibly bore stronger positive selection.
Identification of cis-elements of NBS-LRR genes
The 2,000 bp upstream regions of the initiation codon (ATG) were analyzed to ascertain the potential biological roles of D. officinale NBS-LRR genes using the PlantCARE tool. The cis-elements in the promoter regions were classified into three categories: hormone-related (74.9%), stress-responsive (19.1%) and plant growth (6.0%) (Fig. 5, Table S8). In the hormone-related category (161/215), TCA-element was involved in SA responsiveness and distributed in Dof002831, Dof002838, Dof008997, Dof012439, Dof013262, Dof018917 and Dof020135. In the stress-responsive category (41/215), various elements related to defense and stress responsiveness (14, 6.5%), drought responsiveness (15, 7.0%), low temperature responsiveness (10, 4.7%) and wound responsiveness (2, 0.9%). Only a few cis-elements (13/215) were related to plant growth (Table S8). The above results revealed that there were plenty of hormone-related and stress-responsive cis-elements in the promoter regions of D. officinale NBS-LRR genes, which were consistent with Asparagus officinalis NBS-LRR genes [30].
Functional annotation of D. officinale NBS-LRR genes
The 22 D. officinale NBS-LRR genes were annotated with GO and KEGG databases to explore the role of NBS-LRR genes in D. officinale. All genes had the molecular function of ADP binding, which was due to the conserved structure of the NB-ARC domain (Table S9). Eight genes (Dof002831, Dof002838, Dof008501, Dof026347, Dof019188, Dof020135, Dof020136 and Dof020138) could be playing a role in the plant-pathogen interaction pathway, which belongs to the ETI system and responds to bacterial effectors. The results revealed that CNL-type and NL-type NBS-LRR genes were widely distributed in the D. officinale ETI system. Moreover, six genes (Dof010683, Dof013257, Dof013259, Dof013262, Dof013264 and Dof020566) may regulate gene expression by taking part in the Ras signaling pathway. All results indicated that NBS-LRR genes participated in the D. officinale immune systems upstream and downstream.
NBS-LRR gene expression profiles in response to SA
SA can regulate the expression levels of R genes to activate the resistance response to biotic stress [12]. To evaluate whether D. officinale NBS-LRR genes were in response to SA treatment, NBS-LRR gene expression patterns were investigated. From the transcriptome sequencing, a total of 145,498,271 clean reads were obtained, and all of the Q30 base percentages were above 94.4% (Table S10A; BioProject accession: PRJNA851113). In addition, the clean reads mapped to the D. officinale reference genome ranged from 90.96% to 91.57% (Table S10B), and 1,677 DEGs were identified (Table S10C). The up-regulated and down-regulated DEGs were evenly annotated to biological processes, cellular components and molecular functions (Fig. 6A and B), while the DEGs mainly belonged to the metabolism pathways, including biosynthesis of other secondary metabolites, lipid metabolism, amino acid metabolism, carbohydrate metabolism, metabolism of other amino acids, energy metabolism, metabolism of cofactors and vitamins, metabolism of terpenoids and polyketides, glycan biosynthesis and metabolism and nucleotide metabolism (Fig. 6C and D). The relative expression levels were represented by FPKM values, which were calculated with transcriptome data. The results showed that the expression levels of six NBS-LRR genes (Dof013264, Dof020566, Dof019188, Dof019191, Dof020138 and Dof020707) were significantly up-regulated (foldchange > 1.5 ×) (Fig. 7). Dof020138 and Dof019188 both belonged to the plant-pathogen interaction pathway (Table S9), while there were no TCA-elements (salicylic acid responsiveness) found in the promoter regions (Table S8), which suggested that Dof020138 and Dof019188 may be indirectly up-regulated by SA in the D. officinale ETI system.
WGCNA of D. officinale genes by SA treatment
Weighted gene co-expression network analysis (WGCNA) was performed with transcriptome data to explore the immune response network of D. officinale. The results showed that the turquoise module containing the D. officinale NBS-LRR gene Dof020138 was most positively correlated to SA treatment (Fig. S2, Table S11B). The genes in the turquoise module were classified into five categories: pathogen identification, plant hormone signal transduction, biosynthetic pathway, energy metabolism and MAPK signaling pathway (Fig. 8A, Table S11A). Remarkably, there were 15 genes belonging to the pathogen identification category, among which 11 genes belonged to the PTI system and four genes belonged to the ETI system (Fig. 8B, Table S11). The quantitative PCR results proved that the expression levels of nine genes, except for Dof013547, Dof005640, Dof015798, Dof017381, Dof004597 and Dof017452, were significantly up-regulated by SA treatment (Table S12). These nine genes are widely distributed in PTI (CNGCs, CDPK and CaMCML) and ETI (EDS1 and RPS2) systems, which suggested that the D. officinale PTI and ETI systems, will be activated by the plant hormone, salicylic acid [31].
Discussion
NBS genes are highly variable in Dendrobium
NBS genes originated before the last common ancestor of green plants [19]. Nearly all NBS-LRR genes, of which the proteins contain both NB-ARC and LRR domains, with known functions, are involved in plant immunity [32]. The NBS-LRR genes are divided into three subfamilies: TIR-NBS-LRR (TNL), CC-NBS-LRR (CNL) and RPW8-NBS-LRR (RNL) [19, 33], and the divergence of them should at least predate the divergence of chlorophytes and streptophytes [19]. TNL and CNL proteins are mainly responsible for recognizing specific pathogens, while RNL proteins may play an auxiliary role in downstream defense signal transduction [25, 29]. The NB-ARC domain can bind ATP/GTP, resulting in phosphorylation to transmit disease resistance signals downstream [34]. The LRR domain is typically involved in protein–protein interactions, which generally takes the role of pathogen recognition [35]. Consequently, the sequences of the NB-ARC domain encoded by different NBS-LRR genes are highly conserved, while the LRR domain is highly variable [36].
NBS genes not only expand greatly in plant genomes [25] but also degenerate rapidly [30, 37], which leads that the numbers of NBS genes varying greatly among different species. For example, there are over 2,000 NBS genes in the extremely large wheat genome, but these genes are extremely scarce in orchids, which is in accord with our results [18, 23, 38]. As a result, plants have a wide variety of NBS genes that can identify more pathogens and thus improve their ability to induce defense responses [39].
In this study, phylogenetic analysis, syntenic gene analysis and gene homology analysis were performed to speculate on the evolution of Dendrobium NBS genes. All results showed that Dendrobium NBS genes are highly variable in structures. On the one hand, the types of homologous NBS genes in Dendrobium are changing generally. For example, the 62 of 66 homologous lineages, which widely distributed in 13 chromosome lineages, cannot remain the same type throughout the lineages (Fig. 3). On the other hand, A large number of NBS genes are degenerating, which refers to the loss of the NB-ARC domain. Firstly, CNL genes degenerated in branches a and b (Fig. 1). Secondly, among 66 homologous lineages, 40 homologous lineages were not complete (Fig. 3), which indicated that most NBS genes were degenerating. It is assumed that large numbers of NBS gene expansions from orthologs and duplication events are the basic premise of NBS diversity, which adapted plants to identify more pathogens [24, 39]. In conclusion, NBS genes are highly variable in the genus Dendrobium.
NBS-LRR genes play important roles in D. officinale immune systems and signal transduction pathways
D. officinale is a valuable TCM and is known as “The first of China’s nine immortal herbs” [5]. With the development of D. officinale industrialization, it is urgent to improve the resistance of tissue culture seedlings by genetic engineering. It is common for D. officinale to be exposed to various pathogens, including fungus, bacteria, and viruses during industrial cultivation [6,7,8,9]. For example, the common and destructive fungal pathogen, F. oxysporum, always causes stem rot of D. officinale and has a high incidence of 30% to 50% [8, 40]. The medicinal part of D. officinale is the stem segment, so pathogen infection in stem segments can lead to huge economic losses.
To explore the roles of NBS-LRR genes in D. officinale, structure feature, cis-elements and functional annotation analyses were performed on the D. officinale NBS-LRR genes. All results suggested that D. officinale NBS-LRR genes were homologous to proteins in immune systems and signal transduction pathways. In the plant hormone signal transduction map, Dof008997 and Dof024492 were annotated as DELLA proteins, which promote stem growth and induce germination in the gibberellin signaling pathway (Table S9). In the Ras signaling pathway, Dof010683, Dof013257, Dof013259, Dof013262, Dof013264 and Dof020566 were all annotated as SHOC2 proteins, which regulate the MAPK signaling pathway upstream (Table S9). Remarkably, there were two R genes, RPM1 (Dof002831, Dof002838, Dof008501, Dof026347 and Dof019188) and RPS2 (Dof020135, Dof020136 and Dof020138), annotated in the D. officinale ETI system, both of which respond to bacterial effectors to activate hypersensitive response (Table S9). In conclusion, most NBS-LRR genes (16/22) may play roles in Dendrobium immune systems and signal transduction pathways.
Dof020138 is indirectly activated by SA in the D. officinale ETI system
The plant immune systems are activated by signaling transduction networks, such as calcium (Ca2+), reactive oxygen species (ROS) and hormones [31, 41]. In addition to Ca2+ and ROS, plant hormones, such as SA, JA and ABA, could be the primary signaling molecules that function in the regulation of plant immunity [42]. SA signaling, which might have originated in the last common ancestor of land plants [12, 43], participates in the resistance response to biotrophic pathogens by regulating the expression levels of R genes [44, 45]. In previous studies, NBS-LRR genes that can be up-regulated by SA have been found in many species, such as Arachis hypogaea (AhRRS5, AhRAF4) [46, 47], Gossypium hirsutum (GbaNA1) [48], Zea mays (ZmNBS25) [49], Triticum aestivum (TaRPM1) [50], Manihot esculenta (MeLRRs) [51] and Glycine max (SRC7) [52]. To explore the molecular basis of disease resistance in D. officinale, healthy one-year seedlings were treated with SA. The results of transcriptome data and qPCR showed that the expression levels of several genes in the PTI and ETI systems were up-regulated, and a large number of genes related to biomolecule synthesis and energy metabolism were mobilized in plant cells. Remarkably, Dof020138 (RPS2) and Dof019188 (RPM1) may be indirectly up-regulated by SA in the ETI system (Table S9, Table S12), but only Dof020138 were uncovered in the turquoise module from the WGCNA results.
The expression level of RPS2 protein, which receives signals from the effector protein AvrRpt2 by being antagonized to RIN4 protein, was significantly up-regulated in the ETI system [53]. Afterward, the protein of Dof020138, which was suppressed by RIN4 protein, triggers a hypersensitive response by transducing signals to downstream proteins, such as RAR1, SGT1 and HSP90 (Table S9, Fig. 8B) [54,55,56,57]. However, whether Dof020138 protein performs the function of RPS2 protein and the detailed mechanism of Dof020138 regulated by SA needs further study. At the same time, Dof020138 has comprehensive associations with other genes in the pathogen identification pathways, MAPK signaling pathways, plant hormone signal transduction pathways, biosynthetic pathways and energy metabolism pathways, which suggests that Dof020138 may perform a non-negligible function in the overall mobilization of the D. officinale immune system by SA. In conclusion, the SA can indirectly activate the D. officinale NBS-LRR gene Dof020138 in the ETI system.
Conclusions
In this study, the genomes of six orchids and A. thaliana were used to identify the NBS genes, and the CNL-type NBS proteins were used to reconstruct ML phylogenetic trees. We found that the NBS genes in Orchidaceae species were degenerating generally. The Dendrobium NBS gene homology analysis showed that the Dendrobium NBS genes were diversified. The D. officinale NBS-LRR genes were used for gene structure and conserved motif analyses, cis-elements analysis and functional annotation analysis, which revealed that NBS-LRR genes take parts in the ETI system, plant hormone signal transduction pathway and Ras signaling pathway. In addition, SA treatment transcriptome data was used for exploring the molecular basis of D. officinale immune systems. All results indicated that Dendrobium NBS genes are highly variable during long-term expansion and degeneration events, and the D. officinale NBS-LRR gene Dof020138, which may have important breeding value, is indirectly activated by SA in the ETI system.
Methods
Identification of NBS genes in Orchidaceae
The newest genome data of Dendrobium officinale Kimura et Migo (ID: 31,795), Dendrobium nobile Lindl. (ID: 17,836), Dendrobium chrysotoxum Lindl. (ID: 41,833), Vanilla planifolia Andrews (ID: 17,745), Apostasia shenzhenica Z.J.Liu & L.J.Chen (ID: 66,931), Phalaenopsis equestris (Schauer) Rchb. (ID: 11,403) and Arabidopsis thaliana (L.) Heynh. (ID: 4) were downloaded from the public databases (NCBI). Two strategies, HMM and BLAST searches [58, 59], were performed to identify NBS genes in these six orchids and A. thaliana. Firstly, the protein sequences were mapped and trained against the model of the NB-ARC (PF00931), Toll-Interleukin receptor (TIR, PF01582), Leucine-rich repeat (LRR, PF00560, PF07723, PF07725, PF12799, PF13306, PF08191 and PF13855) and RPW8 (PF05659) domains using hmmer3.0 with default parameters (Table S1A). Secondly, the reference protein sequences were downloaded from the NCBI protein database to contain as many known NBS genes as possible by searching GeneBank with the keywords: “Arabidopsis NB-ARC”, “Arabidopsis LRR”, “Arabidopsis TIR” and “Arabidopsis RPW8”. The 121 sequences of the Arabidopsis proteins with typical features of NBS genes were treated as seed sequences (Table S1B) and aligned as queries to the corresponding genome using BLASTP (Table S1C).
The HMM and BLASTP results were filtered and classified by the Conserved Domain website (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (Table S1D) [60], the Pfam database (http://pfam.xfam.org/) (Table S1E) [61] and the SMART website (http://smart.embl-heidelberg.de/) [62]. The genes that contained significant NB-ARC domains were retained as the putative NBS genes. For the identification of coil-coiled (CC) motifs, the DeepCoil2 program (https://toolkit.tuebingen.mpg.de/tools/deepcoil2) was performed with a threshold value of 0.5 [63]. The types of NBS genes were determined according to the orders of NB-ARC (N), TIR (T), CC (C), LRR (L) and RPW8 (R) domains.
Sequence alignment and phylogenetic analysis
The 52 cp genes and ITS sequences of 25 Orchidaceae species, two Araceae species and A. thaliana were used to reconstruct the Maximum Likelihood (ML) and BI phylogenetic trees (Table S2). The sequences were aligned using MAFFT 7.220 [64]. Under the rule of the Akaike Information Criterion (AIC), the optimum base substitution model calculated by Modeltest 3.7 was GTR + I + Γ [65]. The ML phylogenetic tree was constructed using RAxML 7.4.2 with 1,000 rapid bootstrap inferences [66], and the outgroup was A. thaliana. The BI analysis was made using MrBayes 3.2.7 with 1,000,000 generations [67]. Trees were sampled every 1,000 generations, and the first 25% of these were discarded. The remaining trees were used to build the Bayesian tree of posterior probabilities.
The alignments of CNL-type NBS protein sequences were performed using ClustalX2.1 with the complete alignment [68]. After removing the seven genes (KAH0456733.1, Dof019191, KAH0457269.1, Dof026347, Dof019188, Dof020566 and KAI0514091.1), which lacked the conserved regions, 88 CNL genes were used to reconstruct phylogenetic trees. The phylogenetic trees were estimated using MEGA X by the ML method with the following parameters: Poisson model, pairwise deletion and 1,000 bootstrap replicates [69].
Gene duplication analysis
The MCScanX software was performed to search for gene duplication events between four chromosome-level genomes (D. officinale, D. nobile, D. chrysotoxum and V. planifolia) [70]. All the protein sequences were compared using all-vs-all BLASTP with parameters: V = 10, B = 100, filter = seg, E-value < 1e-10, and the output format was set as tabular format (-m 8). The resulting blast hits were incorporated along with chromosome coordinates as input for MCScanX analysis. The chromosomes were renamed according to the chromosome lengths (Table S3).
Prediction of homologous genes
For the prediction of Dendrobium NBS gene origins, the MCScanX results were used to determine the orthologous genes first. The paralogous genes of other NBS genes were conjectured by the BLASTP results (homochromosomal duplication and heterochromosomal duplication).
Gene structure and conserved motif analyses in D. officinale
The CDS information was shown to investigate the structural variations of D. officinale NBS-LRR genes using the online program Gene Structure Display Server (http://gsds.gao-lab.org/) [71]. The protein sequences of 22 NBS-LRR genes were submitted to the motif analysis using the online tool MEME Suite (https://meme-suite.org/meme/) [72] with the following settings: (1) optimum motif width was set to 6 and 50; (2) number of motifs was eight with an E-value < 1e-10.
Cis-elements analysis
The promoter sequences (2,000 bp upstream of the translational start site) of D. officinale NBS-LRR genes were obtained. Afterward, the online software PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [73] was employed to investigate putative cis-elements in the promoter regions.
Functional annotation of NBS-LRR genes
The D. officinale NBS-LRR genes were functionally annotated based on the publicly available databases including GO and KEGG databases with default parameters [74,75,76].
Plant treatment, RNA extraction and sequencing
D. officinale one-year cultivated seedlings (voucher specimen: Yang202201) without obvious disease infection were selected for SA treatment with 1 mM SA, and SA-free individuals were used as the control. The treatment group and control group were set with three independent replicates, respectively. The leaves from SA treatment and the control were collected 7 d after treatment. All samples were frozen immediately in liquid nitrogen and stored at -80 ℃ until use.
The total RNA was extracted using MiniBEST Plant RNA Extraction Kit (Takara). RNA sequencing was performed using a high-throughput sequencing platform, Illumina HiSeq2500. The clean reads obtained from RNA-Seq were mapped to the D. officinale genome and assembled using Hisat2 and Stringtie, respectively. The differential expression genes (DEGs) were identified using the DEseq2 package in R with the standard of the adjusted p-value of 0.05 and the foldchange more than 1.5 × [77].
Weighted gene co-expression network analysis
Weighted gene co-expression network analysis (WGCNA) was performed for gene co-expression network construction based on the transcriptome data. It is assumed that genes that have related functions may have similar expression profiles [78]. For the gene network, the parameters for dynamic tree cutting were as follows: maxBlockSize: 2000, minModuleSize: 30, deepSplit: 2. The network map of co-expressed genes was drawn based on the software Cytoscape [79]. The position of NBS-LRR proteins in the Plant-pathogen interaction pathway was displayed using map04626 of KEGG database [74,75,76].
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was used to measure the expression levels of 15 genes (Dof008571, Dof024904, Dof000577, Dof010081, Dof010899, Dof013547, Dof005640, Dof006104, Dof014321, Dof015798, Dof017381, Dof004597, Dof017452, Dof018039 and Dof020138), which belonged to D. officinale PTI and ETI systems. The treatment concentration and treatment time of SA were the same as above. The PrimeScript II 1st Strand cDNA Synthesis Kit (TaKaRa) was used for reverse transcription of the extracted total RNA, and LightCycler 96 real-time fluorescent quantitative PCR instrument was used for quantitative analysis. The total volume of each reaction was 20 μL, including SYBR Green I 10 μL, each primer 0.4 μL, cDNA 2 μL and ddH2O 7.2 μL. Temperature Cycles were set to default and three replicates per sample. The gene GAPDH was used as the internal reference gene. Primer sequences are presented in Table S4.
Availability of data and materials
all of the raw data used in this study have been deposited in NCBI (BioProject accession: PRJNA851113, website: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA851113). The voucher specimen (Yang202201) was identified by D.X.Y. and stored in the Institute of Plant Resources and Environment, College of Life Sciences, Nanjing Normal University.
Abbreviations
- TCM:
-
Traditional Chinese Medicine
- R genes:
-
Disease resistance genes
- SA:
-
Salicylic acid
- PTI:
-
Pathogen-associated molecular patterns triggered immunity
- ETI:
-
Effector-triggered immunity
- NB-ARC domain:
-
Nucleotide binding sites domain; LRR domain: C-terminal leucine-rich repeats domain; cp: chloroplast
- WGCNA:
-
Weighted gene co-expression network analysis
- ROS:
-
Reactive oxygen species
- CC:
-
Coil-coiled
- ML:
-
Maximum Likelihood
- AIC:
-
Akaike Information Criterion
- DEGs:
-
Differentially expressed genes
- qPCR:
-
Quantitative real-time PCR
- CNL genes:
-
CNL-type NBS-LRR genes
References
Xiang XG, Schuiteman A, Li DZ, Huang WC, Chung SW, Li JW, Zhou HL, Jin WT, Lai YJ, Li ZY, Jin XH. Molecular systematics of Dendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences. Mol Phylogenet Evol. 2013;69(3):950–60.
Niu Z, Zhu F, Fan Y, Li C, Zhang B, Zhu S, Hou Z, Wang M, Yang J, Xue Q, Liu W, Ding X. The chromosome-level reference genome assembly for Dendrobium officinale and its utility of functional genomics research and molecular breeding study. Acta Pharm Sin B. 2021;11(7):2080–92.
Feng S, Jiang Y, Wang S, Jiang M, Chen Z, Ying Q, Wang H. Molecular Identification of Dendrobium Species (Orchidaceae) Based on the DNA Barcode ITS2 Region and Its Application for Phylogenetic Study. Int J Mol Sci. 2015;16(9):21975–88.
Liu JJ, Liu ZP, Zhang XF, Si JP. Effects of Various Processing Methods on the Metabolic Profile and Antioxidant Activity of Dendrobium catenatum Lindley Leaves. Metabolites. 2021;11(6):351.
Li S, Wu ZG, Zhou Y, Dong ZF, Fei X, Zhou CY, Li SF. Changes in metabolism modulate induced by viroid infection in the orchid Dendrobium officinale. Virus Res. 2022;308:198626.
Kondo H, Maeda T, Shirako Y, Tamada T. Orchid fleck virus is a rhabdovirus with an unusual bipartite genome. J Gen Virol. 2006;87(8):2413–21.
Koh KW, Lu HC, Chan MT. Virus resistance in orchids. Plant Sci. 2014;228:26–38.
Xiao C, Li R. Detection and Control of Fusarium oxysporum from Soft Rot in Dendrobium officinale by Loop-Mediated Isothermal Amplification Assays. Biology (Basel). 2021;10(11):1136.
Cao P, Zheng Z, Fang Y, Han X, Zou H, Yan X. First Report of Stem Rot Caused by Fusarium kyushuense on Dendrobium officinale in China. Plant Dis. 2022. https://doi.org/10.1094/PDIS-12-21-2719-PDN.
Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol. 2012;12(2):89–100.
Zipfel C. Plant pattern-recognition receptors. Trends Immunol. 2014;35(7):345–51.
Han GZ. Origin and evolution of the plant immune system. New Phytol. 2019;222(1):70–83.
Dangl JL, Horvath DM, Staskawicz BJ. Pivoting the plant immune system from dissection to deployment. Science. 2013;341(6147):746–51.
Césari S, Kanzaki H, Fujiwara T, Bernoux M, Chalvon V, Kawano Y, Shimamoto K, Dodds P, Terauchi R, Kroj T. The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J. 2014;33(17):1941–59.
Luo S, Peng J, Li K, Wang M, Kuang H. Contrasting evolutionary patterns of the Rp1 resistance gene family in different species of Poaceae. Mol Biol Evol. 2011;28(1):313–25.
Song W, Wang B, Li X, Wei J, Chen L, Zhang D, Zhang W, Li R. Identification of Immune Related LRR-Containing Genes in Maize (Zea mays L.) by Genome-Wide Sequence Analysis. Int J Genomics. 2015;2015:231358.
Yang X, Wang J. Genome-Wide Analysis of NBS-LRR Genes in Sorghum Genome Revealed Several Events Contributing to NBS-LRR Gene Evolution in Grass Species. Evol Bioinform Online. 2016;12:9–21.
Shao ZQ, Xue JY, Wu P, Zhang YM, Wu Y, Hang YY, Wang B, Chen JQ. Large-Scale Analyses of Angiosperm Nucleotide-Binding Site-Leucine-Rich Repeat Genes Reveal Three Anciently Diverged Classes with Distinct Evolutionary Patterns. Plant Physiol. 2016;170(4):2095–109.
Shao ZQ, Xue JY, Wang Q, Wang B, Chen JQ. Revisiting the Origin of Plant NBS-LRR Genes. Trends Plant Sci. 2019;24(1):9–12.
Cesari S, Thilliez G, Ribot C, Chalvon V, Michel C, Jauneau A, Rivas S, Alaux L, Kanzaki H, Okuyama Y, Morel JB, Fournier E, Tharreau D, Terauchi R, Kroj T. The rice resistance protein pair RGA4/RGA5 recognizes the Magnaporthe oryzae effectors AVR-Pia and AVR1-CO39 by direct binding. Plant Cell. 2013;25(4):1463–81.
Zhang Y, Zhang GQ, Zhang D, Liu XD, Xu XY, Sun WH, Yu X, Zhu X, Wang ZW, Zhao X, Zhong WY, Chen H, Yin WL, Huang T, Niu SC, Liu ZJ. Chromosome-scale assembly of the Dendrobium chrysotoxum genome enhances the understanding of orchid evolution. Hortic Res. 2021;8(1):183.
Xu Q, Niu SC, Li KL, Zheng PJ, Zhang XJ, Jia Y, Liu Y, Niu YX, Yu LH, Chen DF, Zhang GQ. Chromosome-Scale Assembly of the Dendrobium nobile Genome Provides Insights Into the Molecular Mechanism of the Biosynthesis of the Medicinal Active Ingredient of Dendrobium. Front Genet. 2022;13:844622.
Xue JY, Zhao T, Liu Y, Liu Y, Zhang YX, Zhang GQ, Chen H, Zhou GC, Zhang SZ, Shao ZQ. Genome- Wide Analysis of the Nucleotide Binding Site Leucine-Rich Repeat Genes of Four Orchids Revealed Extremely Low Numbers of Disease Resistance Genes. Front Genet. 2020;10:1286.
Qian LH, Zhou GC, Sun XQ, Lei Z, Zhang YM, Xue JY, Hang YY. Distinct Patterns of Gene Gain and Loss: Diverse Evolutionary Modes of NBS-Encoding Genes in Three Solanaceae Crop Species. G3 (Bethesda). 2017;7(5):1577–85.
Liu Y, Zeng Z, Zhang YM, Li Q, Jiang XM, Jiang Z, Tang JH, Chen D, Wang Q, Chen JQ, Shao ZQ. An angiosperm NLR Atlas reveals that NLR gene reduction is associated with ecological specialization and signal transduction component deletion. Mol Plant. 2021;14(12):2015–31.
Kim YK, Jo S, Cheon SH, Joo MJ, Hong JR, Kwak M, Kim KJ. Plastome Evolution and Phylogeny of Orchidaceae, With 24 New Sequences. Front Plant Sci. 2020;11:22.
Yang J, Zhang F, Ge Y, Yu W, Xue Q, Wang M, Wang H, Xue Q, Liu W, Niu Z, Ding X. Effects of geographic isolation on the Bulbophyllum chloroplast genomes. BMC Plant Biol. 2022;22(1):201.
Aköz G, Nordborg M. The Aquilegia genome reveals a hybrid origin of core eudicots. Genome Biol. 2019;20(1):256.
Yu X, Zhong S, Yang H, Chen C, Chen W, Yang H, Guan J, Fu P, Tan F, Ren T, Shen J, Zhang M, Luo P. Identification and Characterization of NBS Resistance Genes in Akebia trifoliata. Front Plant Sci. 2021;12:758559.
Die JV, Castro P, Millán T, Gil J. Segmental and Tandem Duplications Driving the Recent NBS-LRR Gene Expansion in the Asparagus Genome. Genes (Basel). 2018;9(12):568.
Wang L, Liu S, Gao M, Wang L, Wang L, Wang Y, Dai L, Zhao J, Liu M, Liu Z. The Crosstalk of the Salicylic Acid and Jasmonic Acid Signaling Pathways Contributed to Different Resistance to Phytoplasma Infection Between the Two Genotypes in Chinese Jujube. Front Microbiol. 2022;13:800762.
Kourelis J, van der Hoorn RAL. Defended to the Nines: 25 Years of Resistance Gene Cloning Identifies Nine Mechanisms for R Protein Function. Plant Cell. 2018;30(2):285–99.
Collier SM, Moffett P. NB-LRRs work a “bait and switch” on pathogens. Trends Plant Sci. 2009;14(10):521–9.
Bent AF. Plant Disease Resistance Genes: Function Meets Structure. Plant Cell. 1996;8(10):1757–71.
Dangl JL, Jones JD. Plant pathogens and integrated defence responses to infection. Nature. 2001;411(6839):826–33.
DeYoung BJ, Innes RW. Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol. 2006;7(12):1243–9.
Song H, Guo Z, Hu X, Qian L, Miao F, Zhang X, Chen J. Evolutionary balance between LRR domain loss and young NBS-LRR genes production governs disease resistance in Arachis hypogaea cv. Tifrunner BMC Genomics. 2019;20(1):844.
Andersen EJ, Nepal MP, Purintun JM, Nelson D, Mermigka G, Sarris PF. Wheat Disease Resistance Genes and Their Diversification Through Integrated Domain Fusions. Front Genet. 2020;11:898.
Die JV, Román B, Qi X, Rowland LJ. Genome-scale examination of NBS-encoding genes in blueberry. Sci Rep. 2018;8(1):3429.
Oren L, Ezrati S, Cohen D, Sharon A. Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl Environ Microbiol. 2003;69(3):1695–701.
Musetti R, Buxa SV, De Marco F, Loschi A, Polizzotto R, Kogel KH, van Bel AJ. Phytoplasma-triggered Ca(2+) influx is involved in sieve-tube blockage. Mol Plant Microbe Interact. 2013;26(4):379–86.
Verhage A, van Wees SC, Pieterse CM. Plant immunity: it’s the hormones talking, but what do they say? Plant Physiol. 2010;154(2):536–40.
Wang C, Liu Y, Li SS, Han GZ. Insights into the origin and evolution of the plant hormone signaling machinery. Plant Physiol. 2015;167(3):872–86.
Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66.
Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69(4):473–88.
Zhang C, Chen H, Cai T, Deng Y, Zhuang R, Zhang N, Zeng Y, Zheng Y, Tang R, Pan R, Zhuang W. Overexpression of a novel peanut NBS-LRR gene AhRRS5 enhances disease resistance to Ralstonia solanacearum in tobacco. Plant Biotechnol J. 2017;15(1):39–55.
Deng Y, Chen H, Zhang C, Cai T, Zhang B, Zhou S, Fountain JC, Pan RL, Guo B, Zhuang WJ. Evolution and characterisation of the AhRAF4 NB-ARC gene family induced by Aspergillus flavus inoculation and abiotic stresses in peanut. Plant Biol (Stuttg). 2018;20(4):737–50.
Li NY, Ma XF, Short DPG, Li TG, Zhou L, Gui YJ, Kong ZQ, Zhang DD, Zhang WQ, Li JJ, Subbarao KV, Chen JY, Dai XF. The island cotton NBS-LRR gene GbaNA1 confers resistance to the non-race 1 Verticillium dahliae isolate Vd991. Mol Plant Pathol. 2018;19(6):1466–79.
Xu Y, Liu F, Zhu S, Li X. The Maize NBS-LRR Gene ZmNBS25 Enhances Disease Resistance in Rice and Arabidopsis. Front Plant Sci. 2018;9:1033.
Wang J, Tian W, Wang J, Shang H, Chen X, Xu X, Hu X. TaRPM1 Positively Regulates Wheat High-Temperature Seedling-Plant Resistance to Puccinia striiformis f. sp. tritici. Front Plant Sci. 2020;10:1679.
Zhang H, Ye Z, Liu Z, Sun Y, Li X, Wu J, Zhou G, Wan Y. The Cassava NBS-LRR Genes Confer Resistance to Cassava Bacterial Blight. Front Plant Sci. 2022;13:790140.
Yan T, Zhou Z, Wang R, Bao D, Li S, Li A, Yu R, Wuriyanghan H. A cluster of atypical resistance genes in soybean confers broad-spectrum antiviral activity. Plant Physiol. 2022;188(2):1277–93.
Bent AF, Kunkel BN, Dahlbeck D, Brown KL, Schmidt R, Giraudat J, Leung J, Staskawicz BJ. RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science. 1994;265(5180):1856–60.
Shirasu K, Lahaye T, Tan MW, Zhou F, Azevedo C, Schulze-Lefert P. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell. 1999;99(4):355–66.
Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P. SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol Cell. 1999;4(1):21–33.
Chang SC, Erwin AE, Lee AS. Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors. Mol Cell Biol. 1989;9(5):2153–62.
Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10(3):272–82.
Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD. HMMER web server: 2018 update. Nucleic Acids Res. 2018;46(W1):W200–4.
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421.
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A. CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res. 2020;48(D1):D265–8.
Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, Finn RD, Bateman A. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–9.
Letunic I, Khedkar S, Bork P. SMART: recent updates, new developments and status in 2020. Nucleic Acids Res. 2021;49(D1):D458–60.
Ludwiczak J, Winski A, Szczepaniak K, Alva V, Dunin-Horkawicz S. DeepCoil-a fast and accurate prediction of coiled-coil domains in protein sequences. Bioinformatics. 2019;35(16):2790–5.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–80.
Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14(9):817–8.
Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–90.
Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4.
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 20. Bioinformatics. 2007;23(21):2947–8.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018;35(6):1547–9.
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee TH, Jin H, Marler B, Guo H, Kissinger JC, Paterson AH. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40(7): e49.
Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–7.
Bailey TL, Johnson J, Grant CE, Noble WS. The MEME Suite. Nucleic Acids Res. 2015;43(W1):W39–49.
Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–7.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28(11):1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51.
Varet H, Brillet-Guéguen L, Coppée JY, Dillies MA. SARTools: A DESeq2- and EdgeR-Based R Pipeline for Comprehensive Differential Analysis of RNA-Seq Data. PLoS ONE. 2016;11(6):e0157022.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Acknowledgements
We would like to thank the College of Life Sciences, Nanjing Normal University for supporting this work. We are also grateful to the Jiangsu Provincial Engineering Research Center for Technical Industrialization for Dendrobiums for technical support.
Funding
Our work was funded by the National Natural Science Foundation of China (Grant No. 31900268 and 32070353), Natural Science Foundation of Jiangsu Province (BK20190699), Forestry Science and Technology Innovation and Promotion Project of Jiangsu Province (LYKJ[2021]12). None of these funding bodies have any relationship with the publication of this manuscript.
Author information
Authors and Affiliations
Contributions
D.X.Y., N.Z.T. and Y.J.P. designed the study. Y.J.P., X.C.J., L.S.Y. and Z.C. performed the experiments. L.L.L, X.Q.Y. and L.W. were responsible for preparing materials. Y.J.P., X.C.J. and L.S.Y. analyzed the data. Y.J.P. wrote the manuscript. The voucher specimen (Yang202201) was identified by D.X.Y. and made by Y.J.P. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study does not involve any human tissue materials or animal tissue materials. It does not require ethical approval. We declare that the D. officinale individuals used in this study are cultivated species, and do not involve the conservation of wild and endangered resources. The D. officinale cultivated seedlings used in this study were collected from Anhui, China. Experimental researches with D. officinale species comply with Nanjing Normal University guidelines (http://bwc.njnu.edu.cn/info/1085/1433.htm), preserving the genetic background of the species used. The authors' organizations (College of Life Sciences, Nanjing Normal University and Jiangsu Provincial Engineering Research Center for Technical Industrialization for Dendrobiums) approved the publication of this paper.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Xiong, C., Li, S. et al. Evolution patterns of NBS genes in the genus Dendrobium and NBS-LRR gene expression in D. officinale by salicylic acid treatment. BMC Plant Biol 22, 529 (2022). https://doi.org/10.1186/s12870-022-03904-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03904-2