Abstract
Background
Bougainvillea is a popular ornamental plant with brilliant color and long flowering periods. It is widely distributed in the tropics and subtropics. The primary ornamental part of the plant is its colorful and unusual bracts, rich in the stable pigment betalain. The developmental mechanism of the bracts is not clear, and the pathway of betalain biosynthesis is well characterized in Bougainvillea.
Results
At the whole-genome level, we found 23,469 protein-coding genes by assembling the RNA-Seq and Iso-Seq data of floral and leaf tissues. Genome evolution analysis revealed that Bougainvillea is related to spinach; the two diverged approximately 52.7 million years ago (MYA). Transcriptome analysis of floral organs revealed that flower development of Bougainvillea was regulated by the ABCE flower development genes; A-class, B-class, and E-class genes exhibited high expression levels in bracts. Eight key genes of the betalain biosynthetic pathway were identified by homologous alignment, all of which were upregulated concurrently with bract development and betalain accumulation during the bract initiation stage of development. We found 47 genes specifically expressed in stamens, including seven highly expressed genes belonging to the pentose and glucuronate interconversion pathways. BgSEP2b, BgSWEET11, and BgRD22 are hub genes and interacted with many transcription factors and genes in the carpel co-expression network.
Conclusions
We assembled protein-coding genes of Bougainvilea, identified the floral development genes, and constructed the gene co-expression network of petal, stamens, and carpel. Our results provide fundamental information about the mechanism of flower development and pigment accumulation in Bougainvillea, and will facilitate breeding of cultivars with high ornamental value.
Similar content being viewed by others
Introduction
Bougainvillea originates in South America and belongs to the family Nyctaginaceae in the order Caryophyllales. The genus Bougainvillea Juss has 18 species, but only three species, B. glabra Choisy, B. spectabilis Wild and B. peruviana H. & B., have high ornamental values, from which B. glabra Choisy and B. spectabilis are the most showy ornamentals, that are widely distributed in the tropics and subtropics of the word [1, 2]. As an ornamental crop, Bougainvillea has not only bright and colorful flowers, but also has the effect of purifying air and noxious gas removal [3]. Meanwhile the extracts of roots and leaves of Bougainvillea contains antiviral proteins with antiviral characteristics, especially for tobacco mosaic virus (TMV) [4] and tomato spotted wilt tospovirus (TSWV) [5]. Plants in this genus also have been used in traditional medicine. Extracts of Bougainvillea has antinociceptive and anti-inflammatory effects [6], antihyperglycemic activity with the antidiabetic potential [7,8,9], and effective ingredients in prevention of neurological disorders [10,11,12].
The inflorescences of Bougainvillea are axillary, three-flowered umbel, and each floral unit consist of three abnormal leaves—bracts and three flowers, and each flower pedicel attached to the central midrib of the bract [2]. The abnormal floral organs may be regulated by the genes from floral ABCDE model, which proposes that floral organs identity are defined by five classes of genes (A, B, C, D, and E) from MADS-box and AP2/ERF gene family [13, 14]. Generally, the main ornamental organ is petals in angiosperms, which are controlled by A, B and E classes genes [15,16,17]. The B and C classes organ identity genes require the activities of E class genes, and they initiate in stamen development. C and E classes genes regulate carpel development [17]. The D class genes regulate ovule development [18, 19].
The organs with ornamental values of Bougainvillea are the floral bracts enriched with betalains, which are water-soluble and stored in vacuoles, showing brilliant color in flowers or fruits of species in the order Caryophyllalles [20]. They are usually used as natural colorant extracting from red beet because they are more stable than anthocyanins. The betalains are derive from tryrosine, and finally form betaxanthin and betacyanin after a series of enzymatic and spontaneous reaction [21].
The L-DOPA and betalamic acid are crucial substrate for betaxanthin and betacyanin. L-DOPA is catalyzed by tryrosine hydroxylation and tyrosinase or PPO form tryrosine in two ways [22,23,24,25]. Betalamic acid is catalyzed by DOPA 4,5-dioxygenase (DODA), which convert L-DOPA into 4,5-seco-dopa and spontaneous chemical reaction [22]. When L-DOPA is catalyzed by DOPA decarboxylase form dopamine, it enters the betaxanthin synthesis pathway. Dopamine directly binds with betalamic acid or it is catalyzed by catechol O-methyltransferase (COMT) after a spontaneous reaction and then forming miraxanthin-V and 3-methoxytyramain-betaxanthin, respectively. They are a form of betaxanthin [26, 27]. After L-DOPA is catalyzed by tryrosine oxidation or CYP76AD1 (R) produce Cyclo-Dopa, they are involved in glucosylation by the action of GTs [25, 28]. Betanidin 6-O-glucosyltransferase (B6GT) modified betanidin form Gomphrenin-I, and Betanidin 5-O-glucosyltransferase (B5GT) modified betanidin to betanin and form Lampranthin-II, while cyclo-DOPA 5-O-glucosyltransferase (cDOPA-5GT) converts Cyclo-Dopa into Cyclo-Dopa 5-O-glucoside and forms Lampranthin-II and Celosianin-II. These products are betacyanin [20, 29,30,31,32]. Besides biosynthesis pathway genes, some transcription factors are involved in regulation of betalain biosynthesis. In beet, BvMYB1 (Y) is involved in betalain pathway by regulating BvDODA1 and BvCYP76AD1 [33].
To date, no reference genome of Bougainvillea has been reported. Similarly, genetic studies on the mechanisms underlying floral organ morphology and development as well as betalain biosynthesis in Bougainvillea are scarce. In this study, we generated PacBio Iso-Seq and Illumina RNA-Seq data obtained from various organs and stages in petals, stamens, carpels, and bracts, and de novo assembled the expressed protein-coding genes to provide novel insights into key floral genes from the ABCE model participating in bract development; we also identified genes involved in betalain biosynthesis in Bougainvillea, and ascertained the gene co-expression network of petals, stamens, and carpels.
Materials and methods
Genome size analysis, plant RNA-Seq library construct and HiSeq 2500 sequencing
The urban greening commercial cultivar in southern China, Bougainvillea glabra “New River”, was maintained at FAFU greenhouse. It was used for genome size analysis, transcriptome analysis. Fresh young leaves of Bougainvillea “New River” were used for genome size analysis by flow cytometry. The genome size of each sample was estimated with four replications, and the details of the method and data analysis were described in the published article [34]. The use of plants in the study are comply with relevant institutional, national, and international guidelines and legislation.
The petals, stamens, carpels and five development stages (the length of bract with 0.5 cm, 1.0 cm, 2.0 cm, 2.5 cm, and 3.5 cm) of bracts with 2 replicates were sampled and isolated total RNA with total RNA extract kit (TianGen, DP430). The total RNA were quantified and the quality was assessed using an Agilent 2100 Bioanalyzer (Agilent, http://www.agilent.com). Then the RNA samples were used to construct RNA-seq libraries, and follow the protocol of kit (NEBNext Ultra RNA library Prep Kit). These libraries were sent to Beijing Novegene Bioinformatics Technology Co., Ltd for Illumina HiSeq 2500 high through sequence.
Iso-Seq library preparation, PACBIO sequencing and analysis
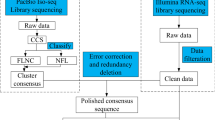
The RNA of leaves, bracts and flowers were extracted separately, and then mixed equally according to total RNA amount of each sample for construct one SMRT cell library (0.5–10 kb). The samples were sent to Beijing Novegene Bioinformatics Technology Co., Ltd for library construct and Pacbio RSII sequence, and the library underwent SMRT sequencing using two SMRT cells. Subreads were filtered and subjected to CCS using the SMRT Analysis Server 2.2.0 (Pacific Biosciences of California, Inc). CD-hit (v4.6.8) [35] with identify 0.85 were used to filter redundancy isoforms.
Coding genes annotation and differentially expression genes analysis
The RNA-seq data from HiSeq 2500 were filter by NGS QC Toolkit. Trinity-v2.8.4 [36] were used to assemble transcriptome data and analyze differentially expressed genes of 5 bract development stages. We assembled and annotated coding genes in Bougainvillea using RNA-Seq and Iso-Seq data from HiSeq 2500 and Pacbio RSII platform, respectively. We got 498,155 isoforms from RNA-Seq data (bract, petal, stamen, and carpel) by trinity assembly, and 257,704 isoforms were kept by CD-hit (v 4.6.8) with similarity thresholds of 0.85 to remove redundancy sequence. And there are 24,840 long reads from Iso-Seq (flowers and leaves tissues) after filter low quality sequence and corrected by short reads from RNA-Seq data. And 10,916 long reads were kept after CD-hit (i_0.85). Then the 257,704 isoforms from RNA-Seq data and 10,916 long reads form Iso-Seq platform were combine and redundancy sequence were removed. And 52,678 proteins were kept by TransDecoder predicated based on Pfam and Swissprot databases (https://github.com/TransDecoder/TransDecoder/wiki). Then the 55,988 proteins were further blastp by NR database and themselves in order to remove similar and negative proteins, and some filter jobs were made by perl scripts in linux.
The differentially expressed genes were analyzed by Trinity (v.2.8.6) and calculated by edgeR (p < 0.001) [37]. All heatmaps were drawn by R script (pheatmap(log2(Exp+1), scale="row”, cluster_cols=F, cellwidth=30, cellheight=15, fontsize=12, color = colorRampPalette (c(“white”,“red”)) (100))) or c(“green”,“white”,“red”), the parameters of cell width, cell height, font size were changed according to the number of genes and samples.
Comparative genome analysis
The evolutionary history of Bougainvillea was not reported before, and proteins of Bougainvillea and other reported seven species were used to speculate the evolutionary time.Proteins and cds of seven species (Spinacia oleracea, Beta vulgaris, Carica papaya, Arabidopsis thaliana, Vitis vinifera, Nelumbo nucifera, Oryza sativa) were chosen to construct phylogenetic tree, and all data were download at ncbi (https://ftp.ncbi.nlm.nih.gov/genomes/refseq/plant/). The single-copy orthologous genes of all species were identified using OrthoMCL (v.2.0) [38]. The phylogenetic tree of seven species was construct by iqtree (v.1.6.3) [39] with single-copy orthologous genes, and ultrametric tree was drawn by the Café software (v.4.2) [40]. The Arabidopsis thaliana and Carica papaya divergence time (68–72 million years ago) [41] was applied as calibrators.
Expression experiment
Real-time qPCR was designed to verify the expression trend of A, B, C, D, and E classes genes and then they were compared with transcriptome data. Mature leaves and five development stages of bracts, petals, stamens, and carpels were collected for Real-time qPCR. The primers were designed by Primer 5.0, and the primer sequences were listed in Table S1. Real-time qPCR (RT-qPCR) was performed on an Roche LightCycler 480 with SYBR Premix ExTaqII (Takara), and relative expression data were quantified by 2−△△CT method [42] and normalized by 18s rRNA.
Co-expression network analysis
The weighted gene correlation network analysis (WGCNA) (v 1.61) package in R [43, 44] was used to construct the gene co-expression network analysis for Bougainvillea based on the gene-level FPKM data from differentially expressed genes during the 5 bract developmental stages. Module detection was performed using the TOM-based similarity measure and the dynamic tree cutting algorithm to cut the hierarchal clustering tree and defined modules as branches from the tree cutting. The minimum number of genes per module was set as 30 genes by default, and the threshold of module merging correlation for eigengene similarity was 0.8. For the module-tissue association analysis, the eigengene value was calculated for each module and used to test the association with each tissue type. The total connectivity and intramodular connectivity, kME, and kME-p-value were calculated and represent the Pearson correlation between the expression level of that gene and the ME. Additionally, genes with highest degree of intramodular connectivity within a module are referred to as hub genes. The networks were visualized using Cytoscape_v.3.8.0 [45].
Results
De novo assembly of transcripts and annotation of coding genes
The mean genome size of cultivar species Bougainvillea glabra “New River” was about 3.04 Gb (n = 4) (Supplementary Figure S1). We present a transcriptomic analysis by combining PacBio Iso-Seq and Illumina sequencing technologies, aiming to (a) obtain accurate and full length transcripts; and (b) providing quantitative information for expression analysis. The coding genes number of RNA-Seq assembled and Iso-Seq data annotated were 257,704 isoforms and 24,840 long reads, respectively. The integrity of proteins from RNA-Seq assembled and Iso-Seq was 91.2 (S:65.2%; D:26.0%) and 40.6% (S:20.7%; D:19.9%), respectively. In addition, 23,469 proteins were retained after merging all proteins from the two platforms and removing redundancy (Table 1).
Phylogenetics analysis of representative angiosperm species
The evolutionary history of Bougainvillea has not yet been reported. We therefore used proteins of Bougainvillea and seven other species to elucidate its evolutionary history. Bougainvillea belongs to the Nyctaginaceae family; however, no genome or protein library for this family has yet been reported. Therefore, the closest family protein libraries of spinach (Spinacia oleracea) from Chenopodiaceae and beet (Beta vulgaris) from Amaranthaceae were used to construct species trees. Four other species from four different families of the eudicot clade, including papaya (Carica papaya), Arabidopsis (Arabidopsis thaliana), grape (Vitis vinifera), lotus (Nelumbo nucifera). Monocot model species rice (Oryza sativa) was used as an outgroup species to construct the phylogenetic tree. The results showed that Bougainvillea was earlier than beet and spinach, and they diverged approximately 41.46 million years ago (MYA) (Fig. 1).
Expression of ABCE model genes during bract development
The ABCE model genes play pivotal roles in floral organ development. We found 12 homologous genes of the ABCE flowering organ identity genes in Bougainvillea, as shown in Fig. 2. Among them, ten genes encoded MADS-box transcription factors; two genes were of AP2 transcription factors (A-class). Expression profile analysis showed that APETALA1 (BgAP1) in Bougainvillea showed high expression levels in bracts and petal tissues. The two homologous genes of AP2 showed varying expression patterns. BgAP2a was highly expressed in bracts, petals, and carpels, whereas BgAP2b was also highly expressed in stamens. The B-class genes including BgAP3 and BgPI. They showed the highest expression level in stamens by RNA-seq analysis, and BgAP3 also showed high expression in petals and carpels by calculating the relative expression level (Supplementary Figure S2). The C-class gene BgAG, and D-class gene BgSHP showed higher expression levels in petals and stamens, and low or no expression in bract tissues. We also found they showed higher expression level in carpels by RT-qPCR (Supplementary Figure S2). There were four homologous genes from E-class, among which BgSEP2a and BgSEP2b showed high expression levels in bracts and petals tissues (Fig. 2B).
The expression analysis of flower organs in Bougainvillea A. The flower tissues samples, including 5 development stages of bract, petal, stamen, and carpel. B. The expression profile of ABCE model genes in floral organs (FPKM). C. The relative expression of ABCE model genes infloral tissues of Bougainvilleaby RT-qPCR
Transcriptomes of bracts at different developmental stages
The bract is the main ornamental element of Bougainvillea, and the mechanisms underlying its development are not yet clear. We calculated the expression levels of 23,469 genes from the whole-genome analysis of Bougainvillea, and 1882 differentially expressed genes (DEGs) were found during the five developmental stages of bracts (p < 0.001). They formed three subclusters depending on their differential expression trends. A total of 574 genes were significantly upregulated during bract development (Fig. 3D), including 36 transcription factors from 17 families. Among them, 12 transcription factors showed significantly higher expression levels in adult bract—bract 3.5 compared to other flower tissues, including three bHLH, two C2H2, two ERF, two HD-ZIP, one MYB, one GATA, and one WRKY transcription factors (Fig. 3E).
The expression trend and profile of 1882 differentially expression genes (DEGs) in bract. A-C. The three clusters of DEGs, and 574 genes in the up-regulated cluster. D. The expression profile of 574 up-regulated genes at 5 developmental stages of bracts, petal, stamen and carpel. E. The expression profile of 36 transcription factors of the 574 up-regulated genes. The data in the heatmap was normalized by log2(FPKM+1)
Kyoto Encyclopaedia of Genes and Genomes (KEGG) enrichment analysis revealed that the upregulated genes were mainly involved in amino acid metabolism (tyrosine metabolism and valine, leucine, and isoleucine degradation), biosynthesis of other secondary metabolites (phenylpropanoid biosynthesis, tropane, piperidine and pyridine alkaloid biosynthesis, anthocyanin biosynthesis), carbohydrate metabolism (galactose metabolism) (10), energy metabolism (carbon fixation in photosynthetic organisms and nitrogen metabolism), and metabolism of terpenoids and polyketides (carotenoid biosynthesis, monoterpenoid biosynthesis, and zeatin biosynthesis) (p < 0.05) (Supplementary Figure S3). The genes in the phenylpropanoid biosynthesis (14) and galactose metabolism (10) pathways were more highly enriched than those in other pathways.
There were eight genes of the phenylpropanoid biosynthesis pathway, and six of them (Gene.3747, BgPRX52-2, BgCAD4, Bg4CL1, Gene.5465, and CYP98A3) showed significantly higher expression in bracts than in other flower tissues (Supplementary Figure S4). BgCAD4 and Bg4CL1 are crucial genes for lignin biosynthesis, and lignin is the main component of the secondary cell wall of vascular tissues [46, 47]. Two genes (Gene.26858 and BgBGAL12) out of ten were involved in galactose metabolism. One (Gene.38630) out of two anthocyanin biosynthesis genes and one (NCED4) out of three carotenoid biosynthesis genes showed particularly high expression levels in bracts.
Highlights of betalain biosynthesis-related genes in bract of Bougainvillea
We further identified 15 homologous genes encoding eight enzymes in the tyrosine-based biosynthetic pathway of betalain, including four genes encoding DODA, two BgCYP76AD1 genes, two encoding DOPA decarboxylase, two encoding cDOPA5GT, two encoding B6GT, and two encoding COMT. Fourteen of these genes showed increased expression concurrent with bract development at the initial developmental stages (Fig. 4). In detail, BgCYP76AD1 and the genes encoding B6GT, COMT, DDC, and DODA showed increased along with bract development and significantly high expression levels in the fourth (Bract 2.5) or fifth (Bract 3.5) developmental stages of bract; three genes (DDC, B5GTb, and CYP76AD1a) were found in the up-regulated cluster. The TYR gene encoding tyrosinase showed the highest expression peak at the third stage (Bract 2.0), while B5GT showed a declining trend during bract development. cDOPA5GT was expressed at all five developmental stages.
The expression analysis of betalain biosynthetic genes at 5 developmental stages of bract (from left to right was bract 0.5, bract 1.0, bract 2.0, bract 2.5, bract 3.5). The color from white to red indicated the expression level from low to high. The schematic diagram of betalain biosynthetic pathway was edited according to KEGG and reported reference[25]. “*” steads of spontaneous chemical reaction steps. The yellow borders represent the product of betaxanthin and the red borders represent the product of betacyanin. DODA: 4,5-DOPA-extradiol-dioxygenase; B6GT: Betalain 6-O-glucosyltransferase; B5GT: betanidin 5-O-glucosyltransferase; cDOPA5GT: Cyclo-DOPA 5-O-glucosyltransferase; COMT: Catechol O-methyltransferase; DDC: DOPA decarboxylase
Weighted gene co-expression network analysis (WGCNA) of different flower organs in Bougainvillea
A total of 12,987 genes (FPKM ≥ 3) were used to construct the co-expression network (Fig. 5). The MEdarkslateblue module (0.98) was chosen to analyse petal-specific co-expression genes; the MElightsteelblue1 module (0.96) was chosen to analyse stamen-specific co-expression genes; the MEgreen module (0.99) was chosen to analyse carpel-specific co-expression genes.
The petal-specific module contains 1103 genes, 640 of which showed higher expression in petals than in other flower tissues. KEGG enrichment analysis indicated that 640 genes were mainly involved in the spliceosome (15), plant hormone signal transduction (11), ubiquitin-mediated proteolysis (9), and circadian rhythms (5) (Supplementary Figure S5). Three plant hormone genes were involved in the plant hormone signal transduction pathway, including auxin (AUX), ethylene (ETH), and gibberellin (GA). ETHYLENE INSENSITIVE 3 (EIN3) and two of its binding F-box proteins (EBF) were also found in this pathway. Seven genes showed significantly higher expression levels than other genes: BgEIN3, which is involved in plant hormone signal transduction and MAPK signalling, Gene.47914, BgHSP70 and BgSR34-2 of the spliceosome, BgGBSS1 of starch and sucrose metabolism, and BgLACS2 and BgFAD2 of the fatty acid metabolism pathway. Furthermore, five genes related to the circadian clock were found in the module, including circadian clock gene BgELF3, photoperception genes BgPHYA, BgPIF3, BgCOP1-1 and BgCOP1-2 (homologous to COP1 in Arabidopsis). BgPHYA and BgELF3 are hub genes that interact with other genes (Fig. 6 A).
The co-expression network and express profile of petal high expression genes. A. Co-expression network of KEGG pathway genes (Pvalue <0.05). The size of circle indicated the expression level from high to low. The genes with octagon indicated hub genes, the color from red to yellow indicated the degree from high to low. B. The expression profile of 25 petal specific high expression genes.
There were 25 genes that showed significantly high expression in petals (not expressed, or FPKM < 3, in other flower tissues), including seven transcription factors, two ERFs (Gene.47813 and BgANT), two ARFs (Gene.12201 and Gene.36476), one Dof (Gene.68808), one TFII (BgTAF2), and one MADS-box (BgAGL32). BgCYS2 and BgPLAT3 showed significantly higher expression levels than the other genes in petals (Fig. 6B).
The stamen co-expression module contains 2,094 co-expression genes (113 TFs). A total of 1412 genes (75 TFs) exhibited higher expression levels in the stamen; upon analysis, they were found to mainly be enriched in genetic information processing and metabolism processes (P <0.05), including pentose and glucuronate interconversions (14), glycolysis/gluconeogenesis (16), fructose and mannose metabolism (9), proteasome (9), arginine and proline metabolism (8), cyanoamino acid metabolism (7), and lipoic acid metabolism (3) (Figure S6). There were 47 genes that showed specific expression in the stamen, 15 genes involved in KEGG pathways, and seven genes in the pentose and glucuronate interconversions pathway, including four genes (Gene.1030, Gene.8930, BgPME3, and BgVGDH2) encoding pectinesterase, two genes (Gene.2513 and Gene.10944) encoding pectate lyase, and one gene (BgPGA4) encoding exopolygalacturonase. Co-expression network analysis (weight > 0.3) identified 44 transcription factors that interact with these specific expression genes (Fig. 7 A). The eight hub genes, including two transcription factors (BgSPTb and BgNAC025), also showed high expression levels in the stamen (Fig. 7B); the other six hub genes were stamen-specific genes. Expression analysis found that BgVGDH2, BgPGA4, Gene.10944, and Gene.1031 of the pentose and glucuronate interconversion pathways, as well as BgFLA3 and Gene.5605 exhibited higher expression levels among these specific expression genes in the stamen (Fig. 7 C).
The carpel-specific module contains 380 genes, including 30 transcription factors, and 272 genes with higher expression levels in petals than in other flower tissues. These genes are involved in the MAPK signalling pathway (6), biosynthesis of secondary metabolites (17), and plant-pathogen interactions (5) (Figure S7). Thirty out of 272 genes showed significantly higher expressions in the carpel, and five genes (BgRD22, BgCCOAMT, BgSEP2C, BgSWEET11, and BgPP2-B15) showed the highest expression level in carpels as compared to other genes (more than four-fold) (Fig. 8 A). Two genes (Gene.9736 and Gene.9737) encoding pectate lyase showed no expression in bract and significantly higher expression levels in carpel (4–12-fold higher than in the petal or stamen). The GA biosynthesis-related gene GA2OX8 exhibited an exceedingly high expression level in the carpel (11–88-fold that of other flower tissues). The co-expression network (weight > 0.2) identified 16 transcription factors that interacted with these 30 carpel-specific high expression genes. BgSEP2b, Gene.12985, BgSWEET11, BgGA2OX8, and BgRD22 were the top five hub genes in the network (Fig. 8 B).
The co-expression network and expression profile of transcription factors and stamen specific expression genes. A The co-expression network of transcription factors and stamen specific expression genes. B The expression profile of transcription factors in the co-expression network. C The expression profile of 47 stamen specific expression genes
The expression profile of 30 carpel specific high expression genes and co-expression network. A The expression profile of 30 carpel specific high expression genes. The heatmap was drawn by R with log2(FPKM+1). B The co-expression network of transcriptions factors and carpel specific genes. The top 5 nodes (about 1%) were set to find hub genes. The color of hub genes from red to yellow indicated the degree from high to low
Discussion
In flowering plants, the development of the main ornamental parts is regulated by genes belonging to the classes A, B, and E [16, 17, 34]. The results showed that the expression profiles of floral organ-regulated genes of ABCE slightly differed from that of typical flowering plants. Bract formation related to A, B, and E class genes; A-class genes such as BgAP2 showed lower expression levels in petals, indicating the possible formation mechanism of large colourful bracts and small dull petals. STK and SHP as D class genes specific to regulate ovule identification [17]. In Bougainvillea, we couldn’t identify any STK orthologous gene. The STK orthologous gene may be lost in Bougainvillea, or simply not annotated; this requires further genome sequencing for verification.
The Bougainvillea plant shows self-incompatibility. Previous reports have shown that B, C, and E-class genes regulate stamen development; C and E proteins complexes identify carpels; and D-class proteins specify ovule identity by interacting with E class proteins [15, 18]. In Bougainvillea, we calculated the expression level of A, B, C, D, and E-class genes by RNA-seq data and real time-qPCR. The expression analysis showed that B-class (BgAP3a, BgAP3b, and BgPI), C-class (BgAG), and D-class (BgSHP) genes showed high expression levels in stamens and carpel, while three out of four (BgSEP1, BgSEP2b, BgAGL6) E-class genes exhibited their highest expression levels in carpels, followed by stamen tissues (Fig. 3). From the expression profile, we found that C and D class genes showed lower expression levels in the carpel, while we proved they showed high expression in carpel by real time-qPCR (Fig. 3 C). E-class genes showed the highest expression in carpel tissues, which was consistent with real time-qPCR, indicating that the D-class genes may be functionally redundant in ovule identification, and E-class genes may play crucial roles in carpel identification. Due to the different calculate methods, the expression trend of some genes were inconsistent between RNA-seq data and real time-qPCR, but the overall gene expression trend of ABCE model genes were consistent as flowering plant.
Betalain is the main pigment in Bougainvillea flowers, which is more stable than anthocyanins in different PH [20]. However, the enzymes involved in the biosynthetic pathway are not as well understood as those of other plant pigments. Only eight homologous enzyme proteins were found in Bougainvillea. They showed different expression modes during the five bract development stages, which may be related to their different locations in the biosynthetic pathway of betalain. Transcription factors, such as MYB–bHLH–WD repeat (MBW) [48], participate in pigment biosynthesis in plant kingdom by regulating key genes in biosynthetic pathways. In betalain biosynthesis, MYB and WRKY transcription factors are also involved. For example, BvMYB regulates CYP76AD1/R and BvDODA1 in beet [33], and HmoWRKY40 regulates HmoCYP76AD1 in Pitaya [49].
In petal-specific module, we found plant hormone signal transduction and circadian rhythms genes’ specific expression in petals. Two ERFs (Gene.47813 and BgANT) and two ARFs (Gene.12201 and Gene.36476) showed specifically high transcriptional level in petal, indicating the crucial role of ethylene and auxin in regulating petal development in Bougainvillea. Photoperception genes BgCOP1-1 and BgCOP1-2 were homologous with E3 ubiquitin-ligase COP1 regulating flowering in Arabidopsis [50], and they were found in petal-specific module, indicating their importance in petal development in Bougainvillea.
Stamen-specific model contains 47 specifically expressed genes, and seven of them encoding pectinesterase, pectate lyase, and exopolygalacturonase in Bougainvillea. The genes encoding pectinesterase are specifically expressed in stamens in wheat, and are involved in the regulation of male fertility [51]. The genes encoding exopolygalacturonase in maize are expressed in tissues associated with pollen development [52]. Pectate lyase degrades pectin in plants and contributes to the mechanical strength and physical properties of the primary cell walls [53]; some genes of this family are specifically expressed in pollen, such as Late Anther Tomato 56 and Late Anther Tomato 59, expressed at maximal levels in mature anthers [54].
Previous research had demonstrated that SEP2 was organ-identity genes and required for development of petals, stamens, and carpels in Arabidopsis [17]. In carpel co-expression network analysis, BgSEP2b showing high expression level in carpels and it was a hub gene interacted with many transcription factors and carpel-associated genes, suggesting it was a crucial gene in carpel development in Bougainvillea.
Conclusions
We obtained 23,469 coding genes using RNA-seq and Iso-Seq data assembly. The genome evolution analysis showed that Bougainvillea was most closely related to spinach of the plant species analysed, and the two diverged approximately 52.7 MYA. Genes in ABCE model of flower development and betalain biosynthetic pathway genes were identified by homologous comparison. Bracts, the main ornamental organs of flowers, are regulated by A, B, and E-class genes. Betalain accumulation along with flower development, and the genes in betalain biosynthetic pathwayshowed an upregulated trend concurrent with bract development. Transcription factors, phenylpropanoids, and pigment biosynthesis pathway genes are involved in bract development. The genes of the spliceosome, plant hormone signal transduction, ubiquitin-mediated proteolysis, and photoperception and circadian rhythm pathways were co-expressed in petals. We found 47 stamen-specific genes in the stamen co-expression module; the genes encoding pectinesterase, pectate lyase, and exopolygalacturonase play crucial roles in stamen development. The stress response genes BgRD22, lignin biosynthetic gene BgCCOAMT, floral gene BgSEP2b, sugar transporter BgSWEET11, and BgPP2-B15 showed higher levels of expression in carpels than did other genes. Finally, we found that BgSEP2b, BgSWEET11, and BgRD22 as hub genes interacted with many transcription factors and genes in the carpel co-expression network.
Availability of data and materials
All RNA-seq data of Bougainvilean reported in this paper have been deposited in the Genome Sequence Archive [55] in National Genomics Data Center [56], China National Center for Bioinformation / Beijing Institute of Genomics, Chinese Academy of Sciences, under accession number CRA004850 that are publicly accessible at https://ngdc.cncb.ac.cn/gsa.
References
Mabberley DJ. In: The plant book. Cambridge: Cambridge Univ. Press; 1997. p. 1–706.
López HA, Galetto L. Flower Structure and Reproductive biology of Bougainvillea stipitata (Nyctaginaceae). Plant biology. 2002;4:508–14.
Sangthong S, Suksabye P, Thiravetyan P. Air-borne xylene degradation by Bougainvillea buttiana and the role of epiphytic bacteria in the degradation. Ecotoxicology and Environmental Safety. 2016;126:273–80.
Murthy NS, Nagarajan K, Sastry AB. Effect of prophylactic sprays of leaf extracts on the infection of tobacco by tobacco mosaic virus. Indian J Agri Sci. 1981;51:792.
Balasaraswathi R, Sadasivam S, Ward M, Walker JM. An antiviral protein from Bougainvillea spectabilis roots; purification and characterisation. Phytochemistry. 1998;47:1561–5.
Alvarez Perez Gil AL, Barbosa Navarro L, Patipo Vera M, Petricevich VL. Anti-inflammatory and antinociceptive activities of the ethanolic extract of Bougainvillea xbuttiana. Journal of Ethnopharmacology. 2012;144:712–9.
Joshi DD, Mujumdar AM, Narayanan CR. Anti-inflammatory activity of Bougainvillea spectabilis leaves. Indian Journal of Pharmaceutical Sciences. 1984;46:187–8.
Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Poloniae Pharmaceutica. 2010;67:113–8.
Purohit A, Sharma A. Blood glucose lowering potential of Bougainvillea spectabilis Willd. leaf extract in streptozotocin induced type - I diabetic albino rats. Indian Drugs. 2006;43:538–42.
Henry C, Wilson JA. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Lancet. 1998;351:1965–6.
Teravainen H, Rinne U, Gordin A. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Adv Neurol. 2001;86:311–25.
Abdel-Salam OME, Youness ER, Ahmed NA, El-Toumy SA, Souleman AMA, Shaffie N, et al. Bougainvillea spectabilis flowers extract protects against the rotenone-induced toxicity. Asian Pac J Trop Med. 2017;10:478–90.
Theissen G, Saedler H. Plant biology. Floral quartets. Nature. 2001;409:469–71.
Rijpkema AS, Vandenbussche M, Koes R, Heijmans K, Gerats T. Variations on a theme: changes in the floral ABCs in angiosperms. Semin Cell Dev Biol. 2010;21:100–7.
Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature. 1992;360:273–7.
Jofuku KD, Boer BG den, Montagu MV, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. The Plant Cell. 1994;6:1211–25.
Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature. 2000;405:200–3.
Kramer EM, Jaramillo MA, Di Stilio VS. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics. 2004;166:1011–23.
Colombo L, Battaglia R, Kater MM. Arabidopsis ovule development and its evolutionary conservation. Trends Plant Sci. 2008;13:444–50.
Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008;54:733–49.
Jain G, Gould KS. Are betalain pigments the functional homologues of anthocyanins in plants? Environ Exp Bot. 2015;119:48–53.
Gandia-Herrero F, Garcia-Carmona F. Characterization of recombinant Beta vulgaris 4,5-DOPA-extradiol-dioxygenase active in the biosynthesis of betalains. Planta. 2012;236:91–100.
Gandia-Herrero F, Escribano J, Garcia-Carmona F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta. 2005;222:586–93.
Gandia-Herrero F, Escribano J, Garcia-Carmona F. Betaxanthins as substrates for tyrosinase. An approach to the role of tyrosinase in the biosynthetic pathway of betalains. Plant Physiol. 2005;138:421–32.
Hatlestad GJ, Sunnadeniya RM, Akhavan NA, Gonzalez A, Goldman IL, McGrath JM, et al. The beet R locus encodes a new cytochrome P450 required for red betalain production. Nat Genet. 2012;44:816–20.
Schliemann W, Kobayashi N, Strack D. The decisive step in betaxanthin biosynthesis is a spontaneous reaction1. Plant Physiol. 1999;119:1217–32.
Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628.
Sullivan ML. Beyond brown: polyphenol oxidases as enzymes of plant specialized metabolism. Front Plant Sci. 2015;5:783.
Sciuto S, Oriente G, Piattelli M, Impelizzeri G, Amico V. Biosynthesis of amaranthin in Celosia plumosa. Phytochemistry. 1974;13:947–51.
Vogt T, Grimm R, Strack D. Cloning and expression of a cDNA encoding betanidin 5-O-glucosyltransferase, a betanidin- and flavonoid-specific enzyme with high homology to inducible glucosyltransferases from the Solanaceae. Plant J. 1999;19:509–19.
Vogt T. Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis. Planta. 2002;214:492–5.
Sasaki N, Abe Y, Wada K, Koda T, Goda Y, Adachi T, et al. Amaranthin in feather cockscombs is synthesized via glucuronylation at the cyclo-DOPA glucoside step in the betacyanin biosynthetic pathway. J Plant Res. 2005;118:439–42.
Hatlestad GJ, Akhavan NA, Sunnadeniya RM, Elam L, Cargile S, Hembd A, et al. The beet Y locus encodes an anthocyanin MYB-like protein that activates the betalain red pigment pathway. Nature Genetics. 2015;47:92–6.
Dolezel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc. 2007;2:2233–44.
Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–2.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat Biotechnol. 2011;29:644–52.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40.
Li L, Stoeckert CJ, Roos DS. OrthoMCL: Identification of Ortholog Groups for Eukaryotic Genomes. Genome Res. 2003;13:2178–89.
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Molecular Biology and Evolution. 2015;32:268–74.
De Bie T, Cristianini N, Demuth JP, Hahn MW. CAFE: a computational tool for the study of gene family evolution. Bioinformatics. 2006;22:1269–71.
Ming R, Hou S, Feng Y, Yu Q, Dionne-Laporte A, Saw JH, et al. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus). Nature. 2008;452:991–6.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Langfelder P, Zhang B, Horvath S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics. 2008;24:719.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. Bmc Bioinformatics. 2008;9:559.
Su G, Morris JH, Demchak B, Bader GD. Biological Network Exploration with Cytoscape 3. Curr Protoc Bioinformatics. 2014;47:8.13.1.
Voelker SL, Lachenbruch B, Meinzer FC, Strauss SH. Reduced wood stiffness and strength, and altered stem form, in young antisense 4CL transgenic poplars with reduced lignin contents. New Phytologist. 2011;189:1096–109.
Özparpucu M, Rüggeberg M, Gierlinger N, Cesarino I, Vanholme R, Boerjan W, et al. Unravelling the impact of lignin on cell wall mechanics: a comprehensive study on young poplar trees downregulated for CINNAMYL ALCOHOL DEHYDROGENASE (CAD). Plant J. 2017;91:480–90.
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. The Plant Journal. 2008;53:814–27.
Zhang L, Chen C, Xie F, Hua Q, Zhang Z, Zhang R, et al. A Novel WRKY Transcription Factor HmoWRKY40 Associated with Betalain Biosynthesis in Pitaya (Hylocereus monacanthus) through Regulating HmoCYP76AD1. Int J Mol Sci. 2021;22:2171.
Yu J-W, Rubio V, Lee N-Y, Bai S, Lee S-Y, Kim S-S, et al. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell. 2008;32:617–30.
Chen Y, Jia Y, Niu F, Wu Y, Ye J, Yang X, et al. Identification and validation of genetic locus Rfk1 for wheat fertility restoration in the presence of Aegilops kotschyi cytoplasm. Theor Appl Genet. 2021;134:875–85.
Barakate A, Martin W, Quigley F, Mache R. Characterization of a multigene family encoding an exopolygalacturonase in maize. J Mol Biol. 1993;229:797–801.
Willats WGT, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Mol Biol. 2001;47:9–27.
Wing RA, Yamaguchi J, Larabell SK, Ursin VM, McCormick S. Molecular and genetic characterization of two pollen-expressed genes that have sequence similarity to pectate lyases of the plant pathogen Erwinia. Plant Mol Biol. 1990;14:17–28.
Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Geno, Prot & Bioinfo. 2021;S1672-0229:00163–7.
CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2021. Nucleic Acids Res. 2021;49:D18–28.
Acknowledgements
This work was supported by a startup fund from Fujian Agriculture and Forestry University. Natural Science Foundation of Fujian Province, China (Grant No. 2019J05055). Science and Technology project of Xiamen, China (Grant No. 3502Z20199008).
Funding
This work was supported by a startup fund from Fujian Agriculture and Forestry University. Natural Science Foundation of Fujian Province, China (Grant No. 2019J05055). Science and Technology Project of Xiamen, China (Grant No. 3502Z20199008).
Author information
Authors and Affiliations
Contributions
R. M. and Wanqi. Z. conceived this genome project and coordinated research activities; R. M., W. Z. and Q. Z. designed experiments; X.S. and L.H. extracted RNA and constructed the RNA library. W. Z., and H.Y. assembled RNA-Seq data and annotated the proteins; Weimin. Z. analyzed the Iso-Seq data; W. Z., J. L., X. M., F.D., and Y. Y. analyzed genome, genes expression, and genes network; Y. L. did the RT-qPCR experiment; W. Z., and R.M. wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: TableS1.
The real time-qPCR primers sequence of ABCE model genes. Figure S1. The genomic sizeof Bougainvillea glabra “New river”. The genomic size was about3.035Gb by the average of 4 replicates measure using flow cytometer. FigureS2. Therelative expression of ABCE model genes infloral tissues of Bougainvilleaby real time-qPCR. Figure S3. KEGGenrichment analysis of 574 up-regulated genes at fivedevelopmental stages of bract. These genes mainly rich in metabolicpathway, biosynthesis of secondary metabolites, phenylpropanoid biosynthesis,etc. Figure S4. The expressionprofile of phenylpropanoid biosynthesis, galactose metabolism, anthocyaninbiosynthesis and carotenoid biosynthesis genes. The genes with red border indicatedthey had high expression level in that pathway. Figure S5. The top 20 ofKEGG enrichment pathway of 640 petal high expression genes. Figure S6. The top 20 ofKEGG enrichment pathway of 1412 stamen high expression genes. Figure S7. The top 20 ofKEGG enrichment pathways of 272 carpel high expression genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, W., Zhou, Q., Lin, J. et al. Transcriptome analyses shed light on floral organ morphogenesis and bract color formation in Bougainvillea. BMC Plant Biol 22, 97 (2022). https://doi.org/10.1186/s12870-022-03478-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-022-03478-z