Abstract
Background
The plant U-box (PUB) proteins are a family of ubiquitin ligases (E3) enzymes that involved in diverse biological processes, as well as in responses to plant stress response. However, the characteristics and functional divergence of the PUB gene family have not yet been previously studied in the Chinese white pear (Pyrus bretschneideri).
Results
In the present study, we identified 62 PbrPUBs in Chinese white pear genome. Based on the phylogenetic relationship, 62 PUB genes were clustered into five groups. The results of conserved motif and gene structure analysis supported the classification phylogenetic tree. The PbrPUB genes were unevenly distribution on 17 pear chromosomes, chromosome 15 housed most member of PUB family, with eight PUB genes. Cis-acting element analysis indicated that PUB genes might participate in diverse biological processes, especially in the response to abiotic stresses. Based on RNA-data from ‘Dangshansuli’ at seven tissues, we found that PUB genes exhibited diverse of expression level in seven tissues, and qRT-PCR experiment further supported the reliable of RNA-Seq data. To identify candidate genes associated with resistance, we conducted qRT-PCR experiment the expression level of pear seed plant under four abiotic stresses, including: ABA, dehydration, salt and cold treatment. One candidate PUB gene associated with dehydration stress was selected to conduct further functional experiment. Subcellular localization revealed PbrPUB18 protein was located on cell nucleus. Furthermore, heterologous over-expression of PbrPUB18 in Arabidopsis indicated that the over-expression of PbrPUB18 could enhance resistance in drought treatment. In conclusions, we systematically identified the PUB genes in pear, and provided useful knowledge for functional identification of PUB genes in pear.
Similar content being viewed by others
Background
Plants are frequently exposed to various abiotic stresses such as drought, salt and low temperature during their life cycles. Several stresses often lead to oxidative damage and have adverse impacts on plant growth and development. To adapt to unfavorable environmental conditions, plants have evolved complex and efficient mechanisms [1]. Previous studies have identified four signal transduction pathways in response to abiotic stress, including transcriptional regulation, post-transcriptional modifications, epigenetic regulation, and post-translational modifications [2]. And ubiquitination is one of the most significant post-translational modifications. The ubiquitin/26S proteasome system (UPS) pathway is a pervasive and effective route for protein removal in eukaryotes [3, 4]. UPS include ubiquitin (Ub), ubiquitin-activating enzyme (E1), ubiquitin- conjugating enzyme (E2), ubiquitin ligase (E3), and the 26S proteasome. The central component of UPS is the highly conserved, 76 amino acid protein ubiquitin. Ubiquitin is bound to specific proteins and functions in the degradation of target proteins in an E1–E2–E3 multienzyme cascade manner [5,6,7,8]. In the pathway, E3 enzymes are clearly the key factors that define substrate specificity. According to their reaction mechanism and subunit compositions, four main types were classified: Ubox, HECT (Homology to E6-Associated Carboxy-Terminus), RING (Really Interesting New Gene) and Cullin–RING ligases (CRLs) [4]. U-box ubiquitin ligases are characterized by a conserved U-box motif of about 70 amino acids. And U-box ubiquitin ligases were firstly discovered among E3 ubiquitin ligases, and was first clarified from ubiquitin fusion degradation protein-2 (UFD2) in yeast [9].
In comparison with the 2 and 21 U-box (PUB) genes identified in Saccharomyces cerevisiae and Homo sapiens genomes, respectively, more U-box genes were widely distributed in plants. In Arabidopsis thaliana, about 61 plant U-box genes were predicted [9, 10], while 77 were found in Oryza sativa [11], 62 in Solanum lycopersicum [12], 93 in Gossypium raimondii [13], 91 in Musa acuminate [14], 61 in Medicago truncatula [15] 101 in Brassica rapa [16] and 125 in soybean [17]. In apple[18], 69 PUB genes were identified, and were divided into seven subgroups based on phylogenetic tree of PUB proteins from apple and Arabidopsis thaliana. Many previous studies have shown that PUB proteins are involved in biological processes such as plant hormone signaling regulations [6], self-incompatible or pseudo-self-compatibility regulations [19] as well as in biotic stress [20,21,22] and abiotic stress [5, 23, 24].
Drought is one of most threatening factors influencing the yield of agronomic crops in the world. Thus, it is certainly need to comprehend the molecular mechanisms of plant response to drought stress and develop drought resistant crops. The signaling pathways induced by drought stress include signal perception, signal transduction and response, as well as the activation of metabolic and physiological reactions [25, 26]. E3 ubiquitin ligases may play a role by inhibiting the drought stress signaling pathway under favorable growth conditions. They may eliminate negative regulators of the stress signaling pathway in response to stimulation or reduce, and eliminate the signaling pathway in time after stress conditions disappear to maintain plants further growth. It is also possible that E3 ubiquitin ligases may act as a positive feedback factor to enhance stress signaling [27]. In Arabidopsis, PUB11 negatively modulated drought responses by ubiquitin mediated degradation of the receptor like protein kinases LRRR1 (LEUCINE‐RICH REPEAT PROTEIN1) and KIN7 (KINASE7) [28]. A previous study shown that PUB12 and PUB13 affect ABA-mediated drought tolerance through targeting ABI1 (ABA-INSENSITIVE 1) [29].
In generally, the activity of transcription factors were regulated by upstream components. After modifications of sumoylation and ubiquitination, they form a complex regulatory network to effect the expression level of genes involved in stress, and then regulate several metabolic and physiological processes [30, 31]. PUB25 and PUB26, two U-box type E3 ubiquitin ligases, trigger cold signaling negative regulator MYB15 to promote plant freezing tolerance [32]. In a number of previous studies, U-box genes acted as regulators in diverse abiotic stress responses including drought, low temperature and salinity conditions. In Arabidopsis thaliana, AtPUB18 and AtPUB19 are negative regulators of ABA signaling by inducing ABA hypersensitivity, and AtPUB22/AtPUB23 are negative regulators in drought stress responses in an ABA-independent pathway [33, 34]. AtPUB44 ubiquitinates the AAO3 (abscisic aldehyde oxidase 3) via 26 proteasome and affects the ABA biosynthesis [35]. Furthermore, AtPUB46 and AtPUB48 were found to be more sensitive to drought [36]. In rice, OsPUB15 has been implicated in positive regulating plant tolerance to salinity and drought stresses [37]. In apple, MdPUB29 may positively regulate salt tolerance [38].
The plant PUB family has been widely studied for abiotic stresses, mainly in model plants such as Arabidopsis, rice and tomato, and less on woody plants such as pear. Pear belongs to the Pyrus genus in the Rosaceae family, and is one of the most important fruit crops and widely distributed fruits in the world. However, due to the effects of abiotic stresses, yield of pear frequently came down. And these abiotic stresses affect growth and development of pear trees, furthermore limit pear crop productivity [39]. Therefore, it is significant to identify genetic determinants associated with drought, cold and salinity stresses tolerance in pear for agricultural development. Based on the Chinese white pear (Pyrus bretschneideri) genome [40], we conducted systematic characterization of PUB genes, and further verified the function of PbrPUB18 associated with drought stress. These results provided new insights for function verification of PUB gene in future.
Results
Identification of PbrPUB gene family members
In our study, we used a strictly pipeline to identify PUB genes in pear genome (See Methods). As a result, a total of 91 candidate PUB genes were identified in pear genome. SMART tools were performed to verify the accuracy of 91 candidate PUB genes, and we deleted 29 PUB genes lacked of U-box domain. At last, 62 PUB genes with complete U-box domain were obtained for further analysis. The number of PUB genes in pear are similar to the number of PUB genes in apple (69) [18]. Based on the location order of PUB genes, we named 62 PbrPUB genes (Table 1). The molecular weight (MW) for the PbrPUB gene family range from 39.33 kDa to 151.30 kDa (Kilodalton) and the isoelectric points (pI) range from 4.99 to 8.83, with an average of 6.78. Subcellular localization of PbrPUBs were also predicted by Cell-PLoc 2.0, and we found that most PUB proteins were located on cell nucleus, except six located in cytoplasm and three located in cell membrane (Table 1).
Phylogenetic analysis of PbrPUB gene family members
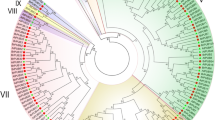
To investigate the evolutionary history of PUB genes in pear, we constructed a phylogenetic tree (NJ, neighbor-joining) using the Mega-X tool based on the PUB proteins from pear (62 members), tomato (62 members) and Arabidopsis (61 members) (Fig. 1a). The protein sequences of PUB genes of tomato and Arabidopsis were obtained from previous study [10, 12]. Based the result of phylogenetic tree, 185 members of PUB genes from these three species were clustered into five subgroups, including Group I, Group II, Group III, Group IV and Group V. The member number of Group III was the largest in five subgroups, and it harboured 64 PUB genes. However, Group IV harboured the least PUB genes, with 10 PUB genes. In generally, the PUB genes of pear and tomato were clustered into one subclade, suggesting that pear and tomato exhibited relatively closer relationship compared to Arabidopsis.
Phylogenetic tree analysis of PUB gene family. a A Neighbor–Joining (NJ) tree of PUB proteins from three species, including pear, tomato and Arabidopsis. The phylogenetic tree was constructed by Mega-X software with 1000 boot strap. The red star, green triangle and blue tick represents the PUB proteins in Arabidopsis, pear and tomato, respectively. All of 185 PUB proteins from three species were clustered into five subgroups, named Group I, II, III, IV and V; b Five pie plots represented the percentage of PUB genes of three species in five groups. The orange part represented the percentage of PUB genes in pear, and the blue part represented the percentage of PUB genes in tomato, and the purple part presented the percentage of PUB genes in Arabidopsis
It is interesting to note that the number of PUB gene family in these three species is similar. This result indicated that the number of PUB genes in these three species is conserved. To explore which group of pears had occurred expansion or lost during evolution process, we measure the number of PUB genes of each species in each group. In pear, Group I, II, III, IV and V contain 11, 21, 21, 3 and 6 PbrPUB gene family members, respectively. In tomato, Group I, II, III, IV and V contain 12, 21, 21, 3, and 5 SIU-box genes, respectively. In Arabidopsis, Group I, II, III, IV and V contain 20, 12, 22, 4 and 3 AtPUB genes, respectively (Fig. 1b). The member number of each group in pear and tomato is almost equal, suggesting that pear had not undergone expansion or lost compared to tomato. However, compared to pear and tomato, the group I of Arabidopsis had undergone rapid expansion, while the group II of Arabidopsis had undergone rapid lost.
Analysis of PbrPUB gene family conserved motifs and gene structures
To further verify the classification results of phylogenetic tree, we investigated the conserved motifs and gene structures of PbrPUB genes in pear. Totally, twenty motifs were estimated using MEME (Multiple Em for Motif Elicitation) software, and we named as motif 1–20 (Fig. 2a, b, Additional file 1: Figure S1). Among them, motif 1, 3 and 5 were found in all groups, indicating that were highly conserved in all PbrPUB proteins. Based on the SMATR website, we determined that the U-box was comprised of Motif 1, Motif 3 and Motif 5 (Additional file 2: Figure S2). This result provided evidence to support the accuracy of PUB genes set identified in our study. Based on the SMATR website, we also found the other conserved domain: ARM and Pkinase domin. The ARM is comprised of motif 2, 4 and 7; the Pkinase is comprised of motif 11, 13 and 20. Generally, most PbrPUB members in the same groups had similar conserved motifs. For example, most of the members in Group II contained motif 6, 10 and 8. This result indicated that these three motifs might be key functional domain of Group II PbrPUB proteins, suggesting that these proteins might have conservative functions.
The conserved motifs and gene structure analysis of PUB gene family in pear. a A Neighbor–Joining (NJ) phylogenetic tree of 62 pear PUB genes. The phylogenetic tree was constructed by Mega-X with 1000 bootstrap. The red branches indicated group I; the blue branches indicated group II; the orange branches indicated group III; the green branches indicated group IV; the purple branches indicated group V; b The conserved motifs analysis of PbrPUB genes in pear. A total of 20 motifs were predicated by MEME tool, named Motif 1–20. The scale bar indicates 200 aa; c The gene structure analysis of PUB genes in pear, including UTR, intron and exon. The green rectangles represented UTR; the yellow rectangles represented Exon; the grey lines presented Intron. The scale bar indicates 2 kb
To explore the gene structures of PbrPUB genes in pear, we extracted the exon–intron information of 62 PbrPUB genes from pear database using in-house scripts. Based on the information, TBtools software was preformed to show the gene structures of PbrPUB (Fig. 2c). The number of exon in PbrPUB genes was greatly divergent, ranging from 1 to 20. Among 62 PUB genes in pear, PbrPUB24 contained the greatest number of exons (20), while 16 PUB genes (25.81%) only contained one exon. Furthermore, the lengths of the exon and intron were differential. There are 30 PUB genes have been found to contain untranslated regions (UTR). Similarly, to the result of motif analysis, the PUB genes with similar gene structures were cluster into same subclade. For example, most members of Group II only housed one exon. This result indicated that the members of same groups exhibited similar gene structures and conserved motifs. These results from conserved motifs and gene structure analysis provided strong evidence to support the accuracy of the classification result of phylogenetic tree.
Chromosomal localization and homologous gene analysis of PbrPUB genes
The chromosome distribution pattern of PbrPUBs on genome was predicted by TBtools (Fig. 3a). The location information of PUB genes in pear were extracted by our in-house scripts. As a result, a total of 50 PbrPUB genes (82.26%) were unevenly mapped on the 17 pear chromosomes, and no member of PbrPUB gene family was mapped on chromosome 8. Therefore, we didn’t show chromosome 8 in our Fig. 3. In addition, 12 genes were located on scaffold contigs, and we also didn’t show them in our Fig. 3. Chromosome 15 had the most PbrPUB genes, with eight PbrPUB genes, followed by chromosome 5 with 6 genes. Chromosome 1, 2, 12 each contained 4 PbrPUB genes. Two or three PbrPUB genes were mapped on chromosomes 3, 6, 7, 9, 10, 11, 13, 14, and 16. Chromosome 4 and 17 contained only one gene. We also identified the homologous genes of PUB gene family using MCScanX software. As result indicate that 16 homologous gene pairs were identified in pear PUB gene family, which contained 26 homologous genes. Three homologous gene pairs were detected between chromosomes 5 and chromosomes 10 (Fig. 3b).
The location distribution and synteny analysis of PUB genes in pear genome. a The distribution pattern of PbrPUBs in 17 pear chromosomes. Due to no PUB genes were mapped on Chromosomes 8, we didn’t show it in the Fig. 3; b The distribution pattern synteny analysis of PUB genes family. The red lines indicated the synteny gene pairs of PUB gene family
Cis-acting elements predication of PbrPUB genes
Cis-acting elements were important clues for the prediction of gene functions. Transcription factors cloud effect the expression level of target genes by binding to the cis-acting element of terget genes in specific biological processes [12]. To further investigate the function of PbrPUB genes, we predicted the cis-acting element of the putative promoter region of PbrPUB genes using PlantCARE databse. As a result, a total of 62 cis-acting elements were identified (Additional file 5: Table S2.), and we selected 15 interesting cis-acting elements for further analysis. These 15 cis-acting were associated with stress, hormone, plant growth and development. As shown in Fig. 4a, some diverse distribution patterns of cis-acting elements were observed in the promoter region of PbrPUB genes, indicating that the PUB gene family of pear particular in various different biology process. Meanwhile, we found that all PbrPUB genes contained the cis-acting related to hormone regulation, such as salicylic acid, gibberellin (GA), auxin and methyl jasmonate (MeJA) responsiveness elements. Previous study had reported that DSG1, which encodes a U-box domain, could regulate cell division and elongation by responding to multiple hormones, such as auxin, salicylic acid and ethylene [41]. ABA responsive element, named as ABRE, is one of most important cis-acting element in the promoter sequence of ABA-inducible genes response to ABA treatment [42]. In our study, 55 PbrPUB genes were identified as the responsiveness elements of ABA, suggesting that PUB gene family might particular in resistance under ABA treatment (Fig. 4b). And in Arabidopsis, AtPUB9, AtPUB18, AtPUB19, and AtPUB44 were identified to involve in ABA response [12]. It is notable that the element related to MYB binding site involved in drought was predicted in 44 PbrPUB genes, suggesting that these 44 PbrPUB genes might mediated by MYB genes response to drought stress. Moreover, there were 30 PbrPUB genes have cis-acting elements related to cold, suggesting that these 30 PbrPUB genes might particular in resistance under low temperature treatment. As we all know, flavonoid biosynthesis is one of the most important phenomenon during the process of response to stress in plant. In this study, we found PbrPUB10, PbrPUB24 and PbrPUB5 contained MYB binding site involved in flavonoid biosynthetic.
The cis-acting elements analysis of putative promoter of 62 PbrPUB genes. a The distribution pattern of 15 cis-acting elements of putative promoter of PUB gene family in pear. The phylogenetic tree is same with the phylogenetic tree in Fig. 2a. b The number of 15 cis-acting elements of putative promoter of PbrPUB genes. The color scale at the top right indicated the number of cis-acting elements. Green color indicated the number of cis-acting elements on PUB member. 15 cis-acting elements including: (I) Abscisic acid responsiveness; (II) MeJA-responsiveness; (III) Gibberellin-responsive element; (IV) Light responsive element; (V) MYB binding site involved in drought-inducibility; (VI) Salicylic acid responsiveness; (VII) Anaerobic induction; (VIII) Auxin-responsive element; (IX) Zein metabolism regulation; (X) Defense and stress responsive element; (XI) Low-temperature responsiveness; (XII) MYB binding site involved in light responsiveness; (XIII) Endosperm expression; (XIV) Wound-responsive element; (XV) MYB binding site involved in flavonoid biosynthetic genes regulation
Tissues-specific expression analysis of PbrPUB genes
To further explore the tissues-specific expressions of PbrPUB genes, we collected RNA-seq data of seven tissues of ‘Dangshansuli’ pear from previous study [43]. We used RPKM (reads per kilobase per million) values to estimate the expression level of PbrPUB genes. Then, we investigated the expression level of 62 PbrPUB genes. Pheatmap, an R package, was used to show the expression patterns of 62 PbrPUB genes (Fig. 5a). Based on the expression patterns of 62 PUB genes, they were clustered into four main classes. Genes in Class IV exhibited highly expression level in all of seven examined tissues, while genes in Class II exhibited almost no expression in all of seven tissues. Class I was specifically expressed in pear leaf, and a diversity of expression pattern were detected in Class III (Fig. 5a). Among the 62 PbrPUB genes, 52 genes (83.87%) were at least expressed in one tissue, even though the transcript abundance of several genes was relatively lower for certain tissues. Approximately 10 non-expressed PUB genes (RPKM value less than 1) were identified in all of seven tissues, and they may lost the function during the evolution process of PUB gene family in pear. 29 PbrPUB genes were expressed in all seven different tissues, indicating that they have various roles in the development of different tissues. Interesting, we found 28 PUB genes exhibited highest expression in leaf, suggesting that these 28 genes might involve the development of leaf. Due to leaf is an important plant organ involved resistance, we referred that these 28 PUB genes might particular in resistance in the process of pear growth and development.
The expression pattern analysis of PbrPUB gene family in seven different tissues. a The heatmap of expression level of PbrPUBs in seven different tissues, including stem, ovary, petal, sepal, bud, fruit and leaf. Pheatmap, an R package, were used to generate the heatmap. The color scale represented the RPKM values normalized by log2(RPKM + 1). Red color represented high expression, while blue represented low expression; b The expression levels of 15 randomly selected PbrPUBs in seven different tissues. Seven tissues are comprised of bud, stem, ovary, petal, sepal, fruit and leaf. The x-axes represented seven different tissues; the y-axes represented the relatively expression of PUB genes
To verify the transcriptome sequences analysis was reliable, 15 PbrPUB genes were randomly selected to conduct a quantitative real-time PCR (qRT-PCR) experiment to investigate the expression levels in seven different tissues of the ‘Dangshansuli’ pear (Fig. 5b). We found that all of 15 PbrPUB genes exhibited a diversity of expression patterns in the seven different tissues, suggesting that PbrPUB gene family may function in different tissues and participate in diverse metabolic processes. Seven genes (PbrPUB1, PbrPUB3, PbrPUB7, PbrPUB9, PbrPUB18, PbrPUB36 and PbrPUB38) exhibited a similar expression pattern with a high expression level in leaf tissues, suggesting that PbrPUB genes play critical functions during leaf development. And all of these seven genes exhibited highly expression level in leaf in transcriptome data. These results provided further evidence to support our transcriptome sequences analysis was reliable. Interestingly, most of 15 PUB genes were highly expressed in reproductive organs, suggesting that PbrPUB genes might associate with the development of reproductive organs.
The expression pattern of PbrPUB genes under abiotic stresses
Previous study had extensively reported PbrPUB gene family involved in various abiotic stresses [44]. To explore the functions of PUB gene family in pear, we detected the expression level of PbrPUB in seedling samples (Pyrus betulaefolia) subjected to four different stress treatments, including dehydration, low temperature, ABA and salt. 11 PbrPUB genes were randomly selected to conduct qRT-PCR experiment. 11 genes are comprised of 2 from group I (PbrPUB1 and PbrPUB14), 4 from group II (PbrPUB12, PbrPUB18, PbrPUB36 and PbrPUB38), 2 from group III (PbrPUB3 and PbrPUB25), 2 from group IV (PbrPUB7 and PbrPUB48) and 1 from group V (PbrPUB34).
Among the eleven PUB genes, 9 PUB genes were up-regulated expressed and one PUB gene (PbrPUB7) was down-regulated expressed under dehydration stress (Fig. 6a). However, PbrPUB14 was not significantly differential expressed under dehydration stress. Among the 9 up-regulated genes, PbrPUB18 exhibited highly increased expression level during the process of dehydration treatment, while PbrPUB12, PbrPUB3 and PbrPUB36 were up-regulated expressed during 12 h dehydration treatment and recovered to normal levels at 24 h. PbrPUB1, PbrPUB38 and PbrPUB25, exhibited highest expression level at 1 h, where PbrPUB12, PbrPUB14, PbrPUB3 and PbrPUB36 exhibited highest expression level at 12 h under dehydration treatment. These results suggested that PbrPUB1, PbrPUB38 and PbrPUB25 respond to dehydration treatment faster than that of PbrPUB12, PbrPUB14, PbrPUB3 and PbrPUB36. Therefore, PUB gene family in pear play vital role in the process of dehydration stress response.
The expression level of 11 randomly selected PbrPUB genes in four abiotic stresses. a For dehydration treatment, the shoots were placed on dry filter papers for 0, 1, 6, 9, 12 and 24 h; b For cold stress, the seedlings were placed in the chamber set at 4 °C for 0, 1, 6, 9, 12, 24, 48 and 96 h; c For salt stress, the seedlings were placed in solution containing 200 mM NaCl solution for 0, 2, 4, 6, 8, 12 and 36 h; d For ABA stress, The seedlings were dipped in solution containing 100 μM ABA for 0, 1, 3, 6, 9, 12 and 36 h. The x-axes represented time after treatment; the y-axes represented the relatively expression of PbrPUB genes
In low temperature treatment (Fig. 6b), we found that 4 genes (PbrPUB12, PbrPUB3, PbrPUB36 and PbrPUB48) were up-regulated expressed under cold stress, suggesting that those PbrPUB genes might respond to low temperature. PbrPUB12, PbrPUB48 and PbrPUB36 were highly increased during the 48 h low temperature exposure. The expression level of PbrPUB3 was reached to double peak at 1 h and 48 h.
In the salt treatment (Fig. 6c), we found that all of the selected 11 PbrPUB genes were significantly up-regulated expressed under the 200 mM salt stress treatment. The expression level of PbrPUB14, PbrPUB25, PbrPUB3, PbrPUB48 and PbrPUB7 were highly increased during the 12 h salt exposure. Moreover, PbrPUB1, PbrPUB12, PbrPUB18, PbrPUB34, PbrPUB36 and PbrPUB38 were highest expressed at 4 h under salt stress, suggesting that these 6 PbrPUB genes respond to salt treatment actively. We focus on the expression level of PbrPUB18. During the 4–8 h, the expression level of PbrPUB18 was significantly increased, and then it was down-regulated at 12 h, finally recovered normal level at 36 h.
Previous study had reported that PUB gene involved in ABA-mediated drought stress responses. In ABA treatment (Fig. 6d), all of 11 PUB genes were respond to the ABA stress, and these genes were unregulated expressed at first, then were down-regulated at 36 h after ABA treatment. These results indicated that PUB genes play important roles in ABA-regulated pathway. The expression levels of three genes (PbrPUB1, PbrPUB25, and PbrPUB36) were reached to peak at 1 h, suggesting that these three genes were actively responded to ABA stress. Interestingly, we found that PbrPUB18 was expressed in 6 h and 12 h after ABA treatment.
Subcellular localization of PbrPUB18 protein
To further verify the biologic function of PbrPUB genes in pear under drought stress, PbrPUB18 was selected to perform subcellular localization experiment. The green fluorescence of GFP control was found in the membrane and the nucleus (Fig. 7a). In contrast, 35S: PbrPUB18-GFP protein was only existed in the nucleus and integrated perfectly with DAPI (4′, 6-diamidino-2-phenylindole) regime (Fig. 7b), suggesting that PbrPUB18 protein was located in the nucleus, which was consistent with our prediction in Table 1.
The subcellular localization of PbrPUB18. a Tobacco leaf epidermal cells were transiently transformed with constructs containing 35S:GFP vector alone as control; b Transient expression of fusion plasmid (35S: PbrPUB18-GFP) in tobacco leaf epidermal cells. The nucleus was identified by DAPI staining. Green fluorescence images, DAPI staining mages, blight field images and the merged images are shown from left to right. Scale bars = 20 μm
Assessment of drought tolerance in transgenic lines of PbrPUB18
To further confirm the biologic function of PbrPUB18 gene under drought stress, Arabidopsis Col-0 plants (WT) were transformed by the floral dip method [45]. Two overexpression lines OE-4 and OE-5 of PbrPUB18 were screened out by PCR identification and semi-quantitative PCR at mRNA level. QRT-PCR also verified the expression of PbrPUB18 in OE-4 and OE-5 far above in WT (Additional file 3: Figure S3). To assess the function of overexpression PbrPUB18 in Arabidopsis on drought tolerance, 20-day-old WT and transgenic lines were conducted to same drought environment (12 days without water). There was no morphological difference between WT and the transgenic lines in the normal condition. After 12 days without water, the two transgenic lines showed more tolerance to the drought stress, as manifested by lesser leaf-wilting symptoms compared with the WT plants (Fig. 8a). In addition, chlorophyll fluorescence measurements were recorded to further verify drought tolerance of WT and the transgenic lines (Fig. 8b). The maximum quantum efficiency of the photochemistry (Fv/Fm) values was not affected by species and growth conditions, but under stress conditions, this parameter decreased significantly. After 12 days drought treatment, the Fv/Fm values of WT was significantly lower than of the two transgenic lines, suggesting WT showed more sensitivity to the drought stress (Fig. 8e). Electrolyte leakage (EL) is extensively used to estimate the cell injury level of plant after drought stress. The EL of two transgenic lines were only approximate 15%–20% compared to WT (37.3%), suggesting that WT suffered more severe membrane damage than transgenic lines of Arabidopsis by overexpressing PbrPUB18 (Fig. 8c). The transgenic plants displayed significantly lower malondialdehyde (MDA) contents than WT exposure to drought condition (Fig. 8d). These results indicated that two transgenic lines of PbrPUB18 suffered to relatively lighter extent oxidative stress.
Drought tolerance assay of transgenic Arabidopsis plants overexpressing PbrPUB18. a Phenotypes of 20-day-old transgenic plants and WT before and after 12 days drought stress; b Images of (Fv/Fm). The false color code depicted on the right of the image ranges from 0 (black) to 1.0 (purple); Electrolyte leakage (c), MDA contents (d) in the WT, OE-4 and OE-5 after drought treatment; e Fv/Fm of WT, OE-4 and OE-5 before and after drought stress; f Histochemical staining with DAB and NBT for detection of H2O2 and O2−, respectively, in WT, OE-4 and OE-5 after drought stress for 12 days; Levels of H2O2 (g) and anti-O2− (h) in Arabidopsis WT, OE-4 and OE-5 after drought treatment. Asterisks indicate that the value is significantly different from that of the WT at the same time point (**P < 0.01; ***P < 0.001)
Histochemical staining shown that the leaves of WT exhibited more deeper staining compared with that of OE-4 and OE-5 after drought stress (Fig. 8f), suggesting that WT type accumulated more ROS (H2O2 and O2−). Similar to staining results, quantitative measurements further demonstrated that two transgenic lines exhibited significant lower H2O2 contents than that of WT type (Fig. 8g). Moreover, anti O2− contents in the two transgenic lines were significantly higher than those of WT (Fig. 8h). These results indicated that PbrPUB18 could enhance drought resistance.
Discussion
Genome-wide and phylogenetic analysis of PbrPUB genes in pear
As a family of ubiquitin ligases, U-box genes encode a conserved U-box motif of about 70 amino acids and regulated the ubiquitination of the substrates [23]. U-box genes were widely distributed in the plants and reported to participate in many biological processes including plant hormone signaling regulations [6], self-incompatible or pseudo-self-compatibility regulations [18] as well as in biotic stress[19,20,21] and abiotic stress [5, 22, 23]. Due to PUB gene play an important role during plant development, PUB genes have been identified in different plant species, such as Arabidopsis thaliana (61) [9, 10], rice (77) [11], tomato (62) [12], cotton (93) [13], and banana (91) [14]. Pear, one of Rosaceae fruit trees, is widely cultivated all over the world. However, the analysis related to PUB genes in pear was poor until now. In the present study, 62 genes were identified as PUB gene family in pear using bioinformatics analysis, and the number of PUB gene in pear is similar to that of Arabidopsis thaliana (61), tomato (62) and apple (69).
Phylogenetic tree analysis indicated that a total of 185 PUB protein members in these three species (containing 62 pear, 62 tomato, and 61 Arabidopsis) were categorized into five groups (I-V). The results of phylogenetic tree were similar with other species [12, 14]. For example, 125 GmPUB genes in soybean proteins were classified into six groups using phylogenetic tree analysis [17]. In apple, 69 U-box genes were clustered into seven groups [18]. Through the phylogenetic relationship analysis, it showed that PbrPUBs exhibited closer relations with SIU-boxs compared with AtPUBs. This result was consistent with the fact that pear and tomato exhibited closely relationship than Arabidopsis. Although the number of PUB genes was similar in three species, we found that the Arabidopsis genes of Group I of had undergone rapid expansion and of Group II had undergone rapid lost. In addition to the U-box domain, 62 PbrPUB proteins are found to bind to different domains including armadillo (ARM) repeats, the tetratricopeptide (TPR) domain and WD40 repeats. The majority of PUB proteins that have been elucidated for biological functions are from the U-box proteins with ARM repeats [18]. The ARM repeats have been shown primarily mediating the interaction with substrates, suggesting that interaction make the substrates available for ubiquitination [23]. 25 member of PUB genes in pear only housed U-box domain, and 25 members housed both U-box and ARM domain. Moreover, TPR domain was found in PbrPUB14 gene and WD40 repeats was found in PbrPUB40 gene.
The function predication of PUB gene family in pear based on cis-acting and specific-tissues expression analysis
The cis-acting analysis of putative promoter indicated that the PUB gene family was involved in stress-related mechanisms, hormonal regulation, growth and development. Previous studies had reported that PUB were responded with ABA. For instance, AtPUB44 could regulate the biosynthesis of ABA through ubiquitinating the AAO3 (abscisic aldehyde oxidase 3) via 26 proteasomes [35]. In additional, one transcription factor of ABI3 was regulated by AtPUB9 and increased the ABA sensitivity of Arabidopsis during seedling germination [46]. AtPUB18, AtPUB19 and AtPUB44 were found to directly interrupt the biosynthesis of ABA. In our study, 55 genes contained the ABA responsive element on the putative promoter region. Especially, we found that eight ABA responsive elements were identified in the promoter region of PbrPUB43. This result indicated that PUB gene might play significant role during ABA signal transduction in pear. In Arabidopsis and Nicotiana, the expression of PUB genes was regulated by abiotic and biotic stress [18]. Previous study had reported that MYB transcription factor could regulated the expression level of resistance genes. For example, PbrMYB21 can specifically bind to the MYB recognition sites in promoters of PbrADC and played a positive role in drought tolerance [47]. In here, we also found MYB binding site involved in drought induction responsive element was identified in the promoter region of PUB genes in pear, suggesting that PUB genes might be regulated by related transcription factors mediating the drought stress signaling. We also found abscisic acid responsive element, defense and stress responsive element, low temperature responsive element, wound responsive element in the promoter region of PUB genes in pear. These results indicated that PUB genes might involve in a diverse of biology process during pear growth and development.
Based on RNA-Seq data and qRT-PCR experiment, we investigate the expression level of PbrPUBs in seven tissues. Among the 62 members of PUB gene family in pear, 29 PbrPUB genes were expressed in all seven different tissues. Additionally, 72.58% of PbrPUBs were expressed in pear sepal. Whereas of PbrPUBs expressed in all tissues, 45.16% were highest in leaves, suggesting these genes may function in the development of pear leaves. qRT-PCR analysis shown that 15 PbrPUBs have highly expression level in ovary, leaf, sepal and petal, suggesting that PbrPUB genes may function in the development of these four tissues.
Roles of PbrPUB genes in response to different abiotic stresses
Previous studies have reported that PUB genes involved in the process of stress responses [32, 33, 48, 49]. A large number of PUB genes were induced expressed during abiotic stress conditions [19]. In this study, the differential expression levels of 11 PbrPUB genes under various abiotic stresses were investigated by using qRT-PCR, including dehydration, low temperature salt and ABA stress. From the result, PbrPUB12, PbrPUB3, PbrPUB36 and PbrPUB48 were significantly up-regulated expressed under four treatment, suggesting these three genes could response to dehydration, ABA, cold and salt stress. PbrPUB7 was down-regulated expressed under dehydration stress, suggesting that PbrPUB7 might negatively regulate the response process of dehydration.
In Arabidopsis, the function of most PUB members from Group II were widely investigated in abiotic stresses process of plant. AtPUB22/AtPUB23 are negative regulators mediating drought responses in the ABA-independent pathway [34]. AtPUB25 and AtPUB26 participated in plant response to low temperature signal by regulating the protein stability of MYB15, a negative transcription factor in CBF signaling pathway [32]. AtPUB30 negatively regulates salt tolerance by facilitating BRI1 KINASE INHIBITOR 1 (BKI1) degradation [50]. In addition, MdPUB29, highly homologous with AtPUB29, may positively regulate salt tolerance [38]. We inferred PbrPUBs in Group II may also related to abiotic stress. Drought is one of most critical stresses and could significantly affect the growth of plant. And in our study, we found the expression level of PbrPUB genes from Group II (PbrPUB12, PbrPUB18, PbrPUB36 and PbrPUB38) were significant up-regulated after dehydration treatment. In order to verify the role of PbrPUBs in drought stress, PbrPUB18 was selected for further functional identification.
Subcellular localization experiment suggested that PbrPUB18 protein was located on the cell nucleus. This result indicated that PbrPUB18 might act biology function at the cell nucleus. Previous study indicated that MYB15 act as a negative regulator factor during freezing stress, and PUB25 and PUB26 can improve the resistance in cold stress by accelerate the degradation of MYB15 [32]. These results suggesting that PbrPUB18 might degradate transcription factors to positively regulate the plant resistance, for example, MYB transcription factors. Heterologous over-expression of PbrPUB18 in Arabidopsis shown better physiological traits, such as lower MDA content, lower EL and higher Fv/Fm than WT in 12 days drought treatment suggesting that overexpression of PbrPUB18 could enhance drought resistance. ROS content analysis indicated that lower levels of H2O2 and higher levels of anti O2− in the transgenic lines, suggesting that the tolerance may be ascribed to more robust activation of ROS scavenging system. AtPUB19 and AtPUB18 act negative roles on ABA signaling pathway downstream of H2O2 [34]. But the cellular mechanism by what PbrPUB18 regulating drought responses remained unclear, and needed to be explored in the future study. Summary, we systematically identified the PUB gene family in pear, and further function identification laid a foundation for the functional study of PUB genes of pear in future.
Conclusions
In our study, a total of 62 PbrPUB members were identified in Chinese white pear (Pyrus bretschneideri) genome, and were unevenly distributed on 17 pear chromosomes. According to phylogenetic tree analysis, PbrPUBs were divided into five groups. The conserved motifs and gene structures analysis provided strong evidence to support the result of classification. Cis-acting element analysis indicated that PUB genes might participate in diverse biological processes, especially in response to abiotic stresses and phytohormones. Transcription sequencing data from different seven tissues exhibited diverse of expression level of PbrPUB genes. Further qRT-PCR was used to identify candidate genes associated with abiotic stresses. In addition, PbrPUB18 was cloned and functionally identified. Subcellular localization revealed PbrPUB18 protein was located on cell nucleus. Heterologous over-expression of PbrPUB18 in Arabidopsis indicated that the over-expression of PbrPUB18 could enhance resistance in drought treatment. But the cellular mechanism of PbrPUB18 regulating drought responses was needed to be explored in the future study.
Materials and methods
Genome identification of PUB gene family members in Chinese white pear
To identify the potential members of the PUB gene family, we firstly downloaded the pear genome (Pyrus bretschneideri) from NCBI database [40]. Then, the seed file of U-box domain (PF04564) was used to search the candidate PUB genes in pear protein database using HMMsearch software. All candidate PbrPUB proteins obtained from the result of HMMsearch were further submitted to SMART website (http://smart.embl-heidelberg.de/) to determine completeness of U-box conserved domain. In addition, the pI and MW of PbrPUBs protein were calculated by ExPASy. Then, we also investigated subcellular localization of PbrPUBs using Cell-PLoc 2.0 [51].
Phylogenetic analysis of PbrPUB proteins
Firstly, we collected PUB protein sequences of Arabidopsis, tomato and pear [10, 12]. A total of 185 PUB protein sequences were download. Second, ClustalW function of MEGA-X software was used to perform sequence alignment using these 185 PUB protein sequences. Third, the phylogenetic tree was constructed by MEGA_X (Method, NJ; Bootstrap, 1000) [52]. Finally, we used Evolview tool (https://evolgenius.info//evolview-v2/#login) to edit the phylogenetic tree of PUB proteins [53].
Gene structures, motif analysis and cis-acting elements analysis
To identify and visualize the structural organization (introns, exons and UTR) of the pear PUB gene family, the information of gene structure was extracted from whole genome database of pear using in-house scripts. The novel conserved motifs of PbrPUB genes were identified by MEME suite (http://meme-suite.org/tools/meme). A total of 20 motifs and a width limit of 200 amino acids were used for the analysis with other default parameters. TBtools were used to visualize the results of gene structure and conserved motif analysis [54]. The 2000-bp region of upstream of PbrPUBs (same strand) were defined as putative promoter sequence. We obtained the promoter sequence of PbrPUBs using getfasta function in Bedtools [55]. Cis-acting elements of PbrPUBs were predicted by PlantCare tools [56]. According to function annotation of cis-acting element (Additional file 5: Table S2), the interesting elements were obtained for further study, and the cis-acting element with same function annotation were integrated to same group.
Synteny analysis and chromosomal localization
The homologous gene pairs of PbrPUBs were identified by blast software with all-vs-all blast strategy. Then, the synteny regions were identified by MCScanX using the result of all-vs-all blast [57]. We plotted circos picture to show the distribution of synteny gens pairs [58]. The chromosome location analysis was conducted by TBtools [54].
Gene expression analysis of PbrPUB on the RNA-Seq Data
The RNA-seq data of the ‘Dangshansuli’ in seven different tissues were download from NCBI [43]. Fastp software was used to perform quality control and filter. Bowtie2 and Tophat2 software were used to perform reads mapping. The RPKM values were measured by featureCount software and in-house python scripts. Then, we used Heatmap.2 package to show the expression pattern of PbrPUBs (log2 (RPKM + 1)) [59].
Plant materials and stress treatments
The seeds of Pyrus betulaefolia were collected from our experimental field (the pear germplasm orchard of the Center of Pear Engineering Technology Research situated at Hushu in Nanjing). Then, the seeds of Pyrus betulaefolia were cultivated in the National Center of Pear Engineering Technology Research (Nanjing Agricultural University, Nanjing). To further explore the function of PbrPUBs during abiotic stresses, the seedlings were subjected to four different abiotic stresses, including dehydration, low temperature, salt and ABA treatment. The method of abiotic treatment was development from our previous method with minor revision [60, 61]. In dehydration treatment, six time points (0, 1, 6, 9, 12 and 24 h) were selected to collected leaves of pear seeding under stress. In cold treatment, eight time points were selected (0, 1, 6, 9, 12, 24, 48 and 96 h). In salt treatment, seven time points were selected (0, 2, 4, 6, 8, 12 and 36 h). In ABA treatment, seven time points were selected (0, 1, 3, 6, 9, 12 and 36 h).
QRT-PCR analysis
Total RNA of leaves materials under stress was extracted using Plant Total RNA Isolation Kit Plus (FOREGENE, China). Then, PrimeScript™ RT reagent kit (Takara Bio, China) was used to reverse transcribe RNA to cDNA. QRT-PCR analysis was conducted on Roche LightCycler® 480 II (Roche, Mannheim, Germany) using LightCycler® SYBR GREEN I Master Mix kit (Roche, China). We designed fifteen pairs specific primers (Additional file 4: Table S1) using Primer5.0 software and checked by using NCBI online software (https://www.ncbi.nlm.nih.gov/). The reaction system and protocol of qRT-PCR were consistent with our previously study [59, 62]. The relatively expression level of PbrPUBs were estimated using 2−∆∆CT method [63]. The pear Tubulin gene (No. AB239681) was selected as an internal reference for Pyrus betulaefolia, and the Actin gene (No. AY063980) was selected as an internal reference for Arabidopsis.
Subcellular localization
The open read frame (ORF) of PbrPUB18 lacked of stop codon were cloned from the cDNA of Pyrus betulaefolia using primer pairs (GSP16, Additional file 4: Table S1). We conducted a 35S: PbrPUB18-GFP fusion vector based on previous study [64]. We transformed 35S: PbrPUB18-GFP fusion vector into Agrobacterium tumefaciens strain GV3101, and we also transformed 35S: GFP as control group[64]. The fluorescence signal was observed with a confocal laser scanning microscope (LSM800, Germany) after 72 h post infiltration and the position of nucleus was revealed by staining with DAPI.
Arabidopsis transformation and characterization of transgenic plants
Arabidopsis thaliana ecotype Columbia Col–0 plants were transformed for heterologous over-expression PbrPUB18 by using the floral dip method [45]. And Agrobacterium tumefaciens suspension containing the vector 35S: PbrPUB18-GFP (OD600 = 0.80) was used for transformation. T0 seeds were identified by Murashige and Skoog (MS) solid mediumwith 20 mg·L−1 hygromycin and then verified by PCR analysis using specific primers pair (GSP17, Additional file 4: Table S1). According to previous research [60, 61], semi-quantitative RT-PCR and qRT-PCR was used to further analyze the transcript levels of PbrPUB18 in T1 plants with primers pair (GSP18 and GSP5, Additional file 4: Table S1). Two overexpressing lines (OE-4 and OE-5) of PbrPUB18 were choosed to cultivate T3 homozygous plants for the further drought tolerance assay.
Assessment of drought tolerance in transgenic lines
To verifiy the drought tolerance of transgenic lines of PbrPUB18, the seedlings (20 days) of transgenic lines of PbrPUB18 and WT (control) were exposed to drought treatment (withholding water) for 12 days. Then, we collected the leaves samples from WT and transgenic lines for estimating phonotype data, including EL, MDA, ROS content. Electrolyte leakage was measured by conductivity monitor (TOADKK, Japan) [65]. Following the instructions of manufacturer, we measured the MDA and ROS (H2O2 and O2−) content by specific analytical kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China), repectively. To further observed ROS (H2O2 and anti-O2−) level, we also used DAB and NBT to perform histochemical staining [66]. The IMAGING-PAM chlorophyll fluorometer was concucted to monitor the level of the chlorophyll fluorescence using ImagingWin software (Walz; Effeltrich, Germany). The detail parameters and the estimate method of Fv/Fm values were described by Woo et al. [67].
Statistical analysis
In our study, each phonotype data of abiotic stress and expression profile of qRT-PCR were repeated at least three times. The data were shown in figures as mean ± standard error (SE). All statistical analyses were performed in R language. T-test function in R were used to test the significance levels of phonotype data between treatment and control groups (*P < 0.05, **P < 0.01 and ***P < 0.001).
Availability of data and materials
The transcriptome sequencing raw data from seven different pear tissues have been deposited at NCBI (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA498777).
Abbreviations
- PUB:
-
Plant U-box gene
- UPS:
-
Ubiquitin/26S proteasome system
- Ub:
-
Ubiquitin
- E1:
-
Ubiquitin-activating enzyme
- E2:
-
Ubiquitin-conjugating enzyme
- E3:
-
Ubiquitin ligase
- HECT:
-
Homology to E6-Associated Carboxy-Terminus
- RING:
-
Really Interesting New Gene
- CRLs:
-
Cullin–RING ligases
- UFD2:
-
Ubiquitin fusion degradation protein-2
- LRRR1:
-
LEUCINE‐RICH REPEAT PROTEIN1
- KIN7:
-
KINASE7
- ABI1:
-
ABA-INSENSITIVE 1
- AAO3:
-
Abscisic aldehyde oxidase 3
- UTR:
-
Untranslated regions
- ABA:
-
Abscisic acid
- MeJA:
-
Methyl jasmonate
- GAs:
-
Gibberellin
- ABRE:
-
ABA responsive element
- RPKM:
-
Reads per kilobase per million
- qRT-PCR:
-
Quantitative real-time PCR
- GFP:
-
Green fluorescent protein
- DAPI:
-
4′, 6-Diamidino-2-phenylindole
- Fv/Fm:
-
The maximum quantum efficiency of the photochemistry
- WT:
-
Wide type
- MDA:
-
Malondialdehyde
- EL:
-
Electrolyte leakage
- DAB:
-
3, 3′-Diaminobenzidine
- NBT:
-
Nitro-blue tetrazolium chloride
- ROS:
-
Reactive oxygen species
- TPR:
-
Tetratricopeptide
- ARM:
-
Armadillo
References
Gong Z, Xiong L, Shi H, Yang S, Herrera-Estrella LR, Xu G, et al. Plant abiotic stress response and nutrient use efficiency. Sci China Life Sci. 2020;63(5):635–74. https://doi.org/10.1007/s11427-020-1683-x.
Hirayama T, Shinozaki K. Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J. 2010;61(6):1041–52. https://doi.org/10.1111/j.1365-313X.2010.04124.x.
McClellan AJ, Tam S, Kaganovich D, Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol. 2005;7(8):736–41. https://doi.org/10.1038/ncb0805-736.
Smalle J, Vierstra RD. The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol. 2004;55(1):555–90. https://doi.org/10.1146/annurev.arplant.55.031903.141801.
Zhang ZY, Li JH, Liu HH, Chong K, Xu YY. Roles of ubiquitination- mediated protein degradation in plant responses to abiotic stresses. Environ Exp Bot. 2015;114:92–103. https://doi.org/10.1016/j.envexpbot.2014.07.005.
Santner A, Estelle M. The ubiquitin-proteasome system regulates plant horm-one signaling. Plant J. 2010;61(6):1029–40. https://doi.org/10.1111/j.1365-313X.2010.04112.x.
Jansen AH, Reits EA, Hol EM. The ubiquitin proteasome system in glia and its role in neurodegenerative diseases. Front Mol Neurosci. 2014;7:73. https://doi.org/10.3389/fnmol.2014.00073.
Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10(5):319–31. https://doi.org/10.1038/nrm2673.
Azevedo C, Santos-Rosa MJ, Shirasu K. The U-box protein family in plants. Trends Plant Sci. 2001;6(8):354–8. https://doi.org/10.1016/s1360-1385(01)01960-4.
Wiborg J, O’Shea C, Skriver K. Biochemical function of typical and variant Arabidopsis thaliana U-box E3 ubiquitin-protein ligases. Biochem J. 2008;413(3):447–57. https://doi.org/10.1042/BJ20071568.
Zeng LR, Park CH, Venu RC, Gough J, Wang GL. Classification, expressi-on pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol Plant. 2008;1(5):800–15. https://doi.org/10.1093/mp/ssn044.
Sharma B, Taganna J. Genome-wide analysis of the U-box E3 ubiquitin ligase enzyme gene family in tomato. Sci Rep. 2020;10(1):9581. https://doi.org/10.1038/s41598-020-66553-1.
Lu X, Shu N, Wang D, Wang J, Chen X, Zhang B, et al. Genome-wide identification and expression analysis of PUB genes in cotton. BMC Genomics. 2020;21(1):213. https://doi.org/10.1186/s12864-020-6638-5.
Hu H, Dong C, Sun D, Hu Y, Xie J. Genome-wide identification and analysis of U-box E3 ubiquitin-protein ligase gene family in banana. Int J Mol Sci. 2018;19(12):3874. https://doi.org/10.3390/ijms19123874.
Song J, Mo X, Yang H, Yue L, Song J, Mo B. The U-box family genes in Medicagotruncatula: Key elements in response to salt, cold, and drought stresses. PLoS ONE. 2017;12(8):e0182402. https://doi.org/10.1371/journal.pone.0182402.
Wang C, Duan W, Riquicho AR, Jing Z, Liu T, Hou X, et al. Genome-wide survey and expression analysis of the PUB family in Chinese cabbage (Brassica rapa ssp. pekinesis). Mol Genet Genom. 2015;290(6):2241–60. https://doi.org/10.1007/s00438-015-1075-x.
Wang N, Liu Y, Cong Y, Wang T, Zhong X, Yang S, et al. Genome-wide identification of soybean U-box E3 ubiquitin ligases and roles of GmPUB8 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 2016;57(6):1189–209. https://doi.org/10.1093/pcp/pcw068.
Wang K, Yang Q, Lanhuang B, Lin H, Shi Y, Dhanasekaran S, et al. Genome-wide investigation and analysis of U-box Ubiquitin-Protein ligase gene family in apple: Expression profiles during Penicilliumexpansum infec-tion process. Physiol Mol Plant Pathol. 2020;111:101487. https://doi.org/10.1016/j.pmpp.2020.101487.
Yee D, Goring DR. The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates. J Exp Bot. 2009;60(4):1109–21. https://doi.org/10.1093/jxb/ern369.
Zeng LR, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, et al. Spotted leaf 11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell. 2004;16(10):2795–808. https://doi.org/10.1105/tpc.104.025171.
Yang CW, Gonzalez-Lamothe R, Ewan RA, Rowland O, Yoshioka H, Shenton M, et al. The E3 ubiquitin ligase activity of Arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defense. Plant Cell. 2006;18(4):1084–98. https://doi.org/10.1105/tpc.105.039198.
Orosa B, He Q, Mesmar J, Gilroy EM, McLellan H, Yang C, et al. BTB-back domain protein POB1 suppresses immune cell death by targeting ubiquitin E3 ligase PUB17 for degradation. PLoS Genet. 2017;13(1):1–26. https://doi.org/10.1371/journal.pgen.1006540.
Kai S, Yang W. E3 Ubiquitin Ligases: Ubiquitous actors in plant develop-ment and abiotic stress responses. Plant Cell Physiol. 2017;9:1461–76. https://doi.org/10.1093/pcp/pcx071.
Trujillo M. News from the PUB: plant U-box type E3 ubiquitin ligases. J Exp Bot. 2018;69(3):371–84. https://doi.org/10.1093/jxb/erx411.
Pérez-Clemente R, Vives V, Zandalinas S, et al. Biotechnolog-ical approaches to study plant responses to stress. Biomed Res Int. 2013;2013:654120. https://doi.org/10.1155/2013/654120.
Liu JH, Peng T, Dai WS. Critical cis-acting elements and interacting trans-cription factors: Key players associated with abiotic stress responses in plan-ts. Plant Mol Biol Report. 2014;32(2):303–17. https://doi.org/10.1007/s11105-013-0667-z.
Lyzenga WJ, Stone SL. Abiotic stress tolerance mediated by protein ubiqu-itination. J Exp Bot. 2012;63(2):599–616. https://doi.org/10.1093/jxb/err310.
Chen X, Wang T, Rehman AU, Wang Y, Gong Z. Arabidopsis U-box E3 ubiquitin ligase PUB11 negatively regulates drought tolerance by degrading the receptorike protein kinases LRR1 and KIN7. Integr Plant Biol. 2020;63:494–509. https://doi.org/10.1111/jipb.13058.
Kong L, Cheng J, Zhu Y, Ding Y, Meng J, Chen Z, et al. Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat Commun. 2015;6:8630. https://doi.org/10.1038/ncomms9630.
Bhargava S, Sawant K. Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed. 2013;132(1):21–32. https://doi.org/10.1111/pbr.12004.
Rohit J, Wani SH, Balwant S, Abhishek B, Dar ZA, Lone AA, et al. Transcription factors and plants response to drought stress: current understan-ding and future directions. Front Plant Sci. 2016;7:1029. https://doi.org/10.3389/fpls.2016.01029.
Wang X, Ding Y, Li Z, Shi Y, Wang J, Hua J, et al. PUB25 and PUB26 promote plant freezing tolerance by degrading the cold signaling negativereg-ulator MYB15. Dev Cell. 2019;51(2):222–35. https://doi.org/10.1016/j.devcel.2019.08.008.
Liu YC, Wu YR, Huang XH, Sun J, Xie Q. AtPUB19, a U-box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana. Mol Plant. 2011;4(6):938–46. https://doi.org/10.1093/mp/ssr030.
Dong HS, Ryu MY, Jammes F, Hwang JH, Turek M, Kang BG, et al. R-oles of four Arabidopsis U-Box E3 Ubiquitin Ligases in negative regulation of Abscisic acid-mediated drought stress responses. Plant Physiol. 2012;160(1):556–68. https://doi.org/10.1104/pp.112.202143.
Raab Sabine, Drechsel Gabriele, Zarepour Maryam, et al. Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J. 2009;59:39–51. https://doi.org/10.1111/j.1365-313X.2009.03846.x.
Adler G, Konrad Z, Zamir L, Mishra AK, Raveh D, Bar-Zvi D. The Arabidopsis paralogs, PUB46 and PUB48, encoding U-box E3 ubiquitin ligases, are essential for plant response to drought stress. BMC Plant Biol. 2017;17(1):8. https://doi.org/10.1186/s12870-016-0963-5.
Park JJ, Yi J, Yoon J, Cho LH, Ping J, Jeong HJ, et al. OsPUB15, an E3ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 2011;65(2):194–205. https://doi.org/10.1111/j.1365-313X.2010.04416.x.
Han PL, Dong YH, Jiang H, Hu DG, Hao YJ. Molecular cloning and fun-ctional characterization of apple U-box E3 ubiquitin ligase gene MdPUB29 reveals its involvement in salt tolerance. J Integr Agric. 2019;18(7):1604–12. https://doi.org/10.1016/S2095-3119(19)62594-3.
Huang X, Li K, Xu X, Yao Z, Jin C, Zhang S. Genome-wide analysis of WRKY transcription factors in white pear (Pyrusbretschneideri) reveals evo-lution and patterns under drought stress. BMC Genomics. 2015;16(1):1104. https://doi.org/10.1186/s12864-015-2233-6.
Wu J, Wang Z, Shi Z, Zhang S, Ming R, Zhu S, et al. The genome of the pear (Pyrusbretschneideri Rehd.). Genome Res. 2013;23(2):396–408. https://doi.org/10.1101/gr.144311.112.
Wang N, Xing Y, Lou Q, Feng P, Liu S, Zhu M, et al. Dwarf and short grain 1, encodinga putative U-box protein regulates cell division and elonga-tion in rice. J Plant Physiol. 2017;209:84–94. https://doi.org/10.1016/j.jplph.2016.11.012.
Grill E, Himmelbach A. ABA signal transduction. Curr Opin Plant Biol. 1998;1(5):412–8. https://doi.org/10.1016/s1369-5266(98)80265-3.
Li Qionghou, Qiao Xin, Yin Hao, et al. Unbiased subgenome evolution following a recent whole-genome duplication in pear (Pyrusbretschneideri Rehd.). Hortic Res. 2019;6(1):34. https://doi.org/10.1038/s41438-018-0110-6.
Stone SL. The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front Plant Sci. 2014;5(5):135. https://doi.org/10.3389/fpls.2014.00135.
Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–43. https://doi.org/10.1046/j.1365-313x.1998.00343.x.
Samuel MA, Mudgil Y, Salt JN, Delmas F, Ramachandran S, Chilelli A, et al. Interactions between the sdomain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiol. 2008;147(4):2084–95. https://doi.org/10.1104/pp.108.123380.
Li K, Xing C, Yao Z, Huang X. PbrMYB21, a novel MYB protein of Pyrusbetulaefolia, functions in drought tolerance and modulates polyamine levels by regulating arginine decarboxylase gene. Plant Biotechnol J. 2017;15(9):1186–203. https://doi.org/10.1111/pbi.12708.
Bergler J, Hoth S. Plant U-box armadillo repeat proteins AtPUB18 and AtPUB19 are involved in salt inhibition of germination in Arabidopsis. Plant Biol. 2011;13(5):725–30. https://doi.org/10.1111/j.1438-8677.2010.00431.x.
Cho SK, Chung HS, Ryu MY, Park MJ, Lee MM, Bahk YY, et al. Heterologous expression and molecular and cellular characterization of CaPUB1 encoding a hot pepper U-Box E3 ubiquitin ligase homolog. Plant Physiol. 2006;142(4):1664–82. https://doi.org/10.1104/pp.106.087965.
Zhang M, Zhao J, Li L, Gao Y, Zhao L, Patil SB, et al. The Arabidopsis U-box E3 ubiquitin ligase PUB30 negatively regulates salt tolerance by facilitating BRI1 kinase inhibitor 1 (BKI1) degradation. Plant Cell Environ. 2017;40(11):2831–43. https://doi.org/10.1111/pce.13064.
Chou KC, Shen HB. Cell-PLoc: a package of Web servers for predicting subcellular localization of proteins in various organisms. Nat Protoc. 2008;3(2):153–62. https://doi.org/10.1038/nprot.2007.494.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecu-lar Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013;30(12):2725–9. https://doi.org/10.1093/molbev/mst197.
Zhang H, Gao S, Lercher MJ, Hu S, Chen WH. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucleic Acids Res. 2012;40(W1):W569–72. https://doi.org/10.1093/nar/gks576.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13(8):1194–202. https://doi.org/10.1016/j.molp.2020.06.009.
Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparinggenomic features. Bioinformatics. 2010;26(6):841–2. https://doi.org/10.1093/bioinformatics/btq033.
Rombauts S, Dehais P, Van Montagu M, Rouze P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999;27(1):295–6. https://doi.org/10.1093/nar/27.1.295.
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinear-ity. Nucleic Acids Res. 2012;40(7):e49. https://doi.org/10.1093/nar/gkr1293.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45. https://doi.org/10.1101/gr.092759.109.
Song B, Tang Z, Li X, Li J, Zhang M, Zhao K, et al. Mining and evolution analysis of lateral organ boundaries domain (LBD) genes in Chinese white pear (Pyrusbretschneideri). BMC Genom. 2020;21:644. https://doi.org/10.1186/s12864-020-06999-9.
Liu Y, Yang TY, Lin ZK, Gu BJ, Xing CH, Zhao LY, et al. A WRKY transcription factor PbrWRKY53 from Pyrusbetulaefolia is involved in drought tolerance and AsA accumulation. Plant Biotechnol J. 2019;17(9):1770–87. https://doi.org/10.1111/pbi.13099.
Zhao L, Yang T, Xing C, Dong H, Qi K, Gao J, et al. The beta-amylase PbrBAM3 from pear (Pyrusbetulaefolia) regulates soluble sugar accumulation and ROS homeostasis in response to cold stress. Plant Sci. 2019;287:110184. https://doi.org/10.1016/j.plantsci.2019.110184.
Gong X, Zhao L, Song X, Lin Z, Gu B, Yan J, et al. Genome-wide analyses and expression patterns under abiotic stress of NAC transcription factors in white pear (Pyrusbretschneideri). BMC Plant Biol. 2019;19(1):161. https://doi.org/10.1186/s12870-019-1760-8.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Method. 2001;25(4):402–8. https://doi.org/10.1006/meth.2001.1262.
Kumar KR, Kirti PB. A mitogen-activated protein kinase, AhMPK6 from peanut localizes to the nucleus and also induces defense responses upon tra-nsient expression in tobacco. Plant Physiol Biochem. 2010;48(6):481–6. https://doi.org/10.1016/j.plaphy.2010.03.010.
Dahro B, Wang F, Peng T, Liu JH. PtrA/NINV, an alkaline/neutral inverta-se gene of Poncirustrifoliata, confers enhanced tolerance to multiple abioticstresses by modulating ROS levels and maintaining photosynthetic efficiency. BMC Plant Biol. 2016;16(1):76. https://doi.org/10.1186/s12870-016-0761-0.
Huang XS, Liu JH, Chen XJ. Overexpression of PtrABF gene, a bZIP tran-scription factor isolated from Poncirustrifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 2010;10(1):230. https://doi.org/10.1186/1471-2229-10-230.
Woo NS, Badger MR, Pogson BJ. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods. 2008;4(1):27. https://doi.org/10.1186/1746-4811-4-27.
Acknowledgements
Not applicable.
Funding
This work has been supported by the National Key Research and Development Program of China (2019YFD1000102), the National Science Foundation of China (31872070; 32072538), the Jiangsu Agriculture Science and Technology Innovation Fund (CX(18)3065), the Fundamental Research Funds for the Central Universities of Nanjing Agricultural University (KYZ201607), the College of Horticulture SRT project of the Nanjing Agriculture University (202011YX05), and the Undergraduate Training Program for Innovation and Entrepreneurship (S20190040).
Author information
Authors and Affiliations
Contributions
CMW and XSH designed and carried out the experiments, and CMW carried out all bioinformatics analysis and wrote the manuscript. YQD and BBS contributed to genes expression analysis. BBS and XSH directed and revised the manuscript. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The genome database of Chinese white pear (Pyrus bretschneideri), is applied for our research under the permission of Center of Pear Engineering Technology Research. The test materials ‘Pyrus betulaefolia’ were collected from the pear germplasm orchard of the Center of Pear Engineering Technology Research situated at Hushu in Nanjing under the permission of Center of Pear Engineering Technology Research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
The logos of 20 conserved motifs predicted in our study.
Additional file 2: Figure S2.
The distribution of domain of PUB genes in pear. The conserved domains were predicted by SMART tools.
Additional file 3: Figure S3.
Molecular identification of transgenic Arabidopsis plants overexpressing PbrPUB18. (a) Genomic PCR identification of the plants using specific primers of PbrPUB18. M, DNA marker (DL 5000); + , positive control (gene plasmid); WT, untransformed plants. Numbers on the top of the gel panels indicate the transgenic lines; (b) Semi-quantitative RT-PCR analysis of the transcript levels of PbrPUB18 in six transgenic lines and WT. M, DNA marker (DL 2000); WT, untransformed plants; (c) The expression level of PbrPUB18 in WT and two transgenic lines. Actin was used as an internal control gene for normalizing the expression levels; Asterisks indicate that the value is significantly different from that of the WT at the same time point (* < 0.05; **P < 0.01; ***P < 0.001).
Additional file 4: Table S1.
Primer sequences used for expression analysis, cloning, subcellular localization, vector construction and transgenic confirmation.
Additional file 5: Table S2.
The cis-acting element analysis of PbrPUB gene family in pear.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, C., Song, B., Dai, Y. et al. Genome-wide identification and functional analysis of U-box E3 ubiquitin ligases gene family related to drought stress response in Chinese white pear (Pyrus bretschneideri). BMC Plant Biol 21, 235 (2021). https://doi.org/10.1186/s12870-021-03024-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-021-03024-3