Abstract
Background
Crop productivity is challenged by abiotic stresses, among which drought stress is the most common. NF-Y genes, especially NF-YA genes, regulate tolerance to abiotic stress.
Results
Soybean NF-Y gene GmNFYA5 was identified to have the highest transcript level among all 21 NF-YA genes in soybean (Glycine max L.) under drought stress. Drought-induced transcript of GmNFYA5 was suppressed by the ABA synthesis inhibitor naproxen (NAP). GmNFYA5 transcript was detected in various tissues at vegetative and reproductive growth stages with higher levels in roots and leaves than in other tissues, which was consist with the GmNFYA5 promoter: GUS fusion assay. Overexpression of GmNFYA5 in transgenic Arabidopsis plants caused enhanced drought tolerance in seedlings by decreasing stomatal aperture and water loss from leaves. Overexpression and suppression of GmNFYA5 in soybean resulted in increased and decreased drought tolerance, respectively, relative to plants with an empty vector (EV). Transcript levels of ABA-dependent genes (ABI2, ABI3, NCED3, LEA3, RD29A, P5CS1, GmWRKY46, GmNCED2 and GmbZIP1) and ABA-independent genes (DREB1A, DREB2A, DREB2B, GmDREB1, GmDREB2 and GmDREB3) in transgenic plants overexpressing GmNFYA5 were higher than those of wild-type plants under drought stress; suppression of GmNFYA5 transcript produced opposite results. GmNFYA5 probably regulated the transcript abundance of GmDREB2 and GmbZIP1 by binding to the promoters in vivo.
Conclusions
Our results suggested that overexpression of GmNFYA5 improved drought tolerance in soybean via both ABA-dependent and ABA-independent pathways.
Similar content being viewed by others
Background
Crop plants are commonly affected by abiotic stresses which cause soil destruction and crop losses worldwide [1, 2]. Water stress, either caused by drought or salt, is a major challenge. Drought is more widespread and damaging than salt stress [3]. Adaptation to drought involves complex regulatory networks involving control of water flux and cellular osmotic adjustment [4,5,6]. ABA regulates drought tolerance in plants by regulating stomatal aperture and modulating drought-responsive genes such as AP2/ERF and MYB family members that play important roles under drought stress [7,8,9]. ABA synthesis inhibitors such as naproxen and tungstate show the importance of ABA [10, 11].
The CCAAT box binding factor (CBF), also known as Nuclear Factor Y (NF-Y) or Heme Activator Protein (HAP), is composed of subunits NF-YA, NF-YB and NF-YC [12,13,14]. In monocotyledonous and dicotyledonous plants, just one of these subunits is encoded by tens of genes, whereas only one or two genes are present in animals [13]. Members of the different NF-Y families have diverse functions in regulating plant development and growth, such as flowering time, plant height, root elongation, embryogenesis, and seed germination [15,16,17,18,19,20,21,22,23,24,25,26].
The NF-Y genes are regulators of abiotic stress-induced responses. For example, overexpression of AtNFYA5 in Arabidopsis caused enhanced resistance to drought stress by affecting the expression of many drought stress-related genes [27]. Transgenic rice lines carrying extra copies of OsNFYA7 showed improved drought tolerance through the ABA-independent pathway [28]. Transgenic rice overexpressing OsHAP2E [29] and bermudagrass Cdt-NFYC1 [11] displayed improved resistance to drought and salt stresses. Several stress-related measures showed that overexpression of ZmNFYB2 resulted in drought resistance in transgenic maize plants [30]. However, most reports showing that NF-Ys play important roles under water stress conditions relate to rice and Arabidopsis. The functions of few NF-Y members were confirmed in soybean. GmNFYA3 and GmNFYB1 were induced by drought and transgenic Arabidopsis lines with those genes showed increased tolerance to drought stress [31, 32]. However, the biological functions of many other NF-Y members in soybean remain to be verified.
In this study, GmNFYA5, a soybean member of the NF-YA family, was induced by drought and ABA. Transgenic Arabidopsis and soybean lines overexpressing GmNFYA5 showed enhanced tolerance to drought stress through both ABA-dependent and ABA-independent pathways. Considering these results, we suggest that GmNFYA5 is an excellent candidate gene for genetic improvement to enhance drought tolerance of soybean.
Results
Isolation and characterization of GmNFYA5
NF-YA genes mediate in drought stress tolerance [27, 28, 31]. Twenty one NF-YA genes were investigated in seedling leaves using qRT-PCR and most of them were induced by drought stress. GmNFYA5 transcript was highest among all genes at 2 h after drought stress (Fig. 1A) and transcription of GmNFYA5 was significantly induced by ABA (Fig. 1B). Detached leaves were used to understand whether ABA could affect the drought-induced transcript of GmNFYA5 by using NAP, an inhibitor of ABA biosynthesis. The GmNFYA5 transcript under drought treatment for 2 h was similar to the above pattern relative to the control (Fig. 1A and C). However, pretreatment with NAP suppressed the level of expression of GmNFYA5 (Fig. 1C).
GmNFYA5 expression as affected by drought stress and ABA. (A) Transcripts of 21 NF-YA genes were analyzed using qRT-PCR in soybean under drought stress. Expression levels were normalized to that of GmCYP2. GmNFYA5 transcript at 0 h was set at 1.0, and SD for three biological replicates is represented by error bars. (B) Transcript of GmNFYA5 analyzed in response to ABA treatment in soybean. Expression levels were normalized to that of GmCYP2. GmNFYA5 transcript at 0 h was set at 1.0, and SD for three biological replicates is represented by error bars. (C) qRT-PCR of GmNFYA5 transcript in soybean plants in response to drought stress with the treatment of 1 mM NAP. Control, drought and drought + NAP are indicated by CTR, D and D + NAP, respectively. The level of GmNFYA5 transcript under control conditions was set at 1.0, and the internal control was GmCYP2. SD for three biological replicates is represented by error bars. Significant differences at P < 0.05 are indicated by different letters above the columns
GmNFYA5 encodes a 37.68 KD polypeptide of 303 amino acid residues with an isoelectric point (pI) of 8.86. GmNFYA5 contains highly conserved core regions in common with Arabidopsis NF-YA proteins; these consist of two subdomains: an NF-YB/C and DNA binding subdomains connected by a linker and required for association with the NF-YB/C heterodimer, and CCAAT binding sequences. The amino acids indicated by asterisks in Fig. S1A are critical, conserved residues. Alignments of GmNFYA5 and Arabidopsis NF-YA proteins showed that it had the highest identity with AtNFYA5 in the conserved domain. Phylogenetic analysis based on the amino acid sequences in the conserved domain showed that GmNFYA5 clustered with Arabidopsis AtNFYA6 and AtNFYA5 (Fig. S1B).
Tissue-specific expression analysis and subcellular localization
The expression of GmNFYA5 in different tissues, including roots, stems, cotyledon, leaves, apical buds, flower buds and flowers was evaluated by qRT-PCR at vegetative and reproductive growth stage under normal conditions. GmNFYA5 transcripts were detected in every tissue at both developmental stages, with a significant increase in leaves at flowering compared with seedling levels (Fig. 2A). Transcript abundance in roots and leaves was higher than in other tissues (Fig. 2A). To investigate the expression patterns in greater detail, transgenic Arabidopsis T3 lines overexpressing GmNFYA5 promoter: GUS were produced. Expression of GUS was detected in various tissues (Fig. 2B). GmNFYA5 expression was highest in the floral tissues, but also prominent in the leaf vascular system and roots. As to leaves, the lower level of GUS staining in seedlings and higher level of staining at the flowering stage (Fig. 2B) were consistent with the results of the qRT-PCR.
Tissue specific expression analysis and subcellular localization of GmNFYA5. (A) Expression abundance of the GmNFYA5 gene in soybean tissues at the seedling and flowering stages. Soybean tissues under normal conditions were used to extract total RNA. The relative transcript levels of GmNFYA5 in soybean tissues are indicated by vertical columns. GmNFYA5 transcript in roots was set at 1.0, and the internal control was GmCYP2. SD for three biological replicates is represented by error bars. Significant differences at P < 0.05 are indicated by different letters above the columns. (B) Expression pattern of GmNFYA5 promoter: GUS in various tissues of transgenic Arabidopsis plants. a 5-day-old seedling, b rosette leaf, c cauline leaf, d root, e flower, f silique. Scale bar, 2 mm (C) Subcellular localization of GmNFYA5. Fluorescence of GFP-GmNFYA5 and mCherry-GmNFYA3 fusion proteins in transformed cells was localized exclusively to the nucleus. Scale bar, 10 μm
To investigate the localization of GmNFYA5 within cells, recombinant vectors p16318GFP:GmNFYA5 and mCherry:GmNFYA3 were co-transformed into Arabidopsis protoplasts. GFP and mCherry fluorescence of the two fusion protein in transformed cells were localized exclusively to the nuclei (Fig. 2D), whereas the control GFP was uniformly distributed across protoplast cells (Fig. 2C), thus showing that GmNFYA5 was a nuclear-localized protein.
Sensitivity of 35S:GmNFYA5 Arabidopsis plants to exogenous ABA and PEG
Transgenic Arabidopsis plants overexpressing GmNFYA5 were generated to elucidate functions of the gene. Three homozygous transgenic lines (35S:GmNFYA5–1, 35S:GmNFYA5–2 and 35S:GmNFYA5–5) detected using qRT-PCR were selected for functional analysis (Fig. 5B). To determine whether ABA sensitivity was affected by GmNFYA5, seeds from 35S:GmNFYA5 and WT Arabidopsis plants were germinated on 1/2 MS medium with different concentrations of ABA. Germination rates were similar under control conditions (Fig. 3A). In the presence of 0.5 and 0.8 μM ABA seed germination was significantly suppressed in both 35S:GmNFYA5 and WT lines, but the inhibition of WT germination was much less than 35S:GmNFYA5 lines; germination of WT seeds reached 73% at 36 h compared with 12 to 19% for 35S:GmNFYA5 lines treated with 0.5 μM ABA (Fig. 3A). Only 7 to 13% germination was detected in 35S:GmNFYA5 lines compared to 62% for WT seeds at 36 h in treatments with 0.8 μM ABA (Fig. 3A).
Germination and root growth of WT and 35S:GmNFYA5 Arabidopsis plants with ABA treatment. (A) Seed germination on 1/2 MS agar plates with 0, 0.5 and 0.8 μM ABA. Each measurement represents the average germination of 64 seeds ± SD. (B) and (C) Root growth of WT and 35S:GmNFYA5 Arabidopsis plants on media with/without ABA. Three-day-old seedlings from 1/2 MS medium were transferred to media containing 0, 0.5 and 1 μM ABA; and photographed 7 days later. Scale bar, 2 cm. Each measurement represents the average root length of 30 seedlings ± SD. Significant differences P < 0.05 are indicated by different letters above the columns
Differential inhibition of root growth between WT and 35S:GmNFYA5 seedlings was also observed following treatment with ABA. Three-day-old seedlings under normal conditions were transferred to1/2 MS medium with 0.5–1 μM ABA. After one week, roots lengths of 35S:GmNFYA5 plants were shorter than WT plants (Fig. 3B and C).
The effects of different concentrations of PEG on germination of WT and 35S:GmNFYA5 lines were also assessed. Germination of WT seeds was more sensitive to PEG than 35S:GmNFYA5 lines. Compared to 53–59% germination of 35S:GmNFYA5 Arabidopsis seeds at 48 h in 8% PEG, there was only 25% germination of WT seeds (Fig. 4A). Germination rates of WT seeds were further delayed relative to 35S:GmNFYA5 Arabidopsis seeds in 10% PEG (Fig. 4A). Seedling growth was also differentially inhibited by PEG (Fig. 4B and C).
Germination rates and root growth of WT and 35S:GmNFYA5 Arabidopsis plants treated with PEG. (A) Seed germination on 1/2 MS agar plates with 8 and 10% PEG. Each measurement represents the average germination of 64 seeds ± SD. (B) and (C) Root growth of WT and 35S:GmNFYA5 Arabidopsis plants on media with/without PEG. Three-day-old seedlings from 1/2 MS medium were transferred to media containing 0, 10 and 12% PEG; photographed after 7 days. Scale bar, 2 cm. Each measurement represents the average root length of 30 seedlings ± SD. Significant differences at P < 0.05 are indicated by different letters above the columns
Tolerance of 35S:GmNFYA5 Arabidopsis seedlings to drought stress
To determine whether overexpression of GmNFYA5 enhanced drought tolerance, 3-week-old 35S:GmNFYA5 and WT Arabidopsis seedlings were deprived of water. The soil water potential (SWP) of WT seedlings fell more quickly than 35S:GmNFYA5 lines (Fig. 5G). The SWP of WT and 35S:GmNFYA5 lines declined to their minimum levels after drought stress for 7 and 9 days, respectively. Detached leaves of 35S:GmNFYA5 plants also lost water more slowly than the WT (Fig. 5D). Concentrations of ABA in the 35S:GmNFYA5 lines were higher than in WT plants after drought treatment for 7 days, and there was no significant difference when the plants were grown under normal conditions (Fig. 5D). Consistent with these results, the stomatal apertures of detached leaves from 35S:GmNFYA5 lines were significantly smaller than those from the WT under the treatment with 10 μM ABA compared with no significant difference in non-treated leaves (Fig. 5h and i).
Improved drought tolerance and stomatal aperture analysis in 35S:GmNFYA5 Arabidopsis plants. (A) Assessment of drought tolerance in 35S:GmNFYA5 Arabidopsis plants. Three-week-old plants were grown without water for 14 days, followed by re-watering for 7 days. Drought resistance of 35S:GmNFYA5 Arabidopsis plants was assayed by capability to resume growth when returned to normal conditions after drought stress. (B) GmNFYA5 transcript detected in 35S:GmNFYA5 Arabidopsis lines. Expression levels were normalized to that of Tub8. Transcript of GmNFYA5 in WT plants was set at 1.0; SD for three biological replicates is represented by error bars. (C-G) Measurements of survival rate, concentrations of ABA, RWC, water loss, and SWP in 35S:GmNFYA5 Arabidopsis and WT lines. (H-I) Stomatal apertures in 35S:GmNFYA5 Arabidopsis and WT plants with zero and 10 μM ABA treatments. Width/length of the stomatal aperture was measured using the ruler tool in Adobe Photoshop CS5. Scale bar, 5 μm. Data represent mean SD for three biological replicates (n = 54). Significant differences P < 0.05 are indicated by different letters above the columns
Drought treatment for 14 days produced more extreme wilting in WT plants than the 35S:GmNFYA5 lines (Fig. 5A). The 35S:GmNFYA5 plants maintained 65–69% RWC and 54–62% ion leakage compared to 24% RWC and 79% ion leakage in leaves of WT plants. No significant difference was detected among 35S:GmNFYA5 and WT controls under normal conditions (Fig. 5E and Fig. S3). After re-watering for 7 days, most WT plants failed to recover (19% survival), whereas most 35S:GmNFYA5 plants maintained turgor and showed higher recovery (56–86% survival) (Fig. 5C). These results showed that overexpression of GmNFYA5 enhanced drought tolerance of transgenic Arabidopsis lines.
Drought tolerance of 35S:GmNFYA5 Arabidopsis lines is maintained for the entire growth cycle
GmNFYA5 overexpression increased drought tolerance of 35S:GmNFYA5 Arabidopsis lines at the seedling stage. However, this increase had to be translated to enhanced drought tolerance all over an entire growth cycle and ultimately lead to increased yield of dry matter or seed. Under continued drought treatment the shoot and pot lengths of 35S:GmNFYA5 plants were markedly longer (Fig. 6A-D), and pod numbers per plant and seed numbers per pod were significantly higher than the WT (Fig. 6E and F). There was no difference between 35S:GmNFYA5 and WT plants grown under normal conditions. These results showed that GmNFYA5 overexpression in Arabidopsis plants could improve drought tolerance and seed yields relative to the WT control.
Improved drought tolerance in 35S:GmNFYA5 Arabidopsis plants all over an entire growth cycle. (A) Drought tolerance assessed in 35S:GmNFYA5 Arabidopsis over a full growth cycle. Scale bar, 4 cm. (B) Pod lengths in 35S:GmNFYA5 and WT Arabidopsis plants were from control and drought conditions. Scale bar, 4 mm. (C-F) Shoot length, pod length, pod number per plant and seed number per pod were measured in 35S:GmNFYA5 and WT plants under control and drought conditions. Data represent mean SD for three biological replicates (n = 18). Significant differences at P < 0.05 are indicated by different letters above the columns
Transcription profiles of stress-related genes in 35S:GmNFYA5 Arabidopsis
To examine the role of GmNFYA5 in regulation of tolerance to drought stress, 9 stress-related genes including 6 ABA-dependent genes (ABI2, ABI3, NCED3, LEA3, RD29A and P5CS1) and 3 ABA-independent genes (DREB1A, DREB2A and DREB2B) were assayed in three 35S:GmNFYA5 Arabidopsis lines in comparison with WT plants under drought, drought + NAP and control treatments. Under control conditions transcripts of DREB1A, DREB2A, DREB2B, ABI2, ABI3, LEA3, RD29A and P5CS1 in 35S:GmNFYA5 plants were significantly higher than in WT plants; levels of NCED3 transcripts were not different (Fig. 6). Moreover, all genes clearly produced much higher transcript levels in 35S:GmNFYA5 plants than in the WT under drought stress (Fig. 7). However, NAP significantly suppressed the drought-induced transcripts of all genes, especially transcripts of ABI2, ABI3, LEA3, RD29A and P5CS1 in the drought + NAP treatment (Fig. 7E-I).
Relative transcript levels of DREB1A, DREB2A, DREB2B, ABI2, ABI3, NCED3, LEA3, RD29A and P5CS1 in 35S:GmNFYA5 and WT Arabidopsis plants under three conditions. Relative transcript level is indicated by the vertical column and normalized to that of Tub8. Transcripts of stress-related genes in WT plants under normal conditions were set at 1.0. Control, drought and drought pretreated with NAP are indicated by CTR, D and D + NAP, respectively. Data represent mean SD for three biological replicates. Significant differences P < 0.05 are indicated by different letters above the columns
Tolerance of transgenic soybean plants to drought stress
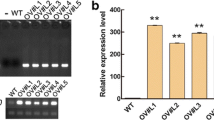
Three transgenic soybean lines OE, EV and RNAi were generated by A. rhizogenes-mediated hairy roots transformation (Fig. 8D) to investigate the functions of GmNFYA5 in soybean. Relative mRNA levels of GmNFYA5 in OE plants measured by qRT-PCR were significantly higher than in plants with the EV, which in turn were higher than in RNAi plants (Fig. 8E). Then the plants that had positive hairy roots were planted into the soil for 7 days to establish the normal growth. These plants were used to explore the drought tolerance following deprivation of water.
Drought tolerance in transgenic soybean plants. (A-D) Drought tolerance was evaluated in transgenic soybean plants. Transgenic soybean plants with positive hairy roots were transferred to plastic pots containing a mixture of peat and vermiculite (1:1, v/v) and grown for 7 days. Water deprivation for 16 days was followed by re-watering for 3 days. The hairy roots phenotype is shown in Fig. 8D. Drought resistance of transgenic soybean plants was assayed by the capability to resume growth when returned to normal conditions after drought stress. Scale bar, 5 cm. (E) Relative transcript of GmNFYA5 was detected in three transgenic soybean lines. Transcript of GmNFYA5 in EV plants was set at 1.0, and the expression levels were normalized to that of GmCYP2. (F-K) Survival rate, RWC, ion leakage, MDA, ABA concentration and SWP in transgenic soybean lines. Data represent mean SD for three biological replicates (n = 18). Significant differences P < 0.05 are indicated by different letters above the columns
The assay showed that the rate of decline in SWP was fastest in RNAi lines with minimum levels in RNAi, EV and OE lines being reached at 7, 8 and 9 days after initiation of drought treatment (Fig. 8K). Assuming that ABA induced in the roots was transferred to other tissue [33], the ABA concentration in soybean leaves was measured after withholding water for 7 days. The ABA concentration of OE plants was higher than in EV plants, and the opposite effect was detected in RNAi plants under drought stress. There was no difference among the lines under control conditions (Fig. 8J).
RNAi plants displayed severe wilting by withholding water for 16 days, with less extreme wilting in EV plants and a near-healthy appearance of OE plants (Fig. 8b). Under drought stress, there were much higher levels of ion leakage and MDA and decreased RWC in RNAi plants relative to EV plants, and opposite effects for OE plants (Fig. 8G-I). After re-watering for 3 days, survival of OE plants was significantly higher than that of EV plants, whereas nearly all RNAi plants were unable to recover (Fig. 8C and Fig. 8F).
Transcription profiles of stress-related genes in transgenic soybean lines
The transcript levels of 6 stress-responsive genes including 3 ABA-dependent genes (GmDREB1, GmDREB2 and GmDREB3) and 3 ABA-independent genes (GmWRKY46, GmNCED2 and GmbZIP1) were analyzed by qRT-PCR with treatments of control, drought and drought + NAP. Under control conditions, significantly higher expression levels of GmDREB1, GmDREB2, GmDREB3, GmWRKY46 and GmbZIP1 were detected in soybean OE hairy roots, and opposite effects were obtained for RNAi lines compared with EV lines. GmNCED2 transcript showed no significant difference between RNAi and EV plants (Fig. 9A-F). Drought-induced expression levels of all 6 genes were significantly higher in OE lines, but much lower in RNAi lines compared with those in EV lines. NAP suppressed the levels of drought-induced transcripts of all genes, especially GmWRKY46 and GmbZIP1 (Fig. 9E and F).
Relative transcript levels of GmDREB1, GmDREB2, GmDREB3, GmNCED2, GmWRKY46 and GmbZIP1 in transgenic soybean and EV plants under three conditions. Relative transcript level is indicated by the vertical column and normalized to that of GmCYP2. Transcript level of stress-related genes in EV plants under normal condition was set at 1.0. Control, drought and drought + NAP are indicated by CTR, D and D + NAP, respectively. Data represent mean SD for three biological replicates. Significant differences P < 0.05 are indicated by different letters above the columns
Transcriptional activation assays in Arabidopsis protoplasts
The promoters of 15 stress-responsive genes were analyzed by the PLACE program (http://www.dna.affrc.go.jp/PLACE/signalscan.html). One to six CCAAT elements were found in each gene (Table S2). Two stress-related genes (GmDREB2 and GmbZIP1) were selected to determine whether GmNFYA5 could bind to the promoters in vivo in an Arabidopsis protoplast transient expression system. Recombinant pGreenII 0800:GmDREB2p/GmbZIP1p vector was co-transformed into Arabidopsis protoplasts with an empty p16318GFP vector or a p16318GFP:GmNFYA5 vector (Fig. 10A). Protoplasts expressing GFP-GmNFYA5 exhibited significantly higher expression levels of LUC reporter gene compared with the GFP (Fig. 10B and C).
GmNFYA5 increased LUC reporter gene activity by binding the promoters of GmDREB2 and GmbZIP1. (A) The structures of effector and reporters. (B) and (C) Ratio of LUC to REN indicates the activity of GmNFYA5 on the transcript of the GmDREB2 and GmbZIP1 promoters. Data represent mean SD for three biological replicates. Significant differences P < 0.05 are indicated by different letters above the columns
Discussion
Drought is the most widespread and damaging abiotic stress [3]. Multiple transcriptional cascades mediate gene regulation under drought stress [34, 35]. Transcription factor genes induced in these cascades in turn regulate related downstream genes to resist the effects of drought stress. Our results showed that most NF-YA members responded to drought stress in soybean (Fig. 1A), consistent with previous observations [36]. Among the NF-YA genes the transcript level of GmNFYA5 was the highest following drought treatment. Tissue-specific expression analysis showed that transcript abundance of GmNFYA5 in roots was higher than in other tissues except the leaves (Fig. 2A), which implied that GmNFYA5 was connected with drought tolerance. Likewise, several genes conferring drought tolerance in transgenic plants maintained their highest transcript abundance in roots under normal conditions [11, 27, 37].
In addition to being induced by drought stress, GmNFYA5 transcript levels were up-regulated by ABA and suppressed by NAP (Fig. 1B and C), indicating that GmNFYA5 transcription in response to drought depended on ABA signaling cross-talk. Under drought stress, the levels of ABA rise and suppress the activity of phosphatase 2Cs, followed by stomatal closure via phosphorylation and other pathways [38,39,40]. As expected, overexpression of GmNFYA5 conferred drought tolerance to transgenic Arabidopsis plants via the ABA-dependent pathway (Fig. 5). Under drought treatment, overexpression of GmNFYA5 increased the expression level of NCED3 to enhance ABA biosynthesis (Fig. 5D and Fig. 7D), and increased ABA accumulation led to reduced stomatal apertures (Fig. 5H H and Fig. 5I), causing reduced water loss from leaves (Fig. 5F). Consistent with these results, the SWP of 35S:GmNFYA5 lines declined more slowly than in the WT (Fig. 5G). Reports that overexpression of GmNFYA3 [31], Cdt-NFYC1 [11], RING-H2 [41] and GmTGA17 [42] conferred increased ABA sensitivity and drought tolerance in transgenic lines, were confirmed in this study (Fig. 3). Moreover, some physiological indices, especially the SWP, were not reported in previous reports [6, 27, 32, 37].
As an important signaling intermediate, ABA controls the expression of many stress-induced genes [43, 44], such as ABI2, ABI3, LEA3, RD29A and P5CS1, which functioned as positive regulators of drought tolerance [45,46,47,48]. These genes maintained higher transcript levels in 35S:GmNFYA5 Arabidopsis plants compared with WT plants under normal and drought conditions (Fig. 7E-I). With the introduction of NAP into the drought treatment, reduced transcripts levels of these genes proved that ABA played a key role in regulating drought tolerance of plants. However, GmNFYA5 overexpression functioned in enhancing drought tolerance via other pathways by up-regulating DREB1A, DREB2A and DREB2B (Fig. 7A-C), overexpression of which improved drought tolerance of Arabidopsis through an ABA-independent pathway [49,50,51,52]. Most importantly, we showed that overexpression of GmNFYA5 led to significantly higher seed yield of 35S:GmNFYA5 Arabidopsis plants compared with WT plants under drought conditions (Fig. 6).
GmNFYA5 functioned in positive regulation of drought tolerance in transgenic soybean plants; RNAi in those plants caused significant changes in several physiological indices (Fig. 8). The functions of GmNFYA3 and GmNFYB1 were previously studied only in transgenic Arabidopsis seedlings [32, 37]. In OE soybean plants, ABA-dependent genes (GmbZIP1 and GmWRKY46) and ABA-independent marker genes (GmDREB1, GmDREB2 and GmDREB3), which functioned in up-regulating drought tolerance in plants [45, 49, 51, 53, 54], maintained higher transcription levels compared with EV lines, whereas opposite effects occurred in RNAi plants (Fig. 9). The results showed that GmNFYA5 conferred drought tolerance in transgenic Arabidopsis and soybean plants via both ABA-dependent and ABA-independent pathways.
One to six CCAAT cis-acting elements were detected in the promoters of all the marker genes used in this study (Table S2). Transcriptional activation assays showed that the promoters of GmDREB2 and GmbZIP1 were bound by GmNFYA5 to enhance the expression level of the LUC reporter gene in vivo (Fig. 10 b and c). The results give further insights into the regulation of drought tolerance by GmNFYA5 through both ABA-dependent and ABA-independent pathways in Arabidopsis and soybean.
Conclusions
Transgenic soybean and Arabidopsis plants overexpressing GmNFYA5 exhibited enhanced drought tolerance as determined by physiological indices and transcript levels of drought-related genes. GmNFYA5 should be a positive gene that can increase drought resistance and therefore has potential for use in molecular breeding of soybean.
Methods
All experiments were performed in three biological replications.
Isolation of GmNFYA5
Total RNA was isolated from soybean (Glycine max L. Merr.) cultivar Williams 82 by a previously described method [37]. The full-length cDNA of GmNFYA5 was obtained by PCR with KOD-Plus DNA polymerase (TOYOBO, Japan) and the following primers: F 5′-GTAAGTGCGACTCTAAGCAAGCCT-3′ and R 5′-TAATGTAAATGAGCCAAGGATGACT-3′. The amplified products for sequencing were purified and cloned into the pEASY-Blunt vector (TransGen, China).
Plant growth and treatments
Cultivar Williams 82 was grown in plastic pots (15 cm diameter, 20 cm depth) containing a mixture of peat and vermiculite (1:1, v/v) in a greenhouse at 28/18 °C day/night, 70% relative humidity and a 14/10 h light/darkness photoperiod [55]. Twenty-day-old seedlings were used for evaluation of expression patterns. For drought treatment, the whole plant was removed, washed and placed in a laminar flow hood for gradual drought exposure for 12 h [11]. For ABA treatment, the roots were subjected to 100 μM ABA for 12 h. For both treatments, the soybean leaves were collected at 0, 1, 2, 4, 8 and 12 h timepoints and immediately frozen by liquid nitrogen for isolation of RNA. To understand whether ABA was involved in drought-induced transcription of GmNFYA5, detached leaves were placed in H2O for 1 h to eliminate the influence of the wound stress, and then treated with distilled water or 1 mM naproxen (ABA synthesis inhibitor) for 3 h, followed by drought treatment for 2 h [11]. Leaves floated in H2O for the entire period constituted the normal control. The soybean leaves were collected and immediately frozen by liquid nitrogen for isolation of RNA.
To assay transcript levels of GmNFYA5 in various tissues, the roots, stems, cotyledon, leaves and apical buds of 20-day-old soybean seedlings were sampled and immediately frozen by liquid nitrogen for isolation of RNA. Roots, stems, cotyledon, leaves, apical buds, flower buds and flowers of 50-day-old soybean plants were also sampled and immediately frozen for isolation of RNA. All the experiments were carried out in a greenhouse at 28/18 °C day/night, 70% relative humidity and a 14/10 h light/darkness photoperiod.
Generation and drought treatment of 35S:GmNFYA5 Arabidopsis lines
The CDS of GmNFYA5 was cloned into the NcoI site of a vector named pCAMBIA1302 and driven by CaMV35S promoter using primer set 5′-GGGACTCTTGACCATGATGAAGAACTTATGTGAG-3′ and 5′-TCAGATCTACCATGGCCATAAGGACTGATAGACG-3′. The pCAMBIA1302:GmNFYA5 construct was introduced into Agrobacterium tumefaciens strain GV3101 which was used to infect Arabidopsis using the floral dip method. Positive transgenic Arabidopsis lines were screened by Hygromycin (Roche, Germany) to select T1 plants and T2 homozygotes.
Transgenic Arabidopsis lines (35S:GmNFYA5–1, 35S:GmNFYA5–2 and 35S:GmNFYA5–5) and ecotype Columbia-0 (WT) seedling were used in this study. Seeds were surface-sterilized with 70% ethanol and thrice washed with sterile water, followed sterilization with 1% sodium hypochlorite for 15 min and again washing three times with sterile water. The seeds were sown on half-strength Murashige and Skoog medium (1/2 MS, 2% sucrose, 0.8% agar). After 2 days at 4 °C in darkness they were placed in a tissue culture room at 22 °C and 70% relative humidity with a 16/8 h light/darkness photoperiod. For drought treatment, 3-week-old seedlings which had been transferred to plastic pots (8 cm in length, width and depth) containing a mixture of peat and vermiculite (1:1, v/v) for 7 days were deprived of water until they became wilted, after which they were irrigated and recovered for 7 days.
To investigate the transcript levels of marker genes under conditions of control, drought and drought + NAP in 35S:GmNFYA5 Arabidopsis plants, leaves of 3-week-old 35S:GmNFYA5 and WT seedlings were placed into H2O for 1 h to eliminate the influence of the wound stress, following by placement in H2O or 1 mM NAP solution for 3 h, and then transferred to a laminar flow hood for 2 h as drought treatment. The leaves of 35S:GmNFYA5 and WT seedlings floated in H2O for the entire period were the normal control. Leaves were sampled and immediately frozen by liquid nitrogen for isolation of RNA. All the treatments were performed in a greenhouse at 22 °C and 70% relative humidity with a 16/8 h light/darkness photoperiod.
To further explore the functions of GmNFYA5, 35S:GmNFYA5 Arabidopsis lines were investigated under normal and drought conditions. For the normal control, 3-week-old 35S:GmNFYA5 and WT Arabidopsis plants were planted with well-watered treatment and 0.35 g H2O g− 1 dry soil (soil water potential is − 70 kPa) was maintained as the soil water content. For drought treatment, Soil water content was deprived of water to 0.20 g H2O g− 1 dry soil (soil water potential is − 280 kPa). The pots in both treatments were weighed daily and adjusted with water to maintain the target soil water potential until harvest [56]. All the treatments were performed in a greenhouse at 22 °C and 70% relative humidity with a 16/8 h light/darkness photoperiod.
The soybean seeds were provided by Dr. Li-Juan Qiu, Institute of Crop Science, Chinese Academy of Agricultural Sciences. The seeds of Arabidopsis were purchased from ABRC (https://abrc.osu.edu/researchers).
Subcellular localization
The coding sequence (CDS) of GmNFYA5 without the termination codon was fused in frame to the N-terminus of GFP in the vector p16318GFP, and ligated with BamHI site to generate a p16318GFP:GmNFYA5 fusion construct under the control of CaMV35S promoter using primer set 5′-TATCTCTAGAGGATCCATGAAGAACTTATGTGAG-3′ and 5′-TGCTCACCATGGATCCCATAAGGACTGATAGACG-3′. The CDS of GmNFYA3 encoding a nuclear-localized protein [31] was cloned into the EcoRI site of a vector pLVX-IRES-mCherry using primer set 5′-TCTATTTCCGGTGAATTCATGCAAACTGTTTATCTT-3′ and 5′-ACTAGTCTCGAGGAATTCAACTTTAAGGTTGCAGCA-3′. The GmNFYA3:mCherry fusion protein was used as a nuclear marker. Arabidopsis protoplasts were prepared as described [57]. Transfected protoplasts were incubated in darkness at 22 °C for 16–18 h, GFP fluorescence signals were observed with a confocal laser scanning microscope (Zeiss, LSIM 700) [58].
Promoter:GUS analysis
The promoter of GmNFYA5 was amplified from the DNA of soybean cultivar Williams 82 with primers F 5′-AAGAGGAACACAGAAGTCTATGAGT-3′ and R 5′-GCACATCAGATTCAGAGGAAGTCCC-3′. The products were introduced into a reconstructive pCAMBIA1305 vector (GFP coding region was replaced by GUS coding region) incorporating EcoRI and NcoI sites with the forward primer 5′-CCATGATTACGAATTCAAGAGGAACACAGAAGTC-3′ and reverse primer 5′-CTCAGATCTACCATGGCTCACATAAGTTCTTCAT-3′. The construct was introduced into A. tumefaciens strain GV3101 and transferred into Arabidopsis Col-0 plants by the floral dip method. Positive transgenic Arabidopsis lines were screened by Hygromycin to obtain homozygous lines.
Germination and root growth assays
Sterilized seeds were sown on 1/2 MS medium with 8–10% PEG 6000 (PEG) and 0.5–0.8 μM ABA respectively and placed in a tissue culture room at 22 °C and 70% relative humidity with a 16/8 h light/darkness photoperiod after stratification at 4 °C for 2 d in darkness. The germination rates were recorded every 12 h until completion. To investigate root growth of the 35S:GmNFYA5 Arabidopsis lines, 3-day-old seedlings were exposed to 1/2 MS medium with 10–12% PEG and 0.5–1 μM ABA. A week later, the root lengths were measured.
A. rhizogenes-mediated transformation of soybean hairy roots
The CDS of GmNFYA5 was inserted into the pCAMBIA3301 vector incorporating NcoI and BstEII sites with the following primers: F 5′-GGACTCTTGACCATGATGAAGAACTTATGTGAG-3′ and R 5′-ATTCGAGCTGGTCACCCATAAGGACTGATAGACG-3′. A 635 bp synthetic RNAi hairpin fragment (Fig. S2) was introduced into the pCAMBIA3301 vector and ligated with the same restriction sites. The pCAMBIA3301:GmNFYA5, pCAMBIA3301 empty vector and pCAMBIA3301:RNAi-GmNFYA5 construct were introduced into A. rhizogenes K599 and used to infect the hypocotyls of 5-day-old soybean seedlings in a tissue culture room at 28/18 °C day/night, 70% relative humidity and a 14/10 h light/darkness photoperiod, and then hairy roots were induced for 2 weeks [59]. The positive hairy roots transformants were screened using a QuickStix kit for PAT/bar (EnviroLogix, America) and qRT-PCR. Transgenic soybean lines having positive hairy roots were named OE-GmNFYA5 (OE), empty vector (EV) and RNAi-GmNFYA5 (RNAi) respectively and transferred to plastic pots (11 cm depth, 13.5 cm diameter) containing a peat and vermiculite mixture (1:1, v/v) to grow for 7 days, followed by deprivation of water until wilting.
To analyze the transcription of drought-related genes under conditions of drought and drought + NAP in transgenic soybean plants, three transgenic hairy roots lines were placed into H2O for 1 h to eliminate the wound stress, following by placement in H2O or 1 mM NAP solution for 3 h, prior to transfer to a laminar flow hood for 2 h as drought treatment. Roots floated in H2O for the entire period were the normal control. All treatments were carried out in a tissue culture room at 28/18 °C day/night, 70% relative humidity and a 14/10 h light/darkness photoperiod. Roots were sampled and immediately frozen by liquid nitrogen for isolation of RNA.
Analysis of transcript levels
Transcript levels were measured by qRT-PCR performed with TransStart Top Green qPCR SuperMix (TransGen, China) (20 μl) according to the manufacturer’s instructions on an Applied Biosystems 7500 real-time PCR system. Gene-specific primers designed by https://biodb.swu.edu.cn/qprimerdb/ and used for qRT-PCR assays are listed in Table S1.
Measurement of water loss
Leaves of 3-week-old Arabidopsis lines were detached, and the weight was measured every 30 min in a tissue culture room at 22 °C and 70% relative humidity. Percentages of initial fresh weight at 9 timepoints were used to represent water loss in transgenic and WT plants.
ABA concentration and stomatal aperture analysis
Following deprivation of water for 7 day, the ABA concentrations of leaves of soybean and Arabidopsis plants were measured by means of an ABA ELISA assay kit (Jiancheng, China) as described [60].
Leaves of 3-week-old 35S:GmNFYA5 and WT Arabidopsis seedlings were treated for 3 h in stomatal opening buffer (5 mM MES, 10 mM KCl, 50 mM CaCl2, pH 5.6) as described previously [61]. The leaves were then transferred to H2O or 10 μM ABA solution for 2 h in a greenhouse at 22 °C and 70% relative humidity. Stomatal apertures of stomatal complexes with no surrounding mesophyll cells were measured.
Measurement of malondialdehyde (MDA), relative water content (RWC) and ion leakage
RWC and ion leakage were measured in 3-week-old Arabidopsis plants following drought treatment for 14 days. MDA contents, RWC and ion leakage in transgenic soybean plants were measured following drought treatment for 16 days. MDA contents were measured and calculated as described previously [58, 62]. RWC and ion leakage were determined as described previously [63, 64].
Measurement of soil water potential (SWP)
When the transgenic soybean and Arabidopsis plants were shut off water supply, the SWP was measured in drought treatment trials until it reduced to the minimum level using WP4-T dewpoint meter (Decagon Devices, USA) according to the manufacturer’s instructions. WP4-T dewpoint meter had been used in research due to its accuracy in several reports [56, 65].
Transcriptional activation assays
The promoters of GmDREB2 (ABA-related gene) and GmbZIP1 (ABA-unrelated gene) were cloned into the LUC reporter vector pGreen II 0800 containing the Renilla luciferase (REN) gene driven by the CaMV 35S promoter and used as an internal control. The effector and reporter plasmids were extracted and introduced into Arabidopsis protoplasts using PEG4000-mediated transformation. The assays were performed as described [6].
Statistical analysis
All the measurements in this study were replicated three times biologically. Variance analyses of all data were preformed using SPSS Statistics 22 (IBM, USA) for a completely randomized design model. Duncan’s tests was used to evaluate differences among plant lines or treatments at P = 0.05.
Availability of data and materials
Soybean genes sequences in this research were downloaded from the plant genomics resource (https://phytozome.jgi.doe.gov/pz/portal.html) and National Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/). Arabidopsis genes sequences in this research were downloaded from The Arabidopsis Information Resource (TAIR) (http://www.arabidopsis.org/). The primers for qRT-PCR used in this research were designed in https://biodb.swu.edu.cn/qprimerdb/. The gene accessions and special primers for qRT-PCR are listed in Table S1.
Arabidopsis plants and soybean plants used in this research were treated following the guidelines of ABRC and Dr. Li-Juan Qiu, respectively.
Abbreviations
- ABA:
-
abscisic acid
- GFP:
-
Green fluorescent protein
- MDA:
-
malondialdehyde
- NAP:
-
naproxen
- RWC:
-
relative water content
References
Golldack D, Luking I, Yang O. Plant tolerance to drought and salinity: stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011;30(8):1383–91.
Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF. Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep. 2012;39(2):969–87.
Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73.
Golldack D, Li C, Mohan H, Probst N. Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci. 2014;5:151.
Xu ZS, Chen M, Li LC, Ma YZ. Functions of the ERF transcription factor family in plants. Botany. 2008;86(9):969–77.
Cui XY, Gao Y, Guo J, Yu TF, Zheng WJ, Liu YW, Chen J, Xu ZS, Ma YZ. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019;180(1):605–20.
Zhang LC, Zhao GY, Xia C, Jia JZ, Liu X, Kong XY. A wheat R2R3-MYB gene, TaMYB30-B, improves drought stress tolerance in transgenic Arabidopsis. J Exp Bot. 2012;63(16):5873–85.
Dai XY, Xu YY, Ma QB, Xu WY, Wang T, Xue YB, Chong K. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol. 2007;143(4):1739–51.
Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li LC, Zhao YX, Lu Y, Ni ZY, Liu L, et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol. 2007;65(6):719–32.
Han SY, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K, Yoshida S, Asami T. A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol. 2004;135(3):1574–82.
Chen M, Zhao Y, Zhuo C, Lu S, Guo Z. Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol J. 2015;13(4):482–91.
Thirumurugan T, Ito Y, Kubo T, Serizawa A, Kurata N. Identification, characterization and interaction of HAP family genes in rice. Mol Gen Genomics. 2008;279(3):279–89.
Petroni K, Kumimoto RW, Gnesutta N, Calvenzani V, Fornari M, Tonelli C, Holt BF 3rd, Mantovani R. The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell. 2012;24(12):4777–92.
Laloum T, De Mita S, Gamas P, Baudin M, Niebel A. CCAAT-box binding transcription factors in plants: Y so many? Trends Plant Sci. 2013;18(3):157–66.
Ballif J, Endo S, Kotani M, MacAdam J, Wu Y. Over-expression of HAP3b enhances primary root elongation in Arabidopsis. Plant Physiol Biochem. 2011;49(6):579–83.
Cai X, Ballif J, Endo S, Davis E, Liang M, Chen D, DeWald D, Kreps J, Zhu T, Wu Y. A putative CCAAT-binding transcription factor is a regulator of flowering timing in Arabidopsis. Plant Physiol. 2007;145(1):98–105.
Hackenberg D, Keetman U, Grimm B. Homologous NF-YC2 subunit from Arabidopsis and tobacco is activated by photooxidative stress and induces flowering. Int J Mol Sci. 2012;13(3):3458–77.
Hou X, Zhou J, Liu C, Liu L, Shen L, Yu H. Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat Commun. 2014;5:4601.
Kwong RW. LEAFY COTYLEDON1-LIKE defines a class of regulators essential for embryo development. Plant Cell. 2002;15(1):5–18.
Miyoshi K, Ito Y, Serizawa A, Kurata N. OsHAP3 genes regulate chloroplast biogenesis in rice. Plant J. 2003;36(4):532–40.
Mu J, Tan H, Zheng Q, Fu F, Liang Y, Zhang J, Yang X, Wang T, Chong K, Wang XJ, et al. LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 2008;148(2):1042–54.
Myers ZA, Kumimoto RW, Siriwardana CL, Gayler KK, Risinger JR, Pezzetta D, Holt Iii BF. NUCLEAR FACTOR Y, subunit C (NF-YC) transcription factors are positive regulators of Photomorphogenesis in Arabidopsis thaliana. PLoS Genet. 2016;12(9):e1006333.
Stephenson TJ, McIntyre CL, Collet C, Xue GP. TaNF-YB3 is involved in the regulation of photosynthesis genes in Triticum aestivum. Funct Integr Genomic. 2011;11(2):327–40.
Wei X, Xu J, Guo H, Jiang L, Chen S, Yu C, Zhou Z, Hu P, Zhai H, Wan J. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 2010;153(4):1747–58.
Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, Samach A, Coupland G. CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell. 2006;18(11):2971–84.
Xu ML, Jiang JF, Ge L, Xu YY, Chen H, Zhao Y, Bi YR, Wen JQ, Chong K. FPF1 transgene leads to altered flowering time and root development in rice. Plant Cell Rep. 2005;24(2):79–85.
Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, Lu XY, Cui X, Jin H, Zhu JK. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20(8):2238–51.
Lee DK, Kim HI, Jang G, Chung PJ, Jeong JS, Kim YS, Bang SW, Jung H, Choi YD, Kim JK. The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 2015;241:199–210.
Alam MM, Tanaka T, Nakamura H, Ichikawa H, Kobayashi K, Yaeno T, Yamaoka N, Shimomoto K, Takayama K, Nishina H, et al. Overexpression of a rice heme activator protein gene (OsHAP2E) confers resistance to pathogens, salinity and drought, and increases photosynthesis and tiller number. Plant Biotechnol J. 2015;13(1):85–96.
Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci U S A. 2007;104(42):16450–5.
Ni Z, Hu Z, Jiang Q, Zhang H. GmNFYA3, a target gene of miR169, is a positive regulator of plant tolerance to drought stress. Plant Mol Biol. 2013;82(1–2):113–29.
Li W, Mallano AI, Bo L, Wang T, Nisa Z, Li Y. Soybean transcription factor GmNFYB1 confers abiotic stress tolerance to transgenic Arabidopsis plants. Can J Plant Sci. 2017;97(3):501–15.
Yoshida T, Christmann A, Yamaguchi-Shinozaki K, Grill E, Fernie AR. Revisiting the basal role of ABA - roles outside of stress. Trends Plant Sci. 2019;24(7):625–35.
Yamaguchi-Shinozaki K, Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol. 2006;57:781–803.
Xu ZS, Chen M, Li LC, Ma YZ. Functions and application of the AP2/ERF transcription factor family in crop improvement. J Integr Plant Biol. 2011;53(7):570–85.
Quach TN, Nguyen HT, Valliyodan B, Joshi T, Xu D, Nguyen HT. Genome-wide expression analysis of soybean NF-Y genes reveals potential function in development and drought response. Mol Gen Genomics. 2015;290(3):1095–115.
Ni ZY, Hu Z, Jiang QY, Zhang H. GmNFYA3, a target gene of miR169, is a positive regulator of plant tolerance to drought stress. Plant Mol Biol. 2013;82(1–2):113–29.
Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. In vitro reconstitution of an abscisic acid signalling pathway. Nature. 2009;462(7273):660–U138.
Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. P Natl Acad Sci USA. 2009;106(50):21425–30.
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324(5930):1064–8.
Ko JH, Yang SH, Han KH. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. 2006;47(3):343–55.
Li B, Liu Y, Cui XY, Fu JD, Zhou YB, Zheng WJ, Lan JH, Jin LG, Chen M, Ma YZ, et al. Genome-wide characterization and expression analysis of soybean TGA transcription factors identified a novel TGA gene involved in drought and salt tolerance. Front Plant Sci. 2019;10:549.
Hu XJ, Chen D, Lynne Mclntyre C, Fernanda Dreccer M, Zhang ZB, Drenth J, Kalaipandian S, Chang H, Xue GP. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ. 2018;41(1):79–98.
Koornneef M, Leon-Kloosterziel KM, Schwartz SH, Zeevaart JAD. The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem. 1998;36(1–2):83–9.
Luo X, Bai X, Sun X, Zhu D, Liu B, Ji W, Cai H, Cao L, Wu J, Hu M, et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J Exp Bot. 2013;64(8):2155–69.
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol. 1999;17(3):287–91.
Zhang X, Zhang B, Li MJ, Yin XM, Huang LF, Cui YC, Wang ML, Xia X. OsMSR15 encoding a rice C2H2-type zinc finger protein confers enhanced drought tolerance in transgenic Arabidopsis. J Plant Biol. 2016;59(3):271–81.
Mittal A, Gampala SS, Ritchie GL, Payton P, Burke JJ, Rock CD. Related to ABA-Insensitive3(ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol J. 2014;12(5):578–89.
Chen M, Wang QY, Cheng XG, Xu ZS, Li LC, Ye XG, Xia LQ, Ma YZ. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem Biophys Res Commun. 2007;353(2):299–305.
Chen M, Xu Z, Xia L, Li L, Cheng X, Dong J, Wang Q, Ma Y. Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean (Glycine max L.). J Exp Bot. 2009;60(1):121–35.
Jin T, Chang Q, Li W, Yin D, Li Z, Wang D, Liu B, Liu L. Stress-inducible expression of GmDREB1 conferred salt tolerance in transgenic alfalfa. Plant Cell Tissue Organ Cult. 2009;100(2):219–27.
Nakashima K, Shinwari ZK, Sakuma Y, Seki M, Miura S, Shinozaki K, Yamaguchi-Shinozaki K. Organization and expression of two Arabidopsis DREB2 genes encoding DRE-binding proteins involved in dehydration- and high-salinity-responsive gene expression. Plant Mol Biol. 2000;42(4):657–65.
Wang RK, Wang CE, Fei YY, Gai JY, Zhao TJ. Genome-wide identification and transcription analysis of soybean carotenoid oxygenase genes during abiotic stress treatments. Mol Biol Rep. 2013;40(8):4737–45.
Gao SQ, Chen M, Xu ZS, Zhao CP, Li L, Xu HJ, Tang YM, Zhao X, Ma YZ. The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol Biol. 2011;75(6):537–53.
Mutava RN, Prince SJK, Syed NH, Song L, Valliyodan B, Chen W, Nguyen HT. Understanding abiotic stress tolerance mechanisms in soybean: a comparative evaluation of soybean response to drought and flooding stress. Plant Physiol Biochem. 2015;86:109–20.
Bresson J, Varoquaux F, Bontpart T, Touraine B, Vile D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. New Phytol. 2013;200(2):558–69.
Zhao SP, Xu ZS, Zheng WJ, Zhao W, Wang YX, Yu TF, Chen M, Zhou YB, Min DH, Ma YZ, et al. Genome-wide analysis of the RAV family in soybean and functional identification of GmRAV-03 involvement in salt and drought stresses and exogenous ABA treatment. Front Plant Sci. 2017;8:905.
Liu P, Xu ZS, Lu PP, Hu D, Chen M, Li LC, Ma YZ. A wheat PI4K gene whose product possesses threonine autophophorylation activity confers tolerance to drought and salt in Arabidopsis. J Exp Bot. 2013;64(10):2915–27.
Kereszt A, Li DX, Indrasumunar A, Nguyen CDT, Nontachaiyapoom S, Kinkema M, Gresshoff PM. Agrobacterium rhizogenes - mediated transformation of soybean to study root biology. Nat Protoc. 2007;2(4):948–52.
Wang Y, Li T, John SJ, Chen M, Chang J, Yang G, He G. A CBL-interacting protein kinase TaCIPK27 confers drought tolerance and exogenous ABA sensitivity in transgenic Arabidopsis. Plant Physiol Biochem. 2018;123:103–13.
Tian W, Hou CC, Ren ZJ, Pan YJ, Jia JJ, Zhang HW, Bai FL, Zhang P, Zhu HF, He YK, et al. A molecular pathway for CO2 response in Arabidopsis guard cells. Nat Commun. 2015;6.
Lv S, Yang A, Zhang K, Wang L, Zhang J. Increase of glycinebetaine synthesis improves drought tolerance in cotton. Mol Breed. 2007;20(3):233–48.
Lu S, Su W, Li H, Guo Z. Abscisic acid improves drought tolerance of triploid bermudagrass and involves H2O2- and NO-induced antioxidant enzyme activities. Plant Physiol Biochem. 2009;47(2):132–8.
Guo Z, Ou W, Lu S, Zhong Q. Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem. 2006;44(11–12):828–36.
Arthur E, Tuller M, Moldrup P, Resurreccion AC, Meding MS, Kawamoto K, Komatsu T, de Jonge LW. Soil specific surface area and non-singularity of soil-water retention at low saturations. Soil Sci Soc Am J. 2013;77(1):43–53.
Acknowledgements
We are grateful to Dr. Li-Juan Qiu (Institute of Crop Science, Chinese Academy of Agricultural Sciences) for providing the soybean seeds.
Funding
This research was financially supported by the National Transgenic Key Project of the Ministry of Agriculture of China (2018ZX0800909B) for designing and performing experiments, and the National Natural Science Foundation of China (31871624) for data analysis, writing and revising the manuscript.
Author information
Authors and Affiliations
Contributions
ZSX and JHZ coordinated the project, conceived and designed experiments, and edited the manuscript; XJM performed experiments and wrote the first draft; TFY, XHL, WJZ, XYC and JM conducted the bioinformatic work and performed experiments; JC, YBZ and MC provided analytical tools and managed reagents; YZM coordinated the project; XJM contributed with valuable discussions. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This research was not applicable for ethics approval and consent to participate.
Consent for publication
This research is not applicable to consent for publication.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Fig. S1.
Sequence alignment of the conserved domains of GmNFYA5 and members of NF-YA family in Arabidopsis. (A) Sequence alignment of the conserved domains in GmNFYA5 and 10 members of NF-YA family in Arabidopsis. Two subdomains and the linker are underlined. Asterisks indicate critical amino acids. (B) Phylogenetic analysis of GmNFYA5 with 10 members of NF-YA family in Arabidopsis. The unrooted neighbor joining tree was constructed using MEGA 7.0.
Additional file 2: Fig. S2.
Sequence of RNAi-GmNFYA5. The hairpin structure is composed of three sequences: the positive sequence of RNAi-GmNFYA5 in blue, the reverse complementary sequence in green, and intron of GmNFYA5 in purple. Restriction sites NcoI and BstEII are in red above the horizontal line. The sequence was inserted into the pCAMBIA3301 vector to generate a pCAMBIA3301:RNAi-GmNFYA5 construct.
Additional file 3: Fig. S3.
Ion leakage in 35S:GmNFYA5 Arabidopsis plants at the seedling stage under normal and drought conditions. Data represent mean SD for three biological replicates. Significant differences at P < 0.05 are indicated by different letters above the columns.
Additional file 4: Table S1.
List of primers for qRT-PCR used in this study.
Additional file 5: Table S2.
Promoter sequence analysis of genes up-regulated by GmNFYA5 in transgenic Arabidopsis and soybean lines.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, XJ., Yu, TF., Li, XH. et al. Overexpression of GmNFYA5 confers drought tolerance to transgenic Arabidopsis and soybean plants. BMC Plant Biol 20, 123 (2020). https://doi.org/10.1186/s12870-020-02337-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-020-02337-z