Abstract
Background
Sodium formononetin-3ʹ-sulphonate (Sul-F) may alleviate I/R injury in vivo with uncertain mechanism. Endoplasmic reticulum (ER) stress-mediated apoptosis participates in the process of cerebral ischemia‐reperfusion (I/R) injury. Our aim is to figure out the effect of Sul-F on cerebral I/R injury and to verify whether it works through suppressing ER stress-mediated apoptosis.
Results
The cerebral lesions of middle cerebral artery occlusion (MCAO) model in SD rats were aggravated after 24 h of reperfusion, including impaired neurological function, increased infarct volume, intensified inflammatory response and poor cell morphology. After intervention, the edaravone (EDA, 3 mg/kg) group and Sul-F high-dose (Sul-F-H, 80 mg/kg) group significantly alleviated I/R injury via decreasing neurological score, infarct volume and the serum levels of inflammatory factors (TNF-α, IL-1β and IL-6), as well as alleviating pathological injury. Furthermore, the ER stress level and apoptosis rate were elevated in the ischemic penumbra of MCAO group, and were significantly blocked by EDA and Sul-F-H. In addition, EDA and Sul-F-H significantly down-regulated the ER stress related PERK/eIF2α/ATF4 and IRE1 signal pathways, which led to reduced cell apoptosis rate compared with the MCAO group. Furthermore, there was no difference between the EDA and Sul-F-H group in terms of therapeutic effect on cerebral I/R injury, indicating a therapeutic potential of Sul-F for ischemic stroke.
Conclusions
Sul-F-H can significantly protects against cerebral I/R injury through inhibiting ER stress-mediated apoptosis in the ischemic penumbra, which might be a novel therapeutic target for ischemic stroke.
Similar content being viewed by others
Introduction
Ischemic stroke is a common central nervous system disease and a common cause of death and disability around the world [1]. Ischemic stroke accounts for about 70% of all strokes, which is caused by insufficient blood supply and results in the immediate depletion of oxygen and glucose in brain tissue [2]. Timely restoration of blood flow and reoxygenation remain the widely accepted methods, although considerable progress has been made in the treatment of cerebral ischemia [3].
In terms of treatment, tissue plasminogen activator (t-PA) is the only thrombolytic drug approved by the Food and Drug Administration for the therapy of ischemic stroke, which dissolves thrombus by activating a proteolytic enzyme [4]. Early use of thrombolytic agents is beneficial for the recovery, rehabilitation and prognosis of acute ischemic stroke patients. However, there are still many obstacles that blocked the application of the thrombolytic drugs, such as narrow therapeutic window and high risk of hemorrhagic transformation [5, 6]. In addition, the cerebral I/R injury may occur after the restoration of blood circulation in the ischemic brain, seriously affecting neurons and ultimately leading to neuronal apoptosis [7]. Therefore, it is still necessary to explore drugs with wide clinical adaptability and high safety.
In China, traditional Chinese medicine (TCM) is widely used in the management of ischemic stroke, based on the advantage of promoting blood circulation to dissipate blood stasis. Buyang Huanwu Decoction (BHD), a classic prescription for stroke treatment, plays a protective role in cerebral I/R injury in vivo by promoting neurogenesis [8, 9], inhibiting neural apoptosis and alleviating inflammation [10], stimulating angiogenesis and improving cerebral circulation [11]. Accumulating evidences have revealed that multiple components of BHD could ameliorate the negative effect of stroke [12,13,14,15,16]. Formononetin (C16H12O4), an isoflavone compound separated from BHD, has demonstrated diverse pharmacological capabilities, including neuroprotection [17], anti-inflammation [18], anti-oxidative stress [19], anti-apoptotic [20], and anti-tumor [21, 22]. However, poor water solubility limited the bioavailability of formononetin in central nervous system.
Subsequently, Sul-F (C16H11O7SNa, Chinese patent: ZL200710017326.5), a sulfonated derivative of formononetin, has been synthesized and overcomes the above disadvantage. Recent researches revealed that Sul-F exerted beneficial effects in multiple cardiovascular and cerebrovascular diseases, including acute myocardial infarction [23]and stroke [24] in animal models. Moreover, Sul-F can reduce permeability of blood brain barrier (BBB) after cerebral ischemic injury as well as possess the effect of anti-apoptosis and anti-thrombosis [25]. These studies suggested that Sul-F maybe not only a potentially effective drug for the treatment of ischemic stroke, but also a gospel of patients with ischemic stroke. Thus, it is of high importance to evaluate the benefits of Sul-F administration and the underlying mechanisms.
The mechanisms related to cerebral I/R injury are still far from clear and an effective prevention for cerebral I/R injury has not been established yet. Recent findings have shown that endoplasmic reticulum (ER) stress is an important signal pathway of neuronal injury caused by cerebral I/R injury [26,27,28,29]. ER plays vital roles in protein translocation, modification and folding, which is an essential organelle in eukaryotic cells [30]. When subjected to various strong stimulating factors, including nutrient deficiencies, Ca2+ metabolic imbalance, toxin stimulation and sustained oxidative stress stimulation, the cell homeostasis will be broken, which further leads to the massively accumulation of the misfolded and unfolded proteins in ER. Thus, the ER stress and unfolded protein response (UPR) will be initiated to help the misfolded and unfolded proteins restore to its normal structure through the activation of PERK, IRE1, and ATF4. By these processes, ER stress rebalances intercellular homeostasis and protects cells from various stimulus. However, overly-activated ER stress and UPR can cause damages [31]. Increased ER stress is observed in the ischemic penumbra of cerebral I/R injury rat model [32, 33]. Besides, inhibiting ER stress with compounds can significantly protect neurons against ischemic injury [26, 29, 34]. Even though evidences indicate that Sul-F is involved in the neuron protection against focal cerebral I/R injury [1], whether it can maintain ER homeostasis and reduce ER stress mediated neuronal apoptosis in cerebral I/R injury rat is still unknown.
In this study, we investigated whether Sul-F treatment could protect neuron against cerebral I/R injury. Further, we explored the protective effects of Sul-F through inhibiting apoptosis in penumbra. Finally, the underlying mechanism of its anti-apoptosis ability was further revealed.
Materials and methods
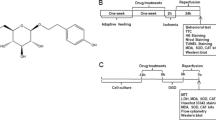
Chemicals
Edaravone injection was purchased from Sinopharm Group Guorui Pharmaceutical Co. LTD (China). Sul-F (> 95% pure) was bought from Shijiazhuang Hairui Pharmaceutical Technology Co. LTD (China). 2,3,5-triphenylte-trazolium chloride (TTC) was acquired from Sigma (USA). Hematoxylin-eosin staining (HE) kit and terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling (TUNEL) kit were purchased from Biyuntian Biotechnology Co. LTD (China). Bcl-2, Caspase3, Bax, CHOP, p-PERK, p-eIF2α, p-IRE1, Caspase12 and ATF4 primary antibodies were purchased from Affinity (China).
Animals
All male Sprague-Dawley (SD) rats (grade SPF) were purchased from the Sibford Co. LTD (Beijing, China). The rats of 290-310 g (8-10 weeks old) were supplied with freely accessible food and water, and were housed in an environment with standard lighting conditions (12 h light/dark cycle), controlled temperature (20-25 °C) and humidity (40-60%). Before building MCAO model, all rats were fasted 12 h with freely accessible water.
Middle cerebral artery occlusion (MCAO)
MCAO rat model was established with an intraluminal filament method as previously described [35]. After the rat was anesthetized with 2% sodium pentobarbital (0.3 mL/100 g) intraperitoneally (i.p), the anterior cervical region was exposed and opened along the midline of the neck to isolate the left common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). The ECA was ligated and CCA was clipped with an arterial clamp. In order to block the blood supply of the left middle cerebral artery, the monofilament nylon suture with a round tip was inserted into ICA via CCA with a depth of 18 ~ 20 mm. After 2 h of ischemia, the suture plug was removed about 0.5 cm and the blood perfusion was restored. The sham operation group was objected to the same operation without inserting a monofilament. The presence of neurological deficit was measured by Zea-Longa method and score point of 1-3 indicated successful modeling and inclusion in the experiment. The specific scoring criteria was as follows: 0, no neurological deficit; 1, failed to fully extend their left forepaw; 2, circling to the left when walking; 3, falling to the left when walking; 4, unable to walk spontaneously or has stroke-related death.
Animal grouping and drug administration
Based on experimental target and the principle of randomization, all the rats were divided into 5 groups including sham group (Sham group), ischemia-reperfusion group (MCAO group), edaravone group (EDA group, 3 mg/kg), Sul-F high dose group (Sul-F-H group, 80 mg/kg) and Sul-F low dose group (Sul-F-L group, 40 mg/kg). All rats were given the drug for the first time at 0 h of reperfusion by tail vein injection with a volume of 4 mL/kg. The concentration of EDA injection was 30 mg/40 mL, and the dose was 3 mg/kg. The concentration of Sul-F low dose group was 200 mg/20 mL and the dose was 40 mg/kg. Sul-F high-dose group was 400 mg/20 mL and 80 mg/kg. Rats in MCAO group and Sham group were injected with equal volume of normal saline via tail vein. Rats in each group were given the second dose at 12 h reperfusion.
Neurological impairment score
According to the blind principle, neurological deficiency was evaluated by the trained investigators after 24 h of reperfusion. Neurological behaviors of all rats were evaluated by a 5-point scale, as referred previously [36]. The higher the score, the more serious nerve function injury is.
Histopathological examination
At 24 h post reperfusion, the rats were anesthetized with 2% sodium pentobarbital (0.3 mL/100 g) i.p and then underwent quick decapitation. A portion of rats’ brain tissue was dissected out and fixed in 4% paraformaldehyde for 48 h. At the end of fixation, tissue processing was done to dehydrate in ascending grades of alcohol, clearing in xylene and embedded in paraffin wax. Paraffin wax embedded tissue blocks were sectioned at 4 µm thickness with the Rotary Microtome (Leica, Germany). All the slides of brains were stained with HE. Then all the pathological changes were observed under optical microscope (Leica, Germany).
TTC staining
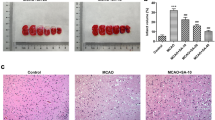
The experiment was conducted according to previous study [1]. When neurological deficit examination was completed, the rats were deeply anesthetized with 2% sodium pentobarbital, then brains were taken out and sectioned coronally with a thickness of 2 mm after freezing in -20 °C refrigerator for 20 min. Before fixed with 4% paraformaldehyde overnight at room temperature, the brain tissue slices were stained with 2% 2, 3, 5-triphenyl tetrazolium chloride (TTC) for 0.5 h at room temperature in the dark. The results showed that the surviving part of the brain section was red, while the dead part was pale. Image J (Version 1.49) was used to measure the infarct area and the whole area of each brain slice (Fig. 1A). The infarct volume ratio was calculated as follows: infarct volume ratio % = (infarct volume / whole brain volume) × 100%.
The effects of Sul-F treatment on infarct volume, neurological score and pathological changes of I/R injured brains. A Infarct volume was determined with TTC staining. The white area defined the infarct area (n = 6). B The infarct volume was expressed as the ratio of (infarct volume / the whole brain volume) × 100% (n = 6). C Neurological score (n = 15). The neurological function of the rats after 24 h of reperfusion was evaluated according to the Zea-Longa score standard. The higher the score, the more severe the neurological impairment is. Neurological score data was presented as M (P25 ~ P75). **P < 0.01 compared with the Sham group, △△P < 0.01 compared with the MCAO group. Data were presented as mean ± SEM from at least three independent experiments. **P < 0.01 compared with Sham group, △P < 0.05 and △△P < 0.01 compared with the MCAO group. D Histopathological characteristics (n = 6). After 24 h reperfusion, the ischemic penumbra area of brain tissue was stained with HE, which was observed at 100 × and 400 × , respectively. Scale bar = 100 μm
ELISA analysis
The blood collected from the aortaventralis was clotted in centrifugal tube (Henan Wenmei Experiment Co. Ltd, Henan, China) in room temperature for 20 min and centrifuged at 12,000 rpm at 4 ℃ for 15 min to obtain the serum. The levels of TNF-α, Interleukin-6 (IL-6), and IL-1β in the serum were measured using ELISA kits (Beijing Bioske Biomedical Technology Co., Ltd, Beijing, China) according to the manufacturer’s instructions.
TUNEL staining
TUNEL staining was used to detect neuronal apoptosis. Briefly, the brain slices were dewaxed and rehydrated. Then, in order to block endogenous peroxidase activity, the sections were incubated with a methanol solution containing 3% H2O2 for 10 min at room temperature. Afterward, treated with TUNEL reaction mixture, the brain sections were maintained in a 37 °C incubator for 1 h. The fluorescence was captured with a laser confocal microscopy (Leica, Germany). The results were presented as apoptosis ratio = (TUNEL positive cells)/(DAPI cells) × 100%.
Western blot analysis
Ischemic penumbra samples were obtained from ischemic hemisphere and preserved at -80 ℃. The BCA protein assay kit (MDL, Beijing, China) was used to measure the protein concentration of brain samples after the tissue was homogenized and lysed. Equal amounts of proteins were separated by sodium dodecyl sulfate polyacrylamide gel and transferred to polyvinylidene fluoride (PVDF) membrane. After blocking with 5% non-fat milk, PVDF membrane was incubated with primary antibody in 4 ℃ overnight. Then, the membrane was washed three times with Tris-buffered saline with Tween 20 (TBST). Thereafter, membranes were incubated with horseradish peroxidase-labeled secondary antibody for 2 h at room temperature. After incubation, the membranes were washed three times again with TBST. Afterwards, chemiluminescence imaging system (Clinx, Shanghai, China) was used to image and detect blots. All protein bands were quantitated by Image-Pro Plus.
Quantitative real‑time polymerase chain reaction (qRT-PCR) analysis
Trizol (Invitrogen, USA) was used to extract total RNA from ischemic penumbra tissue and SuperScript III Reverse Transcription Kit (ABI-invitrogen, USA) was used to prepare cDNA. All qRT-PCR reactions were conducted by ABI PRISM 7500 Sequence Detector System (Applied Biosystems, CA, USA). Relative gene expression was quantified via the 2−ΔΔCt approach. The primer sequences used in the qRT-PCR were as Additional file 1: Table S1.
Statistical analysis
All data was displayed in mean ± standard error of the mean (SEM) and analyzed by SPSS (version 20.0, Chicago, USA). The normality of the data was checked with Shapiro-Wilk normality test, while P > 0.05 was considered to fit a normal distribution. Student’s t-test was used to analyze the statistical significance between two groups, one-way analysis of variance (ANOVA) was used to analyze the statistical significance among three or more groups. P value < 0.05 was considered statistically significant (Additional files 2, 3).
Results
Effect of Sul-F on cerebral I/R injury
TTC assay was used to detect the brain infarct volume and Zea-Longa score was applied to evaluate the neurological deficiency. HE staining was used to investigate the histopathological changes in the brain. Evidently, the infarct volume and neurological score significantly increased in the MCAO group (47.88 ± 14.07% vs. 4.67 ± 2.94% of Sham group, P < 0.01; 3(3 ~ 3) vs. 0(0 ~ 0) of Sham group, P < 0.01). Compared with the MCAO group, the administration of EDA and Sul-F significantly decreased the infarct volume and neurological score (18.10 ± 5.08 and 21.26 ± 5.06% vs. 47.88 ± 14.07%, P < 0.05; 2(1 ~ 2) and 1(1 ~ 2) vs. 3(3 ~ 3), P < 0.01) (Fig. 1B, C). There was no significant difference between the EDA group and the Sul-F-H group (18.10 ± 5.08 vs. 21.26 ± 5.06, P > 0.05; 2(1 ~ 2) vs. 2(1 ~ 2), P > 0.05).
As shown in Fig. 1D (red narrow), swelling and pyknosis in cytoplasm and morphologic changes of apoptosis with karyopyknosis, karyorrhexis and apoptotic body were found in the MCAO group. The intervention of EDA and Sul-F retained the basic structure of neurons and significantly reduced the morphologic changes of nerve cells. Moreover, high dose Sul-F exhibited a better effect than that of low dose.
Effects of Sul-F on inflammation
The levels of TNF-α, IL-1β and IL-6 were increased when cerebral I/R injury occurred (TNF-α: 61.45 ± 2.76 vs. 39.82 ± 3.13 of Sham group, P < 0.01; IL-1β: 51.38 ± 7.03 vs. 27.58 ± 3.82 of Sham group, P < 0.01; IL-6: 93.53 ± 5.03 vs. 29.43 ± 1.54 of Sham group, P < 0.01). Further, the levels of TNF-α, IL-1β and IL-6 were decreased in the EDA, Sul-F-H (80 mg/kg) and Sul-F-L (40 mg/kg) groups, compared to the MCAO group (Fig. 2, P < 0.01).
Sul-F treatment significantly alleviated the I/R-induced serum levels of inflammatory factors including TNF-α, IL-6 and IL-1β, detected by ELISA (n = 13). A The concentration of IL-6 in serum of rats. B The concentration of TNF-α in serum of rats. C The concentration of IL-1β in serum of rats. Data were presented as mean ± SEM from at least three independent experiments. **P < 0.01 compared with the Sham group, △△P < 0.01 compared with the MCAO group
Sul-F attenuated cell apoptosis via inhibiting ERS induced by MCAO
Damage of nerve cells and impairment of neurological function were observed in MCAO rats and Sul-F alleviated the injury of nerve cells and improved the neurological function (Fig. 1). Furthermore, we examined cell apoptosis in the ischemic penumbra by TUNEL staining and Western blot. As shown in Fig. 3A, B, compared with the Sham group, abundant apoptotic cells were found in the penumbra of rats in the MCAO group (52.26 ± 2.06 vs. 10.99 ± 1.36, P < 0.01). However, the apoptotic rate was significantly reduced after the administration of Sul-F and EDA.
Sul-F treatment significantly alleviated the I/R-induced apoptosis in the penumbra of brain after 24 h reperfusion. A TdT-mediated dUTP Nick-End Labeling (TUNEL) staining of I/R-induced apoptosis in penumbra, which was imaged at 100 × . TUNEL cells (red) and the nuclei (DAPI, blue). B The I/R-induced apoptosis presented as ratio = (TUNEL positive cells) / (DAPI cells) × 100% (n = 5). C Representative western blot analysis of CHOP, Bax (pro-apoptotic protein) and Bcl-2 (anti-apoptotic protein) in the penumbra of brain tissue (n = 6). D Histogram showing quantification of images in C (n = 6). The results were normalized to β-actin expression. E Histogram showing quantification of qRT-PCR (n = 6). Data were expressed as the mean ± SEM from different assays. **P < 0.01 compared with the Sham group, △P < 0.05 and △△P < 0.01 compared with the MCAO group, #P < 0.05 and ##P < 0.01 compared with the EDA group, aaP < 0.01 compared with the Sul-F-H group
To further detect the molecular mechanism of Sul-F on apoptosis, we measured the expression of proteins associated with apoptosis (pro-apoptotic protein: Bax; anti-apoptotic protein: Bcl-2; the marker of apoptosis induced by ER stress: CHOP) by Western blot. Compared with the Sham group, the protein expression levels of Bax, and CHOP significantly increased (Bax: 0.76 ± 0.04 vs. 0.13 ± 0.03 of Sham group, P < 0.01; CHOP: 0.71 ± 0.08 vs. 0.14 ± 0.02 of Sham group, P < 0.01), and those of Bcl-2 decreased in the MCAO group (0.13 ± 0.02 vs. 0.61 ± 0.05 of Sham group, P < 0.01). Sul-F-H treatment resulted in the reduction of Bax (0.41 ± 0.02 vs. 0.76 ± 0.04 of MCAO group, P < 0.01), and CHOP (0.38 ± 0.04 vs. 0.71 ± 0.08 of MCAO group, P < 0.05), as well as the increase of Bcl-2 (0.40 ± 0.04 vs. 0.13 ± 0.02 of MCAO group, P < 0.01) level compared with the MCAO group (Fig. 3C, D). In addition, the mRNA expression levels of CHOP, Bax and Bcl-2 were detected by qRT-PCR in all specimens (n = 5). Increasing levels of CHOP mRNA (1.84 ± 0.01 vs. 1.11 ± 0.03 of Sham group, P < 0.01) and Bax mRNA (1.84 ± 0.00 vs. 1.03 ± 0.02 of Sham group, P < 0.01) expression were found in the ischemic penumbra regions while the mRNA relative quantity of Bcl-2 (0.42 ± 0.01 vs. 1.06 ± 0.03 of Sham group, P < 0.01) was decreased significantly in MCAO group. Sul-F-H treatment could significantly suppress CHOP mRNA (1.42 ± 0.01 vs. 1.84 ± 0.01 of MCAO group, P < 0.01) and Bax mRNA (1.44 ± 0.01 vs 1.84 ± 0.00 of MCAO group, P < 0.01) expression and enhance the mRNA relative quantity of Bcl-2 (0.72 ± 0.01 vs. 0.42 ± 0.01 of MCAO group, P < 0.05) (Fig. 3E). These data suggested that Sul-F protected against MCAO-induced neuron injury via inhibition of cell apoptosis; moreover, inhibiting neuronal apoptosis induced by ER stress might be involved in the mechanism of the Sul-F protective effect on cerebral I/R injury.
Sul-F protected against I/R injury induced by MCAO through suppressing apoptosis via the PERK and IRE1 signaling pathways
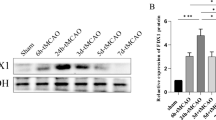
To further investigate whether ERS was involved in the effect of Sul-F on apoptosis in MCAO rats, the expression levels of ERS marker proteins including phosphorated-PERK (p-PERK), phosphorated-eIF2α (p-eIF2α), ATF4, phosphorated-IRE1 (p-IRE1), Caspase12 and Caspase3 were detected by Western blot. As shown in Fig. 4, compared with the Sham group, the relative protein expression level of p-PERK (0.56 ± 0.02 vs. 0.11 ± 0.00 of Sham group, P < 0.01), p-eIF2α (0.60 ± 0.08 vs. 0.08 ± 0.02 of Sham group, P < 0.01), ATF4 (0.68 ± 0.08 vs. 0.13 ± 0.02 of Sham group, P < 0.01), p-IRE1 (0.84 ± 0.03 vs. 0.16 ± 0.01 of Sham group, P < 0.01), Caspase12 (0.71 ± 0.11 vs. 0.12 ± 0.01 of Sham group, P < 0.01), and Caspase3 (0.78 ± 0.07 vs. 0.13 ± 0.01 of Sham group, P < 0.01) were significantly increased in the MCAO group. Sul-F-H treated group significantly decreased the expression levels of p-PERK (0.28 ± 0.01 vs. 0.56 ± 0.02 of MCAO group, P < 0.01), p-eIF2α (0.27 ± 0.02 vs. 0.60±0.08 of MCAO group, P < 0.01), ATF (0.36 ± 0.04 vs. 0.68 ± 0.08 of MCAO group, P<0.01), p-IRE1 (0.49 ± 0.02 vs. 0.84 ± 0.03 of MCAO group, P < 0.01), Caspase12 (0.40 ± 0.05 vs. 0.71 ± 0.0.11 of MCAO group, P < 0.05) and Caspase3 (0.40 ± 0.02 vs. 0.78 ± 0.07 of MCAO group, P < 0.01).
Sul-F treatment significantly attenuated the I/R-induced ER stress (n = 6). A At 24 h post-reperfusion, levels of p-PERK, p-eIF2α, ATF4 were evaluated with western blot. B The histogram showed quantification of images in bar diagram. The relative value of band gray was measured with Image J (1.49 V) and normalized to that of β-actin. C The mRNA levels of PERK, eIF2α and ATF4 were evaluated with qRT-PCR. D Representative western blot results of p-IRE1, Caspase12 and Caspase3 were shown. E The histogram showed quantification of images in bar diagram. The relative value of band gray was measured with Image J (1.49 V) and normalized to that of β-actin. F The mRNA levels of IRE1, Caspase12 and Caspase3 were evaluated with qRT-PCR. Data were expressed as the mean ± SEM from different assays. **P < 0.01 compared with Sham group, △P < 0.05 and △△P < 0.01 compared with the MCAO group, #P < 0.05 and ##P < 0.01 compared with the EDA group, aP < 0.05 and aaP < 0.01 compared with the Sul-F-H group
Further investigation of qRT-PCR for ER stress marker genes revealed that there was a noticeable increase in PERK mRNA (1.82 ± 0.01 vs. 1.08 ± 0.02 of Sham group, P < 0.01), eIF2α mRNA (1.83 ± 0.01 vs. 1.06 ± 0.03 of Sham group, P < 0.01), ATF4 mRNA (1.82 ± 0.01 vs. 1.06 ± 0.03 of Sham group, P < 0.01), IRE1 mRNA (1.83 ± 0.01 vs. 1.09 ± 0.02 of Sham group, P < 0.01), Caspase12 mRNA (1.83 ± 0.01 vs. 1.07 ± 0.07 of Sham group, P < 0.01) and Caspase3 mRNA (1.83 ± 0.01 vs. 1.07 ± 0.03 of Sham group, P < 0.01) expression of the ischemic penumbra regions in MCAO group. Sul-F-H treatment could significantly suppress PERK mRNA (1.44 ± 0.01 vs. 1.82 ± 0.01 of MCAO group, P < 0.01), eIF2α mRNA (1.43 ± 0.01 vs. 1.83 ± 0.01 of MCAO group, P < 0.01), ATF4 mRNA (1.46 ± 0.01 vs. 1.82 ± 0.01 of MCAO group, P < 0.01), IRE1 mRNA (1.43 ± 0.01 vs. 1.83 ± 0.01 of MCAO group, P < 0.01), Caspase12 mRNA (1.45 ± 0.01 vs. 1.83 ± 0.01 of MCAO group, P < 0.01) and Caspase3 mRNA (1.44 ± 0.01 vs. 1.83 ± 0.01 of MCAO group, P < 0.01) expression (Fig. 4E, F). Together, the above data revealed that Sul-F exerted the protective effect in cerebral I/R injury through inhibited ER stress-induced apoptosis of neuron cells in ischemic penumbra.
Discussion
Ischemic stroke causes an estimated 4.4 million deaths each year worldwide, placing a huge physical, emotional and financial burden on patients, families and national health service [37]. Current therapeutic options in stroke are still limited and brain injury caused by cerebral I/R remains a major challenge for the application of conventional management approaches. So there is an urgent need for a comprehensive strategy including neuroprotection and maximizing cerebral reperfusion rate to reduce reperfusion injury, and a comprehensive understanding of the pathophysiological process involved in cerebral I/R injury.
The neuronal damage in the ischemic region (penumbra) after cerebral I/R injury is slow and reversible [38, 39]. Therefore, the key of the clinical treatment is to save the ischemic penumbra of dying neurons and promote damage nerve function recovery. TCM has accumulated a wealth of experience in the treatment of stroke and modern pharmacology studies have shown that many Chinese herbal extracts can protect the neurological function from cerebral I/R injury by reducing penumbra apoptosis in a variety of ways [40,41,42,43]. Sul-F, a synthesized compound of formononetin, exerted beneficial effects in multiple cardiovascular and cerebrovascular diseases, including acute myocardial infarction [23] and stroke [24]. The aim of our study is to figure out the effect of Sul-F on cerebral I/R injury and to verify whether it works through suppressing ER stress-mediated apoptosis.
First of all, we want to figure out whether Sul-F has therapeutic effect on the neuron damage induced by cerebral I/R. To this end, we used MCAO model in rats established by wire embolization to mimic the alterations of cerebral I/R injury [44]. Consistent with previous studies [45, 46], our results indicated that I/R increased the numbers of TUNEL-positive cells and protein expression levels of CHOP and Bax after 24 h of cerebral I/R in the penumbra, which partially indicated that apoptosis was activated in the penumbra. Our results demonstrated that Sul-F alleviated neurological deficits evaluated by Zea-Longa, decreased infarct volume, and ameliorated pathological injury of brain tissue after 24 h of reperfusion. These data suggest that Sul-F could attenuate neuronal damage during cerebral I/R injury by inhibiting apoptosis in the penumbra area.
Inflammatory response plays an important role in cerebral I/R injury. Activation of microglia and astrocytes and exudation of leukocytes are key steps of inflammatory response in the central nervous system [47]. During cerebral I/R injury, the activated inflammatory cells synthesize and release inflammatory mediators, which in turn can further activate inflammatory cells, forming a vicious cycle and aggravating brain injury. IL-1 is secreted by activated astrocytes, oligodendrocytes and infiltrating macrophages after cerebral ischemia, which can promote the expression of adhesion molecules in endothelial cells, thereby aggravating local inflammatory response [48].The level of IL-1β in brain tissue of MCAO model rats began to increase at 6 h and reached the peak at 24 h, indicating that IL-1β was involved in the inflammatory response after cerebral I/R injury [49]. IL-6 plays a dual role in cerebral I/R injury. In the acute phase, IL-6 acts as an inflammatory mediator to promote brain injury, while in the subacute phase, it acts as a neurotrophic mediator to play a neuroprotective role [50]. TNF-α, mainly derives from activated glial cells, especially microglia, has complex biological activities, and its inhibitors can alleviate cerebral I/R injury [51].Previous researches demonstrated that a variety of TCM monomers could exert neuroprotective effects by reducing the levels of the inflammatory mediators TNF-α, IL-1β and IL-6 induced by brain ischemia reperfusion [52,53,54]. In addition, the inflammatory response is related to the endoplasmic reticulum stress signaling pathway, which participate together in the development of cerebral I/R injury [55, 56]. Meanwhile, our result showed that the levels of TNF-α, IL-1β, and IL-6 elevated accompanied with the activation of ERS signaling pathway in the MCAO rats. Interestingly, Sul-F treatment can significantly decrease the concentration of these inflammatory mediators and also inhibit the activation of the ER stress pathway, which suggested that the neuroprotective effects of Sul-F may be associated with the inhibition of neurogenic inflammation through suppressing ER stress pathway.
EDA, a free radical scavenger [57], is an efficacy drug in the therapy of cerebral infarction [58] and has been recommended for AIS treatment by Chinese and Japanese stroke care guidelines [59, 60]. EDA can scavenge many free radicals, such as hydroxyl (-OH), nitric oxide (NO) and peroxynitrite anion (ONOO-), and sequentially relieves cerebral oedema and inhibits delayed neuron death [61]. In addition, we also found that EDA could play a protective role on cerebral ischemia-reperfusion injury by inhibiting apoptosis mediated by ER stress signaling pathway, consistent with the previous study [62]. However, EDA is known to have a fairly short T1/2 and it should not be taken more than twice a day for those with impaired liver and kidney function based on its possible side effects [63]. Therefore, it is imperative to develop new pharmaceuticals. In preliminary study, it has been demonstrated that Sul-F at doses up to 2000 mg has no hepatorenal toxicity [64]. Additionally, we found that there was no difference between EDA (3 mg/kg) group and Sul-F-H (80 mg/kg) group in terms of therapeutic effect on cerebral ischemia–reperfusion injury, indicating the potential of Sul-F to be a clinical drug for the treatment of ischemic stroke.
ER stress is one of the main molecular events underlying the pathology of cerebral I/R injury [65]. Three major transmembrane proteins are involved in the ER stress-activated UPR: IRE1, PERK and ATF4. All these proteins are coupled with the GRP78 and stay inactive under physiological conditions. When the UPR is activated, after the GRP78 dissociation, the IRE1 and PERK oligomerize and phosphorylate to activate their downstream signals, and ATF4 is cleaved by the golgi and moves into the nucleus to act as a transcription promoter [66]. Subsequently, the activated PERK (p-PERK) promotes phosphorylation of eIF2α and activates selective translation of ATF4) [67]. ATF4 is an important mediator of UPR, which can promote cell survival by inducing amino acid metabolism, redox reaction, stress response and ER stress target genes of protein secretion. When cells are in stage of stress for a long time, ATF4 will activate the expression of its downstream target CHOP, a pro-apoptotic gene [68]. A previous study has indicated that CHOP gene transcription is one of the most critical pathways leading to apoptosis, and then, apoptosis can be regulated by regulating the expression of multiple anti-apoptotic and pro-apoptotic genes, such as Bcl-2 and Bax [66]. In addition, activation of IRE1 can promote the downstream Caspase12 signaling pathway to accelerate cell death [69]. Our present results indicated that Sul-F exerted its protection via suppression of Caspase12 signaling pathway.
Therefore, ER stress signaling pathway could be considered as the key molecular or signaling transduction pathway that modulates multiple targets in cerebral I/R injury. In the present study, we observed that I/R significantly activated ER stress evidenced by the increase in ATF4 as well as the hyper-phosphorylation of PERK and eIF2α, which was markedly reversed by Sul-F treatment. In conclusion, Sul-F treatment can rescue neurons against I/R injury through inhibiting PERK/eIF2α/ATF4 and IRE1/Caspase12/Caspase3 associated apoptosis pathways in the penumbra (Fig. 5).
Schematic diagram of the molecular mechanisms underlying the protective effects of Sul-F treatment against cerebral I/R injury. Sul-F treatment alleviated the I/R-induced activation of ER stress, thereby inhibiting multiple downstream pathways, including PERK/eIF2α/ATF4, IRE1/Caspase12/Caspase3 and CHOP/Bcl-2/Bax signal pathway
In summary, Sul-F treatment attenuates cerebral I/R injury by inhibiting ER stress mediated apoptosis in ischemic penumbra through suppression of PERK/eIF2α/ATF4 and IRE1/Caspase12/Caspase3 signaling pathway. Our findings shed light on the novel therapeutic strategy of the administration of Sul-F in ischemic stroke.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files.
Abbreviations
- ER:
-
Endoplasmic reticulum
- I/R:
-
Ischemia‐reperfusion
- Sul-F:
-
Sodium formononetin-3ʹ-sulphonate
- BBB:
-
Blood brain barrier
- MCAO:
-
Middle cerebral artery occlusion
- BHD:
-
Buyang Huanwu Decoction
- UPR:
-
Unfolded protein response
- CCA:
-
Common carotid artery
- ECA:
-
External carotid artery
- ICA:
-
Internal carotid artery
- ATF4:
-
Activating transcription factor 4
- Bcl-2:
-
B-cell lymphoma-2
- Bax:
-
Bcl-2-associated X protein
- Caspase-3:
-
Cysteine aspartic specific protease-3
- Caspase-12:
-
Cysteine aspartic specific protease-12
- CHOP:
-
C-EBP homologous protein
- eIF2α:
-
Eukaryotic initiation factor 2α
- FMN:
-
Formononetin
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- GRP78:
-
Glucose-regulated protein of 78 kDa
- IRE1:
-
Inositol-requiring enzyme 1
- -OH:
-
Hydroxyl
- NO:
-
Nitric oxide
- ONOO-:
-
Peroxynitrite anion
References
Feng D, Wang B, Wang L, Abraham N, Tao K, Huang L, Shi W, Dong Y, Qu Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J Pineal Res. 2017. https://doi.org/10.1111/jpi.12395.
Marciniec M, Sapko K, Kulczyński M, Popek-Marciniec S, Szczepańska-Szerej A, Rejdak K. Non-traumatic cervical artery dissection and ischemic stroke: a narrative review of recent research. Clin Neurol Neurosurg. 2019;187: 105561. https://doi.org/10.1016/j.clineuro.2019.105561.
Pan B, Sun J, Liu Z, Wang L, Huo H, Zhao Y, Tu P, Xiao W, Zheng J, Li J. Longxuetongluo capsule protects against cerebral ischemia/reperfusion injury through endoplasmic reticulum stress and MAPK-mediated mechanisms. J Adv Res. 2021;33:215–25. https://doi.org/10.1016/j.jare.2021.01.016.
Zhang Y, Li M, Li X, Zhang H, Wang L, Wu X, Zhang H, Luo Y. Catalytically inactive RIP1 and RIP3 deficiency protect against acute ischemic stroke by inhibiting necroptosis and neuroinflammation. Cell Death Dis. 2020;11(7):565. https://doi.org/10.1038/s41419-020-02770-w.
Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol. 2018;18(1):8. https://doi.org/10.1186/s12883-017-1007-y.
Zhou Y, Liao J, Mei Z, Liu X, Ge J. Insight into crosstalk between ferroptosis and necroptosis: novel therapeutics in ischemic stroke. Oxid Med Cell Longev. 2021;2021:9991001. https://doi.org/10.1155/2021/9991001.
Wei K, Wan L, Liu J, Zhang B, Li X, Zhang Y, Zhang C, Yao W. Downregulation of TRB3 protects neurons against apoptosis induced by global cerebral ischemia and reperfusion injury in rats. Neuroscience. 2017;360:118–27. https://doi.org/10.1016/j.neuroscience.2017.07.062.
Luo L, Deng S, Yi J, Zhou S, She Y, Liu B. Buyang Huanwu decoction ameliorates poststroke depression via promoting neurotrophic pathway mediated neuroprotection and neurogenesis. Evid Based Complement Alternat Med. 2017;2017:4072658. https://doi.org/10.1155/2017/4072658.
Li H, Peng D, Zhang SJ, Zhang Y, Wang Q, Guan L. Buyang Huanwu Decoction promotes neurogenesis via sirtuin 1/autophagy pathway in a cerebral ischemia model. Mol Med Rep. 2021. https://doi.org/10.3892/mmr.2021.12431.
Li JH, Liu AJ, Li HQ, Wang Y, Shang HC, Zheng GQ. Buyang huanwu decoction for healthcare: evidence-based theoretical interpretations of treating different diseases with the same method and target of vascularity. Evid Based Complement Alternat Med. 2014;2014: 506783. https://doi.org/10.1155/2014/506783.
Zhang ZQ, Song JY, Jia YQ, Zhang YK. Buyanghuanwu decoction promotes angiogenesis after cerebral ischemia/reperfusion injury: mechanisms of brain tissue repair. Neural Regen Res. 2016;11(3):435–40. https://doi.org/10.4103/1673-5374.179055.
Zhang WW, Xu F, Wang D, Ye J, Cai SQ. Buyang Huanwu decoction ameliorates ischemic stroke by modulating multiple targets with multiple components: in vitro evidences. Chin J Nat Med. 2018;16(3):194–202. https://doi.org/10.1016/s1875-5364(18)30047-5.
She Y, Shao L, Zhang Y, Hao Y, Cai Y, Cheng Z, Deng C, Liu X. Neuroprotective effect of glycosides in Buyang Huanwu decoction on pyroptosis following cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. 2019;242: 112051. https://doi.org/10.1016/j.jep.2019.112051.
Gao Q, Tian D, Han Z, Lin J, Chang Z, Zhang D, Ma D. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of buyang huanwu decoction in the treatment of ischemic stroke. Evid Based Complement Alternat Med. 2021;2021:8815447. https://doi.org/10.1155/2021/8815447.
Yan X, Wang S, Yu A, Shen X, Zheng H, Wang L. Cell chromatography-based screening of the active components in buyang huanwu decoction promoting axonal regeneration. Biomed Res Int. 2019;2019:6970198. https://doi.org/10.1155/2019/6970198.
Chen ZZ, Gong X, Guo Q, Zhao H, Wang L. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 α, VEGF and promotion β-ENaC expression. J Ethnopharmacol. 2019;228:70–81. https://doi.org/10.1016/j.jep.2018.09.017.
Sun T, Wang J, Huang LH, Cao YX. Antihypertensive effect of formononetin through regulating the expressions of eNOS, 5-HT2A/1B receptors and α1-adrenoceptors in spontaneously rat arteries. Eur J Pharmacol. 2013;699(1–3):241–9. https://doi.org/10.1016/j.ejphar.2012.10.031.
Li L, Wang Y, Wang X, Tao Y, Bao K, Hua Y, Jiang G, Hong M. Formononetin attenuated allergic diseases through inhibition of epithelial-derived cytokines by regulating E-cadherin. Clin Immunol. 2018;195:67–76. https://doi.org/10.1016/j.clim.2018.07.018.
Mu H, Bai YH, Wang ST, Zhu ZM, Zhang YW. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine. 2009;16(4):314–9. https://doi.org/10.1016/j.phymed.2008.07.005.
Tian Z, Liu SB, Wang YC, Li XQ, Zheng LH, Zhao MG. Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phytother Res. 2013;27(12):1770–5. https://doi.org/10.1002/ptr.4928.
Tay KC, Tan LT, Chan CK, Hong SL, Chan KG, Yap WH, Pusparajah P, Lee LH, Goh BH. Formononetin: a review of its anticancer potentials and mechanisms. Front Pharmacol. 2019;10:820. https://doi.org/10.3389/fphar.2019.00820.
Ong SKL, Shanmugam MK, Fan L, Fraser SE, Arfuso F, Ahn KS, Sethi G, Bishayee A. Focus on formononetin: anticancer potential and molecular targets. Cancers. 2019. https://doi.org/10.3390/cancers11050611.
Zhang S, Tang X, Tian J, Li C, Zhang G, Jiang W, Zhang Z. Cardioprotective effect of sulphonated formononetin on acute myocardial infarction in rats. Basic Clin Pharmacol Toxicol. 2011;108(6):390–5. https://doi.org/10.1111/j.1742-7843.2011.00676.x.
Dong Z, Shi Y, Zhao H, Li N, Ye L, Zhang S, Zhu H. Sulphonated formononetin induces angiogenesis through vascular endothelial growth factor/camp response element-binding protein/early growth response 3/vascular cell adhesion molecule 1 and Wnt/β-catenin signaling pathway. Pharmacology. 2018;101(1–2):76–85. https://doi.org/10.1159/000480662.
Sun Y, Liu N, Wang J, Chen L, Qian X, Chen L, Han Z, Sun J. Effect of formononetin on blood brain barrier integrity after cerebral ischemia reperfusion. Tianjin Pharmacy. 2021;33(01):1–3.
Zhu H, Zou L, Tian J, Lin F, He J, Hou J. Protective effects of sulphonated formononetin in a rat model of cerebral ischemia and reperfusion injury. Planta Med. 2014;80(4):262–8. https://doi.org/10.1055/s-0033-1360340.
Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8(10): e3080. https://doi.org/10.1038/cddis.2017.465.
Sun X, Liu H, Sun Z, Zhang B, Wang X, Liu T, Pan T, Gao Y, Jiang X, Li H. Acupuncture protects against cerebral ischemia-reperfusion injury via suppressing endoplasmic reticulum stress-mediated autophagy and apoptosis. Mol Med. 2020;26(1):105. https://doi.org/10.1186/s10020-020-00236-5.
Guo MM, Qu SB, Lu HL, Wang WB, He ML, Su JL, Chen J, Wang Y. Biochanin a alleviates cerebral ischemia/reperfusion injury by suppressing endoplasmic reticulum stress-induced apoptosis and p38mapk signaling pathway in vivo and in vitro. Front Endocrinol. 2021;12: 646720. https://doi.org/10.3389/fendo.2021.646720.
White A, Parekh RU, Theobald D, Pakala P, Myers AL, Van Dross R, Sriramula S. Kinin B1R activation induces endoplasmic reticulum stress in primary hypothalamic neurons. Front Pharmacol. 2022;13: 841068. https://doi.org/10.3389/fphar.2022.841068.
Qi Z, Chen L. Endoplasmic reticulum stress and autophagy. Adv Exp Med Biol. 2019;1206:167–77. https://doi.org/10.1007/978-981-15-0602-4_8.
Lin YW, Chen TY, Hung CY, Tai SH, Huang SY, Chang CC, Hung HY, Lee EJ. Melatonin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. Int J Mol Med. 2018;42(1):182–92. https://doi.org/10.3892/ijmm.2018.3607.
Li HQ, Xia SN, Xu SY, Liu PY, Gu Y, Bao XY, Xu Y, Cao X. γ-glutamylcysteine alleviates ischemic stroke-induced neuronal apoptosis by inhibiting ros-mediated endoplasmic reticulum stress. Oxid Med Cell Longev. 2021;2021:2961079. https://doi.org/10.1155/2021/2961079.
Lv Z, Liu C, Zhai M, Zhang Q, Li J, Zheng F, Peng M. LPS Pretreatment attenuates cerebral ischaemia/reperfusion injury by inhibiting inflammation and apoptosis. Cell Physiol Biochem. 2018;45(6):2246–56. https://doi.org/10.1159/000488170.
Zhao X, Zhu L, Liu D, Chi T, Ji X, Liu P, Yang X, Tian X, Zou L. Sigma-1 receptor protects against endoplasmic reticulum stress-mediated apoptosis in mice with cerebral ischemia/reperfusion injury. Apoptosis. 2019;24(1–2):157–67. https://doi.org/10.1007/s10495-018-1495-2.
Xu F, Ma R, Zhang G, Wang S, Yin J, Wang E, Xiong E, Zhang Q, Li Y. Estrogen and propofol combination therapy inhibits endoplasmic reticulum stress and remarkably attenuates cerebral ischemia-reperfusion injury and OGD injury in hippocampus. Biomed Pharmacother. 2018;108:1596–606. https://doi.org/10.1016/j.biopha.2018.09.167.
Zheng Y, Hou J, Liu J, Yao M, Li L, Zhang B, Zhu H, Wang Z. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J Pharmacol Sci. 2014;124(3):354–64. https://doi.org/10.1254/jphs.13220fp.
Uzdensky AB. Apoptosis regulation in the penumbra after ischemic stroke: expression of pro- and antiapoptotic proteins. Apoptosis. 2019;24(9–10):687–702. https://doi.org/10.1007/s10495-019-01556-6.
Demyanenko S, Uzdensky A. Profiling of signaling proteins in penumbra after focal photothrombotic infarct in the rat brain cortex. Mol Neurobiol. 2017;54(9):6839–56. https://doi.org/10.1007/s12035-016-0191-x.
Zeng M, Zhou H, He Y, Wang Z, Shao C, Yin J, Du H, Yang J, Wan H. Danhong injection alleviates cerebral ischemia/reperfusion injury by improving intracellular energy metabolism coupling in the ischemic penumbra. Biomed Pharmacother. 2021;140: 111771. https://doi.org/10.1016/j.biopha.2021.111771.
Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018;5(3):245–55. https://doi.org/10.1016/j.gendis.2018.06.001.
Deng Y, Tan R, Li F, Liu Y, Shi J, Gong Q. Isorhynchophylline ameliorates cerebral ischemia/reperfusion injury by inhibiting CX3CR1-mediated microglial activation and neuroinflammation. Front Pharmacol. 2021;12: 574793. https://doi.org/10.3389/fphar.2021.574793.
Yihao D, Tao G, Zhiyuan W, Xiaoming Z, Lingling D, Hongyun H. Ginkgo biloba leaf extract (EGb-761) elicits neuroprotection against cerebral ischemia/reperfusion injury by enhancement of autophagy flux in neurons in the penumbra. Iran J Basic Med Sci. 2021;24(8):1138–45. https://doi.org/10.22038/ijbms.2021.46318.10694.
Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445–54. https://doi.org/10.2147/dddt.S56071.
Zhai M, Liu C, Li Y, Zhang P, Yu Z, Zhu H, Zhang L, Zhang Q, Wang J, Wang J. Dexmedetomidine inhibits neuronal apoptosis by inducing Sigma-1 receptor signaling in cerebral ischemia-reperfusion injury. Aging. 2019;11(21):9556–68. https://doi.org/10.18632/aging.102404.
Zhao L, Li H, Gao Q, Xu J, Zhu Y, Zhai M, Zhang P, Shen N, Di Y, Wang J, et al. Berberine attenuates cerebral ischemia-reperfusion injury induced neuronal apoptosis by down-regulating the CNPY2 signaling pathway. Front Pharmacol. 2021;12: 609693. https://doi.org/10.3389/fphar.2021.609693.
Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33(2):586–92. https://doi.org/10.1161/hs0202.103399.
Wasserman JK, Yang H, Schlichter LC. Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs aged rats. Eur J Neurosci. 2008;28(7):1316–28. https://doi.org/10.1111/j.1460-9568.2008.06442.x.
Caso JR, Moro MA, Lorenzo P, Lizasoain I, Leza JC. Involvement of IL-1beta in acute stress-induced worsening of cerebral ischaemia in rats. Eur Neuropsychopharmacol. 2007;17(9):600–7. https://doi.org/10.1016/j.euroneuro.2007.02.009.
Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J Cereb Blood Flow Metab. 2009;29(3):464–79. https://doi.org/10.1038/jcbfm.2008.141.
Maddahi A, Kruse LS, Chen QW, Edvinsson L. The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat. J Neuroinflammation. 2011;8:107. https://doi.org/10.1186/1742-2094-8-107.
Xie W, Zhu T, Dong X, Nan F, Meng X, Zhou P, Sun G, Sun X. HMGB1-triggered inflammation inhibition of notoginseng leaf triterpenes against cerebral ischemia and reperfusion injury via MAPK and NF-κB signaling pathways. Biomolecules. 2019. https://doi.org/10.3390/biom9100512.
Yang Y, Li X, Zhang L, Liu L, Jing G, Cai H. Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARγ/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol. 2015;8(3):2484–94.
Wang L, Zhao H, Zhai ZZ, Qu LX. Protective effect and mechanism of ginsenoside Rg1 in cerebral ischaemia-reperfusion injury in mice. Biomed Pharmacother. 2018;99:876–82. https://doi.org/10.1016/j.biopha.2018.01.136.
Sprenkle NT, Sims SG, Sánchez CL, Meares GP. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol Neurodegener. 2017;12(1):42. https://doi.org/10.1186/s13024-017-0183-y.
Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454(7203):455–62. https://doi.org/10.1038/nature07203.
Wada T, Yasunaga H, Inokuchi R, Horiguchi H, Fushimi K, Matsubara T, Nakajima S, Yahagi N. Effects of edaravone on early outcomes in acute ischemic stroke patients treated with recombinant tissue plasminogen activator. J Neurol Sci. 2014;345(1–2):106–11. https://doi.org/10.1016/j.jns.2014.07.018.
Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15(3):222–9. https://doi.org/10.1159/000069318.
Wang Y, Liu M, Pu C. Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(3):302–20. https://doi.org/10.1177/1747493017694391.
Shinohara Y, Yanagihara T, Abe K, Yoshimine T, Fujinaka T, Chuma T, Ochi F, Nagayama M, Ogawa A, Suzuki N, et al. Cerebral infarction/transient ischemic attack (TIA). J Stroke Cerebrovasc Dis. 2011;20(4):31–73. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.05.004.
Cheng B, Guo Y, Li C, Ji B, Pan Y, Chen J, Bai B. Edaravone protected PC12 cells against MPP(+)-cytoxicity via inhibiting oxidative stress and up-regulating heme oxygenase-1 expression. J Neurol Sci. 2014;343(1–2):115–9. https://doi.org/10.1016/j.jns.2014.05.051.
Srinivasan K, Sharma SS. Edaravone offers neuroprotection in a diabetic stroke model via inhibition of endoplasmic reticulum stress. Basic Clin Pharmacol Toxicol. 2012;110(2):133–40. https://doi.org/10.1111/j.1742-7843.2011.00763.x.
Ono H, Nishijima Y, Adachi N, Tachibana S, Chitoku S, Mukaihara S, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K, et al. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone a non-controlled study. Med Gas Res. 2011;1(1):12. https://doi.org/10.1186/2045-9912-1-12.
Li G, Yang M, Hao X, Li C, Gao Y, Tao J. Acute toxicity of sodium formononetin-3’-sulphonate (Sul-F) in sprague-dawley rats and beagle dogs. Regul Toxicol Pharmacol. 2015;73(2):629–33. https://doi.org/10.1016/j.yrtph.2015.09.010.
Huang G, Zang J, He L, Zhu H, Huang J, Yuan Z, Chen T, Xu A. Bioactive nanoenzyme reverses oxidative damage and endoplasmic reticulum stress in neurons under ischemic stroke. ACS Nano. 2021. https://doi.org/10.1021/acsnano.1c07205.
Wei J, Wu X, Luo P, Yue K, Yu Y, Pu J, Zhang L, Dai S, Han D, Fei Z. Homer1a attenuates endoplasmic reticulum stress-induced mitochondrial stress after ischemic reperfusion injury by inhibiting the PERK pathway. Front Cell Neurosci. 2019;13:101. https://doi.org/10.3389/fncel.2019.00101.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The Role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16(6):533–44. https://doi.org/10.2174/1566524016666160523143937.
Yang W, Paschen W. Unfolded protein response in brain ischemia: A timely update. J Cereb Blood Flow Metab. 2016;36(12):2044–50. https://doi.org/10.1177/0271678x16674488.
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther. 2012;18(3):250–60. https://doi.org/10.1111/j.1755-5949.2012.00295.x.
Acknowledgements
We thank the staff of the Neurological Laboratory of Hebei Province, The Second Hospital of Hebei Medical University for their contribution to this study. We appreciate the support provided by Chunyan Li, an academician of the Chinese Academy of Engineering.
Funding
This study was funded by the Key Units of Academician Cooperation and Academician Workstation Project of Hebei Science and Technology Agency.
Author information
Authors and Affiliations
Contributions
HH and YL contributed to conception and design of the study. YB, HG and KW carried out the in vivo experiments; ZH, WY and WL carried out the in vitro experiments; YB and WL performed the statistical analysis. YB and YL wrote the first draft of the manuscript. HH and WD reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The animal experiments were carried out in compliance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) and approved by the ethical standards of the Animal Ethics Committee of Kangtai Medical Laboratory Service Company (Hebei, China) (Permit Number: MDL2022-02-16-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The list of primer sequence
Additional file 2:
Real time PCR result
Additional file 3:
Gel electrophoresis map
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bai, Y., He, Z., Duan, W. et al. Sodium formononetin-3'-sulphonate alleviates cerebral ischemia–reperfusion injury in rats via suppressing endoplasmic reticulum stress-mediated apoptosis. BMC Neurosci 23, 74 (2022). https://doi.org/10.1186/s12868-022-00762-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-022-00762-4