Abstract
Background
Mitoxantrone has proved efficacy in treatment of multiple sclerosis (MS). The fact that physical exercise could slow down the progression of disease and improve performance is still a debatable issue, hence; we aimed at studying whether combining mitoxantrone with exercise is of value in the management of MS.
Methods
Thirty-six male rats were divided into sedentary and exercised groups. During a 14-day habituation period rats were subjected to exercise training on a rotarod (30 min/day) before Experimental Autoimmune Encephalomyelitis (EAE) induction and thereafter for 17 consecutive days. On day 13 after induction, EAE groups (exercised &sedentary) were divided into untreated and mitoxantrone treated ones. Disease development was evaluated by motor performance and EAE score. Cerebrospinal fluid (CSF) was used for biochemical analysis. Brain stem and cerebellum were examined histopathological and immunohistochemically.
Results
Exercise training alone did not add a significant value to the studied parameters, except for reducing Foxp3 immunoreactivity in EAE group and caspase-3 in the mitoxantrone treated group. Unexpectedly, exercise worsened the mitoxantrone effect on EAE score, Bcl2 and Bax. Mitoxantrone alone decreased EAE/demyelination/inflammation scores, Foxp3 immunoreactivity, and interleukin-6, while increased the re-myelination marker BDNF without any change in tumor necrosis factor-α. It clearly interrupted the apoptotic pathway in brain stem, but worsened EAE mediated changes of the anti-apoptotic Bcl2 and pro-apoptotic marker Bax in the CSF.
Conclusions
The neuroprotective effect of mitoxantrone was related with remyelination, immunosuppressive and anti-inflammatory potentials. Exercise training did not show added value to mitoxantrone, in contrast, it disrupts the apoptotic pathway.
Similar content being viewed by others
Background
The cytotoxic, synthetic anthracenedione derivative, mitoxantrone, is an antineoplastic, immunomodulatory agent [1]. It has shown to be one of the most effective agents for treatment of relapsing–remitting, progressive relapsing and secondary progressive multiple sclerosis (MS), having the ability to slow down the worsening of neurological disability [2]. However, its effect on neuropathological hallmarks of the disease have been poorly studied [3], likely due to the arrival of new compounds with a safer profile and a higher patients’ compliance. Being an FDA approved disease-modifying agent for patients with relapsing–remitting MS, it is still being used in countries with economic limits instead of the newer immunomodulatory drugs [4, 5]. Its application is limited mainly due to the cardiotoxicity associated with long-term anthracycline therapy. In cancer patients, the occurrence of cardiotoxicity is expected to be about 3% [6], whereas in MS patients, 3.4% of mitoxantrone recipients had a decrease in left ventricular ejection fraction (LVEF) to ≤ 50% following 1 year of monotherapy and at the end of the second year, relevant incidences were 1.9% [2]. Nonetheless, when used as recommended, the risk of considerable myelosuppressive and cardiotoxic effects decline. Therefore, the lifetime collective dose should be firmly restricted to 140 mg/m2 or 2—3 years of therapy and it is not recommended in those with a LVEF of < 50%, those who exhibit a clinically considerable reduction in LVEF throughout treatment, those with a neutrophil count of < 1500 cells/mm3 or those with hepatic impairment [7, 8]. Later, therapy-related acute leukemia in mitoxantrone treated MS patients has also been reported [9, 10].
Exercise training represents a behavioral method for safely managing functional and symptomatic patients, as well as improving their quality of life. It has been found that exercise training can produce small, but important improvements in walking, balance, cognition, fatigue, and depression in MS [11]. Nonetheless, contradictory data has been published on the effects of exercise on molecular pathways in MS patients; while some studies reported a decrease in cytokine levels upon eight weeks of exercise, others reported no effect [12, 13]. In rodents, exercise has shown to increase the release of BDNF, which supports cell proliferation, synaptic plasticity, neuroprotection, and neurogenesis in both physiological and neuroinflammatory conditions [14].
Hence, this study was designed to evaluate the possible influence of exercise with mitoxantrone treatment on the neuronal function and disease progression through an acute relapse of experimental autoimmune encephalomyelitis (EAE).
Materials and methods
Animals
A total of 36 male Sprague–Dawley rats (200–250 g, 2–3 months old) were supplied by the animal house of Pharos University in Alexandria (Alexandria, Egypt). Animals had free access to food and water and stayed in air-conditioned room (23 ± 1 °C) with 12 h light–dark cycle. The Research Ethics Committee of the Faculty of Pharmacy, Cairo University approved the study design (Cairo, Egypt, Permit Number: PT 1978). All procedures comply with ARRIVE guidelines, as well as the National Research Council’s guide for the care and use of laboratory animals. Two blinded individuals completed all observations in an arbitrary routine.
Induction of EAE
Induction proceeded as described previously [15, 16]. Briefly, rats were immunized using a single subcutaneous injection (200 µl) of a mixture of complete Freund’s adjuvant (12 mg/ml) and spinal cord homogenate (50 mg/ml).
Experimental design
A group of 36 rats were distributed between sedentary (n = 18, SED) and exercised (n = 18, EX) rats. Before disease induction (days -14 to -1) exercised rats were trained for 30 min/day to move on a rotarod as described earlier [15].
On day 0, EX and SED rats were divided into control (CNSED; CNEX, n = 6) and untreated EAE (EAESED; EAEEX, n = 12) rats. After induction of EAE, exercised rats maintained their daily rotarod training (20 rpm) till day 17 post EAE induction. On day 13, when all rats developed score 1 of EAE, the EAE (EAESED & EAEEX) rats were divided into four groups (n = 6). Groups I and II were control sedentary (CNSED) and exercised (CNEX) rats. Groups III & IV were untreated EAE rats (EAESED & EAEEX) receiving saline (i.p). Groups V & VI were injected daily with mitoxantrone (MT, 2.5 mg/kg/day; i.p; Santrone©; EIMC United Pharmaceuticals; Alexandria, Egypt) and identified as mitoxantrone sedentary (MTSED) & mitoxantrone exercised (MTEX) [17, 18] starting on day 13 after induction and rats were given the mitoxantrone dose 1 h before the training. Figure 1 shows the experimental outline of the study.
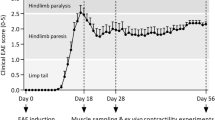
Experimental design timeline. Rats were familiarized to rotarod running before EAE induction (days -14 to -1). After EAE induction (on day 0), control and EAE rats were subjected to daily physical exercise for 17 days. Upon appearance of EAE symptoms (score 1, on day 13) rats were subjected to treatment with mitoxantrone (MT). Finally, all rats were sacrificed on day 18. EAE experimental autoimmune encephalomyelitis, CNSED sedentary control, CNEX exercised control; EAESED = sedentary untreated EAE; EAEEX = exercised untreated EAE rats
After EAE induction, the animal’s overall health has been observed for any changes. Rats were treated for 5 days, from day 13 till the end of the experiment (day 17). Drug treatment was given 1 h before exercise [19].
Assessment of MS progression
EAE score
After EAE induction, rats were monitored daily for the EAE score, as indicator of disease progression and muscular tone. The score was evaluated as described previously [20] and illustrated in Table 1.
Rotarod test
Before EAE induction (days-14 to -1) only rats in the EX-groups were exercised to stay on the rotarod for 30 min (20 rpm). After EAE induction, rats continued to be trained on the metallic rod daily till the end of the experiment (days 0 to 17). The falling time was agreed as indicator of motor performance [19].
Sample processing
On day 18 after EAE induction, rats were euthanized with an overdose of phenobarbital (100 mg/kg). Samples were then collected for further biochemical, histological and immunohistochemistry studies. Rats were then kept frozen till incineration.
The cerebrospinal fluid (CSF) was used to examine the amounts of tumor necrosis factor-α (TNF-α) [CAT# BEK1214] and interleukin-6 (IL-6) [CAT# BEK1110], BDNF as re-myelination indicator (CAT# BEK1013), the apoptosis related markers Bcl-2 [CAT# CSB-E08854r] and Bax [CAT# CSB-EL002573RA]. The ELISA kits were purchased from Chongqing Biospes (Chongqing, PRC) and Cusabio Technology LLC (TX, USA) and used according to manufacturers' instructions.
Histopathological study
The brain stem and cerebellum were separated, washed with phosphate buffered saline (PBS) and immersed in 10% formaldehyde to be processed into paraffin blocks. Samples were then sliced into 5 μm thickness, mounted on glass slides, then stained using hematoxylin and eosin (H&E) to evaluate morphological changes between the different groups. Scoring of inflammation was performed as described previously and illustrated in Table 2 [21].
Luxol fast blue (LFB) staining was used for assessment of demyelination in the cerebellum and brain stem and scored as described previously by Zhang et al. [21] and illustrated in Table 2. Histopathological examination was performed and scored in a blinded fashion.
Immunohistochemistry
For the immunohistochemical staining, 5 µm thick sections were fixed onto positively charged slides, deparaffinized and hydrated in xylene and descending alcohol solutions, and finally rinsed with PBS. Citrate buffer (pH 6.0) was used for heat induced antigen retrieval. Endogenous peroxidase was blocked with H2O2 and endogenous biotin with the aid of a blocking Kit Avidin/Biotin (DAKO #X0909, Glostrup, Denmark). After incubation in blocking buffer, the sections were treated with primary antibodies against Caspase-3 (Cell Signaling Technology, MA, USA, CAT#9662) with a dilution of 1:1000, and Foxp3 (FOXP3 Monoclonal Antibody, Invitrogen, Thermo Fisher Scientific, CA, USA, CAT# 14-5773-82) with a dilution of 5 µg/ml. Reactions were visualized using EnVision + System-HRP Kit (DAKO #K4063, Glostrup, Denmark). DAB (DAKO #K4063 Glostrup, Denmark) was used as chromogen and the tissue was counterstained with Mayer’s Hematoxylin. The number of positive cells in hotspot areas in ten high power fields (HPFs) in areas of demyelination and plaques in the brain stem were counted using the image analysis software (Leica Application Suite Version 4.12.0, Wetzlar, Germany).
Statistical analysis
Data are showed as mean ± standard deviation (S.D). For parametric data, one-way analysis of variance (ANOVA) was used, followed by Tukey’s Multiple Comparisons Test as post hoc. Interaction of exercise and treatment for dependent variables was analyzed using two-way ANOVA, followed by Bonferroni Correction Test. The EAE and histopathology scores were analyzed using Kruskal–Wallis Test, followed by Dunn's post hoc test, and presented as median with range. P < 0.05 was considered the significance limit for all comparisons. All analysis and graphs were performed using Prism computer program (GraphPad software Inc. V5, CA, USA).
Results
General Observation of animal health
The overall health status of sedentary and exercised rats treated with mitoxantrone was declining throughout the study; however, no change in weight was observed. Additionally, and as depicted in Fig. 2A, B porphyrin spots near the eye, as well as (C, D) blue discoloration of testis was observed in the mitoxantrone groups.
Effect of exercise on the animal health, the EAE score and motor performance. A shows representative photographs of mitoxantrone treated rats on day 17 showing porphyrin spots near the eye of exercised and B sedentary rats, as well as C and D blue discoloration of their testes was noted. E represents the EAE score. Data were analyzed using Kruskal–Wallis Test, followed by Dunn's post hoc test and presented as median values with range, p < 0.05. The median values are presented as CNSED = 0, CNEX = 0, EAESED = 4, EAEEX = 4, MTSED = 1.5 and MTEX = 2.5. F shows the percentage to control of motor performance on day 17. Data were analyzed using one-way ANOVA followed by Tukey's Multiple Comparison Test and presented as mean ± S.D (p < 0.05). As compared with CNSED (*), CNEX ($), EAESED (#), EAEEX (%) and MTSED (&) groups. EAE experimental autoimmune encephalomyelitis; groups of CNSED sedentary control, CNEX exercised control, EAESED sedentary untreated EAE; EAEEX exercised untreated EAE; MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone; (n = 6/group)
Effect of mitoxantrone on EAE score
As depicted from Table 3, on day 13 after EAE induction, all rats attained score 1. On day 14, untreated rats (EAESED and EAEEX) weakened reaching score 2, while treated rats stayed at score 1. There was a fluctuation in the scores among groups for the 5 days of treatment till day 17. On day 17, the EAEEX and EAESED rats deteriorated to reach a median score 4. The sedentary rats treated with mitoxantrone had a median score of 1.5; whereas, exercise worsened the case and elevated the median score to 2.5 in the MTEX group to be insignificant from the untreated animals. Figure 2E shows the median (min–max) scores on day 17 post EAE.
Effect of mitoxantrone on motor performance
During the training period (days-14 to -1), a gradual increase in the motor performance of all EX-rats was observed until reaching a steady state of 1800s on day -9 (before EAE induction; Additional file 1) and continued till day 13 after EAE induction. Table 4 presents the changes in motor performance of EAEEX rats. On day 13, an obvious discrepancy was observed between control EX rats and EAE exercised ones. Interestingly, EX animals treated with mitoxantrone have shown fluctuation in motor function till day 15 being almost similar to EX-EAE rats, an effect that vanished later showing a gradual recovery afterwards till reaching almost same values as CNEX on day 17 (Fig. 2F).
Effect of mitoxantrone on histopathological changes in the brain
Histopathological examination (Fig. 3) of the (A–C) cerebellum and (F–H) brain stem of untreated EAE groups showed plaques compared to normal control. Active plaques revealed inflammatory cellular infiltrates with abundant macrophages stuffed with myelin debris, an evidence of ongoing myelin breakdown. Lymphocytic infiltrates were also present, mostly as perivascular cuffs. Small active lesions were often seen centered on small veins and axons were relatively preserved, but reduced in number with microcyst formation. Gliosis and reactive astrocytes were surrounding the plaques. Cerebellar plaques were seen in three areas, leukocortical, intracortical and subpial. Leukocortical plaques showed decrease in the Purkinje cell mass inside the lesions. Brain stem plaques were mainly leukocortical in location.
Histopathological examination of rats’ brain treated with mitoxantrone. A Photomicrograph of control rat cerebellum showing no inflammation and no demyelinating plaques, while photomicrographs of EAE B SED and C EX groups show leukocortical plaques with inflammatory infiltration and decreased neurons in the granular cell layer. Sections of mitoxantrone treated D EAE sedentary and E exercised groups reveal reduced inflammatory cellular infiltrates, preservation of the white matter and grey matter, as well as near complete healing of the plaques. Compared to F normal control brain stem, sections of G sedentary and H exercised EAE groups show an active plaque with reduced numbers of axons and microcyst formation. Reactive astrocytes are surrounding the plaque (black arrows) and lymphocytic infiltrates. Sections of mitoxantrone treated EAE I sedentary and J exercised group reveal reduced inflammatory cellular infiltrates and increased axons with reduced microcyst formation. Reactive astrocytes and gliosis are more pronounced at the periphery. K and L summarize inflammation scores in the cerebellum and brain stem among different groups in exercised and sedentary rats, respectively. Non-parametric data were analyzed using Kruskal–Wallis Test followed by Dunn's Multiple Comparison Test and presented as median with range (p < 0.05). As compared with CNSED (*), CNEX ($), EAESED (#) and EAEEX (%). The median values are presented as CNSED = 0, CNEX = 0, EAESED = 2.5, EAEEX = 2, MTSED = 1 and MTEX = 0. EAE experimental autoimmune encephalomyelitis; groups of CNSED = sedentary control; CNEX exercised control, EAESED sedentary untreated EAE, EAEEX exercised untreated EAE, MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone; (n = 6/group)
Inflammatory infiltration was evaluated and scored revealing severe perivascular cuffing in the untreated group. Figure 3K, L show heavy inflammatory cellular infiltrates in the untreated EAE groups. Inflammatory scores in the EAE group were significantly higher than all other treated groups (p < 0.0001) reaching a median score of 2 in exercised versus 2.5 in sedentary rats. In Fig. 4, the demyelination of (A-C) the cerebellum and (F–H) brain stem was evaluated using LFB staining and median scored 2.5 in both sedentary and exercised untreated EAE rats indicating extensive demyelination.
Effect of mitoxantrone with/without exercise on myelination using luxol fast blue staining. Relative to cerebellum section of A normal control, B EAE sedentary, C EAE exercised untreated groups show light spots of demyelination (black arrows). However, sections of mitoxantrone treated D sedentary/E exercised groups show remyelinated white matter with no evidence of plaques. Similarly, brain stem sections of G EAE sedentary/H exercised groups present pale spots of demyelination (black arrows) compared to F normal control ones. However, sections of mitoxantrone treated I sedentary and J exercised groups show remyelination of plaques (black arrows). The demyelination scores in the cerebellum and brain stem of exercised and sedentary groups are summarized in panels K and L, respectively. Non-parametric data were analyzed using Kruskal–Wallis Test followed by Dunn's Multiple Comparison Test and presented as median values with range (p < 0.05). As compared with CNSED (*), CNEX ($), EAESED (#) and EAEEX (%).The median values are presented as CNSED = 0, CNEX = 0, EAESED = 2.5, EAEEX = 2.5, MTSED = 1 and MTEX = 1.5. EAE experimental autoimmune encephalomyelitis; groups of CNSED = sedentary control; CNEX exercised control, EAESED sedentary untreated EAE; EAEEX = exercised untreated EAE, MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone; (n = 6/group)
Post-administration of mitoxantrone to sedentary and exercised groups smaller patches of demyelination with microcyst formation and lymphocytic infiltrates were seen; and bare unmyelinated axons were fewer (Figs. 3D, E, I, J and 4D, E, I, J). These results were reflected on the inflammatory (Fig. 3K, L) and demyelination (Fig. 4K, L) scores in sedentary and exercised rats, reaching median inflammatory scores of 0 in exercised versus 1 in sedentary rats with median demyelination scores of 1.5 in exercised rats versus 1 in sedentary rats.
Effect of mitoxantrone on the CSF cytokine levels
EAE induction stimulated the inflammatory cascade (Fig. 5), showing increase in (A) TNF-α and (B) IL-6 levels. Treatment with mitoxantrone could not cause a decline in the TNF-α level whether in sedentary (p = 0.8859) or exercised (p = 0.4023) groups. In contrast, treatment with mitoxantrone showed a decline in IL-6 levels compared to untreated EAE exercised (p = 0.0099) and sedentary rats (p = 0.0479). On the other hand, exercise training with mitoxantrone treatment did not show any improvement over its sedentary counterpart (TNF-α p = 0.4747 and IL-6 p = 0.3962). Two-way ANOVA analysis did not reveal any interaction between drug treatment and exercise on TNF-α (F = 2.51, p = 0.0984) nor IL-6 (F = 0.62, p = 0.5438).
Effect of mitoxantrone with/without exercise on cytokines levels in the CSF. A TNF-α, B IL-6. Values are presented as mean ± S.D (n = 6/group). Comparison inside the same group was done using one-way ANOVA followed by Tukey's Multiple Comparison Test. Comparison between sedentary and exercised groups was done using two-way ANOVA followed by Bonferroni Correction Test (p < 0.05). As compared to CNSED (*), CNEX ($), EAESED (#) and EAEEX (%). EAE experimental autoimmune encephalomyelitis; groups of CNSED = sedentary control, CNEX exercised control, EAESED sedentary untreated EAE, EAEEX exercised untreated EAE, MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone
Effect of mitoxantrone on BDNF level in the CSF
As depicted in Fig. 6, the induction of EAE nearly halved (A) the BDNF level in the exercised and sedentary rat groups, compared to their normal groups; however, treatment with mitoxantrone revealed a slight, yet expressive increase in the CSF- BDNF levels compared to untreated control (25% MTSED versus 27% MTEX). Again, mitoxantrone treatment accompanied with exercise did not add value to the treatment protocol (p = 0.1479). Two-way ANOVA analysis did not reveal an interaction between drug treatment and exercise on the BDNF (F = 3.245, p = 0.0626).
Effect of mitoxantrone with/without exercise on A BDNF in the CSF and B–G Foxp3 in the brain stem of EAE rats. B Number of Foxp3 positive cells in the brain stem of exercised and sedentary rats. Immunostaining of Foxp3 showing absence of positively stained lymphocytes in C normal control rats. Sections of EAE untreated rats show positively stained perivascular lymphocytes (arrows) in the active plaques of D sedentary/E exercised groups, whereas treatment with mitoxantrone shows few positively stained lymphocytes in the active plaques of F sedentary/G exercised groups. Values are presented as mean ± S.D (n = 6/group). Comparisons inside the same group was done using one-way ANOVA followed by Tukey's Multiple Comparison Test. Comparison between exercised and sedentary groups was done using two-way ANOVA followed by Bonferroni Correction Test (p < 0.05). As compared to CNSED (*), CNEX ($), EAESED (#), EAEEX (%) and exercised vs sedentary rats (ψ). EAE experimental autoimmune encephalomyelitis; groups of CNSED sedentary control, CNEX exercised control, EAESED sedentary untreated EAE, EAEEX exercised untreated EAE, MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone
Effect of mitoxantrone on Foxp3 in brain stem
An indicator of Treg-cell activity is Foxp3. As shown in Fig. 6, panel (B) summarizes the effect of mitoxantrone with and without exercise on the expression of Foxp3 in the brain stem of untreated exercised and sedentary rats. The photomicrographs, show a marked increase in Foxp3 expression in (D) SED/(E) EX rats as compared to (C) control with a more prominent increase in the sedentary group. On the other hand, mitoxantrone post-administration succeeded in reducing Foxp3 protein expression in (F, G) both treated groups. Exercise did not cause any decrease in the Foxp3 count in rats treated with mitoxantrone, compared to its sedentary counterpart (p = 0.7918). Two-way ANOVA analysis revealed interaction between training and treatment (F = 71.79, p < 0.0001), which is reflected on the EAE untreated exercised rats.
Effect of mitoxantrone on caspase-3 in brain stem
The capase-3 expression (Fig. 7) comes to show the prominent apoptosis in EAE sedentary and exercised groups, where (B) SED and (C) EX rats have shown increased caspase-3 expression, compared to control (A) rats (p < 0.0001). However, mitoxantrone treatment has reduced the caspase-3 expression in both (D) SED and (E) EX rats (p < 0.0001). Panel (F) recapitulates the elevated caspase-3 expression in EAE rats and the anti-apoptotic capability of post-treatment with mitoxantrone. Exercise clearly enhanced the effect of mitoxantrone compared to its sedentary counterpart (p = 0.0205), showing no difference from exercised control (p = 0.0758). This effect induced by exercise and treatment is due to the interaction between both factors as shown using two-way ANOVA analysis (F = 4.766, p = 0.0159).
Effect of mitoxantrone on activated caspase-3 in the brain stem. Compared to the negative immunostaining of caspase 3 in A normal control group, sections of EAE untreated rat show positive staining in the astrocytes and neurons surrounding the plaques (arrows) of B sedentary/C exercised groups. Sections of mitoxantrone treated group show few positively stained astrocytes and neurons in the plaques of D sedentary/E exercised rats. Panel F shows the influence of exercise on the amount of caspase-3 positive cells in brain stem. Values are presented as mean ± S.D. (n = 6/group). Comparison inside the same group was done using one-way ANOVA followed by Tukey's Multiple Comparison Test. Comparison between sedentary and exercised groups was done using two-way ANOVA followed by Bonferroni Correction Test (p < 0.05). As compared with CNSED (*), CNEX ($), EAESED (#), EAEEX (%) and exercised vs sedentary rats (ψ). EAE experimental autoimmune encephalomyelitis; groups of CNSED sedentary control, CNEX exercised control, EAESED sedentary untreated EAE, EAEEX exercised untreated EAE, MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone
Effect of mitoxantrone on Bcl-2 and Bax levels in the CSF
Figure 8 comes to confirm the EAE-induced apoptosis, where it showed a significant inhibition in the anti-apoptotic parameter (A) Bcl-2 and an elevation in the apoptotic marker (B) Bax in both untreated EAE sedentary and exercised rats; these effects were less evident in the exercised group. The (C) Bcl-2/Bax ratio in SED and EX groups confirmed the latter observation. Post-treatment with mitoxantrone has worsened the effect of EAE on the tested markers in the SED and EX groups, showing a decrease in Bcl-2 level (p = 0.0010 and p < 0.0001 respectively), sharp increase in Bax (44% sedentary versus 29% exercised), and hence a decrease in Bcl-2/Bax ratio for both groups (p < 0.001). Exercise made a subtle, yet significant improvement in the mitoxantrone treated groups compared to its SED counterpart (p < 0.0001), where two-way ANOVA shows interaction between mitoxantrone treatment and training (F = 77.7, p < 0.0001 for Bcl-2, F = 146.0, p < 0.0001 for Bax and F = 171.0, p < 0.0001 for Bcl-2/Bax).
Effect of mitoxantrone with/without exercise on CSF levels of A Bcl-2, B Bax and C Bcl-2/Bax ratio. Values are presented as mean ± S.D. (n = 6). Comparison inside the same group was done using one-way ANOVA followed by Tukey's Multiple Comparison Test. Comparison between sedentary and exercised groups was done using two-way ANOVA followed by Bonferroni Correction Test (p < 0.05). As compared with CNSED (*), CNEX ($), EAESED (#), EAEEX (%) and exercised vs sedentary rats (ψ). EAE experimental autoimmune encephalomyelitis; groups of CNSED sedentary control, CNEX exercised control, EAESED sedentary untreated EAE, EAEEX exercised untreated EAE, MTSED sedentary mitoxantrone, MTEX exercised mitoxantrone
Discussion
Our study has emphasized the influence of exercise with mitoxantrone treatment on the neuronal function and disease management during an EAE animal model. Based on previous findings, exercise has shown effectiveness in MS patients, delaying the progression of disease and restoring the overall health status [22, 23]. Mitoxantrone treatment in doses ranging from 0.2 to 5 mg/kg, given before the onset of clinical signs, has previously shown the ability to inhibit the course of the disease significantly in an EAE model, the most commonly used animal model to study immunopathological mechanisms in MS [1, 17, 24, 25].
In rodents, the EAE scoring is equivalent to the Expanded Disability Status Scale (EDSS) in the MS patients, which evaluates disease progression [26]. EAE scoring also associates with CNS injury, as well as areas of inflammatory lesions [27,28,29]. In accordance with earlier data [28, 30] untreated EAE groups in this study showed the highest EAE score on day 17 after EAE induction. Exercise without treatment did not succeed to reduce the acuteness of EAE, where no significant differences were observed between sedentary and exercised untreated EAE rats. Though the EAE score was halved in this study with mitoxantrone treatment on day 17 compared to EAE values without exercise, surprisingly, exercise has worsened the mitoxantrone EAE score to mimic the untreated EAE group. On the contrary, regular exercise of EAE rats treated with mitoxantrone clearly improved the motor performance of the EAE rats on day 17, as expected from earlier studies, which showed that exercise training improved MS performance [27, 30]. The rotarod results of untreated EAE exercised rats has matched previous reports [28, 31]; where the motor performance markedly decreased 13 days post EAE induction and thereafter.
As rotarod performance correlated with central inflammatory lesions and demyelinated motor nerves in the CNS [27], accordingly, physical exercise together with mitoxantrone treatment in this study shows a possible enhancement of motor activity in MS patients, however, worsening of the EAE score confuses this issue. In 2016, van den Berg and his colleagues [27] indicated that compared to the neurological/clinical scoring system, motor performance measured by the rotarod test was more objective and quantitative. They also reported that this motor performance test strongly correlated to the surface area of inflammatory lesions in the motor systems. Such data emphasize that the rotaroad test could be a better indication for the ability of mitoxantrone to resolve EAE motor symptoms, an effect confirmed by the reduction of the inflammatory score assessed microscopically in the brain stem to resolve the puzzle about the aberrant contradictory behavioral observations in the mitoxantrone exercised EAE rats. After finalizing our work, other exercise protocols, like swimming or voluntary running wheel, have been recently introduced to show the effectiveness in several EAE models [32, 33]; these tests hence, could be considered an alternative option with drug treatment, which needs further inspection.
The aberrant enhanced EAE score of mitoxantrone exercised rats could be clarified by the present unchanged demyelination score in LFB stained sections versus EX-EAE alone. The histopathological changes and LFB staining confirmed the destructive effect of EAE to the neurons, where both inflammatory and demyelinating scores were elevated, effects that matched the results of Zhang et al. [21]. Adding to the earlier data in this study, treatment with mitoxantrone has shown an obvious neuronal protective effect, partly by diminishing inflammation and improving neuronal remyelination markers with or without exercise. Nonetheless, the latter was not reflected on demyelination score though it showed a tendency to reduce it without reaching a significant level.
The potential anti-inflammatory effect of mitoxantrone has been evaluated here by measuring the changes in the pro-inflammatory cytokine, TNF-α, which is related to the pathogenesis of MS. Elevated TNF-α levels were associated with disease severity [34, 35], as well as myelin and axonal damage [34, 36]. It has also been considered as one of the causes of delayed remyelination [34]. As reported by Bielekova and Martin, [37] measuring cytokine levels in the CSF, but not peripheral blood, could give a better indication of MS severity, a fact that was formerly proven by Villarroya et al. [38]. In the current study, administration of mitoxantrone did not alter the elevating effect of EAE on TNF-α, neither in exercised groups nor sedentary ones. As noted by Gbadamosi et al.[39], short term treatment of MS patients with mitoxantrone did not alter TNF-α level, a finding that supports the present result to verify that this cytokine does not play a role in mitoxantrone-mediated improvements.
Similar to TNF-α, IL-6 affects demyelination, as well as inflammation [40, 41] and its altitude in CSF of MS patients is associated with the severity of the disease [40, 41]. Measuring IL-6 in the CSF was better correlated to disease severity than TNF-α, where mitoxantrone resulted in a decrease of its CSF levels compared to EAE rats whether sedentary or exercised to explain the present reduced inflammatory and/or demyelination scores with the treatment. Exercise training alone did not have an additional value to the effect of mitoxantrone on IL-6 levels. As a result of less inflammatory infiltrates and cytokines, one can assume that the attenuated EAE course was not only due to increased remyelination, but rather a protection from demyelination, especially considering that the animals were sacrificed at the peak of disease and not in the recovery phase.
Apart from the documented elevation of inflammatory markers in MS, this disease is also correlated with a prominent decline in the neurotrophic factor BDNF in serum of MS patients [34] and EAE animal models [41, 42], facts that support the current findings. In our study, EAE induction has shown an evident decrease in the CSF level of BDNF, whereas treatment with mitoxantrone has shown a clear improvement in BDNF levels in both exercised and sedentary rats. This in turn can partly explain the neuroprotective effect of mitoxantrone as confirmed in the histopathology examination, as well LFB staining and in earlier studies [41, 43]. Unfortunately, exercise training did not have any added value on the BDNF level compared to mitoxantrone sedentary group.
Another factor contributing to neuronal survival is the T cell population. In a previous study, Dombrowski et al. [44], has reported that Treg cells are able to promote myelination and remyelination and its transcriptional factor, Foxp3, is a good indicator of Tregs activity [21, 45]. Nevertheless, this point is debatable, where other studies revealed that Tregs activity is unrelated to Foxp3 count [46, 47]. Unexpectedly, our results showed a marked increase in the Foxp3 positive T lymphocytes in the EAE untreated group relative to normal control group, an effect that was even more evident in the sedentary rats over the exercised ones. This finding can be attributed to the aptitude of mitoxantrone to improve myelination, which did not necessitate the recruitment of Tregs. This notion can be supported by the high expression of Foxp3 in the untreated model possibly as a kind of body compensatory mechanism to control the acute period of the disease as mentioned earlier by Irony-Tur-Sinai et al. [45]. On the other hand, studies that showed an accumulation of Tregs in EAE brain have stated that this was an unsuccessful attempt to counteract local autoimmunity [48], meaning that, despite their brain recruitment, their function is impaired during EAE. Treatment with mitoxantrone, however, caused a sharp decrease in the number of Foxp3 T lymphocytes in the sedentary group to correspond to the results reported by Hanes et al. [49] and D’Arena et al. [50], pointing to the efficacy of mitoxantrone in controlling the acuteness of the disease. Although the exercise did not add any beneficial effect to the mitoxantrone treated groups, yet its impact was obvious in the exercised EAE rats. This group showed that exercise training decreased the overall immunoreactivity in the brain stem, as manifested in the decreased Foxp3 level in exercised EAE rats compared to its sedentary counterpart.
We also evaluated whether mitoxantrone mediates its effect by preserving neuronal cell survival via examining the immunostaining of caspase-3, which is implicated in cell death, axonal damage, neuronal apoptosis and inflammation in EAE [51, 52]. As observed in this study and previously reported, EAE rats showed a prominent increase in caspase-3 positive cells, making it an important target to decrease the axonal-damage and degeneration in MS using specific caspase-3 inhibitors [51]. Post-treatment with mitoxantrone succeeded to decrease the amount of caspase-3 positive cells, effect that was further reduced in the exercised rats receiving mitoxantrone relative to their sedentary counterparts to highlight the role of exercise in protecting neurons against apoptosis. Such influence of exercise in reducing caspase-3 expression has been earlier described in EAE mice hippocampus [53].
The effect of mitoxantrone with/without exercise on the CSF level of the anti-apoptotic biomarker Bcl-2 and the pro-apoptotic biomarker Bax were also evaluated. While sedentary/ exercised untreated EAE groups have shown a sharp decline in Bcl-2 levels, they have increased the Bax levels to concur with earlier findings in MS patients and EAE animal models [54, 55]. The effect of exercise in minimizing cell death was evidenced in results of these markers, comparing exercised with the sedentary values in all treated and untreated groups, such effects have been reported earlier by Kim et al. [53] in the EAE mice hippocampus. Surprisingly, the results of mitoxantrone pointed to its apoptotic potential in this model, where it failed to oppose the EAE effect on Bcl-2 and even worsened the EAE effect on the apoptotic marker Bax. Remarkably, mitoxantrone decreased the Bcl-2/Bax ratio in the CSF confirming its well-known apoptotic pathway [56, 57]; however, it simultaneously decreased caspase-3 level in the spinal cord. Since Bcl-2 and Bax are mainly related to the intrinsic type of apoptosis, in which the mitochondrial function plays a critical role, then this unexpected effect of mitoxantrone could be owed to its ability to cause mitochondrial energetic imbalance that results in ATP decrease, beside other mitochondrial dysfunctions [58]. However, the role of mitochondrial topoisomerase, a critical mitochondrial integrity enzyme, on the effect of mitoxantrone cannot be excluded being a DNA topoisomerase inhibitor [59]. The apoptotic potential of mitoxantrone has also been documented previously [60, 61]. According to this notion, one can speculate that the inhibited caspase-3 may be due to the extrinsic apoptotic event, although TNF-α, which is one element of this pathway, was also not altered by mitoxantrone, a point that needs thorough investigation.
As a further confirmation for the potential toxic effect of mitoxantrone, rats treated with mitoxantrone revealed blue discoloration of their testis and the porphyrin spots around their eyes. Indeed, mitoxantrone pharmacokinetics profile might explain these features, as Batra et al. [62] reported that multiple doses of mitoxantrone in animals showed an extensive distribution into tissues and a slow elimination to clarify the already proven toxicity of mitoxantrone [63]. A previous study by Williams [64] proved that porphyrin‐pigments and lipid‐laden tears were predominantly produced from the Harderian gland as a normal phenomenon, but increases in the case of chromodacryorrhoea as a warning sign of potentially severe systemic disease or physiologic stresses.
Conclusions
In conclusion, mitoxantrone has partially minimized the EAE severity, via enhancing remyelination, protecting from demyelination, augmenting the neurotrophic factor BDNF and reducing IL-6. However, its anti-apoptotic effect needs further investigation; while it succeeded to abate caspase-3 efficiently it failed to correct Bcl2 and Bax. On the other hand, exercise training alone did not add a significant value to most studied parameters, except for a reduction in the brain stem Foxp3 immunoreactivity. To our knowledge, this is the first study examining the effect of exercise with mitoxantrone treatment on the neuronal function and disease management in EAE model. Whether physical exercise improves or deteriorates treatment of MS with mitoxantrone, still needs further investigation and validation with more attention directed towards the apoptotic cascade. A longer observation period of the effect of exercise with mitoxantrone could be also required.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- CSF :
-
Cerebrospinal fluid
- CN :
-
Control
- EAE :
-
Experimental Autoimmune Encephalomyelitis
- EX :
-
Exercised rats
- IL:
-
Interleukin
- LFB :
-
Luxol fast blue
- MS:
-
Multiple sclerosis
- MT :
-
Mitoxantrone
- SED :
-
Sedentary rats
- TNF-α:
-
Tumor necrosis factor-α
References
Boneschi FM, Vacchi L, Rovaris M, Capra R, Comi G. Mitoxantrone for multiple sclerosis. Cochrane Database Syst Rev. 2013(5).
Hartung H-P, Gonsette R, Konig N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. The Lancet. 2002;360(9350):2018–25.
Cotte S, Von Ahsen N, Kruse N, Huber B, Winkelmann A, Zettl UK, et al. ABC-transporter gene-polymorphisms are potential pharmacogenetic markers for mitoxantrone response in multiple sclerosis. Brain. 2009;132(9):2517–30.
English C, Aloi JJ. New FDA-approved disease-modifying therapies for multiple sclerosis. Clin Ther. 2015;37(4):691–715.
Carrá A, Macías-Islas MÁ, Gabbai AA, Correale J, Bolaña C, Sotelo ED, et al. Optimizing outcomes in multiple sclerosis: consensus guidelines for the diagnosis and treatment of multiple sclerosis in Latin America. Ther Adv Neurol Disord. 2011;4(6):349–60.
Dunn CJ, Goa KL. Mitoxantrone. Drugs Aging. 1996;9(2):122–47.
Goldenberg MM. Multiple sclerosis review. Pharm Therap. 2012;37(3):175.
Scott LJ, Figgitt DP. Mitoxantrone. CNS Drugs. 2004;18(6):379–96.
Stroet A, Hemmelmann C, Starck M, Zettl U, Dörr J, Paul F, et al. Incidence of therapy-related acute leukaemia in mitoxantrone-treated multiple sclerosis patients in Germany. Ther Adv Neurol Disord. 2012;5(2):75–9.
Buttmann M, Seuffert L, Mäder U, Toyka KV. Malignancies after mitoxantrone for multiple sclerosis: a retrospective cohort study. Neurology. 2016;86(23):2203–7.
Motl RW, Sandroff BM. Benefits of exercise training in multiple sclerosis. Curr Neurol Neurosci Rep. 2015;15(9):62.
White LJ, Castellano V. Exercise and brain health—implications for multiple sclerosis. Sports Med. 2008;38(2):91–100.
Castellano V, Patel DI, White LJ. Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol. 2008;104(6):1697–702.
Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580–90.
El-Emam MA, El Achy S, Abdallah DM, El-Abhar HS, Gowayed MA. Neuroprotective role of galantamine with/without physical exercise in experimental autoimmune encephalomyelitis in rats. Life Sci. 2021;277:119459.
Wallström E, Olsson T. Rat models of experimental autoimmune encephalomyelitis. In: Michael Conn P, editor. Sourcebook of models for biomedical research. Totowa: Springer; 2008. p. 547–56.
Weilbach F, Chan A, Toyka K, Gold R. The cardioprotector dexrazoxane augments therapeutic efficacy of mitoxantrone in experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2004;135(1):49–55.
Sloboda AE, Oronsky AL, Kerwar S. Studies of the effect of mitoxantrone on adjuvant induced arthritis in rats. Clin Immunol Immunopathol. 1986;40(2):236–43.
Aharoni R, Rosen C, Shezen E, Bar-Lev DD, Golani O, Reisner Y, et al. Assessing remyelination-metabolic labeling of myelin in an animal model of multiple sclerosis. J Neuroimmunol. 2016;301:7–11.
Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis Amelioration of clinical status by M2 activated monocyte administration. Mult Scler J. 2011;17(1):2–15.
Zhang F, Zhang B, Shen R, Xu X, Guo L, Wang Y, et al. The scutellaria baicalensis stem-leaf total flavonoid regulates the balance of Th17/Treg in EAE rats. Int J Clin Exp Med. 2017;10(2):2408–18.
Bernardes D, Oliveira-Lima OC, da Silva TV, Faraco CCF, Leite HR, Juliano MA, et al. Differential brain and spinal cord cytokine and BDNF levels in experimental autoimmune encephalomyelitis are modulated by prior and regular exercise. J Neuroimmunol. 2013;264(1–2):24–34.
Patel DI, White LJ. Effect of 10-day forced treadmill training on neurotrophic factors in experimental autoimmune encephalomyelitis. Appl Physiol Nutr Metab. 2013;38(2):194–9.
Baker D, O’neill J, Davison A, Turk J. Control of immune-mediated disease of the central nervous system requires the use of a neuroactive agent: elucidation by the action of mitoxantrone. Clin Exp Immunol. 1992;90(1):124–8.
Watson CM, Davison AN, Baker D, O’Neill JK, Turk JL. Suppression of demyelination by mitoxantrone. Int J Immunopharmacol. 1991;13(7):923–30.
Al-Izki S, Pryce G, Hankey DJ, Lidster K, von Kutzleben SM, Browne L, et al. Lesional-targeting of neuroprotection to the inflammatory penumbra in experimental multiple sclerosis. Brain. 2013;137(1):92–108.
van den Berg R, Laman JD, van Meurs M, Hintzen RQ, Hoogenraad CC. Rotarod motor performance and advanced spinal cord lesion image analysis refine assessment of neurodegeneration in experimental autoimmune encephalomyelitis. J Neurosci Methods. 2016;262:66–76.
Shaw MA, Gao Z, McElhinney KE, Thornton S, Flick MJ, Lane A, et al. Plasminogen deficiency delays the onset and protects from demyelination and paralysis in autoimmune neuroinflammatory disease. J Neurosci. 2017;37(14):3776–88.
Uzawa A, Mori M, Masuda H, Ohtani R, Uchida T, Kuwabara S. Recombinant thrombomodulin ameliorates experimental autoimmune encephalomyelitis by suppressing high mobility group box 1 and inflammatory cytokines. Clin Exp Immunol. 2018;193(1):47–54.
Bernardes D, Oliveira ALRD. Regular exercise modifies histopathological outcomes of pharmacological treatment in experimental autoimmune encephalomyelitis. Front Neurol. 2018;9:950.
Bernardes D, Oliveira ALR. Comprehensive catwalk gait analysis in a chronic model of multiple sclerosis subjected to treadmill exercise training. BMC Neurol. 2017;17(1):160.
Gentile A, Musella A, De Vito F, Rizzo FR, Fresegna D, Bullitta S, et al. Immunomodulatory effects of exercise in experimental multiple sclerosis. Front Immunol. 2019;10:2197.
Xie Y, Li Z, Wang Y, Xue X, Ma W, Zhang Y, et al. Effects of moderate-versus high-intensity swimming training on inflammatory and CD4+ T cell subset profiles in experimental autoimmune encephalomyelitis mice. J Neuroimmunol. 2019;328:60–7.
Bir SC, Chernyshev OY, Minagar A. Roles of Macrophages and Astrocytes in Pathogenesis of Multiple Sclerosis. Neuroinflammation: Elsevier; 2018. p. 517-28. https://doi.org/10.1016/B978-0-12-811709-5.00028-4
Okada Y, Ochi H, Fujii C, Hashi Y, Hamatani M, Ashida S, et al. Signaling via toll-like receptor 4 and CD40 in B cells plays a regulatory role in the pathogenesis of multiple sclerosis through interleukin-10 production. J Autoimmun. 2018;88:103–13.
Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747.
Bielekova B, Martin R. Development of biomarkers in multiple sclerosis. Brain. 2004;127(7):1463–78.
Villarroya H, Violleau K, Younes-Chennoufi AB, Baumann N. Myelin-induced experimental allergic encephalomyelitis in Lewis rats: tumor necrosis factor α levels in serum and cerebrospinal fluid Immunohistochemical expression in glial cells and macrophages of optic nerve and spinal cord. J Neuroimmunol. 1996;64(1):55–61.
Gbadamosi J, Buhmann C, Tessmer W, Moench A, Haag F, Heesen C. Effects of mitoxantrone on multiple sclerosis patients’ lymphocyte subpopulations and production of immunoglobulin, TNF-alpha and IL-10. Eur Neurol. 2003;49(3):137–41.
Luchtman DW, Ellwardt E, Larochelle C, Zipp F. IL-17 and related cytokines involved in the pathology and immunotherapy of multiple sclerosis: current and future developments. Cytokine Growth Factor Rev. 2014;25(4):403–13.
Katsavos S, Anagnostouli M. Biomarkers in multiple sclerosis: an up-to-date overview. Mult Scler Int. 2013. https://doi.org/10.1155/2013/340508.
Paap BK, Hecker M, Koczan D, Zettl UK. Molecular biomarkers in multiple sclerosis. J Clin Cell Immunol. 2013;10(4):34–65.
Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106(2):163–77.
Dombrowski Y, O’hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674–80.
Irony-Tur-Sinai M, Grigoriadis N, Tsiantoulas D, Touloumi O, Abramsky O, Brenner T. Immunomodulation of EAE by alpha-fetoprotein involves elevation of immune cell apoptosis markers and the transcription factor FoxP3. J Neurol Sci. 2009;279(1–2):80–7.
Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, et al. Foxp3 transcription-factor-dependent and-independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27(5):786–800.
Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci. 2006;103(17):6659–64.
Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–31.
Hanes WM, Olofsson PS, Kwan K, Hudson LK, Chavan SS, Pavlov VA, et al. Galantamine attenuates type 1 diabetes and inhibits anti-insulin antibodies in nonobese diabetic mice. Mol Med. 2015;21(1):702–8.
D’Arena G, Simeon V, D’Auria F, Statuto T, Di Sanzo P, De Martino L, et al. Regulatory T-cells in chronic lymphocytic leukemia: actor or innocent bystander? Am J blood research. 2013;3(1):52.
Ahmed Z, Doward AI, Pryce G, Taylor DL, Pocock JM, Leonard JP, et al. A role for caspase-1 and-3 in the pathology of experimental allergic encephalomyelitis: inflammation versus degeneration. Am J Pathol. 2002;161(5):1577–86.
Meyer R, Weissert R, Diem R, Storch MK, de Graaf KL, Kramer B, et al. Acute neuronal apoptosis in a rat model of multiple sclerosis. J Neurosci. 2001;21(16):6214–20.
Kim T-W, Sung Y-H. Regular exercise promotes memory function and enhances hippocampal neuroplasticity in experimental autoimmune encephalomyelitis mice. Neuroscience. 2017;346:173–81.
Duarte-Silva E, da Rocha Araújo SM, Oliveira WH, de Lós DB, de França MER, Bonfanti AP, et al. Sildenafil ameliorates EAE by decreasing apoptosis in the spinal cord of C57BL/6 mice. J Neuroimmunol. 2018;321:125–37.
Zidan A, Hedya SE, Elfeky DM, Abdin AA. The possible anti-apoptotic and antioxidant effects of acetyl l-carnitine as an add-on therapy on a relapsing-remitting model of experimental autoimmune encephalomyelitis in rats. Biomed Pharmacother. 2018;103:1302–11.
Prokop A, Wieder T, Sturm I, Eβmann F, Seeger K, Wuchter C, et al. Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia. 2000;14(9):1606–13.
Kalantzis ED, Scorilas A, Vassilacopoulou D. Evidence for L-Dopa decarboxylase involvement in cancer cell cytotoxicity induced by docetaxel and mitoxantrone. Curr Pharm Biotechnol. 2018;19(13):1087–96.
Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Prasad SVN, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Investig. 2014;124(2):617–30.
Douarre C, Sourbier C, Dalla Rosa I, Das BB, Redon CE, Zhang H, et al. Mitochondrial topoisomerase I is critical for mitochondrial integrity and cellular energy metabolism. PLoS ONE. 2012;7(7):e41094.
Neuhaus O, Kieseier BC, Hartung H-P. Therapeutic role of mitoxantrone in multiple sclerosis. Pharmacol Ther. 2006;109(1–2):198–209.
Neuhaus O, Wiendl H, Kieseier BC, Archelos JJ, Hemmer B, Stüve O, et al. Multiple sclerosis: mitoxantrone promotes differential effects on immunocompetent cells in vitro. J Neuroimmunol. 2005;168(1–2):128–37.
Batra VK, Morrison JA, Woodward DL, Siverd NS, Yacobi A. Pharmacokinetics of mitoxantrone in man and laboratory animals. Drug Metab Rev. 1986;17(3–4):311–29.
Shenkenberg TD, von Hoff DD. Mitoxantrone: a new anticancer drug with significant clinical activity. Ann Intern Med. 1986;105(1):67–81.
Williams DL. Ocular disease in rats: a review. Veterinary ophthalmology. 2002;5(3):183–91.
Acknowledgements
Dr. Samar O. El-Ganainy (Pharmacology and Toxicology, Department of Pharmacology and Therapeutics, Faculty of Pharmacy, Pharos University in Alexandria) is highly acknowledged for her assistance in doing the training study on the rotarod device.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MG: Conceptualization, Methodology (performed the research), Analysis and interpretation of data, Writing original draft. HA: Conceptualization, Supervision, Writing-Review and Editing, Final approval of the manuscript for submission. ME: Methodology (performed the research), Analysis and interpretation of data. SA: Methodology (performed the histopathology, immunohistochemistry and Luxol fast blue), Analysis and interpretation of data, Writing original draft. DA: Conceptualization, Supervision, Writing-Review and Editing, Final approval of the manuscript for submission, Corresponding author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol was approved by the Research Ethics Committee of the Faculty of Pharmacy, Cairo University (Cairo, Egypt, Permit Number: PT 1978). All procedures comply with the ARRIVE guidelines and the National Research Council’s guide for the care and use of laboratory animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Changes in motor performance of exercised rats during the 14 training days before induction.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
El-Emam, M.A., El Achy, S., Abdallah, D.M. et al. Does physical exercise improve or deteriorate treatment of multiple sclerosis with mitoxantrone? Experimental autoimmune encephalomyelitis study in rats. BMC Neurosci 23, 11 (2022). https://doi.org/10.1186/s12868-022-00692-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12868-022-00692-1