Abstract
Background
Urinary tract infection (UTI) is one of the most prevalent infectious diseases with worldwide health threatening. Antimicrobial resistant strains of Escherichia coli (E. coli) are a common cause of UTI which were identified as a treatment challenge. This study aimed to assay the prevalence of common β-lactam resistance genes including blaTEM, blaSHV, blaCTX-M and blaCMY and phenotypic resistance to commonly used β-lactam and fluoroquinolone antibiotics in UTIs. These factors were evaluated in various phylogenetic groups (phylotypes) of E. coli isolates. Real-time PCR was applied to detect β-lactam resistance genes and conventional PCR was used to determine the phylotypes. Phenotypic resistance against β-lactams (ceftazidime, cefotaxime, aztreonam and ceftriaxone) and fluoroquinolones (ciprofloxacin) were identified by the disc diffusion technique. The ability of extended spectrum β-lactamases (ESBLs) production in E. coli isolates was detected using the combined disc diffusion method.
Results
The prevalence of resistance genes were 89.6% for blaTEM, 44.3% for blaCTX-M, 6.6% for blaSHV and 0.9% for blaCMY. The two high prevalent phylotypes were B2 (29.2%) and D (17.9%) followed by E (14.1%), F (9.4%), C (6.6%) and 10.3% of isolates were unknown in phylotyping. Disc diffusion results showed high prevalence of antibiotic resistance to cefotaxime (88.6%), aztreonam (83%), ceftireaxon (77.3%), ceftazidime (76.4%) and ciprofloxacin (55.6%). Totally, 52.8% of isolates were found as phenotypical ESBL-producers.
Conclusions
This study’s results confirmed an explosion of antibiotic resistance amongst E. coli isolates from UTI against β-lactams and fluoroquinolones. Findings explain the necessity of deep changes in quantity and quality of drug resistance diagnosis and antibiotic therapy strategies. More studies are suggested to better and confident evaluations.
Similar content being viewed by others
Background
Urinary tract infections (UTIs) are amongst the most common infectious diseases, especially in women, caused by different microorganisms, such as Escherichia coli (E. coli), Enterococcus, Staphylococcus, Proteus, Klebsiella, Pseudomonas, etc. [1]. Uropathogenic E. coli (UPEC) pathotype causes more than the three-quarters of UTIs via several virulence factors such as adhesins, capsule, siderophores and toxins [2,3,4].
Several antibiotics are considered for treatment of cystitis and pyelonephritis, including oral β-lactams (amoxicillin–clavulanic acid or third-generation cephalosporin), fluoroquinolones (ciprofloxacin or levofloxacin), nitrofurantoin, fosfomycin or trimethoprim–sulfamethoxazole [5]. It is a public health issue that the widespread use of antibiotics for the treatment of UTIs has led to the growth of antimicrobial resistant UPECs, which makes it harder to treat, prevent, and manage UTIs.
Resistance against β-lactam agents can occur via (i) mutation or expression of alternative penicillin-binding proteins (PBPs) as the drug target, (ii) downregulation of porins to reduce the bacterial permeability against β-lactams, (iii) over-expression of efflux systems which are membrane transport proteins to export drug substrates and (iv) production of β-lactamases that hydrolyze the β-lactam amide [6]. The production of extended spectrum β-lactamases (ESBLs) is a primary β-lactam resistance mechanism in Gram-negative bacteria. There are four distinct classes of ESBLs, termed A, B C and D based on specific sequence motifs and hydrolytic mechanism in Ambler classification system. Some main enzyme families are TEM, SHV, CTX-M and KPC in class A; NDM and VIM in class B; CMY and ADC in class C; and OXA in Class D [6].
Resistance against the quinolones/fluoroquinolones can occur via three mechanisms; (i) chromosomal mutations that change the targets of the drug, such as GyrA subunits of DNA gyrase and ParC of topoisomerase IV, (ii) mutations related to reduce the drug concentration in bacterial cytoplasm via over-expression of efflux pumps and downregulation of porins, and (iii) PMQR (plasmid-mediated quinolone resistance) genes, such as qnr gene responsible to proteins that protect DNA gyrase or topoisomerase IV, aac(6′)-lb-cr gene acetylating quinolones and qepA and oqxAB genes which increase the outflow of the drug molecules through efflux pumps [7].
Antimicrobial resistance genes (ARGs) could be shared among E. coli strains via mobile genetic elements, including conjugative plasmids, transposons, insertion sequences and genomic islands [8]. Transmission of these elements leads to recombination in E. coli strains in a moderate level. Nevertheless, E. coli populations are clonal and could be classified to various phylogenetic groups [9]. Based on presence or absence of four genetic sequences named chuA, yjaA, TspE4 and arpA, E. coli could be categorized into the phylotypes A, B1, B2, D, C, E, F, G and Escherichia cryptic clade I [10].
In this study, the phenotypic antibiotic resistance against β-lactams and fluoroquinolones was first studied in E. coli isolates from women UTIs in Jiroft city of Iran. Then some of the most important β-lactam resistance genes were screened in the isolates. Finally, phylotypes were determined, and all variables were analyzed in relation to each other.
Methods
Sampling, E. coli isolation and confirmation
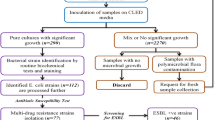
In this study, urine samples were collected in two laboratories in Jiroft city (southeast of Iran) during the spring season of 2021. The total number of UTI referrals to the laboratories was 168 cases. All urine samples belonged to non-hospitalized women with uncomplicated UTI in Jiroft; they were premenopausal and non-pregnant women without urinary tract abnormality which mostly showed acute cystitis and pyelonephritis in clinical examinations by physicians. The women with suspected UTI submitted their samples to medical diagnostic laboratories in Jiroft for microbial examination.
All sampling procedures were done in the medical diagnostic laboratories; the patients washed their hands before collecting the sample. They collected a midstream urine sample in sterile containers without touching the inside of it. Finally, they closed the container and delivered to the laboratory for next steps.
The urine samples were cultured onto MacConkey agar plates and were incubated at 37 °C for 24–48 h. Among 168 urine samples, 106 (63%) MacConkey agar plates showed suspected E. coli colonies. One single smooth and pink colony was selected from each plate and confirmed by IMViC biochemical technique including indole, methyl red, Voges-Proskauer and citrate tests [11].
Phenotypic assessment of E. coli strains
In this study, the antimicrobial resistance was identified using the Kirby Bauer disk diffusion method; the antibiotics were ceftazidime (CAZ; 30 µg), cefotaxime (CTX; 30 µg), cefotzxime clavulanate (CZA; 30 µg), ceftazidime clavulanate (CTC; 30 µg), aztreonam (AZT; 30 µg), ciprofloxacin (CP; 5 μg) and ceftriaxone (CRO; 30 µg). As a test control, E. coli ATCC 25,922 was utilized, and the findings were evaluated according to the Institute of Clinical and Laboratory Standards Institute (CLSI 2018; Table 2). Also, the E. coli strains with a ≥ 5-mm increase in zone diameter for cefotaxime-clavulanate vs the zone diameter of cefotaxime or a ≥ 5-mm increase in zone diameter for ceftazidime-clavulanate vs the zone diameter of ceftazidime were considered as ESBL-producing E. coli strains [12].
DNA extraction
Total genomic DNA of the confirmed E. coli strains was extracted by boiling technique; a single colony from each sample was suspended in 400 µL sterile distilled water and heated at 98–100 °C in a heating block (Eppendorf, Germany) for 10–15 min. Then, lysates were centrifuged (13,000 × g, 2 min), and the supernatants were moved to a new microtube and stored at -20 °C as DNA templates for next steps [13].
Real-time and conventional PCR for β-lactamase genes
Four antimicrobial resistance genes including blaTEM, blaSHV, blaCMY and blaCTX-M, were screened using Real-time polymerase chain reaction (PCR) and the positive samples were reconfirmed by conventional PCR. For Real-time PCR step, the reactions were uniplex and arranged in 25 µL volume for each gene including 2 µL DNA extract, 0.4 µM from each primer [14], 12.5 µL RealQ Plus 2 × Master Mix Green (Ampliqon, Denmark) and distilled water up to volume of reaction. Thermal cycler program was included 95 °C for 15 min followed by 30 cycles of 95 °C for 15 s; 50 °C for 15 s and 70 °C for 20 s. Positive controls were Klebsiella ATCC 700,603 (for blaCTX-M), E. coli ATCC 35,218 (for blaTEM and blaSHV) and we didn’t have positive control for blaCMY. One E. coli strain (without the four resistance genes) was used to negative control. The real-time PCR was done via LightCycler® 96 System (Roche Diagnostics GmbH, Mannheim, Germany).
For more confirmation of the results in previous step, conventional PCR were performed on the positive samples. Uniplex PCR were carried out in 25 µL reactions containing 12.5 µL 2X Taq PCR Master Mix (pars tous, Iran), 0.4 µM of each forward and reverse primer [14], 8 µL of sterile water and 2.5 µL of extracted bacterial DNA. The PCR steps were initial denaturation (95 °C for 10 min), 35 thermal cycles including denaturation (95 °C for 30 s), annealing [55 °C for 30 s (for blaTEM and blaSHV) and 60 °C for 30 s (for blaCTX-M and blaCMY)] and elongation (72 °C for 1 min). Finally, an elongation step was attached at 72 °C for 5 min.
PCR for phylogenetic classification
In this study, the E. coli strains were phylotyped by Clermont et al. (2013) scheme using a quadriplex PCR [10]. The sequences arpA (400 bp), chuA (288 bp), yjaA (211 bp) and TspE4.C2 (152 bp) were targeted to determine phylotypes including A, B1, B2, C, D, E, F and cryptic clade I. The strain EcoR62 was used as positive control in PCR examinations. Samples were subjected to a 35 PCR cycles including 10 s denaturation at 94 °C, 25 s annealing at 59 °C, and 5 s elongation at 72 °C. The PCR products were electrophoresed on 1.3% agarose gel for 60 min at 80 V. The electrophoresed gel was analyzed by gel documentation imaging system (vilber lourmat, France).
All data related to the presence or absence of phylogenetic groups and antibiotic resistance in each isolate were entered into Excel (Microsoft 2016) and SPSS (SPSS 24; IBM) programs to calculate the prevalence percentage in descriptive statistics.
Results
Phenotypic antimicrobial resistance
In this study, antibiotic resistance was assessed against six commonly used antibiotics in the treatment of urinary tract infections related to fluoroquinolones and β-lactam classes. In phenotypic tests, more than half of isolates were ESBL-producer (Fig. 1C). Only three isolates showed no phenotypic resistance against studied antibiotics (Table 1). Also, three isolates were resistant against just one antibiotic and the remaining (103 isolates; 97.1%, 95%CI: 91.9–99.9%) were resistant to more than one antibiotics (Table 2). The most common resistance pattern was related to CP/AZT/CRO/CAZ/CTX (Table 2). The results showed that the resistance against antibiotics cefotaxime and aztreonam were highly prevalent (Table 1 and Fig. 1A). However, ciprofloxacin showed the lowest rates of antibiotic resistance (Table 1 and Fig. 1A).
A: Prevaalence of resistant, intermediate and susceptible E. coli strains to studied antibiotics. B: Prevalence of antimicrobial resistant genes. C: Frequency of EABL-producing E. coli strains. D: The overlapping pattern of phylotypes with ESBL-positve and ESBL-negative E. coli strains. E: Frequency of phylotypes in this study
Prevalence of β-lactamase genes
In this study, among 106 isolates, blaTEM was the most prevalent gene followed by blaCTX-M, blaSHV and blaCMY, respectively (Table 1, Fig. 1B and Fig. 2). Also, the most prevalent resistance gene profiles were blaTEM and blaTEM/blaCTX-M (Table 3).
Odds ratio between AR genes and phenotypes significantly (P < 0.05) revealed that the isolates showing resistance against CTX, CAZ and CRO were 4.59, 3.24 and 8.1 times more likely to the positive for blaCTX-M gene than the isolates which were susceptible to CTX, CAZ and CRO, respectively (Table 4). The ESBL-producing isolates were 24.95 and 6.38 times more likely (P < 0.05) to the positive for blaTEM and blaCTX-M genes, respectively, than the isolates which were negative for ESBL. Other odds ratios between AR genes and phenotypes were not significant (Table 4).
Prevalence of phylotypes
Overall, out of 106 E. coli isolates, phylotypes A, B1, B2, C, D, E, F and unknown (U) strains were identified; frequencies from high to low were B2 > D > E > F > B1 > C > A, respectively (Fig. 1D, 1E and 2). There was significant difference (P < 0.05) between B2 frequency and the other phylotypes.
Discussion
ESBL-producing UPEC strains were widely reported in developing countries as one of the most critical challenges defined by World Health Organization (WHO) [15, 16]. More than half of our E. coli isolates were phenotypical ESBL-producer; this result is approximately similar to the prevalence of ESBL-producing E. coli isolates reported by Iranian studies during 2015–2022 [17, 18]. Our results are very similar to prevalence of ESBL-producing isolates in previous study in Jiroft (2018) [19]. Also, our results are similar to prevalence in China [20], higher than Nigeria [21] and comparable with a wide range of abundance in other African researches [22]. The initial treatment of UTIs with mainly β-lactam antibiotics may be responsible for the high rates of ESBL-producers in our study and other studies. During a surveillance program in Europe, E. coli isolates from UTIs in 18 countries were assessed, and almost one-fifth of all isolates were positive for ESBL phenotypes [23]. Various prevalence rates were reported in North America, showing the annual increase (approximately 8%) in ESBL-producing isolates [24, 25]. ESBL prevalence in this study was compared with some previous studies in Iran and other regions of the world in Table 5.
Many factors are involved in the emergence of ESBL-producing bacteria. Incorrect antibiotic use, self-medication, prescription without antimicrobial susceptibility tests, and consumption of counterfeit drugs may lead to selective pressure in favor of ESBL-producing bacteria and the detriment of susceptible strains to β-lactam antibiotics [26]. ESBL-producing strains could be transferred from one host to another and contaminate the environment at the same time. Therefore, admission to a hospital, surgery, hospitalization, a history of UTI, travel, and swimming are among the most significant risk factors for acquiring ESBL-producing E. coli strains [4, 16].
The prevalence of resistance against antibiotics ceftazidime, cefotaxime, aztreonam and ceftriaxone were considerably high. Approximately, it is in agreement with studies in Ethiopia, Nigeria and Cyprus [21, 27, 28] and higher than the results in Sri Lanka, US and Canada [23,24,25, 29, 30]. Furthermore, resistance towards cefotaxime was more frequent than against ceftazidime, in accordance with the results reported by Gaviria et al. (2022) in Cerdanya [30]. Frequency rate of resistance against ciprofloxacin was found in approximately half of our isolates. According to a retrospective observational study conducted in China, the resistance rate to ciprofloxacin in UPECs varies between 55 and 70% during 8-year, 2012 to 2019 [20]. The fluoroquinolone resistance level in our study was higher than some findings in Europe and North America [23, 24]. Prevalence of phenotypic antimicrobial resistance in this study was compared with some previous studies in Iran and other regions of the world in Table 6. The most common pattern of resistance in our study was related to all six studied antibiotics, including CP/AZT/CRO/CAZ/CTX. This profile is not considered a multidrug-resistant (MDR) pattern because we studied only two antimicrobial categories; MDR means non-susceptibility to at least one agent in three or more antimicrobial classes. Nevertheless, resistance to more than one antimicrobial agent leads to problems in treatment of infectious disease.
Resistance to β-lactams and fluoroquinolones limits options in the treatment of UTI associated with ESBL-producing E. coli. Therefore, choosing the best antibiotic should be based on the severity of UTI and antibiogram tests. Nevertheless, there are some suggestions for treatment of resistant UTIs including piperacillin-tazobactam, meropenem/vaborbactam, imipenem/cilastatin-relebactam, ceftazidime-avibactam, ceftolozane-tazobactam, plazomicin, cefiderocol, fosfomycin, sitafloxacin, finafloxacin, colistin, and tigecycline [31].
The use of antibiotics in veterinary medicine for food-producing animals is an important reason for the development of resistance in E. coli as a member of gut microbiota. It can lead to two phenomena: 1) The emergence of resistant bacteria in animals and transmission of them through the food chain or direct contact. 2) Transmission of antimicrobial agents to humans through animal products containing antibiotic residues and subsequently emergence of resistant bacteria in humans [32]. Since antibiotics such as bacitracin, virginiamycin, colistin, etc. may be used as growth promoters in food-producing animals, the products must be tested to check the antibiotic residue according to the laws in Iran.
In level of antimicrobial resistance genes (ARGs), β-lactamase (bla) genes were studied; the results showed that blaTEM and blaCTX-M genes were positive with a high prevalence rate, but blaSHV and blaCMY had low frequency. Meta-analytic studies showed that the prevalence of ESBL genes was significantly high in different regions of Iran with various rates [33, 34]. Similar to our study, high frequencies of blaTEM and blaCTX-M-positive isolates were reported in other countries and regions, such as northern and eastern Europe [35]. In many countries, blaCTX-M group genes are prevailed, and rapidly disseminating among different Enterobacteriaceae causing UTIs [36]; for example, the rate of this gene was evaluated more than 85% in E. coli–causing bloodstream and urinary tract infections in patients hospitalized in the US [37]. The results of the present study on the genes blaTEM and blaCTX-M were higher than some studies in South Africa and Nigeria [38, 39]. The prevalence of blaSHV was near to the results of Sri Lanka [29]. Prevalence of blaCMY, one of the most common plasmid-mediated AmpC β-lactamase gene, was lower than Sri Lanka [29], and close to the findings in China [40]. ARGs prevalence in this study was compared with some previous studies in Iran and other regions of the world in Table 7.bla genes were originally chromosomal which is incorporated into plasmid and has spread to various Enterobacteriaceae members. These genes usually acquired by the horizontal gene transfer from other bacteria using mobile genetic elements such as conjugative plasmids or transposons [22]. bla genes have been detected in hospitals and clinics worldwide and are often responsible for resistance phenotypes to β-lactam antibiotics [41]. blaTEM is one of the most well-known determinants of resistance with more than 170 variants. It seems that blaSHV, blaCTX-M and blaOXA genes are mutants of classical blaTEM genes [41]; blaTEM was significantly found in ESBL producing strains of present work (Table 4). Also, a significant relationship was found between the presence of the blaCTX-M gene and the resistance phenotype against the antibiotics ceftazidime, cefotaxime and ceftriaxone (Table 4) which is in agreement with previous researches; blaCTX-M encodes enzymes for the hydrolysis of cephalothin, cephaloridine, penicillin, cefotaxime and ceftazidime [42]. Previous studies show that blaSHV is responsible for resistance to penicillins such as ampicillin and piperacillin [42]. blaCMY is a type of AmpC plasmid that may cause antibiotic resistance to ceftazidime, cefotaxime, cefoxitin, azetronam and probably cefepime [43].
E. coli, as one the main member of commensal papulation in the intestinal microflora, have stable genetic structure with a moderate levels of recombination in their genome. This genetic trait result in a clonal status in bacterial population and this is considered as a principle for identification of strong phylogenetic groups [44]. Today, four prevalent phylotype, including A, B1, B2, and D and four scarce phylotypes including C, E, F, and G have identified for E. coli bacterium. Some phenotypic and genotypic differences, including antibiotic resistance, virulence factors and growth rate, have introduced among various phylotypes [45]. For example, strains of UPEC pathotype usually belong to phylotypes B2 and D, but intestinal pathogenic and commensal E. coli strains belong to A and B1 [45]. In the present study, the most common phylotypes were B2 and D which is in agreement with other works in Iran [46] and Ethiopia [27]. Nevertheless, Mohsin et al. (2022) reported interesting results that differed from many studies; phylotyping of E. coli isolates from 500 UTIs in Iraq showed that the most frequent phylotype was F, followed by C > B2 > E > A > D > B1, respectively [47]. Table 8 compares the prevalence of phylotypes in our study with that of previous studies conducted in Iran and other regions of the world. However, it should be noted that there are numerous studies on this topic conducted in different parts of the world that were not included in our analysis.
Halaji et al. (2022) reported an increasing trend for phylotype B2 incidence from 2014 to 2020 among UPEC infections during a meta-analytic systematic review; they have introduced several variables including host species, nutrition types, infection types, geographical regions, methodology, sample size and time of study to explain the variation in phylotype frequency in different researches [46]. Moreover, Touchon et al. (2020) introduced some factors, such as host (species, diet, sex, age and body mass), environment (climate and geographic location) and bacteria (resistance and virulence) for the distribution pattern of phylotypes [45].
There were several limitations and weaknesses in this study, such as the small sample size, the limited number of regions and cities examined, and the absence of patient history, including information on prior infections, recurrent UTIs, and antibiotic use. Therefore, we suggest conducting further studies, such as a survey on antibiotic-resistant E. coli isolates from fecal samples of UTI cases in multiple cities, to provide a comprehensive overview of the antimicrobial resistance prevalence at the national level.
Conclusions
This work shows the high prevalence of ESBL-producing E. coli strains and the predominance of blaTEM and blaCTX-M genes among the strains that mostly belonged to the pathogenic phylotypes B2 and D. Best of our knowledge, blaCMY gene and the phylotypes C, E and F were reported for the first time in Jiroft in this study. Comparison of our findings with some previous studies in different time periods in Iran shows the increasing trend of phenotypic and genotypic antimicrobial resistance prevalence in Iran and Jiroft city (Fig. 2); therefore, this study highlights the need for urgent action to prevent the spread of antibiotic resistance, such as strict rules to control the sale of antibiotics and presence of antibiotic residues in foods produced by animals. This study emphasizes the urgent need for awareness about antibiotic use in the community and implementation of a national surveillance system to monitor antibiotic-resistant bacteria.
Availability of data and materials
All data generated or analysed during this study are included in this published article. Also, our dataset does not include proteomics data and protein sequences, DNA and RNA sequences, genetic polymorphisms, linked genotype and phenotype data, macromolecular structure, gene expression data, and crystallographic data for small molecules. So, our data does not fall under the list of data types that must be deposited in BMC recommended repositories.
References
Bazaid AS, Saeed A, Alrashidi A, Alrashidi A, Alshaghdali K, Hammam SA, et al. Antimicrobial surveillance for bacterial uropathogens in Ha’il, Saudi Arabia: a five-year multicenter retrospective study. Infect Drug Resist. 2021;14:1455.
Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017;8:1566.
Öztürk R, Murt A. Epidemiology of urological infections: a global burden. World J Urol. 2020;38:2669–79.
Larramendy S, Deglaire V, Dusollier P, Fournier J-P, Caillon J, Beaudeau F, et al. Risk factors of extended-spectrum beta-lactamases-producing Escherichia coli community acquired urinary tract infections: a systematic review. Infect Drug Resist. 2020;13:3945.
Chardavoyne PC, Kasmire KE. Appropriateness of antibiotic prescriptions for urinary tract infections. West J Emerg Med. 2020;21:633.
Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, et al. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J Mol Biol. 2019;431:3472–500.
Solano-Gálvez SG, Valencia-Segrove MF, Prado MJO, Boucieguez ABL, Álvarez-Hernández DA, Vázquez-López R. Mechanisms of resistance to quinolones. In: Antimicrobial Resistance-A One Health Perspective. London: IntechOpen; 2020. p. 25–48.
Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31:e00088-e117.
Pettengill JB, Kase JA, Murray MH. The population genetics, virulence, and public health concerns of escherichia coli collected from rats within an urban environment. Front Microbiol. 2021;631761.
Clermont O, Christenson JK, Denamur E, Gordon DM. The C lermont E scherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5:58–65.
Markey B, Leonard F, Archambault M, Cullinane A, Maguire D. Clinical Veterinary Microbiology E-Book. USA: Elsevier Health Sciences; 2013. p. 239–274.
CLSI. Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplement M100. Wayne, PA: Clinical and laboratory standards institute; 2018. p. 30–37.
Jajarmi M, AsadabadiSafat A, Sakhaee E, Ghanbarpour R. Study of the presence of blaTEM, blaSHV and blaCTX-M genes in Escherichia coli strains isolated from sheep in Kerman province. Iran Vet J. 2021;16:16–23.
Roschanski N, Fischer J, Guerra B, Roesler U. Development of a multiplex real-time PCR for the rapid detection of the predominant beta-lactamase genes CTX-M, SHV, TEM and CIT-type AmpCs in Enterobacteriaceae. PLoS ONE. 2014;9: e100956.
Tacconelli, E., 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development, infection control Africa network. South Africa. Retrieved from https://policycommons.net/artifacts/1818147/global-priority-list-of-antibiotic-resistant-bacteria-to-guide-research-discovery-and-development/2555608/ on 21 Apr 2023. CID: 20.500.12592/khnnff.
Stewart AG, Harris PNA, Henderson A, Schembri MA, Paterson DL. Oral cephalosporin and β-lactamase inhibitor combinations for ESBL-producing Enterobacteriaceae urinary tract infections. J Antimicrob Chemother. 2020;75:2384–93.
Mood EH, Meshkat Z, Izadi N, Rezaei M, Jamehdar SA, Nasab MN. Prevalence of quinolone resistance genes among extended-spectrum B-lactamase-producing Escherichia coli in Mashhad Iran. Jundishapur J Microbiol. 2015;8:e16217.
Sadeghi M, Ebrahim-Saraie HS, Mojtahedi A. Prevalence of ESBL and AmpC genes in E coli isolates from urinary tract infections in the north of Iran. New Microbes New Infect. 2022;45:100947.
Mashaiekhi S, Kheirkhah B, Amini K. Molecular study of virulence genes SHV and TEM in antibiotic resistant Escherichia coli strains isolated from urethral specimens in city of Jiroft. Razi J Med Sci. 2018;25:75–82.
Sun J, Du L, Yan L, Dai W, Wang Z, Xu X. Eight-year surveillance of uropathogenic escherichia coli in Southwest China. Infect Drug Resist. 2020;13:1197.
Ejikeugwu PC, Ikegbunam NM, Ugwu CM, Iroha IR, Esimone CO. Extended-spectrum β-lactamase-producing Escherichia coli isolates from suspected community acquired urinary tract infections. Eur J Sci Res. 2012;84:565–71.
Sonda T, Kumburu H, van Zwetselaar M, Alifrangis M, Lund O, Kibiki G, et al. Meta-analysis of proportion estimates of Extended-Spectrum-Beta-Lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob Resist Infect Control. 2016;5:18.
Critchley IA, Cotroneo N, Pucci MJ, Jain A, Mendes RE. Resistance among urinary tract pathogens collected in Europe during 2018. J Glob Antimicrob Resist. 2020;23:439–44.
Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis. 2016;85:459–65.
Kaye KS, Gupta V, Mulgirigama A, Joshi AV, Scangarella-Oman NE, Yu K, et al. Antimicrobial resistance trends in urine Escherichia coli isolates from adult and adolescent females in the United States from 2011 to 2019: rising ESBL strains and impact on patient management. Clin Infect Dis. 2021;73:1992–9.
OucharMahamat O, Lounnas M, Hide M, Dumont Y, Tidjani A, Kamougam K, et al. High prevalence and characterization of extended-spectrum ß-lactamase producing Enterobacteriaceae in Chadian hospitals. BMC Infect Dis. 2019;19:205.
Dadi BR, Abebe T, Zhang L, Mihret A, Abebe W, Amogne W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa. Ethiopia BMC Infect Dis. 2020;20:1–12.
Cantas L, Suer K, Guler E, Imir T. High emergence of ESBL-producing E. coli cystitis: time to get smarter in Cyprus. Front Microbiol. 2016;6:1446.
Perera PDVM, Gamage S, De Silva HSM, Jayatilleke SK, de Silva N, Aydin A, et al. Phenotypic and genotypic distribution of ESBL, AmpC β-lactamase and carbapenemase-producing Enterobacteriaceae in community-acquired and hospital-acquired urinary tract infections in Sri Lanka. J Glob Antimicrob Resist. 2022;30:115–22.
Gaviria LP, Montsant L, Azuaje C, González-Díaz A, Horcajada JP, Limón E, et al. A descriptive analysis of urinary ESBL-producing-escherichia coli in cerdanya hospital. Microorganisms. 2022;10:488.
Bader MS, Loeb M, Leto D, Brooks AA. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad Med. 2020;132:234–50.
Palma E, Tilocca B, Roncada P. Antimicrobial resistance in veterinary medicine: An overview. Int J Mol Sci. 2020;21:1914.
Ghaderi RS, Yaghoubi A, Amirfakhrian R, Hashemy SI, Ghazvini K. The prevalence of genes encoding ESBL among clinical isolates of Escherichia coli in Iran: a systematic review and meta-analysis. Gene Reports. 2020;18: 100562.
Jabalameli L, Beigverdi R, Ranjbar HH, Pouriran R, Jabalameli F, Emaneini M. Phenotypic and genotypic prevalence of extended-spectrum β-Lactamase-producing escherichia coli: a systematic review and meta-analysis in Iran. Microb Drug Resist. 2021;27:73–86.
Sepp E, Andreson R, Balode A, Bilozor A, Brauer A, Egorova S, et al. Phenotypic and molecular epidemiology of ESBL-, AmpC-, and carbapenemase-producing Escherichia coli in Northern and Eastern Europe. Front Microbiol. 2019;10:2465.
Zowawi HM, Harris PNA, Roberts MJ, Tambyah PA, Schembri MA, Pezzani MD, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12:570–84.
Mendes RE, Jones RN, Woosley LN, Cattoir V, Castanheira M. Application of next-generation sequencing for characterization of surveillance and clinical trial isolates: analysis of the distribution of β-lactamase resistance genes and lineage background in the United States. Open Forum Infect Dis. 2019;6:S69–78.
Mohammed Y, Gadzama GB, Zailani SB, Aboderin AO. Characterization of extended-spectrum beta-lactamase from Escherichia coli and Klebsiella species from North Eastern Nigeria. J Clin diagnostic Res JCDR. 2016;10:DC07.
Kubone PZ, Mlisana KP, Govinden U, Abia ALK, Essack SY. Antibiotic susceptibility and molecular characterization of uropathogenic Escherichia coli associated with community-acquired urinary tract infections in urban and rural settings in South Africa. Trop Med Infect Dis. 2020;5:176.
Jia P, Zhu Y, Li X, Kudinha T, Yang Y, Zhang G, et al. High prevalence of extended-spectrum beta-lactamases in Escherichia coli strains collected from strictly defined community-acquired urinary tract infections in adults in china: a multicenter prospective clinical microbiological and molecular study. Front Microbiol. 2021;1611:663033.
Oduro-Mensah D, Obeng-Nkrumah N, Bonney EY, Oduro-Mensah E, Twum-Danso K, Osei YD, et al. Genetic characterization of TEM-type ESBL-associated antibacterial resistance in Enterobacteriaceae in a tertiary hospital in Ghana. Ann Clin Microbiol Antimicrob. 2016;15:1–9.
Shaikh S, Fatima J, Shakil S, Rizvi SMD, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: types, epidemiology and treatment. Saudi J Biol Sci. 2015;22(1):90–101.
Merida-Vieyra J, Colsa-Ranero D, Calderón-Castañeda Y, Aquino-Andrade A. Detection of CMY-type beta-lactamases in Escherichia coli isolates from paediatric patients in a tertiary care hospital in Mexico. Antimicrob Resist Infect Control. 2020;9:1–10.
de Stoppe NC, Silva JS, Carlos C, Sato MIZ, Saraiva AM, Ottoboni LMM, et al. Worldwide phylogenetic group patterns of Escherichia coli from commensal human and wastewater treatment plant isolates. Front Microbiol. 2017;8:2512.
Touchon M, Perrin A, de Sousa JAM, Vangchhia B, Burn S, O’Brien CL, et al. Phylogenetic background and habitat drive the genetic diversification of Escherichia coli. Plos Genet. 2020;16: e1008866.
Halaji M, Fayyazi A, Rajabnia M, Zare D, Pournajaf A, Ranjbar R. Phylogenetic group distribution of uropathogenic escherichia coli and related antimicrobial resistance pattern: a meta-analysis and systematic review. Front Cell Infect Microbiol. 2022;12:790184.
Mohsin AS, Alsakini AH, Ali MR. Outbreak of drug resistance Escherichia coli phylogenetic F group associated urinary tract infection. Iran J Microbiol. 2022;14:341–50.
Acknowledgements
The authors would like to express their gratitude to medical diagnostic laboratories of Jiroft for their kind participation in this research.
Funding
There was no financial support for this research.
Author information
Authors and Affiliations
Contributions
MJ and RG designed the study and analyzed the data; SA performed the main experiments; PM wrote and drafted the manuscript; NA and MB performed the complementary experiments and English edition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the committee for ethics in biomedical research in Veterinary Faculty of Shahid Bahonar University of Kerman, Iran. Also, all methods were carried out in accordance with relevant guidelines and regulations presented by Iran National Committee for Ethics in Biomedical Research. We obtained informed consent from the all persons for sample collection.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Afsharikhah, S., Ghanbarpour, R., Mohseni, P. et al. High prevalence of β-lactam and fluoroquinolone resistance in various phylotypes of Escherichia coli isolates from urinary tract infections in Jiroft city, Iran. BMC Microbiol 23, 114 (2023). https://doi.org/10.1186/s12866-023-02860-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-023-02860-7