Abstract
Background
Fungi associated with insects represent one potentially rich source for the discovery of novel metabolites. However, a comprehensive understanding of the fungal communities of Apis mellifera ligustica remains elusive.
Results
Here, we investigated the phylogenetic diversity and community composition of honeybee-associated fungi using combination of culture-dependent and culture-independent approaches. A total of forty-five fungi were isolated and purified from the Apis mellifera ligustica, royal jelly, and honeycomb, which belonged to four classes and eleven different genera. Furthermore, 28 bacterial 16S rRNA gene sequences were obtained by PCR from the fungal metagenome. High-throughput sequencing analyses revealed that the fungal communities were more diverse, a total of 62 fungal genera were detected in the honeybee gut by culture-independent method, whereas only 4 genera were isolated by culture-dependent method. Similarly, 247 fungal genera were detected in the honeycomb, whereas only 4 genera were isolated. In addition, we assessed the antibacterial and antioxidant activities of fungal isolates. Most fungal crude extracts obtained from the cultivation supernatant exhibited antioxidant activities. Only two fungal crude extracts displayed moderate activity against Escherichia coli and Staphylococcus aureus. Chemical analysis of Chaetomium subaffine MFFC22 led to the discovery of three known compounds, including cochliodinol (1), emodin (2), chrysophanol (3). Among them, cochliodinol (1) showed intense DPPH radical scavenging activity with the 50% inhibitory concentration (IC50) of 3.06 μg/mL, which was comparable to that of the positive ascorbic acid (IC50 = 2.25 μg/mL). Compound 2 displayed weak inhibitory activities against Micrococcus tetragenus and S. aureus.
Conclusions
This research provided a fundamental clue for the complex interactions among honeybees, fungi, bacterial symbionts, and the effects on the honeybee. Furthermore, the diversity of honeybee-associated fungi had great potential in finding the resource of new species and antioxidants.
Similar content being viewed by others
Background
Insects are the most abundant group of animals on the Earth. Over one million species of insects have been named and widely distributed in various habitats [1, 2]. The great diversity of insects nurtures a large microbial community association with the insect. Some microbial species were found valuable functions in nutrition and protection in social insects, such as termites, ants, and bees [3–5]. It is worth mentioning that the relationship between insects and their associated fungi capture researchers' attention, for instance, beetle-fungus farming symbiosis, fungus-cultivating termites, and fungus-farming ants [6–8]. In addition, reports indicate that some bacteria were present in fungal hyphae [9, 10]. It is worth considering to evaluate in many further aspects of the associations between fungi and insects as well as fungi and bacteria.
Insect-associated fungi are vital microbial sources of natural bioactive products. The insect-associated fungi isolated from arthropod cadavers, leaf-cutting ants, and stoneflies were contained new microbial species [11–13]. Insect-associated fungi could enhance insects' fitness by producing bioactive compounds [14]. Furthermore, many novel compounds have been discovered from insect-associated fungi, and these compounds had the potential as immune inhibitors, antibacterial agents, and biofungicides [15–17]. However, the current research in metabolites produced by insect-associated fungi is still not comprehensive.
As a social insect, honeybees belong to the class Insecta and the order Hymenoptera in the phylum Arthropoda. It is a critical species for agricultural production as pollinators [18]. Various parts of honeybee larvae and adults, their food, and honeycomb harbor numerous microorganisms, which play a significant role in food digestion, pollination, and antagonistic effect against different pathogens [19]. Especially, fungi associated with honeybee may provide material for pollen degradation or assist in royal jelly maturation, and also can be a food source [20, 21]. For example, the Brazilian stingless bee larva grows by eating fungal mycelia of Monascus inside the brood cell [22]. Therefore, honeybees are a potential model of fungus-host-symbiont interactions, which is worth exploring. As a high royal jelly-producing honeybee, Apis mellifera ligustica acquired fungi from their diet, the surrounding environment, or mates [23]. However, only a few reports involve fungi isolated from specimens associated with A. mellifera ligustica [24]. Our knowledge about the species, biological activity, and secondary metabolites of these associated fungi are limited until now. Here, we investigated the diversity of fungi from A. mellifera ligustica using culture-dependent and culture-independent methods and explored the bacterial symbionts, biological activity, and secondary metabolites of the fungi isolated from A. mellifera ligustica (larvae, adults), honeycomb, and royal jelly.
Results
Identification of cultivable fungi associated with honeybee
In this study, a total of forty-five fungi were isolated from the honeycomb, royal jelly, larvae, and different parts of honeybee on nine different media (Table 1, Fig. 1). The morphological and microscopic characteristics of the ten representative strains are shown in Fig. 2. Among them, seven strains were isolated from honeycomb, thirteen from royal jelly, ten from larvae, two from honeybee cuticle, seven from gut, three from head, and three from hypopharyngeal gland. ITS sequence analysis of the forty-five isolates revealed that the fungal isolates belonged to two different phyla (Basidiomycota and Ascomycota) and eleven different genera. Twenty-seven isolates (60.0%) were distributed in the Agaricomycetes within the phylum Basidiomycota. The other eighteen isolates were grouped into three classes [Sordariomycetes (26.7%), Dothideomycetes (11.1%), and Eurotiomycetes (2.2%)] within Ascomycota.
Neighbor-joining phylogenetic tree of ITS gene sequences of honeybee-associated fungi. The numbers at the nodes represent bootstrap support, based on a neighbor-joining analysis of 1000 replicates. Only the bootstrap value odes represent bootstrap sbranches.The GeneBank taxa are designated by species name with accession number while our isolates are designated by code name
The morphological and microscopic characteristics of some honeybee- associated fungi. Colony (A1) and conidia (A2) of Alternaria alternata MFYC01; colony (B1) and conidia (B2) of Paraconiothyrium brasiliense MFB02; colony (C1) and conidia (C2) of Irpex lacteus MFT03; colony (D1) and conidia (D2) of Fusarium solani FWJ19; colony (E1) and conidia (E2) of Arthrinium arundinis MFYC08; colony (F1) and conidia (F2) of Chaetomium subaffine MFFC22; colony (G1) and conidia (G2) of Trametes hirsuta FWJ10; colony (H1) and conidia (H2) of Bjerkandera adusta MFYC06; colony (I1) and conidia (I2) of Schizophyllum commune FWJ08; colony (J1) and conidia (J2) of Leiotrametes lactinea MFYC12
Fungal isolates belonging to class Agaricomycetes were assigned to two orders, including the Agaricales (3 isolates) and Polyporales (24 isolates). The three strains in Agaricales were isolated from the honeycomb, royal jelly, and hypopharyngeal gland, respectively, which showed highly similar to Schizophyllum commune with more than 99% identity. The three strains in Agaricales were isolated from the honeycomb, royal jelly, and hypopharyngeal gland, respectively, which showed highly similar to Schizophyllum commune with an identity of more than 99%. The strains of Polyporales were isolated from most of the samples and represented by the family Polyporaceae, Irpicaceae and Phanerochaetaceae. Among them, most strains (17 isolates) belonging to genus Irpex were identified as Irpex lacteus. Notably, the strain FWJ13 showed only 98% similarity to I. lacteus. In addition, two strains isolated from honeybee larvae were identified as Bjerkandera adusta and Leiotrametes lactinea, respectively. Moreover, B. adusta was also found in royal jelly. The last four strains belonging to Trametes genus showed similar to Trametes hirsuta with an identity of 99%.
The other representative class was Sordariomycetes, including three orders (Hypocreales, Sordariales, and Xylariales). Eight isolates belonging to the family Hyporcreaceae exhibited a sequence match of more than 99% to Fusarium solani. Fusarium spp. were found in association with honeybee gut, larvae, and royal jelly. Both strains belonging to Sordariales were isolated from the honeycomb, identified as Chaetomium subaffine with an identity of 100%. The other two strains belonging to the Xylariales were identified as Arthrinium arundinis, which were found to be most related to honeybee intestines and larvae.
The five fungal sequences of Dothideomycetes were grouped into Pleosporales, including Paraconiothyrium brasiliense (3 isolates), and Alternaria alternate (2 isolates). The P. brasiliense with an identity of 99% were isolated from honeybee cuticle and larvae, respectively. The genus Alternaria was only isolated from honeybee larvae.
Finally, only one isolate belonging to class Eurotiomycetes was grouped into the order Eurotiales, which belonged to the genus Aspergillus with a high sequence match to Aspergillus flavus (> 99%). The strain was isolated from honeybee gut.
Culture-independent community
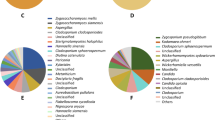
The ITS1 region was performed to analyze the fungal community within the honeybee gut and honeycomb by using Illumina Miseq sequencing. A total of 408,112 high-quality fungal clean reads were generated from two populations with 3 replicates for each population (honeybee gut: MFF1_1, MFF1_2, MFF1_3, honeycomb: FC_01, FC_02, FC_03) (Supplementary Table S1 and S2). A total of 3 fungal phyla were identified in honeybee gut samples, including Ascomycota, Basidiomycota, and unclassified fungi, the average abundance was 93.29, 6.67, 0.04%, respectively (Fig. 3A). Ascomycota (88.81% of the average abundance) and Basidiomycota (7.05%) were identified in honeycomb samples (Fig. 3C). Regarding the composition of fungal community, all samples were dominated at the phylum level by Ascomycota. The proportions of Ascomycota in the honeybee gut were higher than in the honeycomb. The community composition was further analyzed at the genus level. 62, 247 genera were identified across investigated samples of honeybee gut and honeycomb, respectively. Among them, Kodamaea (80.13%), Zygosaccharomyces (8.19%), Wallemia (6.11%), and Wickerhamomyces (3.62%) had higher abundance in the honeybee gut (Fig. 3B). However, Bipolaris (47.07%), Metschnikowia (18.17%), Starmerella (3.85%), Trichoderma (16.77%), Kodamaea (3.35%) had higher abundance in the honeycomb (Fig. 3D). The relative abundance of yeast such as Kodamaea, Zygosaccharomyces, and Wickerhamomyces was higher in the honeybee gut than in the honeycomb. There are differences in fungal communities of honeybee gut and honeycomb samples between the two approaches. Culture-independent community analysis showed more diverse fungal communities than did the culture-dependent method.
Identification of bacterial symbionts
In this study, a total of twenty-eight bacterial symbionts 16S rRNA were observed from genomic DNA extractions of the honeybee-associated fungi (Table2). Among them, nineteen, six, two, and one strain were isolated from the host of the Agaricomycetes, Sordariomycetes, Dothideomycetes, and Eurotiomycetes, respectively. All bacterial symbionts were divided into four genera by initial BLAST comparisons in GenBank and were putatively identified as Bacillus velezensis, Bacillus siamensis, Microbacterium tumbae, Pandoraea sputorum, and Achromobacter xylosoxidans. These bacterial symbionts were mainly identified as B. velezensis and B. siamensis in Bacillaceae. Moreover, B. velezensis was obtained from fungi of different honeybee samples or parts, such as honeybee gut, hypopharyngeal gland, head, cuticle, larvae, honeycomb, and royal jelly.
Antimicrobial activities
The filter paper disc method was used to evaluate the antibacterial activities of 36 fungal crude extracts, which were obtained from the cultivation supernatant (Table3). Most of the fungal crude extracts showed no antibacterial activities. However, I. lacteus FWJ16 displayed moderate activity against E. coli with a disc diameter of inhibition zone diameter (IZD) of 10.13 mm, which was weaker than the positive gentamicin sulfate with the IZD of 26.33 mm. Additionally, P. brasiliense MFYC10 also displayed moderate activity against S. aureus with the IZD of 9.67 mm, which was weaker than the positive gentamicin sulfate with the IZD of 23.92 mm.
Antioxidant activities
The antioxidant activities of 36 fungal crude extracts are shown in Table3. The results revealed that 32 extracts (88.9%) exhibited antioxidant activities under the concentration of 166.67 µg/mL. Among them, scavenging rates between 10–40%, 40–70%, and 70–100% were found in 19 (52.8%), 3 (8.3%), and 5 (13.9%) strains, respectively. Especially, crude extract of MFFC22 showed the strongest antioxidant activity with the DPPH scavenging activity of 91.6%. Thus, the MFFC22 was further selected to evaluate antioxidant activity at different concentrations (Fig.4). The result showed that the MFFC22 had a higher activity with the increasing concentration. Its IC50 on DPPH scavenging activity was 22.11 µg/mL, which was relatively weaker than a commercial natural antioxidant ascorbic acid (Vc, 2.25 µg/mL).
Identification of the secondary metabolites from C. subaffine MFFC22
Three compounds were purified from the liquid fermentation product of C. subaffine MFFC22 and their structures were determined to be cochliodinol (1) [25], emodin (2) [26], and chrysophanol (3) [27] (Fig. 5) by spectroscopic data analyses and comparison of their data in the literature.
Cochliodinol (1): purple solid; HR-ESI–MS: m/z: 505.2109 [M—H]−, calculated for C32H30N2O4 505.2128; 1H NMR (600 MHz, CDCl3) δ: 8.42 (1H, s, 2,5-OH), 8.14 (1H, s, 1´-NH), 7.60 (1H, s, H2´), 7.44 (1H, s, H4´), 7.34 (1H, d 8.3, H7´),7.09 (1H, d 8.3, H6´), 5.40 (1H, m, H11´), 3.48 (1H, d 7.1, H10´), 1.76 (3H, s, 13´-CH3), 1.74 (1H, s, 14´-CH3); 13C NMR (150 MHz, CDCl3): 134.3 s (C8´), 133.7 s (C5´), 131.6 s (C12´), 127.1 d(C2´), 126.2 s (C9´), 124.4 d (C11´), 123.4 d (C6´), 120.8 s (C4´),111.0 d (C7´), 110.7 s (C3, C6), 104.2 s (C3´), 34.6 t (C10´), 25.6 q (C14´), 17.8 q (C13´). Due to the resonance of the benzoquinone ring, the carbonyls resonances (C1, C2, C4, and C5) of cochliodinol were not observed; these carbons can only be observed in THF-d8 or CDCl3 at -75 ℃ [28].
Emodin (2): orange crystals; HR-ESI–MS: m/z: 269.0444 [M—H]−, calculated for C15H9O5, 269.0450; 1H NMR (600 MHz, acetone-d6) δ:12.21 (1H, s, 1-OH), 12.08 (1H, s, 8-OH), 10.15 (1H, s, 3-OH), 7.59 (1H, d 1.5, H5), 7.28 (1H, d 1.5, H4), 7.16 (1H, d 0.6, H7), 6.68 (1H, d 2.0, H2), 2.48 (3H, s, 6-CH3); 13C NMR (150 MHz, acetone-d6): 191.6 (C9), 182.1(C10), 166.5 (C1), 166.3 (C3), 163.3 (C8), 148.8 (C6), 136.9 (C12), 134.5 (C14), 125.0 (C7), 121.7 (C5), 113.8 (C13), 109.8 (C11), 109.7 (C4), 109.0 (C2), 22.2 (CH3).
Chrysophanol (3): orange crystals; HR-ESI–MS: m/z: 253.0521 [M—H]−, calculated for C15H9O4, 253.0501; 1H NMR (600 MHz, CDCl3) δ: 12.12 (1H, s, 1-OH), 12.02 (1H, s, 8-OH), 7.83 (1H, d 7.2, H5), 7.68 (1H, m, H6), 7.66 (1H, s, H4), 7.30 (1H, d 8.4, H7), 7.11(1H, s, H2), 2.47 (3H, s, 3-CH3).
Antibacterial and antioxidant activities of compounds
The antibacterial activities of the compounds isolated from MFFC22 were shown in Table 4. Specifically, compound 2 displayed weak inhibitory activities against M. tetragenus and S. aureus with the IZD of 7.00 and 7.33 mm, respectively. Compound 1 had no inhibition effect on three bacterial strains.
The antioxidant activities of compound 1 isolated from MFFC22 are shown in Fig. 4. Notably, the 50% inhibitory concentration (IC50) of cochliodinol (1) on DPPH scavenging activity was 3.06 μg/mL, which was comparable to that of positive ascorbic acid (2.25 μg/mL).
Discussion
Microbial community is extensive among insects. As a special kind of microbial community, insect-associated fungi have been a source of new microbial resources [29, 30] and biologically active natural products [16, 31, 32]. While fungi were present in A. mellifera ligustica, the leading research mainly focused on the honeycomb, honeybee gut, pollen, and bread [23, 33–36]. To the best of our knowledge, this is the first report on the diversity, antibacterial and antioxidant activities of culturable fungi from honeycomb, honeybee product (royal jelly), larvae, and different parts of A. mellifera ligustica. Forty-five fungi were isolated and characterized by the dilution-plate method and molecular biological identification. Furthermore, three metabolites were purified and characterized from C. subaffine MFFC22. Consequently, honeybee-associated fungi can provide a resource for microbial diversity and natural products.
The same fungus of I. lacteus was isolated and identified from honeybee gut, head, hypopharyngeal gland, larvae, and royal jelly in our study. It suggests that the fungus is widely distributed in A. mellifera ligustica. Some fungi isolated from honeybee larvae, hypopharyngeal gland, honeycomb, and royal jelly were wood-rotting fungi, such as T. hirsuta, S. commune, B. adusta, and L. lactinea [37–39]. Besides, the genus Arthrinium isolated from honeybee gut and larvae has been reported as a plant pathogen [40]. Compared with the fungal diversity of other insects, some fungi isolated from honeybee were the same as those of other different insects. For example, I. lacteus and P. brasiliense were also isolated from termites, and F. solani was isolated from beetles [41, 42]. Aspergillus, Alternaria, Chaetomium, Fusarium, and Arthrinium genera have already been found in the honeybee. For example, Aspergillus, Chaetomium, Fusarium, and Alternaria sp. were isolated from honeybee gut, and Arthrinium sp. was isolated from pollen [23, 33–35]. However, most of these fungi reported in this study are new for this honeybee. For instance, this is the first report of the isolation of T. hirsuta and S. commune from both honeycomb and royal jelly, I. lacteus from all parts of honeybee samples. Additionally, one rare fungus P. brasiliense was found in honeybee cuticle, which was reported previously in the gut of Acrida cinerea [43].
A total of 62 fungal genera were discovered in honeybee gut by culture-independent method. Among them, Kodamaea, Zygosaccharomyces, Wallemia, and Wickerhamomyces were predominant genera. Previous study based on pyrosequencing of ITS region of honeybee gut samples derived from Korea revealed that Saccharomyces and Zygosaccharomyces were dominant [20]. However, Starmerella and Hanseniaspora had high relative abundance in honeybee gut samples collected from Italy and Saudi Arabia [16]. Previous studies on predominant genera were inconsistent with each other, and also differed from our results. Presumably, it was due to the different surrounding environments of the sampling sites. Some genera analyzed by culture-independent approaches were also isolated by culture-dependent method, such as Aspergillus in honeybee gut, Trametes, and Chaetomium in honeycomb. However, culture-independent approaches in general revealed higher microbial composition and diversity compared to culture-dependent method. For example, 247 fungal genera were detected in the honeycomb by culture-independent method, whereas only 4 genera were isolated. Interestingly, as the causative agent of chalkbrood disease in honeybee Reserved literature [44], Ascosphaera spp. was detected in honeybee gut and honeycomb by culture-independent approach, but it has not been isolated yet.
The DPPH radical scavenging assay indicated that most crude extracts from honeybee-associated fungi showed antioxidant activities. Notably, the crude extract of C. subaffine MFFC22 isolated from honeycomb has shown notable antioxidant activity. Chaetomium genus is already known to have strong DPPH scavenging activity, such as C. nigricolor, C. globosum, C. cruentum [26, 44, 45]. Nevertheless, C. subaffine and its metabolites have not reported DPPH scavenging activity. The compound 1 from MFFC22 showed strong DPPH scavenging activity with the IC50 values of 3.06 μg/mL, comparable to the positive ascorbic acid (Vc). Comparing a commercial antioxidant BHT with IC50 of 95.7 μM, Dehghan et al. found that compounds 2 and 3 also exerted moderate antioxidant activities against DPPH with IC50 of 271.2 and 297.0 μM, respectively [46]. The phenolic compounds with the free hydroxyl groups were well known for robust antioxidant activity [47]. Therefore, the antioxidant activity of compounds 1–3 may be attributed to their phenolic hydroxyl groups. Additionally, it was reported that antioxidant compounds produced by fungi could protect their hosts by enhancing tolerance to abiotic stresses [48]. Whether compounds 1–3 have similar effects deserves further exploration. In addition, these isolated compounds have been shown to have other bioactivities. For example, compound 1 was a common fungal metabolite with cytotoxic activities against the KB, MDA-MB-435 and MRC5 cell lines [49]. Compounds 2–3 were anthraquinone derivative and acted as an antimalarial and antiallergic agents [50, 51].
Symbiotic bacteria have occurred in some fungi, which affected fungi function and subsequent host-fungus interactions. However, only a few studies have explored their effects in insect-associated fungi [52, 53]. Here, we found the first insight into the bacterial symbionts in fungi associated with honeybee. The bacterial symbiont from honeybee has already been shown to protect the host by producing inhibitory compounds or providing nutrition [54, 55]. However, whether fungal-bacterial symbionts influence the growth of honeybee-associated fungi, and how they affect tripartite honeybee-fungus-bacterium mutualisms, remains to be explored further.
Conclusions
Here, the diversity of the honeybee-associated fungi was investigated using both culture-dependent and culture-independent analysis methods. This study expands our knowledge of honeybee-associated fungi and further raises the pool of fungal species from A. mellifera ligustica. The results show that several of these fungi have antibacterial and antioxidant activities, among which the fungus C. subaffine MFFC22 was the most prominent. Furthermore, the antioxidant activities of C. subaffine MFFC22 can be attributed to the identified phenolic compounds. Collectively, the culturable honeybee-associated fungi provide insight into the widespread insect symbionts, which have great potential in finding the resource of novel species and antioxidants.
Materials and methods
Sample collection and microbial isolations
A. mellifera ligustica (including larvae, adults), honeycomb, and royal jelly were collected from the Institute of Apicultural Research, Anhui Agricultural University, Hefei, China (GPS: 31◦53ʹN, 117◦20ʹE) between April and July 2021. The honeybee larvae and adults starved for 24 h, and all samples were stored at 4 ℃. Initially, seven larvae, seven adults, and one gram honeycomb were placed separately into 10 mL sterile phosphate-buffered saline (PBS) buffer solution in an autoclaved 50 mL tube to obtain fungi from external isolation. Then, the same tube from the external was filled with 10 mL 75% ethanol for 2 min, followed by rinsing in sterilized 10 mL 1% bleach with 0.1% tween 20 for three times (30 s each). The supernatant was removed and replaced with 10 mL sterile PBS solution. Subsequently, sterile forceps were used to dissect samples of adult honeybee to get head, gut, and hypopharyngeal gland. Each body part was grounded in 10 mL sterile PBS. According to the earlier report, the honeybee larvae, one gram honeycomb, and one gram royal jelly were fully homogenized separately in 10 mL sterile PBS [56]. Then, the homogenates were diluted in a tenfold series (i.e., 10−1, 10−2, 10−3), and aliquots of 100 µL from each dilution were spread onto nine isolation media (Table 5). Pure colonies of fungi from the appropriate dilution were transferred into a new PDA medium and incubated aerobically at 28 ℃. All these isolated fungal strains were preserved on PDA slants at 4 ℃ until use. The fungi were used for freeze-drying preservation by a freeze-dryer (BTP-3ES; SP Scientific, USA) [57], and were stored at our institute.
DNA sequencing
All honeybee-associated fungi were identified by molecular techniques and morphological characteristics [10, 58, 59]. Each fungus was cultured in malt extract medium at 28 ± 0.5 ℃ for 7 days. Then the fungal genomic DNA was extracted using the Fast DNA Extraction Kit (Aidlab Biotechnologies Co., Ltd., Beijing, China) as claimed by the manufacturer's specification. The primers ITS1/ITS4 were used to amplify ITS based on the fungal genomic DNA. Additionally, 16S rRNA was amplified using the primers 27F/1492R by PCR from the fungal metagenome [10, 60]. The quality of PCR products was visualized on 1% agarose gel by electrophoresis. Each product was successfully amplified from a PCR for sequencing (Tsingke Biotechnology Co., Ltd., Beijing, China).
Identification of fungi and bacterial symbionts
As mentioned before [42], all achieved sequences' affiliation returned from Tsingke Biotechnology Company recognized by the available data in BLAST from the National Center for Biotechnology Information (NCBI) database. Sequence alignment and Neighbor-joining phylogenetic analysis were performed using MEGA software version 5.0. Bootstrap analysis of tree construction on the strength of the sequences was habituated to judge the neighbor-joining information based on 1,000 replicates [61]. The obtained ITS sequences were deposited in the GenBank database under accession numbers OK184563-OK184606 and OK285068. The accession numbers of bacterial 16S rRNA sequences were OK147622-OK147645 and OK169608-OK169611.
Culture-independent community analysis
The sample pretreatment was the same as those mentioned above to obtain the honeybee gut and honeycomb. Then, the total genome DNA of samples was extracted using the Fast DNA Extraction Kit, the concentration and purity of DNA were confirmed on 2% agarose gels. Each sample was performed in triplicate. ITS1 genes of regions were amplified using a specific primer (ITS5-1737F and ITS2-2043R) with the barcode. The reaction conditions were 98℃ for 1 min, followed by 30 cycles of 98℃ for 10 s, 50℃ for 30 s, and 72℃ for 30 s, finally 72℃ for 5 min. Mixture PCR products were purified using Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing library was generated using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) according to manufacturer's recommendations and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform using 250 bp pairedend reads.
Raw reads were demultiplexed and quality-filtered using QIIME V1.9.1 [62]. Effective Tags were obtained by comparing with the reference Unite database and using UCHIME Algorithm to remove chimera sequences [63, 64]. Sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs) by using the software Uparse V7.0.1001 [65]. Taxonomy of each representative sequence was assessed by using the MUSCLE software V3.8.31, and comparison against the Unite database based on blast algorithm [66]. Raw data is available from the NCBI Short Read Archive under accession number PRJNA817087 and PRJNA817099.
Cultivation of fungi and preparation of culture extract
Each fungus was inoculated in PDA medium and incubated at 28 ± 0.5 ℃for 3–4 days. Then, the fresh mycelia of each fungus (9 plugs of 5 mm) were inoculated in a 250 mL Erlenmeyer flask containing 150 mL of ME liquid medium and incubated at 28 ± 0.5 ℃ for 7 days in a shaker rotating at 180 rpm. The culture was passed through four layers of cheesecloth to remove the fungal thallus, then the supernatant was exhaustively extracted three times with ethyl acetate (EtOAc, 1:1, v/v). The fungal crude extracts were concentrated in a vacuum to yield culture extract for further experimental use.
Antimicrobial activities
The filter paper disc method was used to screen the antibacterial activity of crude extracts [67]. Three bacterial strains (Escherichia coli (ATCC8739), Micrococcus tetragenus (ATCC35098), and Staphylococcus aureus (ATCC6538) were selected for the test and cultivated on tryptic soy blood agar (TSBA) medium in 37 ℃. Next, sterile filter paper discs (5 mm in diameter) were added to 5 µL of the tested crude extracts, which were dissolved separately in acetone to get a concentration of 18 mg/mL. The filter paper disc treated with acetone alone and gentamicin sulfate was served as a negative and positive control, respectively. The plates were prepared in triplicate and were cultivated at 37 ℃ for 24–36 h. Finally, the diameters of inhibition zone diameter (in mm) were measured for evaluating antimicrobial activity.
Antioxidant activities
The 2,2-diphenyl-1 picrylhydrazyl (DPPH) radical scavenging activity was conducted according to the previous method with some modifications [68]. 1 mL of each crude extract (1 mg/mL) mixed with 5 mL of DPPH solutions in methanol (20 μg/mL). The mixture was incubated at room temperature for 30 min in darkness, and the absorbance was measured at 517 nm using a spectrophotometer (UV-1601; Beijing Beifen-ruili Analytical Instrument Co., Ltd., China). Methanol and ascorbic acid (Vc) were used as negative and positive controls, respectively. Each sample was performed in triplicate. The radical scavenging activity of crude extracts in the DPPH was calculated as follows:
Scavenging rate (SR) (%) = (Ab- As) /Ab*100%
where SR was the scavenging activity of the tested sample (%), Ab was the absorbance without sample, and As was the absorbance in the presence of the samples or a positive substance.
Isolation of compounds from MFFC22
A total of 13 L of the culture broth of MFFC22 was filtered and extracted with EtOAc (3 × 13 L). The EtOAc phase was concentrated under reduced pressure to obtain crude extract (2 g). The crude extract was subjected to column chromatography (CC) using silica gel (100–200 mesh) column with a gradient of CH2Cl2/MeOH (100:0–100:16, v/v) to give six fractions (Fr1-Fr6). Compound 1 (15 mg) was crystallized from the CH2Cl2 solution from the Fr1 (CH2Cl2 /MeOH, 100:0, v/v). Fr1 was further fractionated on a silica gel column, eluting with (CH2Cl2/MeOH, 100:0, 100:1, v/v) to give compounds 2 (0.9 mg) and 3 (0.7 mg).
Structural elucidation of metabolites
The structures of all compounds were primarily analyzed by High Resolution-Mass Spectrometry (HR-ESI–MS) and 1H/13C-nuclear magnetic resonance (NMR) spectroscopies. HR-ESI–MS spectra were measured on a TripeTOF 4600 mass analyzer (Bruker, USA). 1H/13C NMR data were acquired using Agilent DD2 600 Hz spectrometer (Agilent, USA) with tetramethylsilane (TMS) as an internal standard, and chemical shifts (δ) were reported as parts per million (ppm) values.
Antibacterial and antioxidant activities of compounds
The raw data supporting the conclusions of this manuscript will be made available to the authors, without undue reservation, to any qualified researcher. Publicly available datasets were analyzed in this study. This data can be found here: The obtained ITS gene sequences were deposited in the GenBank database under accession numbers OK184563-OK184606 and OK285068. The obtained 16S rRNA gene sequences were deposited in the GenBank database under accession numbers OK147622-OK147645 and OK169608-OK169611. Data of high-throughput sequencing by paired-end Illumina technology of ITS 1 gene amplicons can be retrieved from the NCBI Short Read Archive under accession number PRJNA817087 and PRJNA817099.
Availability of data and materials
The raw data supporting the conclusions of this manuscript will be made available to the authors, without undue reservation, to any qualified researcher. Publicly available datasets were analyzed in this study. This data can be found here: The obtained ITS gene sequences were deposited in the GenBank database under accession numbers OK184563-OK184606 and OK285068. The obtained 16S rRNA gene sequences were deposited in the GenBank database under accession numbers OK147622-OK147645 and OK169608-OK169611. Data of high-throughput sequencing by paired-end Illumina technology of ITS 1 gene amplicons can be retrieved from the NCBI Short Read Archive under accession number PRJNA817087 and PRJNA817099.
Change history
22 December 2022
A Correction to this paper has been published: https://doi.org/10.1186/s12866-022-02740-6
References
Basset Y, Cizek L, Cuénoud P, Didham RK, Guilhaumon F, Missa O, et al. Arthropod diversity in a tropical forest. Science. 2012;388:1481.
Stork NE. How many species of insects and other terrestrial arthropods are there on earth? Annu Rev Entomol. 2018;63:31–45.
Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. Fems Microbiol Rev. 2008;32:723–35.
Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, et al. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450:560–5.
Kwong WK, Engel P, Koch H, Moran NA. Genomics and host specialization of honey bee and bumble bee gut symbionts. Proc Natl Acad Sci USA. 2014;111:11509–14.
Hulcr J, Stelinski LL. The ambrosia symbiosis: from evolutionary ecology to practical management. Annu Rev Entomol. 2017;62:285–303.
Li HJ, Yelle DJ, Li C, Yang MY, Ke J, Zhang RJ, et al. Lignocellulose pretreatment in a fungus-cultivating termite. Proc Natl Acad Sci USA. 2017;114:4709–14.
Li HJ, Sosa-Calvo J, Horn HA, Pupo MT, Clardy J, Rabeling C, et al. Convergent evolution of complex structures for ant-bacterial defensive symbiosis in fungus-farming ants. Proc Natl Acad Sci USA. 2018;115:10720–5.
Hoffman MT, Arnold AE. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl Environ Microb. 2010;76:4063–75.
Shao MW, Lu YH, Miao S, Zhang Y, Chen TT, Zhang YL. Diversity, bacterial symbionts and antibacterial potential of gut-associated fungiisolated from the Pantala flavescens Larvae in China. PLoS ONE. 2015;10: e0134542.
Attili-Angelis D, Duarte APM, Pagnocca FC, Nagamoto NS, de Vries M, Stielow JB, et al. Novel Phialophora species from leaf-cutting ants (tribe Attini). Fungal Divers. 2014;65:65–75.
Jaber S, Mercier A, Knio K, Brun S, Kambris Z. Isolation of fungi from dead arthropods and identification of a new mosquito natural pathogen. Parasite Vector. 2016;9:491.
White MM, Valle LG, Lichtwardt RW, Lichtwardt RW, Siri A, Strongman DB, et al. New species and emendations of Orphella: taxonomic and phylogenetic reassessment of the genus to establish the Orphellales, for stonefly gut fungi with a twist. Mycologia. 2018;110:147–78.
Kandasamy D, Gershenzon J, Andersson MN, Hammerbacher A. Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle ( Ips typographus) with its fungal symbionts. ISME J. 2019;13:1788–800.
Zhang YL, Ge HM, Zhao W, Dong H, Xu Q, Li SH, Li J, et al. Unprecedented immunosuppressive polyketides from Daldinia eschscholzii, a mantis-associated fungus. Angew Chem Int Edit. 2008;47:5823–6.
Zhang YL, Zhang J, Jiang N, Lu YH, Wang L, Xu SH, et al. Immunosuppressive polyketides from the mantis-associated Daldinia eschscholzii. J Am Chem Soc. 2011;133:5931–40.
Zhang SX, Sun FF, Liu LJ, Bao LY, Fang W, Yin CP, et al. Dragonfly-associated Trichoderma harzianum QTYC77 is not only a potential biological control agent of Fusarium oxysporum f. sp. cucumerinum but also a source of new antibacterial agents. J Agric Food Chem. 2020;68:14161–7.
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, et al. Safeguarding pollinators and their values to human well-being. Nature. 2016;540:220–9.
Khan KA, Al-Ghamdi AA, Ghramh HA, Ansari MJ, Ali H, Alamri SA, et al. Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb Pathogenesis. 2019;138: 103793.
Yun JH, Jung MJ, Kim PS, Bae JW. Social status shapes the bacterial and fungal gut communities of the honey bee. Sci Rep. 2019;2018:8.
de Paula GT, Menezes C, Pupo MT, Rosa CA. Stingless bees and microbial interactions. Curr Opin Insect Sci. 2021;44:41–7.
Menezes C, Vollet-Neto A, Marsaioli AJ, Zampieri D, Fontoura IC, Luchessi AD, et al. A Brazilian social bee must cultivate fungus to survive. Curr Biol. 2015;25:1–5.
Callegari M, Crotti E, Fusi M, Marasco R, Gonella E, Noni ID, et al. Compartmentalization of bacterial and fungal microbiomes in the gut of adult honeybees. NPJ Biofilms Microbi. 2021;7:42.
Kwong WK, Medina LA, Koch H, Sing KW, Soh EJY, Ascher JS, et al. Dynamic microbiome evolution in social bees. Sci Adv. 2017;3: e1600513.
Kingsland SR, Barrow RA. Identification of Chaetoviridin E from a cultured microfungus, Chaetomium sp. and structural reassignment of Chaetoviridins B and D. Aust J Chem. 2009;62:269–74.
Mari SH, Varras PC, Atia-tul Wahab, Choudhary IM, Siskos MG, Gerothanassis IP. Solvent-dependent structures of natural products based on the combined use of DFT calculations and 1H-NMR chemical shifts. Molecules. 2019;24:2290.
Liu RM, Li AF, Sun AL. Preparative isolation and purification of hydroxyanthraquinones and cinnamic acid from the chinese medicinal herb rheum officinale baill. by high-speed counter-current chromatography. J Chromatogr A. 2004;1052:217–21.
Xu GB, He G, Bai HH, Yang T, Zhang GL, Wu LW, et al. Indole alkaloids from Chaetomium globosum. J Nat Prod. 2015;78:1479–85.
Pažoutová S, Follert S, Bitzer J, Keck M, Surup F, Šrůtka P, et al. A new endophytic insect-associated daldinia species, recognised from a comparison of secondary metabolite profiles and molecular phylogeny. Fungal Divers. 2013;60:107–23.
Nagam V, Aluru R, Shoaib M, Dong GR, Li Z, Pallaval VB, et al. Diversity of fungal isolates from fungus-growing termite Macrotermes barneyi and characterization of bioactive compound from Xylaria escharoidea. Insect Sci. 2021;28:392–402.
Ren FX, Zhu SM, Wang B, Li L, Liu XZ, Su RB. Hypocriols A-F, heterodimeric botryane ethers from hypocrea sp., an insect-associated fungus. J Nat Prod. 2016;79:1848–56.
Tong ZW, Xie XH, Wang TT, Lu M, Jiao RH, Ge HM. Acautalides A-C, neuroprotective diels−alder adducts from solid-state cultivated acaulium sp. H-JQSF Org Lett. 2021;23:5587–91.
Gilliam M, Prest DB. Fungi isolated from the intestinal contents of foraging worker honey bees. Apis mellifera J Invertebr Pathol. 1972;20:101–3.
Moubasher AH, Abdel-Sater MA, Soliman Z. Yeasts and filamentous fungi inhabiting guts of three insect species in Assiut. Egypt Mycosphere. 2017;8:1297–316.
Zhao YZ, Zhang ZF, Cai L, Peng WJ, Liu F. Four new filamentous fungal species from newly-collected and hive-stored bee pollen. Mycosphere. 2018;9:1089–116.
Disayathanoowat T, Li HY, Supapimon N, Suwannarach N, Lumyong S, Chantawannakul P, et al. Different dynamics of bacterial and fungal communities in hive-stored bee bread and their possible roles: a case study from two commercial honey bees in China. Microorganisms. 2020;8:264.
Lien NTH, Hieu NV, Hong LT, Anh FT, Thao PTH. Identification of white rot fungus CP9 and its potential application in biopulping. J Biotechnol. 2016;14:721–6.
Jović J, Buntić A, Radovanović N, Petrović B, Mojović L. Lignin-degrading abilities of novel autochthonous fungal isolates Trametes hirsuta F13 and Stereum gausapatum F28. Food Technol Biotechnol. 2018;56:354–65.
Horisawa S, Inoue A, Yamanaka Y. Direct ethanol production from lignocellulosic materials by mixed culture of wood rot fungi Schizophyllum commune, Bjerkandera adusta, and Fomitopsis palustris. Fermentation. 2019;5:21.
Crous PW, Groenewald JZ. A phylogenetic re-evaluation of Arthrinium. Ima Fungus. 2013;4:133–54.
Masuya H, Kajimura H, Tomisawa N, Yamaoka Y. Fungi associated with scolytogenes birosimensis (coleoptera: curculionidae) infesting pittosporum tobira. Environ Entomol. 2012;41:255–64.
Xu X, Shao MW, Yin CP, Mao ZC, Shi ZC, Yu XY, et al. Diversity, bacterial symbionts, and antimicrobial potential of termite-associated fungi. Front Microbiol. 2020;11:300.
Liu CX, Wang L, Chen JF, Guo ZY, Tu X, Deng ZS, et al. Paraconfuranones A-H, eight new furanone analogs from the insect-associated fungus Paraconiothyrium brasiliense MZ-1. Magn Reson Chem. 2015;53:317–22.
Dhayanithy G, Subban K, Chelliah J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019;19:22.
Zhao SH, Wu XL, Duan XY, Zhou CX, Zhao ZQ, Chen H, et al. Optimal extraction, purification and antioxidant activity of total flavonoids from endophytic fungi of Conyza blinii H. Lév PeerJ. 2021;9: e11223.
Dehghan H, Salehi P, Amiri MS. Bioassay-guided purification of α-amylase, α-glucosidase inhibitors and DPPH radical scavengers from roots of rheum turkestanicum. Ind Crop Prod. 2018;117:303–9.
Campos JF, de Castro DTH, Damião MJ, Torquato HFV, Paredes-Gamero EJ, Carollo CA, et al. The chemical profile of senna velutina leaves and their antioxidant and cytotoxic effects. Oxid Med Cell Longev. 2016;2016:8405957.
Jia M, Chen L, Xin HL, Zheng CJ, Rahman K, Han T, Qin P. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol. 2016;7:906.
Casella TM, Eparvier V, Mandavid H, Bendelac A, Odonne G, Dayan L, et al. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry. 2013;96:370–7.
Stompor-Gorący M. The health benefits of emodin, a natural anthraquinone derived from rhubarb—a summary update. Int J Mol Sci. 2021;22:9522.
Kim SJ, Kim MC, Lee BJ, Park DH, Hong SH, Um JY. Anti-inflammatory activity of chrysophanol through the suppression on NF-kB/caspase-1 activation in vitro and in vivo. Molecules. 2010;15:6436–51.
Oh DC, Poulsen M, Currie CR, Clardy J. Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat Chem Biol. 2009;5:391–3.
Wyche TP, Ruzzini AC, Schwab L, Currie CR, Clardy J. Tryptorubin A: A polycyclic peptide from a fungus-derived Streptomycet. J Am Chem Soc. 2017;139:12899–902.
Martinson VG, Danforth BN, Minckley RL, Rueppell O, Tingek S, Moran N. A simple and distinctive microbiota associated with honey bees and bumble bees. Mol Ecol. 2011;20:619–28.
Miller DL, Smith EA, Newton IL. G. A bacterial symbiont protects honey bees from fungal disease. mBio. 2021;12:e00503-21.
Chevrette MG, Carlson CM, Ortega HE, Thomas C, Ananiev GE, Barns KJ, et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat Commun. 2019;10:516.
Ryan MJ, Smith D. Cryopreservation and freeze-drying of fungi employing centrifugal and shelf freeze-drying. Methods Mol Biol. 2007;368:127–40.
Li J, Zhong M, Lei XL, Xiao SL, Li AY. Diversity and antibacterial activities of culturable fungi associated with coral Porites Pukoensis. World J Microb Biot. 2014;30:2551–8.
Liu S, Ahmed S, Zhang CG, Liu TS, Shao CL, Fang YW. Diversity and antimicrobial activity of culturable fungi associated with sea anemone Anthopleura xanthogrammica. Electron J Biotechn. 2020;44:41–6.
José RH, Claudia LR, Antonio VN, Alejandro SA, Roberto RM, Holjes SL, et al. A novel intracellular nitrogen-fixing symbiosis made by Ustilago maydis and Bacillus spp. New Phytol. 2015;207:769–77.
Felsenstein J. Confidence limits on phylogenies: an approach using thebootstrap. Evolution. 1985;39:783–91.
Caporaso JG, Kuczynski J, Stombaugh J, Bittingeret K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200.
Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8.
Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7.
Li S, Shao MW, Lu YH, Kong LC, Jiang DH, Zhang YL. Phytotoxic and antibacterial metabolites from Fusarium proliferatum ZS07 isolated from the gut of long-horned grasshoppers. J Agric Food Chem. 2014;62:8997–9001.
Zhang YL, Yin CP, Kong LC, Jiang DH. Extraction optimisation, purification and major antioxidant component of red pigments extracted from Camellia japonica. Food Chem. 2011;129:660–4.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (32011540382, 31770007), Anhui Province Natural Science Funds for Distinguished Young Scholars (2108085J18), the State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University (FAMP202008K), and the University Graduate Science Research Project of Anhui Province (YJS20210200).
Author information
Authors and Affiliations
Contributions
YL-Z designed the research. YL-Z supervised the study. P-C, K-K, Y-Y, ZD-H, SP-S, P-L, N-A and LS-Y. performed the experiments and analyzed the data. P-C and K-K analyzed and wrote the manuscript. All authors revised the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Taxonomic assignment of the OTUs obtained in the study and their relative abundance at each honeybee gut.
Additional file 2: Table S2.
Taxonomic assignment of the OTUs obtained in the study and their relative abundance at each honeycomb.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, P., Kong, K., Yao, Y. et al. Community composition, bacterial symbionts, antibacterial and antioxidant activities of honeybee-associated fungi. BMC Microbiol 22, 168 (2022). https://doi.org/10.1186/s12866-022-02580-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02580-4