Abstract
Background
Cadmium (Cd) contamination in soil poses a serious safety risk for the development of medicine and food with ginseng as the raw material. Microorganisms are key players in the functioning and service of soil ecosystems, but the effects of Cd-contaminated ginseng growth on these microorganisms is still poorly understood. To study this hypothesis, we evaluated the effects of microorganisms and Cd (0, 0.25, 0.5, 1.0, 2.0, 5.0, and 10.0 mg kg-1 of Cd) exposure on the soil microbial community using Illumina HiSeq high-throughput sequencing.
Results
Our results indicated that Cd-contaminated soil affected the soil microbial diversity and composition, and bacterial diversity was affected more than fungal diversity in Cd-contaminated soil, especially according to Shannon indices. The abundance of the soil microbial community decreased and the composition changed according to the relative abundances at the phylum level, including those of Saccharibacteria and Gemmatimonadetes in bacteria and Mortierellomycota in fungi. The LEfSe algorithm was used to identify active biomarkers, and 45 differentially abundant bacterial taxonomic clades and 16 differentially abundant fungal taxonomic clades were identified with LDA scores higher than 4.0. Finally, a heatmap of Spearman's rank correlation coefficients and canonical discriminant analysis (CDA) indicated that some key biomarkers, Arenimonas, Xanthomonadales, Nitrosomonadaceae, Methylophilales, Caulobacterales, Aeromicrobium, Chitinophagaceae, Acidimicrobiales, Nocardioidaceae, Propionibacteriales, Frankiales, and Gemmatimonadaceae, were positively correlated with the total and available Cd (p<0.05) but negatively correlated with AK, AP, and pH (p<0.05) in the bacterial community. Similarly, in the fungal community, Tubaria, Mortierellaceae, and Rhizophagus were positively correlated with the total and available Cd but negatively correlated with AK, AP, TK, and pH.
Conclusion
Cd contamination significantly affected microbial diversity and composition in ginseng-growing soil. Our findings provide new insight into the effects of Cd contamination on the microbial communities in ginseng-growing soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Ginseng, the root and rhizome of Panax ginseng CA Meyer, is mainly cultivated in the northeastern China [1]. Due to its extremely high medicinal value, ginseng has been widely used to treat diseases, such as neurodegenerative diseases, cardiovascular disease, and oxidative damage diseases, for thousands of years [2,3,4]. Previous studies have indicated that ginseng cultivation is often threatened by the addition of fertilizer and organic fertilizer, which causes an increase in heavy metals in soils [5]. Heavy metals may cause functional disorders of soil [6], inhibit plant growth, and even endanger human health through a contaminated food chain [7, 8]. Nevertheless, understanding the mechanisms of these interactions between soil function and heavy metals remains a major challenge in soil ecology.
Soil pollution has become a prominent environmental issue in China due to the excessive use of chemical fertilizers in agricultural activities [9, 10] and air pollution from industrial emissions and vehicle exhaust [11]. Heavy metals in surface soil, such as cadmium (Cd), arsenic (As), lead (Pb), iron (Fe), copper (Cu), and zinc (Zn), can cause soil pollution [12]. Heavy metal pollution in soil is divided into two categories based on the toxicity and necessity for plant growth and development. Some heavy metals with stronger toxicity such as Cd, As and Pb, are well studied because of their persistence, toxicity and nonactive degradation. Others heavy metals are essential elements for plant growth [13], such as Fe, Cu, Zn, but too much of these will cause heavy metal pollution. Cd is one of the most hazardous elements and has been a public concern with increasing risk in soil remediation. Cd pollution poses a major threat to human and animal health due to its continuous release into the environment, especially soil, even at a low concentration.
Most of the large number of studies on heavy metal passivators in croplands focused on the effects of passivators on the proportions of heavy metals in soils and the translocation of heavy metals from soil to crops. However, few have focused on Cd contamination on the soil microbial community in Panax ginseng-growing soil [14]. Soil microorganisms are the most abundant and diverse life forms on earth, and they have important effects on biogeochemical processes and nutrient transformation [15, 16]. Soil microorganisms are an important index for measuring soil quality and are involved in soil organic matter decomposition, mineralization and nutrient utilization. However, they are affected by biotic and abiotic factors, such as pH, soil organic matter, and other toxic metals [17, 18]. When the accumulation of heavy metals in soil exceeds a certain range, they will cause certain toxic effects on microorganisms, and further affect the balance of the soil ecosystem or even destroy the function of the soil environmental system. As an important index representing soil functional stability and ecosystem structure, soil microbial communities can effectively monitor the soil environmental pollution status [19, 20]. The toxic mechanism may be caused by the destruction of microbial cells, changes in the enzyme system, and abruption of the normal metabolic response of cells [21, 22]. Soil microorganisms are used as an index to evaluate the soil pollution level in recently contaminated soil [23].
Cd is a nonessential element for plant growth. Its characteristics include high toxicity, nonbiodegradability and long-term accumulative behaviour, which can cause serious illnesses in humans though the food chain [24]. Cd contamination not only affects soil fertility but also disturbs the microbial community structure and reduces biodiversity [25]. Soil microbial communities can quickly respond to environmental stress in the rhizosphere and further adjust the direction of the dominant microorganism [26]. For example, Deng et al. [27] found that Cd plays a vital role in decreasing alpha diversity in a typical Cd-contaminated farmland ecosystem and that some Cd-tolerant microbes become the dominant microbes. However, other studies showed that AM fungi can adapt to Cd-contaminated soil and help plants adapt to adverse growing conditions by reshaping the microbial community structure [28]. Therefore, some microbes are regarded as environmental monitoring factors.
We investigated that the effects of different Cd contaminated levels on the microbial community structure in ginseng-growing soil, and 16S and ITS1 were analysed using Illumina HiSeq high-throughput sequencing technology. We hypothesized that: (1) the microbial abundance, diversity, and community composition might be affected by the different Cd-contaminated treatments, especially for the bacterial community, We also hypothesized that due to the directed regulation of Cd on microorganisms, biodiversity and some key biomarkers would be the dominant microbes in response to the degree of Cd contamination. Finally, we aimed to identify microbes and fungi related toCd contamination as monitoring factors and apply them in planting management.
Results
Total and available cadmium in soil
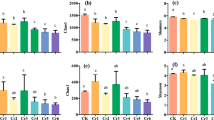
After a complete ginseng growth period, the concentration of total and available Cd was higly variable among the different treatments (Fig. 1). The addition of Cd significantly increased the total and available Cd concentrations in the soil among the seven treatment soils based on Duncan's multiple comparison (p<0.05 or p<0.001). Although the total and available Cd concentrations were not significantly increased in the Cd1 and Cd2 treatments compared with the CK group, but the total and available Cd concentrations in the Cd3, Cd4, Cd5, and Cd6 treatments groups were significantly higher than those in the CK group at the p<0.01 or p<0.001 level.
Sequencing depth and soil microbial community diversity analysis
A total of 1400903 sequence reads in 16S were generated from 21 samples, with an average of 66710 sequences per sample. However, a total of 1678800 sequence reads in ITS1 were generated from 21 samples, with an average of 79943 sequences per sample. The average sequence lengths of these sequences are approximately 418 bps and 319 bps in 16S and ITS1, respectively. The reads were clustered into 1007 OTUs and 714 OTUs in 16S and ITS1, respectively, based on 97% sequence similarity (Fig. S1 and S2).
Three α-diversity indices (Shannon, ACE and Chao1) were used to evaluate the soil microbial community richness and diversity (Fig. 2). Although cadmium addition did not significantly affect fungal taxonomic α-diversity (p>0.05, Fig. 2b), it had strong effects on the bacterial taxonomic α-diversity (p<0.05, p<0.01 or p<0.001, Fig. 2a). The Shannon index was higher in the CK group than in the Cd1 and Cd3 groups (p<0.001, p<0.05), and the index was lowest in the Cd1 group among all the treatment groups. Similarly, the ACE and Chao1 indices were lower in the Cd1 group than in the other treatment groups, especially in the Cd2, Cd3 and Cd4 treatment groups (p<0.05).
The index of Shannon, ACE and Chao1 of the microbial(a) and fungal(b) of ginseng roots in the different Cd addition treatment. The horizontal bars within boxes represent medians. The tops and bottoms of boxes represent the 75th and 25th percentiles, respectively. the symbols *, ** and *** were used only to indicated significantly with p<0.5, p<0.01 and p<0.001, respectively
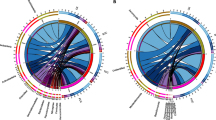
The β-diversity of the soil microbial communities in the Cd-added treatments was evaluated by UPGMA clustering analysis. The microbial communities in the seven treatments differed from each other (Fig. 3a and b), and the soil bacterial and fungal communities clustered into seven groups depending on the Cd concentration. Similarly, this grouping between bacterial and fungal communities was observed in canonical discriminant analysis (CDA) (Fig. 4). Thus, the UPGMA clustering analysis and CDA indicated that the Cd concentration might be directly correlated with the bacterial and fungal community structure.
Correlations among microbial community, environmental parameters and phyla
The microbial community structure might be correlated with environmental parameters in the Cd-supplemented soil. Canonical discriminant analysis (CDA) was carried out on the microbes (bacteria and fungi) to link the abundance of key biomarkers with the main soil physicochemical properties (Fig. 4). The length of an environmental factor arrow indicates the strength of the environmental factors to the overall bacterial communities. Thus, AK, AP, total Cd, available Cd and NO3--N concentrations and pH appear to be the most important environmental factors. The CDA of the relative abundance of the key biomarkers at the different classification levels and environmental factors was performed, Arenimonas, Xanthomonadales, Nitrosomonadaceae, Methylophilales, Caulobacterales, Aeromicrobium, Chitinophagaceae, Acidimicrobiales, Nocardioidaceae, Propionibacteriales, Frankiales, and Gemmatimonadaceae were positively correlated with the total and available Cd, but negatively correlated with AK, AP, and pH. Similarly, a correlation was found between fungal community structure and environmental factors, such as the abundance of Tubaria, Mortierellaceae, and Rhizophagus. These genera were positively correlated with the total and available Cd, but negatively correlated with AK, AP, TK and pH. The results indicated that the microbial community may be mainly regulated by the Cd concentration.
Microbial composition in response to different treatments of cadmium addition
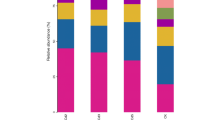
The taxonomic distribution of bacteria and fungi at the phylum level is summarized in Fig. 5. Bacterial sequences were assigned to 24 phyla as shown, and Proteobacteria was the most abundant phylum across all samples, accounting for 45.62–53.63% of the total valid reads in all samples, with an average relative abundance of 49.05%. Actinobacteria was the second most abundant phylum across all samples, accounting for 12.67–28.03% of the total valid reads in all samples, with an average relative abundance of 18.94%. The other dominant phyla were Bacteroidetes (7.96–10.59%, averaging 9.17%), Saccharibacteria (3.41–6.17%, averaging 4.81%), Acidobacteria (2.88–5.00%, averaging 3.67%), Verrucomicrobia (2.10–3.38%, averaging 2.93%), Chloroflexi (1.36–3.72%, averaging 2.51%), Gemmatimonadetes (1.28–4.23%, averaging 2.28%), Cyanobacteria (0.50–4.29%, averaging 1.35%), and Parcubacteria (0.29–1.76%, averaging 1.04%). At the class level, a wide range of classes dominated. Based on the average relative abundance, the most abundant classes were Alphaproteobacteria, Betaproteobacteria, Actinobacteria, Gammaproteobacteria, and Sphingobacteriia.
Similarly, fungal sequences were assigned to 13 phyla as shown. Ascomycota was the most abundant phylum across all samples, accounting for 37.63–61.63% of the total valid reads in all samples, with an average relative abundance of 49.97%. Glomeromycota was the second most abundant phylum across all samples, accounting for 13.80–24.59% of the total valid reads in all samples, with an average relative abundance of 16.24%. The other dominant phyla were Mortierellomycota (3.30–10.89%, averaging 6.21%) and Basidiomycota (2.76–7.80%, averaging 4.88%). At the class level, a wide range of classes dominated. Based on the average relative abundance, the most abundant classes were Sordariomycetes, Dothideomycetes, Glomeromycetes, Mortierellomycetes, and Leotiomycetes.
In contrast, in soils with increased cadmium concentrations, the change in the relative abundance of Acidobacteria (5.00%) was higher than those of Saccharibacteria (3.53%) in the CK group. However, the relative abundance of Saccharibacteria (average 4.81, ranging from 3.41% to 6.17%) was higher than that of Acidobacteria (average 3.67%, ranging from 2.88% to 4.36%) in the cadmium-addition treatment groups, and the composition of Chloroflexi and Gemmatimonadetes was reversed between the CK group and cadmium-addition treatment grous. Similarly, in the fungal species, the fungal composition of Glomeromycota and Mortierellomycota showed the opposite trend. These results were further confirmed by Spearman's rank correlation coefficients analysis, as shown in Fig. 6.
Heatmap of Spearman's rank correlation coefficients between the soil environmental variables and the relative abundance of bacterial (a) and fungal (b) communities at the phylum level, respectively. Note: Horizontal row represents soil physical and chemical properties and Cd, vertical row represents microbial community abundance information, red represents positive correlation, blue represents negative correlation, darker color indicates higher correlation, p value is correlation test result, * in the figure indicates p<0.05, ** indicates p<0.01, and *** indicates p<0.001. Total soil carbon (C); Total soil nitrogen (N); Total soil phosphate (P); Total soil kalium (K); Soil organic carbon (SOC); Available soil phosphate (AP); Available soil potassium (AK); Total soil cadmium (TCd); Available soil cadmium (ACd)
To further study the effects of added Cd in soil microbial abundance and composition, the linear discriminant analysis effect size (LEfSe) method was used to identify significant genes in all samples based on the Wilcoxon rank sum test. A phylogenetic dendrogram of biomarker bacteria (BmB) and fungi (BmF) for different Cd-added treatment samples is shown in Fig. 7. In total, 45 differentially abundant bacterial abundant taxonomic clades and 16 differentially abundant fungal taxonomic clades were identified with an LDA score higher than 4.0.
Discussion
Cd addition changes soil microbial biodiversity
A previous study indicated that Cd addition can cause a decrease in pH and affect the available nutrient supply [29]. In this pot experiment, Cd addition directly increased the total and available Cd contents in the soil. The available Cd content in the Cd6 group was more than 91 times than in the CK group (Fig. 1). The addition of Cd significantly reduced the soil pH and inhibited the supply of available NPK in the soil (Fig. S1). These results are probably due to the fact that soil dissolves organic matter, mainly soil humus, protein, fatty acids, amino acids and citric acid, and this effect is gradually fixed by the excessive available Cd [30].
Both bacterial and fungal alpha diversity were, to some extent, correlated with soil physicochemical properties, especially in the treatment groups with Cd added. These finding suggest that high Cd concentrations affected biodiversity. Our experiments support the hypothesis that Cd contamination (treatments from Cd1 to Cd6) affects the species richness and diversity of soil microbes, especially soil bacteria. Previous studies indicated that soil microorganisms were highly sensitive to stress caused by heavy metals because these contaminants significantly affected their growth and metabolism through functional disturbances, protein denaturation, or cell membrane destruction [31,32,33]. The ACE and Chao1 indices were used for microbial richness, and the Shannon index reflects microbial diversity [34]. Our results showed that the Shannon index of the soil bacteria in the Cd1 treatment group was lower than that in the other treatment groups (p<0.01 or p<0.001). The ACE and Chao1 indices showed the same trend as the Shannon index (Fig. 2), but the Shannon, ACE, and Chao1 indices of the higher Cd treatment groups (from Cd2 to Cd6) were not obviously different from those of the CK group. Although the Cd concentration significantly affected soil bacterial richness and diversity, there were few effects in fungi, suggesting that the effect of Cd contamination on bacterial diversity was greater than that on fungal diversity. This result is consistent with the findings of other researchers [35, 36]. One possible reason is that soil microorganisms adapt to the stress of low Cd concentrations by regulating their physiological metabolism. However, some microbial communities may be sustained and multiply even in the presence of high Cd concentrations. Contaminants can alter some local microbial populations by exerting selective pressure but leave other populations intact [27].
Microbial community composition and key species
Given that both microbial abundance and community composition were highly affected by the Cd contamination, the Spearman's rank correlation coefficients that responded positively to Cd contamination could be groups of taxa that can be rapidly multiplicated (Figs. 2, 3 and 5). the response of the soil microbial community to Cd contamination has been reported in many species, but microbial communities and composition are regulated by many factors, such as heavy metal types and concentrations and plant root exudates [37,38,39]. Soil microbes are an indicator of soil fertility and participate in material transfer and energy exchange, which are usually considered the link between soil and plants [31, 40]. Many studies have confirmed that heavy metal pollution affects microbial abundance, especially microbial assembly and changes in functional microbial community composition [41, 42]. Different concentrations of Cd addition to soils changed the composition and assemblage of functional microbial communities (Figs. 3 and 4). The results indicated that Acidobacteria and Chloroflexi in bacteria and Glomeromycota in fungi were the Cd tolerant microbial communities, and their assemblage could grow normally in the Cd contaminated soil. In addition, the LEfSe results showed that the Cd1 treatment group had the highest number of biomarker bacteria, followed by the CK, Cd4, Cd2, Cd3, and Cd5. Conversely, the Cd4 treatment had the highest amount of biomarker fungi, followed by CK, Cd2, Cd3, Cd1. The results also explained that the Cd-tolerant taxa was promoted and Cd sensitive taxa was disappeared, which may be associated with the differences of microbial function, such as their abilities to absorb nutrients and metabolism differ [43].
A previous study indicated that heavy metal contamination leads to changes in the community structure and function [44,45,46]. The reorganization of the soil microbial community formed a new microbial assembly and changed its relevant function, which may be an adaption for its ecological services in Cd-contaminated soil. A significant decrease in bacterial diversity was found in the treatment groups with higher concentrations of Cd addition in ginseng-growing soil, and this was associated with significant changes in bacterial and fungal composition (Figs. 2 and 5). The possible reason for this is that the number of Cd-sensitive organisms was reduced, so the number of resistant organisms increased in the ginseng growing soil. This could lead to changes in diversity and composition. In addition, sequences belonging to members of the Acidobacteria and Chloroflexi phyla are commonly found in many heavy metal- contaminated soils, including mercury-stressed soils [47], farmland soils [48], sediment soils [49], and Cd/As-contaminated soils [50]. Similarly, in the fungal community, the Glomeromycota community can survive in Cd-contaminated soil. One possible mechanism is that arbuscular mycorrhizal fungi (AMF) belong to Glomeromycota, and AMF have been shown to increase the tolerance of plants to the presence of heavy metals [51, 52]. These shifts in bacterial and fungal community life strategies are significant in predicting the responses of microorganisms and soil biogeochemical cycling to changes in ginseng-growing soils polluted with Cd contamination.
Environmental drivers of microbial communities
Soil microbial community structure is highly sensitive to soil environmental changes and stresses [53, 54] and is extremely affected by heavy metals [55,56,57]. Our results in Cd-contaminated ginseng-growing soil (Figs. 3 and 5) and a recent analysis of agricultural paddy soil microbial community assembly [58] support that the influence of deterministic processes might cause dominant microbe composition changes and increase Cd-tolerant microorganism assembly. Moreover, the bacterial diversity was in agreement with the findings of a previous study [59]. A low concentration of heavy metals promoted bacterial reproduction, but high concentrations led to a reduced bacterial population.
As expected, soil bacterial diversity was markly affected by Cd addition treatments, although there was no significant difference in fungal diversity. These findings indicate soil bacteria were more sensitive to Cd contamination than fungi. This result is consistent with the results from a study on saline-alkaline stress in Cd-contaminated soil [60]. Although Cd concentration is a dominant factor for shaping soil bacterial communities, other soil factors, such as pH, OM, and available NPK, are often even more important because they can reduce the toxicity of Cd contamination [61]. CDA indicated that total and available Cd, AK, AP, pH, NH4+-N, and OM significantly affected soil bacterial and fungal communities, and the environmental factors may be the most important factors explaining both bacterial and fungal community compositions (Fig. 4). A heatmap of Spearman's rank correlation further supported our third hypothesis that some functional microbial groups, such as Verrucomicrobia, Armatimonadetes, Proteobacteria, Parcubacteria, Saccharibacteria and Bacteroidetes in bacteria and Glomeromycota in fungi, were significantly positively correlated with total and available Cd, but significantly negatively correlated with AK, AP and pH. The significant positive correlation between Cd and the key microbes further indicated that microorganisms might generate resistance and enable themselves to survive by increasing the transport of metal ions and excreting metal ions. These results were confirmed by previous studies in bamboo plantations [62], activated sludge systems [63] and smelter-contaminated soil [64]. One possible mechanism may be that taxa are generally considered a dominant part of the metabolically active bacterial community in heavy metal-contaminated soil [65].
These dominant phyla could adapt to extremely contaminated soil. For example, Verrucomicrobia and Proteobacteria are oligotrophic bacteria that contribute to predicting the multifunctional resistance of ecosystems, especially for heavy metal resistance [32]. Actinobacteria can produce a variety of extracellular hydrolytic enzymes that degrade soil organic compounds and enhance the cycling of C, N, and other elements. Similarly, Glomeromycota belong to the fungal community, which is the tolerant to the presence of heavy metals [64].
Conclusion
Cd contamination not only affected soil pH and available NPK, but also shaped microbial diversity and community composition under pot culture conditions in Cd-contaminated ginseng-growing soil. Using Illumina HiSeq rDNA sequence analysis, we found that Cd contamination affected bacterial diversity more than fungal diversity. Additionally, Cd contamination significantly changed the composition of the soil bacterial and fungal communities but had no significant impact on the species of the dominant microbes. Specifically, Saccharibacteria, Gemmatimonadetes and Mortierellomycota may be microbial factors that can monitor microorganisms during the aggravation of Cd contamination. In addition, some key biomarkers were identified at different classification levels in Cd-contaminated soil, which can also be regarded as Cd-contaminated monitoring factors. In the future, the potential application prospects of these tolerant biomarkers (BmB of BmF) should be explored to find the most suitable habitat conditions under different levels of Cd contamination. This could fully expose their unique heavy metal transfer and absorption functions to provide the corresponding reference basis for bioremediation in Cd-contaminated ginseng regions.
Methods
Culture and experimental design
The experiment was conducted in an intelligent cultivation center. The room temperature, light intensity and humidity cultivation were controlled at the Institute of Special Animal and Plant Sciences of CAAS, Changchun, China. These cultures were submitted to a photoperiod of 12 h:12 h light/dark with constant aeration, and the light intensity was set at 95 μmol·m-2·s-1. The temperature of the cultures was kept at 21 °C by controlling room temperature, and the humidity was set at 65% during the experiment.
A pot culture experiment based on a completely randomized design (CRD) was conducted from October 2018 to March 2019 at the Institute of Special Animal and Plant Sciences of CAAS, Changchun, China. The dimensions of the black PVC pot were as follows: bottom diameter of 130 mm and height of 180 mm. The experiment was set up to have seven treatments with three replicates. The pot soil was collected from a three-year abandoned farmland in the town of Changchun City of Jilin Province (E:125°26'24", N:43 °46'24"). The soil texture was sandy loam with 51.26% of sand, 7.67% of clay and 41.07% of silt based on the international soil society classification of soil texture. The basic soil properties were as follows: the pH was 6.64, and organic matter, total phosphate, total potassium, NH4+-N, NO3--N, available phosphate and available potassium were 4.05 g kg-1, 0.98 g kg-1, 14.81 g kg-1, 36.37 mg kg-1, 3.98 mg kg-1, 14.98 mg kg-1 and 270.38 mg kg-1, respectively. In each pot, a piece of nylon filter (130 cm2) with a mesh diameter of 55 mesh was placed at the bottom and then it was filled with 3.0 kg dry soil mixed with different cadmium concentrations. The treatments were as follows: (CK) no cadmium addition, (Cd1) 0.25 mg cadmium per kg dry soil, (Cd2) 0.50 mg cadmium per kg dry soil, (Cd3) 1.00 mg cadmium per kg dry soil, (Cd4) 2.00 mg cadmium per kg dry soil, (Cd5) 5.00 mg cadmium per kg dry soil, and (Cd6) 10.00 mg cadmium per kg dry soil. Each treatment was replicated three times, and each pot was considered a replicate. One week after the Cd was added, five three-year-old ginseng seedlings with consistent weights, shapes, and sizes were chosen and cultivated, and the water content was controlled by weight. Irrigation was conducted if the water content was lower than 18% (60% of FC) during the experiment.
Soil sample preparation
Approximately 110 d of cultivation after the ginseng was planted, fresh rhizosphere soil samples were collected from ginseng roots after the ginseng from one sample withered. A total of 21 samples were collected and immediately passed through a 2 mm sieve. Then, the treated soil was divided into two subgroups: air-dried for chemical property analysis and stored at -80 °C for DNA extraction.
Analysis of soil properties
Total carbon (TC) and total nitrogen (TN) were determined using an element analyser (Vario EL, Germany). Soil organic matter (SOM) was measured via a potassium dichromate oxidation method [66]. Soil pH was measured at a 1:5 (soil:water) ratio with a glass electrode (FE20, Switzerland). Inorganic N (NH4+-N and NO3--N) was determined on a continuous flow analyser (AA3, Germany) after extraction from soil with 2 M KCl. Available phosphorus (AP) and available potassium (AK) were analysed by the analysis method of soil agricultural chemistry [67]. Total phosphate (TP) was measured with molybdenum blue using spectrophotometry (T6, China). Total potassium (TK) was measured using a flame photometer (6400A, China). Total and available Cd concentrations were measured by full spectrum direct-reading inductively coupled plasma emission spectrometry (Varian 710ES, American) based on a previously described method [68].
DNA extraction, PCR amplification and sequencing
Soil DNA was extracted based on the manufacturer's instructions of the Power Soil DNA Isolation Kit (MO BIO Laboratories). DNA quantification and PCR amplification were performed as described in a previous study [5]. Each soil sample was extracted three times and then mixed and sequenced. High-throughput sequencing analysis of bacterial/fungal rDNA was performed using the Illumina HiSeq 2500 platform (2×250 paired ends) at Biomarker Technologies Corporation, Beijing, China.
Bioinformatics analysis
The microbial sequence analyses were completed on the Biomarker biocloud platform (www.biocloud.org). The paired-end reads were merged by FLASH (v1.2.7, http://ccb.jhu.edu/software/FLASH/) [69], and the treated raw tags were filtered and clustered in the next steps. Finally, the tags with an average quality score <20 in a 50 bp sliding window were abandoned using Trimmomatic (http://www.usadellab.org/cms/?page=trimmomatic) [70] and tags shorter than 350 bp were removed. The possible chimaeras were identified by employing UCHIME, which is a tool included in mothur (https://drive5.com/usearch/manual/uchime_algo.html). The denoised sequences were clustered using USEARCH (version 10.0) and tags with similarity ≥97% were regarded as OTUs. Bacterial taxonomy was assigned to all OTUs by searching against the Silva databases (Release128, http://www.arb-silva.de.) using the uclust within QIIME. The fungal taxonomic information was identified using the UNITE database (https://unite.ut.ee/) based on the BLAST tool in QIIME (version 1.9.1) [71].
Data analysis
The Shannon index, ACE and Chao 1 data and fungal taxa abundance were analysed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA), and the significant differences in the microbial alpha diversity between different cadmium treatments were calculated using Duncan's multiple test at p<0.05, p<0.01 or p<0.001, respectively. Total and available cadmium were calculated using SAS version 9.1(SAS Institute Inc., Cary, NC, USA). One-way-analysis of variance (ANOVA) and Spearman's correlations were also calculated using SAS version 9.1(SAS Institute Inc., USA). To determine the potential differences in the bacterial and fungal communities across compartments and chromium-added treatments, a heatmap of Spearman's rank correlation was generated using the package “gpplot” (Oksanen et al., 2012) in R (v3.6.2) based on Bray-Curtis dissimilarity. Canonical discriminant analysis (CDA) was performed to calculate the environmental factors that have the most remarkable influence on the microbial and fungal communities by CANOCO 5.0 (Biometrics Wageningen, Netherlands) [72, 73]. LEfSe analysis was applied using the version at https://huttenhower.sph.harvard.edu/galaxy/ [74].
Availability of data and materials
The datasets supporting the conclusions of this article are available in the NCBI repository [unique persistent identifier and hyperlink to datasets in https://www.ncbi.nlm.nih.gov/sra/PRJNA714690; accession project number: PRJNA714690; and https://www.ncbi.nlm.nih.gov/sra/PRJNA714699; accession project number: PRJNA714699;].
Abbreviations
- Cd:
-
Cadmium
- SOM:
-
Soil organic matter
- TN:
-
Total nitrogen
- TP:
-
Total phosphate
- TK:
-
Total potassium
- AP:
-
Available phosphate
- AK:
-
Available potassium
- CDA:
-
Canonical discriminant analysis
- CRD:
-
Completely randomized design
- ANOVA:
-
Analysis of variance
- UPGMA:
-
Unweighted Pair Group Method with Arithmetic mean
- NCBI:
-
National Center for Biotechnology Information
References
Xiao CP, Yang LM, Zhang LX, Liu CJ, Han M. Effects of cultivation ages and modes on microbial diversity in the rhizosphere soil of Panax ginseng. J Ginseng Res. 2016;40(1):28–37.
Kim KH, Lee D, Lee HL, Kim CE, Jung K, Kang KS. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past finding and future directions. J Ginseng Res. 2018;42(3):239–47.
Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38(3):161–6.
Ye RD, Yang QZ, Kong XW, Han JL, Zhang X, Zhang YX, et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58(3):391–8.
Sun H, Zhang YY, Sun CW, Gao M, He YP, Wang JH, et al. Effects of different manure treatments on heavy metals content (Cr, Cu, Pb and Zn) and their availability in ginseng cultivated soil. J Jilin Agri Univ. 2011;33(4):411–7.
Sebastián MS, Armstrong B, Cordoba JA. Exposures and cancer incidence near oil fields in the Amazon basin of Ecuador. Occup Environ Med. 2001;58:517–22.
Xie Y, Bu HS, Feng QJ, Wassie M, Amee M, Jiang Y, et al. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ Res. 2021;200;111730.
Ugbede FO, Osahon OD, Akpolile AF, Oladele BB. Assessment of heavy metals concentrations, soil-to-plant transfer factor and potential health risk in soil and rice samples from Ezillo rice fields in Ebonyi State, Nigeria. Environ Nonotechnol Monitoring Manag. 2021;16:100503.
Zhou SW, Su S, Meng L, Liu X, Zhang HY, Bi XL. Potentially toxic trace element pollution in long-term fertilized agricultural soils in China: A meta-analysis. Sci Total Environ. 2021;789:147967.
Bayne BL. Measuring the Effects of Pollution at the Cellular and Organism Level. The Role of the Oceans as a Waste Disposal Option. 1986;172:617–34.
Peng JY, Zhang S, Han YY, Bate B, Ke H, Chen YM. Soil Heavy metal pollution of industrial legacies in China and health risk assessment. Sci Total Environ. 2021;21:151632.
Duan QN, Lee JC, Liu YS, Chen H, Hu HY. Distribution of heavy metal pollution in surface soil samples in China: A graphical review. Bull Environ Toxico. 2016;97:303–9.
Tyagi N, Raghuvanshi R, Upadhyay MK, Srivastava AK, Suprasanna P, et al. Elemental (As, Zn, Fe and Cu) analysis and health risk assessment of rice grains and rice based food products collected from markets from different cities of Gangetic basin, India. J Food Composit Analysis. 2020;93:103612.
Zhao HD, Du LY, Wu Y, Wu XN, Han W. Numerical assessment of the passivator effectiveness for Cd-contaminated soil remediation. Sci Total Environ. 2021;779:146485.
Smith JE, Kluber LA, Jennings TN, McKay D, Brenner G, Sulzman EW. Does the presence of large down wood at the time of a forest fire impact soil recovery? Forest Ecol Manag. 2017;391:52–62.
Yu WJ, Lawrence NC, Sooksa-nauan T, Smith SD, Tenesaca C, Howe AC, et al. Microbial linkages to soil biogeochemical processes in a poorly drained agricultural ecosystem. Soil Biol Biochem. 2021;156:108228.
Byers AK, Condron L, O’Callaghan M, Waipara N, Black A. Soil microbial community restructuring and functional changes in ancient kauri (Agathis australis) forests impacted by the invasive pathogen Phytophthora agathidicida. Soil Biol Biochem. 2020;150:108016.
Fu DG, Wu XN, Duan CQ, Zhao LQ, Li B. Different life-form plants exert different rhizosphere effects on phosphorus biogeochemistry in subtropical mountainous soils with low and high phosphorus content. Soil Tillage Res. 2020;199:104516.
Singh JP, Vaidya BP, Goodey NM, Krumins JA. Soil microbial response to metal contamination in a vegetated and urban brownfield. J Environ Manag. 2019;244:313–9.
Xavier JC, Costa PES, Hissa DC, Melo VMM, Falcão RM, Balbino VQ, Mendonca LAR, Lima MGS, Coutinho HDM, Verda LC. Evaluation of the microbial diversity and heavy metal resistance genes of a microbial community on contaminated environment. Applied Geochem. 2019;105:1–6.
Walker CF, Harvey MJ, Bury SJ, Chang FH. Biological and physical controls on dissolved dimethylsulfide over the north-eastern continental shelf of New Zealand. J Sea Res. 2000;43(3–4):253–64.
Lorenz N, Hintemann T, Kramarewa T, Katayama A, Yasuta T, Marschner P, Kandeler E. Response of microbial activity and microbial community composition in soils to long-term arsenic and cadmium exposure. Soil Biol Biochem. 2006;38(6):1430–7.
Renella G, Mench M, Landi L, Nannipien P. Microbial activity and hydrolase synthesis in long-term Cd-contaminated soils. Soil Biol Biochem. 2005;37(1):133–9.
Anetor JI. Rising environmental cadmium levels in developing countries: threat to genome stability and health. Niger J Physiol Sci. 2012;27:103–15.
Zeng L, Liao M, Huang C, Luo Y. Effects of Cd contamination on paddy soil microbial biomass and enzyme activities and rice physiological indices. Biodivers Sci. 2005;13(6):555–65.
Sazykina MA, Minkina TM, Konstantinova EY, Khmelevtsova LE, Azhogina TN, Antonenko M, et al. Pollution impact on microbial communities composition in natural and anthropogenically modified soils of Southern Russia. Microbiol Res. 2022;254:126913.
Deng Y, Fu SD, Sarkodie EK, Zhang SF, Jiang LH, Liang YL, Yin HQ, Bai LY, Liu HW, Jiang HB. Ecological responses of bacterial assembly and functions to steep Cd gradient in a typical Cd-contaminated farmland ecosystem. Ecotoxicol Environ Saf. 2022;229:113067.
Riaz M, Kamran M, Fang YZ, Wang QQ, Cao HY, Yang GL, et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J Hazard Mater. 2021;402:123919.
Xu WJ, Hou SZ, Muhammad A, Chao Y, Xiao LL, Ruan ZB, et al. Effects of water and fertilization management on Cd immobilization and bioavailablility in Cd-polluted paddy soil. Chemosphere. 2021;276:130168.
Wang G, Li JC, Yang PY, Gao SF, Fang L. Study on the species in soil solutions and the availability of cadmium as affected by organic materials. Acta Scientiae Circumstantiae. 2020;20(5):621–6.
Gustave W, Yuan ZF, Li XJ, Ren YX, Feng WJ, Shen HB, et al. Mitigation effects of the microbial fuel cells on heavy metal accumulation in rice(Oryza sativa L.). Environ Pollut. 2020;260:113989.
Zhao XQ, Huang J, Lu J, Sun Y. Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicol Environ Saf. 2019;170:218–26.
Shuaib M, Azam N, Bahadur S. Romman M, Yu Q, Chang X. X. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microbial Pathogenesis. 2021;150:104713.
Lopez LCS, Fracasso MPDA, Mesquita DO, Palma ART, Riul P. The relationship between percentage of singletons and sampling effort: A new approach to reduce the bias of richness estimates. Ecological Indicators. 2012;14:164–9.
Wang F, Zhang WW, Miao LJ, Ji TW, Wang YF, Zhang HJ, et al. The effects of vermicompost and shell powder addition on Cd bioavailablity, enzyme activity and bacterial community in Cd-contaminated soil: A filed study. Ecotoxicol Environ Saf. 2021;215:112163.
Wang L, Zou R, Li YC, Tong ZH, You M, Huo WM, et al. Effect of Wheat-solanum nigrum L. intercropping on Cd accumulation by plants and soil bacterial community under Cd contaminated soil. Ecotoxicol Environ Saf. 2020;206:111383.
Wang GB, Zhang QQ, Du WC, Ai FX, Yin Y, Ji R, et al. Microbial communities in the rhizosphere of different willow genotypes affect phytoremediation potential in Cd contaminated soil. Sci Total Environ. 2021;769:145224.
Feng Y, Yang JJ, Liu W, Yan YB, Wang YH. Hydroxyapatite as a passivator for safe wheat production and its impacts on soil microbial communities in a Cd-contaminated alkaline soil. J Hazard Mater. 2021;404:124005.
Wei XM, Cao P, Wang G, Han JP. Microbial inoculant and garbage enzyme reduces cadmium (Cd) uptake in Salvia miltiorrhiza (Bge.) under Cd stress. Ecotoxicol Environ Saf. 2020;192:110311.
Fierer N, Wood SA, Bueno de Mesquita CP. How microbes can, and cannot, be used to assess soil health. Soil Biol Biochem. 2021;153:108111.
Kaur I, Gaur VK, Regar RK, Roy A, Srivastava PK, Gaur R, et al. Plants exert beneficial influence on soil microbiome in a HCH contaminated soil revealing advantage of microbe-assisted plant-based HCH remediation of a dumpsite. Chemosphere. 2021;280:130690.
Fan DW, Wang SY, Guo YH, Zhu YL, Agathokleous E, Ahmad S, et al. Cd induced biphasic response in soil alkaline phosphatase and changed soil bacterial community composition: the role of background Cd contamination and time as additional factors. Sci Total Environ. 2021;757:143771.
Sarria Carabalí MM, García-Oliva F, Cortés Páez LE, López-Lozano NE. Effect of cadmium contamination on the rhizosphere bacterial diversity of Echinocactus platyacanthus. Rhizosphere. 2020;13:100187.
Chen Y, Jiang YM, Huang HY, Mou LC, Ru JL, Zhao JH, et al. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. 2018;637-638:1400–12.
Macdonald CA, Clark IM, Zhao FJ, Hirsch PR, Singh BK, McGrath SP. Long-term impacts of zinc and copper enriched sewage sludge additions on bacterial, archaeal and fungal communities in arable and grassland soils. Soil Biol Biochem. 2011;43:932–41.
Griffiths BS, Philippot L. Insights into the resistance and resilience of the soil microbial community. Fems Microbiol Rev. 2012;37:112–29.
Li Z, Ma Z, van der KTJ, Yuan Z, Huang L. A review of soil heavy metal pollution from mines in china: pollution and health risk assessment. Sci Total Environ. 2014;468–469:843–53.
Wang X, Fang LC, Beiyuan JZ, Cui YX, Peng Q, Zhu SL, et al. Improvement of alfalfa resistance against Cd stress through rhizobia and arbuscular mycorrhiza fungi co-inoculation in Cd-contaminated soil. Environ Pollution. 2021;277:116758.
Chang F, Jia FG, Lv R, Li Y, Wang Y, Jia QG, et al. Soil bacterial communities reflect changes in soil properties during the tillage years of newly created farmland on the Loess Plateau. Applied Soil Ecol. 2021;161:103853.
Wang CX, Wang YB, Liu PY, Sun YY, Song ZL, Hu XK. Characteristics of bacterial community structure and function associated with nutrients and heavy metals in coastal aquaculture area. Environ Pollution. 2021;275:116639.
Dong YM, Gao ML, Qiu WW, Song ZG. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol Environ Saf. 2021;211:111899.
Sánchez-Castroa G-PV, Cleyet-Marel JC, Baudoin E, Tuinena DV. Glomeromycota communities survive extreme levels of metal toxicity in an orphan mining site. Sci Total Environ. 2017;598:121–8.
He L, Yang HS, Yu ZX, Tang JJ, Xu LG, Chen X. Arbuscular mycorrhizal fungal phylogenetic groups differ in affecting host plants along heavy metal levels. J Environ Sci. 2014;26(10):2034–40.
Li HY, Qiu YZ, Yao T, Han DR, Gao YM, Zhang JG, et al. Nutrients available in the soil regulate the changes of soil microbial community alongside degradation of alpine meadows in the northeast of the Qinghai-Tibet Plateau. Sci Total Environ. 2021;792:148363.
Liu L, Zhu K, Krause SMB, Li SP, Wang X, Zhang ZC, et al. Changes in assembly processes of soil microbial communities during secondary succession in two subtropical forests. Soil Biol Biochem. 2021;154:108144.
Lin YB, Ye YM, Hu Y, Shi HK. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol Environ Saf. 2019;180:557–64.
Cui HB, Shi Y, Zhou J, Chu HY, Cang L, Zhou DM. Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agri Ecosys Environ. 2018;267:165–73.
Hu XS, Liu XX, Qiao LK, Zhang S, Su KW, Qiu ZL, et al. Study on the spatial distribution of ureolytic microorganisms in farmland soil around tailings with different heavy metal pollution. Sci Total Environ. 2021;775:144946.
Luo LY, Xie LL, Jin DC, Mi BB, Wang DH, Li XF, et al. Bacterial community response to cadmium contamination of agricultural paddy soil. Applied Soil Ecol. 2019;139:100–6.
Liu SS, Fu JP, Cai XD, Zhou JM, Dang RL. Effect of heavy metals pollution on ecological characteristics of soil microbes: a review. Ecol Environ Sci. 2018;27(6):1173–8.
Ma WY, Sun T, Xu YM, Zheng SN, Sun YB. In–situ immobilization remediation, soil aggregate distribution, and microbial community composition in weakly alkaline Cd–contaminated soils: A field study. Environ Pollut. 2022;292(part A):118327.
Bian FY, Zhong ZK, Li CZ, Zhang XP, Gu LJ, Huang ZC, et al. Intercropping improves heavy metal phytoremediation efficiency through changing properties of rhizosphere soil in bamboo plantation. J Hazard Mater. 2021;416:125898.
Wang XH, Ya T, Zhang ML, Liu L, Hou PF, Lu SY. Cadmium(II) alters the microbial community structure and molecular ecological network in activated sludge system. Environ Pollut. 2019;255(Part 2):113225.
Ali A, Shaheen SM, Guo D, Li YM, Xiao R, Wahid F, et al. Apricot shell- and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ Pollut. 2020;264:114773.
Wang M, Chen SB, Chen L, Wang D. Responses of soil microbial communities and their network interactions to saline-alkaline stress in Cd-contaminated soils. Environ Pollut. 2019;252(part B):1609-1621.
Li CC, Quan Q, Gan YD, Dong JY, Fang JH, Wang LF, et al. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. Sci Environ. 2020;749:141555.
Bao SD. Soil agrochemical analysis (3rd). Beijing: Agricultural Press; 2000.
Lu RK. The analysis method of soil agricultural chemistry. Beijing: China Agricultural Science and Technology Press; 2000. p. 106–10.
Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–63.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Koljalg U, Koljalg RH, Nilsson K, Abarenkov L, Tedersoo AF, Taylor M, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol. 2013;22(21):5271–7.
Badagliacca G, Laudicina VA, Amato G, Badalucco L, Frenda AS, Giambalvo D, et al. Long-term effects of contrasting tillage systems on soil C and N pools and on main microbial groups differ by crop sequence. Soil Tillage Res. 2021;211:104995.
Das S, Hwang HY, Song HJ, Cho SR, Nostrand JDV, Kim PJ. Soil microbial response to silicate fertilization reduces bioavailable arsenic in contaminated paddies. Soil Biol Biochem. 2021;159:108307.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60.
Acknowledgements
We sincerely thank Dr. Qiuxia Wang for the helpful discussion in the bioinformatic analyses, we also acknowledge Ning Liu for assistance in sample collection.
Funding
Yayu Zhang acknowledges funding from China Agriculture Research System of MOF and MARA and Agricultural Science and Technology Innovation Program of CAAS (CAAS-XTCX20190025-6), Cai Shao acknowledges funding from Agricultural Science and Technology Innovation Program of CAAS (CAAS-ASTIP-ISAPS-2021-013), Hai Sun acknowledges funding from Agricultural Science and Technology Innovation Program of CAAS (CAAS-ASTIP-ISAPS-2021-015), Meijia Li acknowledges funding from Technology Development Program of Jilin Province (20200708024YY) on high throughput sequencing.
Author information
Authors and Affiliations
Contributions
Hai Sun carried out the experimental plan, and revised the manuscript. Chai Shao, Qiao Jin, Meijia Li and Zhenghai Zhang collected the samples and performed experiments. Hao Liang extracted DNA and conducted data analysis. Jiaqi Qian participated in the experimental design, and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments on plants were conducted in accordance with the cultivars protection regulations of the Institute of special animal and plant science of Chinese Academy of Agricultural Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, H., Shao, C., Jin, Q. et al. Effects of cadmium contamination on bacterial and fungal communities in Panax ginseng-growing soil. BMC Microbiol 22, 77 (2022). https://doi.org/10.1186/s12866-022-02488-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02488-z