Abstract
Background
Spodoptera litura (Fab.) (Lepidoptera: Noctuidae) commonly known as tobacco caterpillar is a polyphagous pest that causes significant damage to many agricultural crops. The extensive use of chemical insecticides against S. litura has resulted in development of resistance. In order to find potential biocontrol agents, gut microbes were investigated for insecticidal potential. These microbes live in a diverse relationship with insects that may vary from beneficial to pathogenic.
Results
Enterococcus casseliflavus, Enterococcus mundtii, Serratia marcescens, Klebsiella pneumoniae, Pseudomonas paralactis and Pantoea brenneri were isolated from adults of S. litura. Screening of these microbial isolates for insecticidal potential against S. litura showed higher larval mortality due to K. pneumoniae and P. paralactis. These bacteria also negatively affected the development of insect along with significant decline in relative growth and consumption rate as well as efficiency of conversion of ingested and digested food of insect. The bacteria significantly decreased the reproductive potential of insect. Perturbations in the composition of gut microbiome and damage to gut epithelium were also observed that might be associated with decreased survival of this insect.
Conclusions
Our study reveals the toxic effects of K. pneumoniae and P. paralactis on biology of S. litura. These bacteria may be used as potential candidates for developing ecofriendly strategies to manage this insect pest.
Similar content being viewed by others
Introduction
Spodoptera litura (Fab.) (Lepidoptera: Noctuidae), commonly known as tobacco caterpillar, is one of the most destructive polyphagous pests. It feeds on a wide range of host plants belonging to more than 40 families. Cotton, alfalfa, berseem, maize, tobacco, groundnut, summer legumes, and vegetables like cucurbits, brinjal, potato, sweet potato etc. are among the most preferred host plants [1, 2]. Besides having high reproductive potential and strong migratory ability of adults, S. litura can adapt to wide range of ecological conditions. Thus under favourable conditions, its population increases in large numbers and causes economic losses to many of the commercially important crops [3,4,5]. The female lays eggs in masses, the early instar larvae feed gregariously while later instars spread and feed voraciously causing huge crop losses. The management of this pest is primarily relied on chemical insecticides and because of polyphagous nature; it has been exposed to a number of insecticides over the years. There are reports indicating development of varying levels of resistance in S. litura to different groups of insecticides such as pyrethroids, organophosphates, carbamates, abamectin, emamectin, benzoate, chlorantraniliprole and indoxacarb [6,7,8,9,10]. Moreover, these insecticides have potentially undesirable side effects on environment, humans and other non target species. Thus it becomes imperative to search for alternative ecofriendly strategies of pest management. Recently, considerable emphasis is being laid on the use of biopesticides based on microorganisms or their derivatives and plant products. Microbial pesticides based on fungi, bacteria, viruses and nematodes are gaining popularity due to their species specificity and environmental safety [11, 12].
Among the biocontrol agents, entomopathogenic bacteria and their toxins have been developed as commercial formulations which are being used successfully. Many Bacillus species viz. B. popilliae, B. lentimorbus, B. larvae, B. thuringiensis, B. sphaericus have been recognised as definitive insect pathogens [13, 14]. Apart from Bacillus, there are many other bacteria such as Serratia, Photorhabdus, Xenorahabdus, Streptomyces etc. which have also been reported as insect pathogens [15,16,17,18]. Among these, B. thuringiensis (Bt) is most successful and widely used against insect pests belonging to Diptera, Coleoptera and Lepidoptera. However, there are reports indicating development of resistance to Bt in lepidopteran pests viz. Plutella xylostella (Linnaeus), Spodoptera frugiperda (JE Smith), Helicoverpa zea (Boddie) and Pectinophora gossypiella (Saunders) [19,20,21,22,23]. This necessitates the search for more novel bacteria with insecticidal activity. Nowadays gut microbes isolated from insects have been explored for their insecticidal potential against agricultural pests [24, 25].
Insects are associated with a variety of microbes that play an important role in contributing nutrition, digestion, detoxification etc. [26, 27]. Gut microbiota particularly in termites and cockroaches help in digestion of cellulose while the aphids depend on gut microbes for their requirement of essential amino acids [28, 29]. Besides various beneficial roles, these gut bacteria may become opportunistic pathogens due to physiological or environmental changes that lead to perturbation in the gut microbial diversity [30, 31]. Various studies revealed the pathogenicity of enteric bacteria against insect hosts such as Enterobacter cloacae isolated from S. litura and B. thuringiensis isolated from Spodoptera exigua (Hubner) [24, 32]. Flavobacterium sp. and Klebsiella sp. isolated from Spodoptera littoralis (Boisduval) when tested for their virulence against same insect host exhibited 67% and 77% mortality respectively [33]. Similarly Sevim et al. [34] reported 60% mortality in Agrotis segetum (Denis & Schiffermuller) due to its gut bacteria Enterococcus gallinarum. Most of these entomopathogenic bacteria have been reported to produce diverse toxins with mode of action like B. thuringiensis [35, 36].
In order to develop ecologically sustainable strategies for pest control and to reduce the load of insecticides on environment, there is an increasing interest in finding indigenous bacterial isolates which are more pathogenic and effective against various insect pests. In this respect the present study aimed to explore the insecticidal potential of gut microbes isolated from adults of S. litura.
Results
Screening bioassays
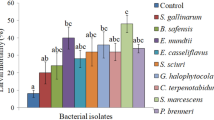
A total of six bacteria i.e. E. casseliflavus, E. mundtii, S. marcescens, K. pneumoniae, P. paralactis and P. brenneri were isolated from adults of S. litura. Screening of these bacterial isolates exhibited varying level of virulence in S. litura. In comparison to control, all the bacterial treatments showed significantly high larval mortality (Fig. 1). Among these, K. pneumoniae and P. paralactis exhibited higher larval mortality i.e. 52% and 56% respectively, thus both were selected for detailed bioassay studies.
Dose-response experiments
Mortality and development period
A significant effect was observed on survival and development of S. litura when the larvae were fed on castor leaves treated with different concentrations of K. pneumoniae and P. paralactis. Both the bacteria caused significantly higher larval mortality in comparison to control. As is evident from Fig. 2, the mortality rate increased in a dose dependent manner. The larvae feeding on leaves treated with different concentrations of K. pneumoniae suffered 38.00-72.00% mortality (F =63.53, p ≤ 0.05). Similarly P. paralactis caused 42.00-70.00% mortality in S. litura larvae (F =57.36, p ≤0.05). The larval mortality started after third day of treatment at higher concentrations i.e. 3.6 × 109 and 5.8 × 109 cfu/ml of K. pneumoniae and at highest concentration (5.0 × 109 cfu/ml) of P. paralactis. However, at lowest concentration the larval mortality started after five days in case of K. pneumoniae and seventh day after treatment in case of P. paralactis (Figs. 3 and 4). The LC50 values were calculated by using Probit analysis. It was found to be 1.2 × 109 cfu/ml for K. pneumoniae and 6.4 × 108 cfu/ml for P. paralactis. The infected larvae showed the symptoms of sluggishness, cessation of feeding and the dead larvae became black in color, flaccid with intact integument due to pathogenic effects of these bacteria (Fig. 5a and b).
Effect of different concentrations of K. pneumoniae (C1=3.2 × 108cfu/ml, C2=8.2 × 108cfu/ml, C3=1.9 × 109cfu/ml, C4= 3.6 × 109cfu/ml and C5=5.8 × 109cfu/ml) and P. paralactis (C1=2.4 × 108cfu/ml, C2=6.8 × 108cfu/ml, C3=1.4 × 109cfu/ml, C4=3.2 × 109cfu/ml and C5=5.0 × 109cfu/ml) on larval mortality of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey’s test P ≤ 0.05
Consumption of bacteria significantly delayed the development of insect (Table 1). Significant differences were observed among the treatments in case of larval development period. In comparison to control, the larvae took 2.38 to 4.74 days more to complete their development at 1.9 × 109 to 5.8 × 109 cfu/ml of K. pneumoniae. Significant effect was also detected on pupal development period. Except for the lowest concentration the total development period of S. litura extended significantly due to consumption of K. pneumoniae. Similarly P. paralactis influenced the development of S. litura. All the concentrations significantly delayed the larval development. With respect to control, the highest concentration i.e. 5.0 × 109 cfu/ml extended the larval period by 8.04 days. Although no significant effect was observed on pupal period except for the highest concentration, but the total development period of S. litura was prolonged significantly at all the concentrations of P. paralactis (Table 1).
Adult emergence and reproductive potential
Higher concentrations of both the bacteria significantly decreased the emergence of adults. In comparison to 91.06% in control, only 70.52 and 71.00% adult emergence was recorded due to K. pneumoniae and P. paralactis at 5.8 × 109 cfu/ml and 5.0 × 109 cfu/ml respectively (Table 1). The adults emerged from treated larvae exhibited morphological deformities such as unequal and crumpled wings (Fig. 5c and d). As is evident from Fig. 6, both the bacterial treatments also influenced the adult longevity with significant effect at higher concentrations. Fecundity of females tended to decrease under the influence of K. pneumoniae, but significant effect was only detected at the highest concentration where the female laid only 556 eggs during its lifetime in comparison to 866.66 eggs/female in control. Higher concentrations of P. paralactis also significantly decreased the fecundity (Fig. 7). Viability of eggs was also adversely affected at higher concentrations of the bacterial cell suspensions (Fig. 8).
Influence of different concentrations of K. pneumoniae (C1 = 3.2 × 108 cfu/ml, C2 =8.2 × 108cfu/ml, C3 =1.9 × 109cfu/ml, C4 = 3.6 × 109 cfu/ml and C5 =5.8 × 109 cfu/ml) and P. paralactis (C1 =2.4 × 108cfu/ml, C2 =6.8 × 108 cfu/ml, C3 =1.4 × 109 cfu/ml, C4 =3.2 × 109 cfu/ml and C5 =5.0 × 109 cfu/ml) on adult longevity of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey’s test P ≤ 0.05
Influence of different concentrations of K. pneumoniae (C1 = 3.2 × 108 cfu/ml, C2 =8.2 × 108 cfu/ml, C3 =1.9 × 109cfu/ml , C4 = 3.6 × 109 cfu/ml and C5 =5.8 × 109 cfu/ml) and P. paralactis (C1 =2.4 × 108 cfu/ml, C2 =6.8 × 108 cfu/ml, C3 =1.4 × 109cfu/ml, C4 =3.2 × 109cfu/ml and C5 =5.0 × 109 cfu/ml) on fecundity of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey’s testP ≤ 0.05
Influence of different concentrations of K.pneumoniae (C1 = 3.2 × 108 cfu/ml, C2 =8.2 × 108 cfu/ml, C3 =1.9 × 109cfu/ml , C4 = 3.6 × 109 cfu/ml and C5 =5.8 × 109 cfu/ml) and P. paralactis (C1 =2.4 × 108cfu/ml, C2 =6.8 × 108 cfu/ml, C3 =1.4 × 109 cfu/ml, C4 =3.2 × 109cfu/ml and C5 =5.0 × 109 cfu/ml) on egg hatching of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey’s test P ≤ 0.05
Effect of K. pneumoniae and P. paralactis on nutritional physiology
The results presented in Table 2, depict reduction in RCR of S. litura larvae feeding on leaves treated with different concentrations of cell suspension of both the bacteria when compared to control. The differences were statistically significant at higher concentrations. Decrease in consumption rate further lead to decrease in relative growth rate of larvae. With respect to control there was 30.30 to 33.33% and 24.24 to 36.36% decrease in RGR due to different concentrations of K. pneumoniae and P. paralactis respectively (F =5.19, p ≤0.05; F =4.88, p ≤0.05). Ingestion of leaves treated with both the bacteria also influenced the efficiency of conversion of ingested and digested food of S. litura. The higher concentrations of K. pneumoniae cell suspension resulted in 2.04 to 2.06 times decrease in ECI and 1.79 to 1.80 times decrease in ECD with respect to control. As is evident from Table 2, all the concentrations of P. paralactis also significantly decreased the values of ECI and ECD with respect to control. However, statistically significant differences were not observed among the concentrations. Higher concentrations of K. pneumoniae significantly decreased the approximate digestibility while no significant effect was detected due to P. paralactis except for the highest concentration.
Effect of K. pneumoniae and P. paralactis on gut microflora of S. litura
Gut microbial diversity of control as well as larvae treated with bacterial suspension of K. pneumoniae and P. paralactis at their LC50 values i.e. 1.2 × 109 and 6.4 × 108 cfu/ml respectively was explored in order to see the difference in culturable bacteria. Gut microbial composition differed in control and treated larvae. E. mundtii, E.casseliflavus and A. hemolyticus were isolated from control larvae having cfu count of 7.4 × 106, 6.9 × 106 and 4.0 × 105 per ml respectively (Table 3). However, when the larvae were fed on castor leaves treated with K. pneumoniae, three cultures i.e. E. mundtii, E. casseliflavus and K. pneumoniae were present with 7.0 × 105, 7.5 × 104 and 8.2 × 107 cfu/ml respectively (Table 3). Similarly E. mundtii, E. casseliflavus and P. paralactis were present with 7.3 × 104, 4.2 × 103 and 6.6 × 106 cfu/ml respectively when the larvae were fed on P. paralactis treated leaves. However, A. hemolyticus was absent in the larvae treated with both the bacterial concentrations. The numbers of Enterococcus colonies were superseded by the number of colonies of K. pneumoniae and P. paralactis in the treated larvae.
Histological studies
The histopathological effects of K. pneumoniae and P. paralactis on the midgut of S. litura larvae were also detected. The midgut cross-sections of treated larvae showed damage of the midgut epithelial cells (Fig. 9). Midgut of larvae fed with K. pneumoniae and P. paralactis showed the vacuolization of the cytoplasm, brush border membrane destruction and complete destruction of membrane at some sites. In contrast, the control S. litura larvae showed a well-preserved layer of epithelial cells with unaffected apical microvilli membrane of the midgut.
Longitudinal section through the midgut of 4th instar S. litura larvae a midgut of larvae fed on control leaf diet showing intact peritrophic membrane (PM), epithelial layers (EL) and muscle layer (ML), b midgut of larvae fed on leaf treated with K. pneumoniae showing lumen (L), peritrophic membrane disruption (PMD), epithelial layers disruption (ELD) and cytoplasmic vacuolation (CV), c midgut of larvae fed on leaf treated with P. paralactis showing cytoplasmic vacuolation (CV), epithelial layers disruption (ELD) and muscle layer disruption (MLD)
Evaluation of the presence of K. pneumoniae and P. paralactis in larval haemolymph
The growth of K. pneumoniae bacteria was observed in the hemolymph of larvae infected with K. pneumoniae, however, no growth was observed in case of P. paralactis treatment as well as in control larvae.
Discussion
Insects live in a symbiotic relationship with various gut microbes that play an important role in nutrition and digestion, development, detoxification of secondary plant metabolites and reproduction of insects [26, 27, 37]. In the present study culturable bacteria viz. E. casseliflavus, E. mundtii, S. marcescens, K. pneumoniae, P. paralactis and P. brenneri were isolated from the gut of adults of S. litura. These bacteria have earlier been reported to be associated with larvae and adults of lepidopterans and other insects [38,39,40]. In order to identify new candidates for biological control, these bacteria were tested for their effect on survival and development of S. litura. Among these K. pneumoniae and P. paralactis induced up to 72 and 70% mortality respectively in the larvae. Pathogenicity of K. pneumoniae and Pseudomonas species have earlier been reported against the same host as well as other insects. Klebsiella sp. isolated from S. littoralis and Bombyx mori (Linnaeus) showed high insecticidal activity when tested against the same host [33, 41]. The pathogenicity of different strains of K. pneumoniae has also been documented against Galleria mellonella (Linnaeus) causing 100% mortality when the larvae were injected with highest concentration (107 cfu) after 24 h of infection [42]. K. pneumoniae isolated from infected pupae of G. mellonella also caused cross pathogenicity in Trichoplusia ni (Hubner) larvae [40].
Pseudomonas is a broad-host-range entomopathogenic bacterium that exhibits insecticidal activity towards agricultural pests. Pseudomonas strains have been found to infect and kill larval stages of Drosophila melanogaster (Meigen), S. littoralis and P. xylostella [36, 43, 44]. Maciel-Vergara et al. [45] documented higher mortality in the larvae of the giant mealworm Zophobas morio (Fabricius) due to P. aeruginosa when injected into the hemocoel in comparison to oral ingestion. Other species of Pseudomonas viz. P. taiwanensis, P. protegens strains and P. chlororaphis have also been reported to have potent insecticidal activity against G. mellonella and Manduca sexta (Linnaeus) larvae [36, 46]. Contrary to this, there are reports indicating the role of these bacteria in fitness of insect. Klebsiella oxytoca helps to reduce the intraspecific competition in age-disparate larval offsprings by affecting the ovipositional behaviour of gravid females of Musca domestica (Linnaeus) [47]. Previous studies also documented the role of Klebsiella spp. in increasing the mating performance in Ceratitis capitata (Wiedemann) [48,49,50]. Similarly Pseudomonas genus has been reported to help in digestion of cellulose, amino acid synthesis and production of siderophores for extraction of iron required in many biochemical reactions, and to overcome iron toxicosis [51,52,53].
The larvae treated with K. pneumoniae and P. paralactis showed the symptoms of sluggishness, cessation of feeding and the dead larvae became black in color, flaccid with intact integument. Similar symptoms have earlier been reported in other insects due to Klebsiella and Pseudomonas infection [42, 45]. Histopathological studies revealed disruption of peritrophic matrix, damage in microvilli and midgut epithelial cells of S. litura larvae due to K. pneumoniae and P. paralactis infection. Damage to peritrophic membrane and disruption of intestinal integrity has earlier been reported due to oral ingestion of Pseudomonas and Bt toxins [44, 54, 55]. The pathogenicity of bacteria may be due to toxemia or septicemia. The larvae treated with P. paralactis did not show any bacterial growth in the hemolymph, indicating that the mortality of larvae may be due to toxin production and not due to its entry into the hemocoel. However, K. pneumoniae had been found to cross the gut epithelial barrier and invade the hemocoel causing septicaemia and ultimately death of the host. Previously Insua et al. [42] documented mortality in G. mellonella due to replication of K. pneumoniae in hemocoel. The high bacterial load present in the hemolymph cause concomitant tissue necrosis due to bacterial toxins [56, 57]. Pseudomonas is known to produce toxins such as extracellular proteinases and metalloproteases causing larval mortality in insects [45, 58]. The gut bacteria persist usually in low numbers inside the insect host without causing any disease; however, they may become pathogenic under stress conditions when the insect immune system gets weakened or due to alterations in the composition of microbiota [59,60,61]. S. marcescens, Pseudomonas and klebsiella are present as a part of normal gut microflora of lepidopterans and other insects [39, 62, 63]. However, the pathogenicity of these bacteria isolated from insects has also been reported against same host as well as other insects [33, 45, 64]. Present study reveals the change in gut microbial diversity between the infected and control larvae. The composition of gut microflora of control larvae consists of three cultures viz. E. mundtii, E. casseliflavus and A. hemolyticus in uniform distribution. However, in case of larvae fed on diet treated with K. pneumoniae and P. paralactis, the number of respective bacteria increased in comparison to E. mundtii and E. casseliflavus and thus become dominant in infected larvae and inhibited the growth of A. hemolyticus. Similar reports have earlier been documented by Thakur et al. [32] and Broderick et al. [61] in lepidopterans pests. Perturbation in gut microbial diversity due to bacterial infection thus lead to death of the host [60, 65,66,67].
In addition to mortality, larval treatment with K. pneumoniae and P. paralactis also delayed the development period of S. litura. It is in line with the previous studies indicating delay in development of S. litura and Bactrocera dorsalis (Hendel) due to infection of gut bacteria Enterobacter cloacae and Lactobacillus lactis [32, 68]. The results also revealed a significant negative effect of K. pneumoniae and P. paralactis on nutritional parameters of S. litura. The relative consumption rate of S. litura significantly decreased when the larvae were fed on diet treated with higher concentrations of bacterial cell suspension. Decreased RCR further led to concomitant decrease in growth rate relative to control. The treated larvae also showed reduction in efficiency of conversion of ingested and digested food and approximate digestibility of insect. Similar inhibitory effects of bacterial infection on nutritional physiology have earlier been documented on S. litura and Cnaphalocrocis medinalis (Guenee) [32, 69]. The decrease in consumption rate may be due to antifeedant effect of bacteria which impairs the larva from feeding or prohibits it from making maximum utilization of the ingested diet that may lead to longer larval developmental time [68]. The bacterial infection also caused damage to epithelial membrane and peritrophic matrix which may further interrupt the digestion and nutrient absorption thereby slowing the growth of larvae as suggested by Buchon et al. [70]. The Pseudomonas bacteria are known to produce chitinases that hydrolyse chitin, which is a common constituent of the insect exoskeleton and midgut peritrophic membrane [71,72,73]. Chitinases have been reported to disrupt the peritrophic membrane and decrease the digestive function [74,75,76]. An extracellular chitinase purified from B. subtilis has been found to negatively affect the nutritional parameters of S. litura [77]. The reduced adult emergence and morphological deformities in adults such as unequal and crumpled wings were observed along with the decrease in reproductive potential of adults. Olcott et al. [43] also documented delay in development as well as morphological defects in adult flies of D. melanogaster due to P. fluorescens infection. Similarly P. aeruginosa infection negatively affected the longevity of C. capitata [38]. These results show that the bacteria isolated from S. litura act as opportunistic pathogens which exert growth inhibition, antifeedant and toxic effects on S. litura.
Conclusions
In conclusion, cultivable bacteria viz. K. pneumoniae and P. paralactis isolated from the gut of S. litura adults exhibited insecticidal potential. Both these bacteria caused significantly higher mortality in S. litura larvae and delayed the development of insect. These bacteria also negatively affected the nutritional physiology and reproductive potential of the insect. Thus both these bacterial isolates appear to be significant candidates for microbial control of this pest. However, further optimization studies on mass production of bacterial cells and their testing under natural conditions need to be done.
Materials and methods
Collection and mass rearing of insect
The egg masses and larvae of S. litura were collected from cabbage and cauliflower fields around Amritsar (Punjab), India. The larvae were reared in the laboratory on fresh castor leaves in plastic jars (15 cm×10 cm) under controlled temperature and humidity conditions of 25 ± 2oC and 60 ± 5% respectively [53]. The larval diet was changed daily till pupation. The pupae were transferred to pupation jars and freshly emerged adults were shifted to oviposition jars. The adults were provided with honey solution (1 part honey: 4 parts water v/v) soaked on a cotton swab. The oviposition jars were lined with filter paper to facilitate egg laying. The newly hatched larvae were transferred to fresh castor leaves for further maintenance of culture. Larvae from the laboratory culture were used for conducting experiments.
Bacterial isolation
The male and female adults (4 days old) obtained from laboratory culture were surface sterilised by rinsing with sterile water followed by 70% (v/v) ethanol, and then thoroughly rinsed with sterilized distilled water to remove the disinfectant. The adults were dissected aseptically with the help of sterilized micro scissors to remove the gut. The dissected guts of male and female adults were then separately homogenized in 1.0 ml Phosphate Buffer Saline (PBS) solution (pH 7.0). Homogenised samples were then serially diluted up to ten times and 100 µl of each diluted sample was then plated on Luria Bertani (LB) plates for isolation of bacteria. The plating was done by spread plate technique. The whole procedure was carried out under the laminar flow cabinet (ESCO, USA). The plates were incubated at 30 °C for 72 h and the morphologically distinct isolates were obtained. The pure bacterial cultures were stored in 50% (w/v) glycerol at -80 °C. Microbial isolates were identified as Enterococcus casseliflavus, Enterococcus mundtii, Serratia marcescens, Klebsiella pneumoniae, Pseudomonas paralactis and Pantoea brenneri (data submitted elsewhere). All these bacterial isolates were present in the females while the males harboured only E. casseliflavus, E. mundtii, S. marcescens and K. pneumoniae.
Preparation of bacterial suspension
Different bacterial cultures were inoculated into LB broth and incubated at 30 °C for 48 h. After incubation each bacterial culture was centrifuged at 4000 rpm and 4˚C for 10 min to obtain the pellet. The pellet was washed once with sterile distilled water and resuspended in PBS. The bacterial density was measured at optical density (OD600) and adjusted to 1.89 (1.8 × 109 cfu/ml approximately) and 10 ml of adjusted culture was further used in bioassays as described by Eski et al. [24] with some modifications.
Screening bioassays
The screening of bacterial cultures for their insecticidal potential was conducted on second instar larvae (6 days old) of S. litura. The larvae were randomly selected and kept in rearing vials. Fresh castor leaves were surface sterilized with 5% (v/v) NaOCl followed by washing with distilled water. These leaves (approximately 10 cm2) were treated by dipping in 10 ml bacterial cell suspension of different isolates prepared as described above. The treated leaves after air drying at room temperature were placed in rearing vials containing larvae. In order to avoid cannibalism, one larva was kept in each rearing tube. Initial screening was done with 50 larvae with 5 replications of each bacterial isolate (10 larvae per replicate). Surface sterilized castor leaves dipped in PBS buffer were fed to control group. The experimental conditions were maintained at 25 ± 2oC temperature and 60 ± 5% relative humidity. The diet was changed after every 48 h and for that larvae were provided with fresh castor leaves treated with freshly prepared bacterial suspension until pupation. Observations were made daily on larval mortality.
Dose response experiments
Dose response experiments were conducted with bacterial isolates, K. pneumoniae and P. paralactis based on their higher larval mortality in S. litura according to screening test. Five different bacterial concentrations of each bacterial isolate were used i.e. C1= 3.2 × 108 cfu/ml, C2=8.2 × 108 cfu/ml, C3=1.9 × 109 cfu/ml, C4=3.6 × 109 cfu/ml and C5=5.8 × 109 cfu/ml in case of K. pneumoniae and C1=2.4 × 108 cfu/ml, C2=6.8 × 108 cfu/ml, C3=1.4 × 109 cfu/ml, C4=3.2 × 109 cfu/ml and C5=5.0 × 109 cfu/ml in case of P. paralactis (based on their OD600 values). Ten ml of each concentration was used to treat the castor leaves (approximately 10 cm2). The leaves dipped in PBS only served as control. Experiments on both the bacterial cultures were conducted on 50 s instar larvae (6 days old) with 5 replications (10 larvae per replicate) for each concentration. After every 48 h diet was changed till pupation. The observations on larval mortality and development of S. litura were recorded daily. The percentage of adult emergence was calculated and the freshly emerged adults from all the treatments and control were transferred to oviposition jar in 2:1 ratio (2 female: 1 male) to observe the longevity and fecundity of adults. One oviposition jar represented one replicate and all the treatments were replicated thrice. Based on larval mortality data, lethal concentration (LC50) values for both the bacteria were determined by Probit analysis using the SPSS 20.0 statistical software.
Nutritional analysis
To evaluate the effect of bacterial cultures, K. pneumoniae and P. paralactis on nutritional physiology, second instar larvae of S. litura were starved for 3–4 h. The larvae were weighed individually and released in rearing vials containing the weighed leaves treated with above mentioned bacterial concentrations. Similarly leaves dipped in PBS served as control. The experiment was performed on 50 s instar larvae in each concentration of both the bacterial cultures. After 72 h of feeding, the weight of larvae, residual diet and faecal matter was recorded and overall change in each variable was compared with the last recorded value. The data obtained were used to calculate nutritional indices on dry weight basis following the procedure of Farrar et al. [78] and Datta et al. [79]. Relative growth (RGR) and consumption rates (RCR) were calculated as G/I (G = change in larval dry weight/day and I = initial larval dry weight) and C/I (C = change in diet dry weight/day and I = initial larval dry weight) respectively. Both are calculated as mg mg−1 d−1. Index of food conversion efficiency (ECI) was calculated as 100 × G/C; where G = dry weight gain of insect and C = dry weight of food consumed. Approximate digestibility (AD) and efficiency of conversion of digested food (ECD) were calculated as C − F/C × 100 (where C = change in diet dry weight/day and F = dry weight of frass/day) and G/C − F × 100 (where G = change in larval dry weight/day, C = change in diet dry weight/day and F = dry weight of frass/day, respectively. Efficiency of conversion of ingested food (ECI), approximate digestibility (AD) and efficiency of conversion of digested food (ECD) were calculated as percent.
Effect of K. pneumoniae and P. paralactis on gut microflora of S. litura
To determine the effect of ingestion of bacteria on gut microbial diversity of S. litura, second instar larvae were fed on LC50 values of K. pneumoniae and P. paralactis. After 96 h of bacterial treatment, ten healthy control larvae and ten infected larvae showing the symptoms of slow growth, reduction in size, black pigmentation on integument were selected. The gut of both infected and control larvae were removed separately with the help of dissection scissors. These larval guts were homogenized in a homogenizer containing 1 ml of 0.1 M phosphate buffer (pH 7.0) under the laminar flow cabinet. The homogenized suspension was diluted up to ten times and 100 µl of each dilution was spread on Luria Bertani (LB) agar plates with the help of spreader. The plates were incubated for 48 h at 30˚C and observed for appearance of bacterial colonies and the cfu/ml of different bacteria was calculated by plate count method.
Histological analysis
The effect of LC50 values of K. pneumoniae and P. paralactis infection on histology of midgut of S. litura was studied on 2nd instar (6 days old) larvae. The leaves treated with bacterial suspensions were fed to larvae for 96 h. The larvae fed on leaves dipped in PBS only served as control. The temperature and humidity conditions were maintained at 25± 2ºC and 60 ± 5% respectively. After 96 h, larvae were dissected aseptically and gut was removed in distilled water. The gut was preserved in 10% formalin until processing of tissue. After fixation, the material was washed with distilled water in a tube and process was repeated many times. Then dehydration of tissue was done by passing through 30-90% grades of alcohol. For each treatment as well as control, the tissue was fixed in paraffin wax. After solidification of wax blocks, thin ribbons from blocks were prepared using the microtome. These thin ribbons having gut sections were placed on slide coated with very thin layer of Mayer’s egg albumin and kept on warm hot plate at 40-45ºC temperature for equal spreading of wax. Again tissue section placed on slide was passed through 30-90% grades of alcohol in ascending and descending way. Then permanent staining of slides was done by using hematoxylin and eosin stain following the methodology of Verma and Srivastava [80]. The slides were observed under the microscope (Evos XL Core) at magnification 400X to study the histology of gut tissue.
Evaluation of the presence of bacteria in larval hemolymph
To evaluate the presence of bacteria in hemolymph of larvae, the second instar larvae were fed on LC50 values of K. pneumoniae and P. paralactis. After 96 h of bacterial treatment, 100 µl of hemolymph was collected from ten infected larvae of bacteria treated groups and ten control larvae. The hemolymph collected was serially diluted and spread on LB agar plates with the help of spreader. Plates were incubated at 30˚C and observed after 48 h for the appearance of bacterial colonies.
Statistical analysis
The larval mortality, development period, adult emergence, adult deformities and all parameters of nutritional analysis were replicated five times (10 larvae/replication) while the experiments on male and female longevities, fecundity and egg hatching were replicated three times. All the values were represented as their mean ± SE. The difference in means were compared by one way analysis of variance (ANOVA) with Tukey’s test at p ⩾ 0.05. SPSS 20.0 software was used for statistical analysis.
Availability of data and materials
Not applicable.
References
Ahmad M, Arif MI, Ahmad M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007;26(6):809–17.
Ahmad M. Observed potentiation between pyrethroid and organophosphorus insecticides for the management of Spodoptera litura (Lepidoptera: Noctuidae). Crop Prot. 2009;28(3):264–8.
Dhaliwal GS, Jindal V, Dhawan AK. Insect pest problems and crop losses: changing trends. Indian J Ecol. 2010;37(1):1–7.
Punithavalli M, Sharma AN, Rajkumar MB. Seasonality of the common cutworm Spodoptera litura in a soybean ecosystem. Phytoparasitica. 2014;42(2):213–22.
Fu X, Zhao X, Xie B, Ali A, Wu K. Seasonal pattern of Spodoptera litura (Lepidoptera: Noctuidae) migration across the Bohai Strait in northern China. J Econ Entomol. 2015;108(2):525–38.
Ahmad M, Sayyed AH, Saleem MA, Ahmad M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 2008;27(10):1367–72.
Saleem M, Hussain D, Ghouse G, Abbas M, Fisher SW. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 2016;79:177–84.
Tong H, Su Q, Zhou X, Bai L. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J Pest Sci. 2013;86(3):599–609.
Sang S, Shu B, Yi X, Liu J, Hu M, Zhong G. Cross-resistance and baseline susceptibility of Spodoptera litura (Fabricius)(Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Manag Sci. 2016;72(5):922–8.
Wang X, Huang Q, Hao Q, Ran S, Wu Y, Cui P, Yang J, Jiang C, Yang Q. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Prot. 2018; 106:110–6.
Valicente FH. Entomopathogenic Viruses. In natural enemies of insect pests in neotropical agroecosystems. Springer, Cham. 2019;137–150.
Fernández-Grandon GM, Harte SJ, Ewany J, Bray D, Stevenson PC. Additive effect of botanical insecticide and entomopathogenic fungi on pest mortality and the behavioral response of its natural enemy. Plants. 2020;9(2):173.
Stahly DP, Andrews RE, Yousten AA. The genus Bacillus-insect pathogens. Prokaryotes. 2006;4:563–608.
Charles JF, Silva-Filha MH, Nielsen-LeRoux C. Mode of action of Bacillus sphaericus on mosquito larvae: incidence on resistance. In Entomopathogenic bacteria: from laboratory to field application. Springer, Dordrecht. 2000. p. 237–252.
Mona HA, Aly NA. Insecticidal activity and genetic characterization of four bacterial isolates of Xenorhabdus and Photorhabdus associated with entomopathogenic nematodes. Pest Technol. 2009;3(1):50–7.
Pineda-Castellanos ML, Rodríguez-Segura Z, Villalobos FJ, Hernández L, Lina L, Nuñez-Valdez ME. Pathogenicity of isolates of Serratia marcescens towards larvae of the scarab Phyllophaga blanchardi (Coleoptera). Pathogens. 2015; 4(2):210–28.
Ruiu L. Insect pathogenic bacteria in integrated pest management. Insects. 2015; 6(2):352–67.
Raymann K, Coon KL, Shaffer Z, Salisbury S, Moran NA. Pathogenicity of Serratia marcescens strains in honey bees. mBio. 2018;9(5):e01649-18.
Tabashnik BE, Cushing NL, Finson N, Johnson MW. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol. 1990;83(5):1671–6.
Zago HB, Siqueira HA, Pereira EJ, Picanço MC, Barros R. Resistance and behavioural response of Plutella xylostella (Lepidoptera: Plutellidae) populations to Bacillus thuringiensis formulations. Pest Manag Sci. 2014;70(3):488–95.
Naik VC, Kumbhare S, Kranthi S, Satija U, Kranthi KR. Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders)(Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manag Sci. 2018;74(11):2544–54.
Yang F, Williams J, Porter P, Huang F, Kerns DL. F2 screen for resistance to Bacillus thuringiensis Vip3Aa51 protein in field populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) from Texas, USA. Crop Prot. 2019;126:104915.
Yang F, Head GP, Price PA, Santiago González JC, Kerns DL. Inheritance of Bacillus thuringiensis Cry2Ab2 protein resistance in Helicoverpa zea (Lepidoptera: Noctuidae). Pest Manag Sci. 2020;76(11):3676–84.
Eski A, Demir I, Güllü M, Demirbağ Z. Biodiversity and pathogenicity of bacteria associated with the gut microbiota of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb. Pathog. 2018;121:350–8.
Noman MS, Liu L, Bai Z, Li Z. Tephritidae bacterial symbionts: potentials for pest management. Bull Entomol Res. 2020;110(1):1–4.
Engel P, Moran NA. The gut microbiota of insects–diversity in structure and function. Rev. 2013;37(5):699–735.
Ceja-Navarro JA, Vega FE, Karaoz U, Hao Z, Jenkins S, Lim HC, Kosina P, Infante F, Northen TR, Brodie EL. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat Commun. 2015;6(1):1–9.
Warnecke F, Luginbühl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450(7169):560–5.
Douglas AE, Francois CL, Minto LB. Facultative ‘secondary’bacterial symbionts and the nutrition of the pea aphid, Acyrthosiphon pisum. Physiol Entomol. 2006;31(3):262–9.
Haloi K, Kalita MK, Nath R, Devi D. Characterization and pathogenicity assessment of gut-associated microbes of muga silkworm Antheraea assamensis Helfer (Lepidoptera: Saturniidae). J Invertebr Pathol. 2016;138:73–85.
Ketola T, Mikonranta L, Laakso J, Mappes J. Different food sources elicit fast changes to bacterial virulence. Biol Lett. 2016;12(1):20150660.
Thakur A, Dhammi P, Saini HS, Kaur S. Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J Invertebr Pathol. 2015;127:38–46.
Cakici FO, Sevim A, Demirbag Z, Demir I. Investigating internal bacteria of Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae) larvae and some Bacillus strains as biocontrol agents. Turk J Agric For. 2014;38(1):99–110.
Sevim A, Demirbag Z, Demir I. A new study on the bacteria of Agrotis segetum Schiff.(Lepidoptera: Noctuidae) and their insecticidal activities. Turk J Agric For. 2010;34(4):333–42.
Perchat S, Buisson C, Chaufaux J, Sanchis V, Lereclus D, Gohar M. Bacillus cereus produces several nonproteinaceous insecticidal exotoxins. J Invertebr Pathol. 2005;90(2):131–3.
Ruffner B, Péchy-Tarr M, Ryffel F, Hoegger P, Obrist C, Rindlisbacher A, Keel C, Maurhofer M. Oral insecticidal activity of plant‐associated pseudomonads. Environ Microbiol. 2013;15(3):751–63.
Ramya SL, Venkatesan T, Srinivasa Murthy K, Jalali SK, Verghese A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz J Microbiol. 2016;47:327–36.
Behar A, Yuval B, Jurkevitch E. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity. <background-color:#CCFF99;bvertical-align:super;>J</background-color:#CCFF99;bvertical-align:super;>. Insect Physiol. 2008;54(9):1377–83.
Chen B, Teh BS, Sun C, Hu S, Lu X, Boland W, Shao Y. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci. Rep. 2016;6(1):1–4.
He Y, Qin Q, DiLegge MJ, Vivanco JM. Isolation of Klebsiella pneumoniae and Pseudomonas aeruginosa from entomopathogenic nematode-insect host relationship to examine bacterial pathogenicity on Trichoplusia ni. Microb Pathog. 2019;135:103606.
Mohanta MK, Saha AK, Saleh DK, Islam MS, Mannan KS, Fakruddin M. Characterization of Klebsiella granulomatis pathogenic to silkworm, Bombyx mori L. 3 Biotech. 2015;5(4):577–83.
Insua JL, Llobet E, Moranta D, Pérez-Gutiérrez C, Tomás A, Garmendia J, Bengoechea JA. Modeling Klebsiella pneumoniae pathogenesis by infection of the wax moth Galleria mellonella. Infect Immun. 2013;81(10):3552–65.
Olcott MH, Henkels MD, Rosen KL, L. Walker F, Sneh B, Loper JE, Taylor BJ. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PloS One. 2010;5(9):e12504.
Chen WJ, Hsieh FC, Hsu FC, Tasy YF, Liu JR, Shih MC. Characterization of an insecticidal toxin and pathogenicity of Pseudomonas taiwanensis against insects. PLoS pathogens. 2014;10(8):e1004288.
Maciel-Vergara G, Jensen AB, Eilenberg J. Cannibalism as a possible entry route for opportunistic pathogenic bacteria to insect hosts, exemplified by Pseudomonas aeruginosa, a pathogen of the giant mealworm Zophobas morio. Insects. 2018;9(3):88.
Péchy-Tarr M, Bruck DJ, Maurhofer M, Fischer E, Vogne C, Henkels MD, Donahue KM, Grunder J, Loper JE, Keel C. Molecular analysis of a novel gene cluster encoding an insect toxin in plant‐associated strains of Pseudomonas fluorescens. Environ. Microbiol. 2008;10(9):2368–86.
Lam K, Babor D, Duthie B, Babor EM, Moore M, Gries G. Proliferating bacterial symbionts on house fly eggs affect oviposition behaviour of adult flies. Anim. Behav. 2007;74(1):81–92.
Ben Ami E, Yuval B, Jurkevitch E. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. The ISME J. 2010;4(1):28–37.
Niyazi N, Lauzon CR, Shelly TE. Effect of probiotic adult diets on fitness components of sterile male Mediterranean fruit flies (Diptera: Tephritidae) under laboratory and field cage conditions. J Econ Entomol. 2004;97(5):1570–80.
Yuval B, Ben-Ami E, Behar A, Ben‐Yosef M, Jurkevitch E. The Mediterranean fruit fly and its bacteria–potential for improving sterile insect technique operations. Appl Entomol. 2013;137:39–42.
Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202(2):199–211.
Sonawane MS, Chaudhary RD, Shouche YS, Sayyed RZ. Insect gut bacteria: a novel source for siderophore production. Proceedings of the National Academy of Sciences, India Section B: Biol. Sci. 2018;88(2):567–72.
Xia X, Lan B, Tao X, Lin J, You M. Characterization of Spodoptera litura gut bacteria and their role in feeding and growth of the host. Front Microbiol. 2020;11:1492.
Song F, Lin Y, Chen C, Shao E, Guan X, Huang Z. Insecticidal activity and histopathological effects of Vip3Aa protein from Bacillus thuringiensis on Spodoptera litura. J Microbiol Biotechnol. 2016;26(10):1774–80.
Deepak T, Shashank AS, Shivaraj Y, Asiya Nuzhat FB. Toxic effect of Bacillus thuringiensis (Serotype 14) bacteria shows behavioural & histological changes on mosquito larvae. J Entomol Zool Stud. 2019;7(2):537–543
Mason KL, Stepien TA, Blum JE, Holt JF, Labbe NH, Rush JS, Raffa KF, Handelsman J. From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. mBio. 2011;2(3):e00065–11.
Jurat-Fuentes JL, Jackson TA. Bacterial Entomopathogens. In: Vega FE, Kaya HK, editors. Insect Pathology. Elsevier; 2012. p. 265–349. https://doi.org/10.1016/B978-0-12-384984-7.00008-7.
Andrejko M, Zdybicka-Barabas A, Cytryńska M. Diverse effects of Galleria mellonella infection with entomopathogenic and clinical strains of Pseudomonas aeruginosa. J Invertebr Pathol. 2014;115:14–25.
Sikorowski PP, Lawrence AM. Microbial contamination and insect rearing. Am Entomol. 1994;40(4):240–53.
Alverdy J, Holbrook C, Rocha F, Seiden L, Licheng R. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232(4):480.
Broderick NA, Robinson CJ, McMahon MD, Holt J, Handelsman J, Raffa KF. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009;7(1):1–9.
Azambuja P, Garcia ES, Ratcliffe NA. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005;21(12):568–72.
Jing TZ, Qi FH, Wang ZY. Most dominant roles of insect gut bacteria: digestion, detoxification, or essential nutrient provision?. Microbiome. 2020;8(1):1–20.
Bidari F, Shams-Bakhsh M, Mehrabadi M. Isolation and characterization of a Serratia marcescens with insecticidal activity from Polyphylla olivieri (Col.: Scarabaeidae). J Appl Entomol. 2018;142(1–2):162–72.
Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319(5864):777–82.
Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb Ecol. 2010;59(2):199–211.
Vacheron J, Péchy-Tarr M, Brochet S, Heiman CM, Stojiljkovic M, Maurhofer M, Keel C. T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. The ISME J. 2019;13(5):1318–29.
Khaeso K, Andongma AA, Akami M, Souliyanonh B, Zhu J, Krutmuang P, Niu CY. Assessing the effects of gut bacteria manipulation on the development of the oriental fruit fly, Bactrocera dorsalis (Diptera; Tephritidae). Symbiosis. 2018;74(2):97–105.
Nathan SS, Chung PG, Murugan K. Effect of biopesticides applied separately or together on nutritional indices of the rice leaffolder Cnaphalocrocis medinalis. Phytoparasitica. 2005;33(2):187.
Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5(2):200–11.
Chen L, Jiang H, Cheng Q, Chen J, Wu G, Kumar A, Sun M, Liu Z. Enhanced nematicidal potential of the chitinase pachi from Pseudomonas aeruginosa in association with Cry21Aa. Sci Rep. 2015;5(1):1–1.
Zhong W, Ding S, Guo H. The chitinase C gene PsChiC from Pseudomonas sp. and its synergistic effects on larvicidal activity. Genet. Mol. Biol<background-color:#CCFF99;bvertical-align:super;>.</background-color:#CCFF99;bvertical-align:super;> 2015;38(3):366–72.
Loper JE, Henkels MD, Rangel LI, Olcott MH, Walker FL, Bond KL, Kidarsa TA, Hesse CN, Sneh B, Stockwell VO, Taylor BJ. Rhizoxin analogs, orfamide A and chitinase production contribute to the toxicity of Pseudomonas protegens strain Pf-5 to Drosophila melanogaster. Environ Microbiol. 2016;18(10):3509–21.
Rao R, Fiandra L, Giordana B, de Eguileor M, Congiu T, Burlini N, Arciello S, Corrado G, Pennacchio F. AcMNPV ChiA protein disrupts the peritrophic membrane and alters midgut physiology of Bombyx mori larvae. Insect Biochem Mol Biol. 2004;34(11):1205–13.
Kabir KE, Sugimoto H, Tado H, Endo K, Yamanaka A, Tanaka S, Koga D. Effect of Bombyx mori chitinase against Japanese pine sawyer (Monochamus alternatus) adults as a biopesticide. Biosci Biotechnol Biochem. 2006;70(1):219–29.
Suganthi M, Senthilkumar P, Arvinth S, Chandrashekara KN. Chitinase from Pseudomonas fluorescens and its insecticidal activity against Helopeltis theivora. J Gen Appl Microbiol. 2017;63(4):222–7.
Chandrasekaran R, Revathi K, Nisha S, Kirubakaran SA, Sathish-Narayanan S, Senthil-Nathan S. Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura Fab. Pestic Biochem Physiol. 2012;104(1):65–71.
Farrar RR, Barbour JD, Kennedy GG. Quantifying food consumption and growth in insects. Ann Entomol Soc Am. 1989;82(5):593–8.
Datta R, Kaur A, Saraf I, Singh IP, Kaur S. Effect of crude extracts and purified compounds of Alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest, Spodoptera litura (Fabricius). Ecotoxicol Environ Saf. 2019;168:324–9.
Verma PS, Srivastava PC. Advanced Practical Zoology. New Delhi: S. Chand and company 1td; 2012.
Acknowledgements
Financial assistance received from University Grants Commission (UGC), Government of India, New Delhi, is duly acknowledged.
Supplementary data
The datasets used and/or analysed during the current study is available from corresponding author on reasonable request.
Funding
University Grants Commission (UGC), Government of India, New Delhi (Grant number 484). The supporter had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Sanehdeep Kaur* and Harvinder Singh Saini conceived and designed the experiments. Sarita Devi performed the experiments, maintained the insect culture, analyzed the data and prepared the manuscript with the help of Sanehdeep Kaur* and Harvinder Singh Saini. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Devi, S., Saini, H.S. & Kaur, S. Insecticidal and growth inhibitory activity of gut microbes isolated from adults of Spodoptera litura (Fab.). BMC Microbiol 22, 71 (2022). https://doi.org/10.1186/s12866-022-02476-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02476-3