Abstract
Background
Probiotics have been reported to reduce total cholesterol levels in vitro, but more evidence is needed to determine the clinical relevance of this activity. Chinese traditional fermented pickles are a good source of lactic acid bacteria. Therefore, pickle samples were collected for screening lactic acid bacteria based on their ability to survive stresses encountered during gastrointestinal passage and cholesterol reducing potency.
Results
Seventy five lactic acid bacteria strains were isolated from 22 fermented pickles. From these bacteria, Lactobacillus plantarum E680, showed the highest acid (85.25%) and bile tolerance (80.79%). It was sensitive to five of the eight antibiotics tested, inhibited the growth of four pathogenic bacteria, and reduced the total cholesterol level by 66.84% in broth culture. In vivo testing using hypercholesterolemic mice fed high-fat emulsion, independent of food intake, found that L. plantarum E680 suppressed body weight gain and reduced total cholesterol and low-density lipoprotein cholesterol levels, with no effect on high-density lipoprotein cholesterol.
Conclusions
Chinese traditional fermented pickles are a good source for probiotics. L. plantarum E680, isolated from pickles, was acid and bile tolerant, sensitive to antibiotics, and reduced cholesterol levels both in vitro and in vivo. Based on these results, L. plantarum E680 may have potential as a novel probiotic for the development of cholesterol-lowering functional food.
Similar content being viewed by others
Background
Hypercholesterolemia, or elevated serum cholesterol levels, can result from a genetic disorder affecting lipoprotein metabolism or from unhealthy lifestyle choices leading to dyslipidemia. Dyslipidemia is defined as elevated levels of low-density lipoprotein (LDL) and/or triglycerides, or low high-density lipoprotein (HDL) levels within plasma. Hypercholesterolemia, resulting from either cause is considered a major risk factor for cardiovascular disease (CVD) [1]. According to the World Health Organization (WHO), CVD is currently the world’s leading cause of death, accounting for more than 17 million fatalities each year [2]. The primary methods to reduce serum cholesterol levels, for prevention and treatment of CVD, are pharmaceuticals, dietary changes, and exercise. Statins, which inhibit hydroxy-methylglutaryl-coenzyme A reductase activity, are commonly used to reduce the levels of serum LDL-cholesterol (LDL-C). However, adverse side effects such as myotoxicity, hepatotoxicity and kidney injury have been reported due to the use of statins due to oxidative stress, and more recently, concerns have arisen in regard to their therapeutic efficacy [3]. Low-fat diets and exercise are effective approaches for lowering serum lipids and prevention of coronary atherosclerosis; however, a gap exists between targeted actual results due to consumer acceptance of low-fat diets [4]. Therefore, researchers are exploring alternative methods for lowering serum cholesterol.
Lactic acid bacteria (LAB) are ubiquitous in nature, and essential microorganisms in the production of fermentated foods, such as kimchi, dadih and cheese. In addition, several species have been investigated as probiotics for their potential to affect physiological functions within humans, including the following: immune regulation [5], alleviation of lactose intolerance [6], prevention of colon cancer and reduction of allergic reactions [7, 8].
The first report of LAB in dairy products associated with a lowering of serum cholesterol was in 1974 [9], and more researchers were attracted for its effect on preventing CVD in humans. More recently, clinical and animal studies have indicated that LAB, specifically Lactobacillus species, may assist in lowering serum cholesterol levels [10,11,12]. L. plantarum 299v (Pro Viva), which was isolated from the human intestinal tract, was reported to decrease viable bacteria translocation, and improve mucosal inflammation in rats [13]. In addition, this bacterium was shown to lower concentrations of LDL-C and fibrinogen in hypercholesterolemic patients [14]. L. plantarum LIP-1, isolated from homemade koumiss products, assimilated 71.47 μg/mL of cholesterol in vitro, and significantly reduced serum total cholesterol (TC), triacylglycerols (TG) and LDL-C levels with a concatenate increase of HDL-C in rats fed a high-fat diet [15]. In contrast to the above results, researchers have reported that some probiotic strains, which lowered cholesterol in vitro, did not significantly alter lipid profiles in vivo [16]. The contradicting results from previous studies supports the need for further exploration into the potential for using LAB as probiotics to lower serum cholesterol and prevent the development of metabolic diseases. Therefore, this study aimed to identify lactic acid bacteria from traditional fermented pickles, and investigate their cholesterol-lowering activity, as probiotic function in vitro. Strains displaying cholesterol-lowering activity will be further assessed for beneficial effects on hypercholesterolemic mice.
Results
Screening of strain with cholesterol-lowering ability
A total of 75 lactic acid bacteria isolates from 22 pickles samples were evaluated for their cholesterol-lowering capability. For the negative control (cholesterol-MRS without fermentation), the cholesterol content is 97.17 ± 2.56 μg/mL, which means the recovery percent (> 97%) of cholesterol is good for GC-MS. Five isolates were selected for further characterization due to their ability reduce cholesterol levels by more than 55% in vitro, which are similar or higher than the positive control (ATCC 43121, Lactobacillus acidophilus, cholesterol-reduction is 58.31%). The isolates were identified by 16S rRNA sequencing and designated as the following: Lactobacillus plantarum E680, Lactobacillus fermentum B02, Lactobacillus plantarum A07, Lactobacillus brevis H05 and Lactobacillus acidophilus K04 (Table 1). Of these strains, L. plantarum E680 displayed the highest level of cholesterol reduction (66.84%); which was significantly higher than the cholesterol-reduction observed from the control strain ATCC 43121 (P < 0.05).
Acid and bile salts tolerance
As shown in the Table 1, acid tolerance for the five LAB isolates ranged from 66.50 to 85.25%. L. plantarum E680 had the highest level of acid tolerance (85.25%), while LA K04 displayed a similar level of tolerance to the control strain L. acidophilus ATCC 43121. LB H05 was the least tolerant (66.50%). Resistance to bile salts was also shown in Table 1, with L. acidophilus ATCC 43121 showing the highest level of resistance at 84.51%. L. plantarum E680 displayed a significantly higher level of bile salts tolerance than other strains isolated in this study at 80.79%.
Antibacterial activity of L. plantarum E680
L. plantarum E680 showed antimicrobial activity against 4 selected pathogens (Fig. 1). Activity against the four pathogens differed significantly with Staphylococcus aureus ATCC 29213 being most sensitive (19.8 mm inhibition zone), and Salmonella Typhimurium CMCC 50335 the least sensitive (14.89 mm inhibition zone). For the negative control (MRS without fermentation), no inhibition zone was found around four pathogens, which means the MRS have no effect on this four pathogens.
Effect of L. plantarum E680 on mice’s body weight
Body weight and food intake were assessed to test the potential probiotic function of L. plantarum E680 (Fig. 2). Initial body weight of mice was not significantly different among those receiving saline (control), a high-fat emulsion (model), or a high-fat emulsion with L. plantarum E680 (E680) (P > 0.05). However, after 2 weeks, all mice given the high-fat emulsion were significantly heavier than the control group. The body weight of L. plantarum E680 groups appeared similar to the control group, and significantly lower than the model group (P < 0.05). The slopes of linear regression equations were 0.16, 0.37 and 0.19 for the control, model, and E680 groups respectively. Differences in body weight were not due to changes in food intake levels (data not shown).
Measure of body weight (BW) within three groups of mice. Control: saline; Model: high-fat emulsion; and E680: high-fat emulsion supplemented with 109 CFU/day L. plantarum E680. Error bars represent standard deviations with different lowercase letters indicating significant differences (P < 0.05, n = 10)
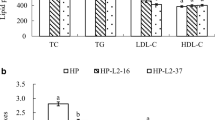
Effect of L. plantarum E680 on serum lipid profiles
Mice fed a high-fat emulsion diet (model) showed a significant increase in total serum cholesterol (Fig. 3a) and LDL-cholesterol (Fig. 3c) when compared to control mice receiving only saline (control). In addition, serum triglycerides levels (Fig. 3b) remained constant, and HDL-cholesterol was significantly decreased (Fig. 3d). Administering L. plantarum E680 with the high-fat emulsion diet prevented elevation of the total cholesterol and LDL levels, which were 10.71 and 16.47% lower than levels observed in the model group respectively (Fig. 3a and c). L. plantarum E680 had no effect on triglycerides levels (Fig. 3b); and was unable to prevent a drop in HDL-C levels (Fig. 3d).
Effects of L. plantarum E680 on serum lipid levels of (a) total cholesterol (TC), (b) triacylglycerols (TG), (c) low-density lipoprotein cholesterol (LDL-C), and (D) high-density lipoprotein cholesterol (HDL-C). Error bars represent standard deviations, different lowercase letters in each index indicate significant differences (P < 0.05, n = 10)
Discussion
Results of this study showed the well cholesterol-lowering activity of L. plantarum E680. Similar to our study, several Lactobacillus species, including L. acidophilus, L. casei, L. delbrueckii subsp. bulgaricus, L. fermentum, L. gasseri, L. paracasei, L reuteri; L. rhamnosus, and L. salivarius subsp. salicinius; as well as other strains of L. plantarum, have been reported to have cholesterol-lowering activity in vitro [17, 18]. In all of these studies, levels of cholesterol reduction were between 20% and 65%, suggesting that L. plantarum E680 is one of the more effective Lactobacillus strains possessing this activity. Although in vitro activity may identify potential probiotic candidates, one study suggested in vitro testing may not accurately reflect the cholesterol lowering activity in vivo, thus emphasizing the need for in vivo models to assess probiotic potential.
Another essential characteristic for a potential probiotic is the ability to survive passage through the gastrointestinal tract (GIT). Specifically, a probiotic must tolerate exposure to acid and bile salts for survival in the stomach and small intestine [19]. Of the strains tested in this study, L. plantarum E680 showed the highest tolerance to acid and bile salts. Although further studies are required to demonstrate acid and bile salt tolerance in vivo; the tolerance observed for L. plantarum E680 appears comparable or better than resistance reported for other L. plantarum strains investigated as potential probiotics. One study reported bile salts tolerance of L. plantarum isolates from dairy origin at 50.93 ~ 69.83% [20], while another reported acid tolerance of L. plantarum S04 and S10 at 73.4% and 63.2% respectively, at pH 2.5 for 3 h [21].
With bacterial antibiotic resistance being a global public health issue, there is concern about probiotics contributing to the problem due to their inherent resistance to some clinically relevant drugs [22]. In this study, L. plantarum E680 was tested for sensitivity to eight antibiotics, representing seven different classes (Table 2). L. plantarum E680 was sensitive to ampicillin (beta-lactam), cefazolin (cephalosporin), erythromycin (macrolide), sulfamethoxazole (sulfonamide), and chloramphenicol (amphenicol), with inhibition zones measuring between 16.09 and 32.41 mm in diameter; and displayed intermediate sensitivity to penicillin (beta-lactam), gentamicin (aminoglycoside) and ciprofloxacin (quinolones).
Resistance to both ciprofloxacin and gentamicin has been reported in Lactobacillus strains used as starter cultures in Norwegian dairy products [24]; and in strains investigated as potential probiotics [25]. In these studies, resistance was considered intrinsic, with the bacteria not considered a source for the spread of antibiotic resistance to other bacterial hosts. Observations in the current study are similar to results reported for L. plantarum dairy isolates which were sensitive to ampicillin, chloramphenicol, erythromycin, and resistant to gentamicin [26]. However, due to the concern that LAB could serves as a potential conduit for the transfer of antibiotic resistance through food, screening for resistance to clinically relevant antibiotics is essential when screening for potential probiotics [27].
Antimicrobial activity is another potential probiotic characteristic, and L. plantarum E680 displayed broad spectrum activity against both Gram-positive and Gram-negative bacteria, with S. aureus (inhibitory zone diameter, 19.79 mm) and E. coli O157:H7 being the most sensitive (inhibitory zone diameter, 17.74 mm). A previous report showed L. plantarum Lp9 had a comparable antibacterial spectrum, however Salmonella Typhi and L. monocytogenes were more sensitive than S. aureus and E. coli [28]. Other studies have also reported that Lactobacillus strains can inhibit the growth S. aureus and/or E. coli [29, 30]. The antibacterial activity of LAB may be due to organic acids (eg. lactic acid, acetic acid), hydrogen peroxide, bacteriocins, and/or exopolysaccharides produced though their metabolism [31].
In vivo analysis of the probiotic activity of L. plantarum E680 relied on the establishment of a hypercholesterolemic mouse model, which was successfully achieved after 14 days intragastric administration of a high-fat emulsion containing 15% cholesterol and 30% fat. The mice displayed increased total cholesterol and LDL-cholesterol levels, with normal triglycerides levels, as expected with hypercholesterolemia [1]. Previous studies have demonstrated that a high-fat emulsion diet results in lipid metabolic disorders, such as obesity, hyperlipidemia, and nonalcoholic fatty liver disease [32, 33]. This study showed that intake of L. plantarum E680 significantly reduced the increase of body weight caused by the high-fat emulsion in mice. Other studies reported similar results, finding that administering Lactobacillus to rats prevented an increase in body weight induced by a high-fat diet [34, 35]. More studies are required to determine the mechanism by which L. plantarum E680 prevented an increase in body weight. Other studies have shown that obesity and lipid metabolic diseases are closely related, and reported that probiotics can help prevent obesity by regulating bile salts metabolism and inhibiting dietary fat absorption in the small intestine [36, 37].
Results were comparable to those observed in previous studies, where L. plantarum PH04 was shown to reduce serum TC by 7% in mice [38], and the administration of L. fermentum M1–16 reduced TC and LDL-C levels by 12.5 and 17.3%, respectively, in rats fed a high-cholesterol diet [39]. In addition, a previous study administering probiotic blends to reduce serum TC in hypercholesterolemic rats didn’t report an increase in serum HDL-C [40], as was the case in this study. On the contrary, other studies have reported increased HDL-C levels that accompany a reduction in TC and LDL-C in hypercholesterolemic animal given probiotics [41]. More work is required to determine if the increase in HDL-C improves probiotic efficacy in preventing the development of cardiovascular disease in animal models.
Results of this study support the potential for using probiotics to control serum cholesterol levels in mice; however, more studies are required to understand the effectiveness of these bacteria in humans. One study reported that a capsule containing a probiotic blend of L. acidophilus and B. longum did not show beneficial effects on plasma lipids in young men and women with normal cholesterol levels [42]; yet a recent meta-analysis of 32 randomized controlled trials concluded that probiotic supplements could significantly reduce serum TC [43]. Several factors could explain the variations reported for the effectiveness of probiotics in controlling serum cholesterol levels, including the following: the probiotic strains being used, the host’s microbiome, and experimental protocols (probiotic dose, method of administering the probiotic, protocol for analysis of serum levels, etc.). Thus, thorough human clinical evaluations are required when trying to determine the true probiotic potential of a bacterium.
Conclusion
In this study, 75 lactic acid bacteria isolates were found from 22 Chinese traditional fermented pickles. L. plantarum E680 isolated from pickles had the highest acid and bile tolerance; was sensitive to antibiotics; and was effective in reducing cholesterol levels in both in vitro and in vivo studies. Based on these results, L. plantarum E680 may have potential as a novel probiotic for the development of cholesterol-lowering functional food.
Methods
Sample collection and isolation of LAB strains
Twenty-two traditional fermented pickles were collected from farmers in Taishun, Wenzhou, Zhejiang. All samples were obtained with the farmers’ permission, and were individually packed in sterile sampling bags (Whirl-Pak® Bags, Nasco, USA) for immediate storage at 4 °C for transport to the laboratory. Ten-fold serial dilutions were prepared in sterile saline (0.85% sodium chloride), with the 10− 5, 10–6, and 10− 7 dilutions (100 μL) spread onto de Man Rogosa and Sharpe (MRS) agar (Land Bridge Technology, Beijing, China). After anaerobic incubation at 37 °C for 48 h, colonies with typical morphological features were picked and subcultured on MRS agar for 3–5 passages. Isolates were further characterized by Gram-stain and catalase assays. Strains identified as lactic acid bacteria were stored in MRS broth (Land Bridge Technology, Beijing, China) with 40% (v/v) glycerol (Land Bridge Technology, Beijing, China) at − 80 °C. Prior to assays, strains were revived and passaged at least three times on sterile MRS agar.
Identification of LAB strains
Genomic DNA was extracted with the Ezup Column Bacteria Genomic DNA Purification kit (Sangon Biotech, Shanghai, China). The 16S rDNA sequences were amplified using a bacterial universal primer set (27f: AGTTTGATCMTGGCTCAG, 1492r: GGTTACCTTGTTACGACTT) following a PCR test. The high quality PCR product obtained was sequenced by Sangon Biotech (Shanghai) Co. (Shanghai, China), and then aligned using the NCBI BLAST sequence database (http://www.ncbi.nlm.nih.gov/) to identify the species of each strain.
In vitro cholesterol-lowering ability
LAB isolates were cultured for 18 h, harvested by centrifugation (5000 x g, 5 min, 4 °C) and resuspended in sterile saline solution at 109 CFU/mL. The bacterial suspension was used to inoculate (2% v/v) MRS broth containing 0.3% (w/v) oxgall Bile (Sigma-Aldrich, USA) and 100 mg/L of water-soluble cholesterol (Sigmae-Aldrich, USA), and incubated anaerobically at 37 °C for 48 h. Uninoculated broth was used as a control. Lactobacillus acidophilus ATCC 43121 was purchased from the American Type Culture Collection (Manassas, VA, USA), and used as a positive control, due to its reported cholesterol-lowering activity [44].
Cholesterol content was determined through modification of a previously reported method [45]. Briefly, cell free supernatant was centrifuged at 5000 x g for 5 min at 4 °C, 1 mL was collected and passed through a 0.22 μm filter into a gas chromatography sample vial (2 mL). Samples were analyzed using an Agilent 7890A gas chromatography system (Agilent, USA) with hydrogen flame ion detector, and HP-5 elastic quartz capillary column (30 m × 0.32 mm × 0.25 μm, Agilent, USA). Split-mode (5:1), direct injection of the sample (0.5 μL) was performed using helium as the carrier gas at a flow rate of 1.5 mL/min. Initial column temperature was 100 °C and increased at a rate of 20 °C/min, reaching 300 °C at the detection port. The cholesterol-lowering activity was calculated using the formula:
C0 is the cholesterol content in the control sterile broth (mg/L); C1 is the cholesterol content in the cell free supernatant (mg/L).
Acid and bile salts tolerance
Acid and bile salts tolerance was determined according to the method described by previous study with minor modifications [21]. Lactobacillus species were cultured for 18 h, then washed twice with sterile saline, and suspended in MRS broth adjusted to pH 2.0 at a concentration of approximately 108 CFU/mL. Cultures were incubated anaerobically at 37 °C for 3 h, with viable counts performed at 0 and 3 h. Separate cultures were prepared in a similar manner in MRS broth adjusted to pH 8.0 and supplemented with 0.3% (w/v) oxgall Bile (Sigmae-Aldrich, USA) at 37 °C for 6 h. Control cultures were prepared in MRS (pH 6.2) without oxgall Bile and incubated anaerobically at 37 °C for 6 h.
Antibiotic susceptibility test
L. plantarum E680 was tested for antibiotic susceptibility using the disk diffusion method [46], with results analyzed according to the Clinical and Laboratory Standards Institute Technical Guidelines (2017) [23]. MRS agar was surface inoculated by evenly spreading 100 μL of bacterial culture (107 CFU/mL). Disks (Table 2) (Hangzhou Microbial Reagent Co., Ltd., Hangzhou, China) were placed onto the agar surface, and plates were incubated anaerobically at 37 °C for 24 h. The diameter of the inhibition zone was measured using a vernier caliper.
Antibacterial activity test
An agar well diffusion assay [47] was used to screen L. plantarum E680 for antibacterial activity against select pathogens: Escherichia coli O 157: H 7 ATCC 25922, Salmonella Typhimurium ATCC 13311 (American Type Culture Collection (ATCC), Manassas, VA, USA), Listeria monocytogenes CMCC 54007, and Staphylococcus aureus CMCC 26003 (China Medical Microorganism Culture Collection (CMCC), Guangdong, China). Molten Luria-Bertani (LB) agar (Land Bridge Technology, Beijing, China) was inoculated with a select pathogen (final concentration 106 CFU/mL) and plates were poured with precast wells (8.00 ± 0.01 mm in diameter). Wells were filled with 150 μL of cell-free supernatant (18 h). Uninoculated MRS (pH 6.2) was used as control. Antibacterial activity was identified by the presence of inhibition zones.
Animal experiments
All experiments were performed in accordance with the NIH and Zhejiang University guidelines for Laboratory Animals Care and Use and approved by the Committee for the Care and Use of Laboratory Animals at the Laboratory Animal Center, Zhejiang University (ZJU-2017-03-1).
A modified high-fat emulsion was prepared using a previously reported protocol [48]. Solid lard (30 g) was melted, and 15 g of cholesterol (Sigma-Aldrich, USA) was added, stirring constantly until fully dissolved. The solution was further supplemented with 1 g of methylthiouracil and 15 mL of Tween-80, resulting in an oil mixture. An aqueous solution was prepared by mixing 10 mL of propylene glycol and 2 g of sodium deoxycholate in 30 mL of distilled water, and heating to 60 °C. The aqueous solution was combined with the oil mixture to generate the high-fat emulsion, which was stored at 4 °C. Prior to use, the emulsion was heated to 40 °C in a water bath and homogenized.
Thirty male ICR mice, 1 month old, weighing 25 ± 2 g, were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). During the experimental period, the mice were housed in an animal room under controlled environmental conditions at a temperature of 22 ± 2 °C, relative humidity of 50 ± 5%, and a 12-h light/dark cycle, with food and water readily available. The commercial diet (Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) was composed of 20.5% crude protein, 4% fat, 5% crude fiber, 10% moisture, and 8% coarse ash.
After 3 days of adaptation, mice were assigned to three groups (Control, Model and E680 groups) randomly, each consisting of ten mice, according to their body weight (BW). A gavage of high-fat emulsion was given to mice as previously described [49, 50]. The animal experiment scheme is illustrated in Fig. 4. Specifically, the control group was given sterile saline twice a day (0.5 mL/mouse in the morning, and 15 mL/kg BW in the evening (10 h later) by gavage. The model group received 0.5 mL of sterile saline per mouse in the morning, and intragastrical administration of 15 mL/kg BW of high-fat emulsion in the evening. The probiotic group was orally treated with a dose of 0.5 mL sterile saline containing L. plantarum E680 (2 × 109 CFU/mL) each morning, followed by 15 mL/kg BW of high-fat emulsion in the evening. Mice were fed these diets for 14 days. Health of the mice was monitored daily, with food intake and body weight measurements recorded every 2 days.
Following 14 d of treatment, all animals were fasted for 12 h and then anaesthetized with ether (4%), the mice were sacrificed by cervical dislocation after anesthesia, and the experimental animals were not given pain during the whole process. Blood samples were collected from the orbital venous plexus into a heparinized test tube, stored at 37 °C for 1 h, and then kept at 4 °C for 30 min. Serum was obtained by centrifugation (3000 g, 10 min), and stored at − 80 °C for subsequent lipid analysis.
The concentration of serum lipids including total cholesterol (TC), triacylglycerols (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured by commercial kits (Jiancheng, Nanjing, China) according to the manufacturer’s instructions. Ten μL of sample was mixed with working solution (37 °C, 5–10 min), then determined absorbance (510 nm) by microplate reader (Multiskan™ FC, Thermo Scientific, USA). The concentration of serum lipids was calculated based on the standard curve made from the standards.
Statistical analysis
All experiments were repeated three times in duplicate and the results were expressed as mean ± SD. One-way ANOVA was performed on all data using SPSS professional (version 17.0). Statistical analysis was conducted using Duncan’s multiple range test (DMRT), with differences at P < 0.05 considered statistically significant.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- TC:
-
Cholesterol
- TG:
-
Triacylglycerols
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- CVD:
-
Cardiovascular disease
- BW:
-
Body weight
References
Martinez-Hervas S, Ascaso JF. Hypercholesterolemia. In: Huhtaniemi I, Martini L, editors. Encyclopedia of Endocrine Diseases. Second ed. Oxford: Academic Press; 2019. p. 320–6.
WHO. Global health estimates 2016: Deaths by cause, age, sex, by country and by region, 2000-2016. Geneva: World Health Organization; 2018.
Liu AM, Wu QH, Guo JC, Ares I, Rodriguez JL, Martinez-Larranaga MR, Yuan ZH, Anadon A, Wang X, Martinez MA. Statins: adverse reactions, oxidative stress and metabolic interactions. Pharmacol Therapeut. 2019;195:54–84.
Aydaş SB, Aslim B. Chapter 54 - The Cholesterol-Lowering Effects of Probiotic Bacteria on Lipid Metabolism. In: Watson RR, Preedy VR, editors. Probiotics, Prebiotics, and Synbiotics: Academic Press; 2016. p. 699–722.
Surono IS, Martono PD, Kameo S, Suradji EW, Koyama H. Effect of probiotic L. plantarum IS-10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre-school children. J Trace Elem Med Bio. 2014;28(4):465–9.
Oak SJ, Jha R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci. 2019;59(11):1675–83.
Prakoeswa CRS, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati AN, Indramaya DM, Kusumowidagdo ER, Surono IS. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef Microbes. 2017;8(5):833–40.
Mendes MCS, Paulino DSM, Brambilla SR, Camargo JA, Persinoti GF, Carvalheira JBC. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J Gastroentero. 2018;24(18):1995–2008.
Mann GV. Studies of a surfactant and cholesteremia in the Maasai. Am J Clin Nutr. 1974;27(5):464–9.
Anderson JW, Gilliland SE. Effect of fermented milk (yogurt) containing Lactobacillus acidophilus L1 on serum cholesterol in hypercholesterolemic humans. J Am Coll Nutr. 1999;18(1):43–50.
Steinmetz KA, Childs MT, Stimson C, Kushi LH, Mcgovern PG, Potter JD, Yamanaka WK. Effect of consumption of whole Milk and skim Milk on blood lipid profiles in healthy-men. Am J Clin Nutr. 1994;59(3):612–8.
Ha CG, Cho JK, Lee CH, Chai YG, Ha YA, Shin SH. Cholesterol lowering effect of Lactobacillus plantarum isolated from human feces. J Microbiol Biotechnol. 2006;16(8):1201–9.
Molin G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am J Clin Nutr. 2001;73(2 Suppl):380S.
Bukowska H, Pieczul-Mroz J, Jastrzebska M, Chelstowski K, Naruszewicz M. Decrease in fibrinogen and LDL-cholesterol levels upon supplementation of diet with Lactobacillus plantarum in subjects with moderately elevated cholesterol. Atherosclerosis. 1998;137(2):437–8.
Wang J, Zhang H, Chen X, Chen Y, Menghebilige BQ. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J Dairy Sci. 2012;95(4):1645–54.
Lin SY, Ayres JW, Winkler W Jr, Sandine WE. Lactobacillus effects on cholesterol: in vitro and in vivo results. J Dairy Sci. 1989;72(11):2885–99.
Madani G, Mirlohi M, Yahay M, Hassanzadeh A. How much in vitro cholesterol reducing activity of lactobacilli predicts their in vivo cholesterol function? Int J Prev Med. 2013;4(4):404–13.
Ren DY, Li C, Qin YQ, Yin RL, Du SW, Ye F, Liu CX, Liu HF, Wang MP, Li Y, et al. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe. 2014;30:1–10.
Ashraf R, Smith SC. Commercial lactic acid bacteria and probiotic strains- tolerance to bile, pepsin and antibiotics. Int Food Res J. 2016;23(2):777–89.
Zago M, Fornasari ME, Carminati D, Burns P, Suarez V, Vinderola G, Reinheimer J, Giraffa G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011;28(5):1033–40.
Guo CF, Zhang S, Yuan YH, Yue TL, Li JY. Comparison of lactobacilli isolated from Chinese suan-tsai and koumiss for their probiotic and functional properties. J Funct Foods. 2015;12:294–302.
Imperial ICVJ, Ibana JA. Addressing the antibiotic resistance problem with probiotics: reducing the risk of its double-edged sword effect. Front Microbiol. 2016;7:1983.
CLSI: Performance Standards for Antimicrobial Susceptibility Testing. CLSI - M100-S27 2017, 37:282.
Katla AK, Kruse H, Johnsen G, Herikstad H. Antimicrobial susceptibility of starter culture bacteria used in Norwegian dairy products. Int J Food Microbiol. 2001;67(1–2):147–52.
Huang RH, Tao XY, Wan CX, Li SJ, Xu HY, Xu F, Shah NR, Wei H. In vitro probiotic characteristics of Lactobacillus plantarum ZDY 2013 and its modulatory effect on gut microbiota of mice. J Dairy Sci. 2015;98(9):5850–61.
Mathara JM, Schillinger U, Kutima PM, Mbugua SK, Guigas C, Franz C, Holzapfel WH. Functional properties of Lactobacillus plantarum strains isolated from Maasai traditional fermented milk products in Kenya. Curr Microbiol. 2008;56(4):315–21.
Mermelstein NH. Combating antibiotic resistance. Food Technol-Chicago. 2018;72(3):62–8.
Kaushik JK, Kumar A, Duary RK, Mohanty AK, Grover S, Batish VK. Functional and Probiotic Attributes of an Indigenous Isolate of Lactobacillus plantarum. PLoS One. 2009;4(12):e8099.
Strus M, Malinowska M, Heczko PB. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med. 2002;47(1):41–6.
Abushelaibi A, Al-Mahadin S, El-Tarabily K, Shah NP, Ayyash M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. Lwt-Food Sci Technol. 2017;79:316–25.
Garcia-Cano I, Rocha-Mendoza D, Ortega-Anaya J, Wang K, Kosmerl E, Jimenez-Flores R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl Microbiol Biot. 2019;103(13):5243–57.
Yang ZW, Wang J, Li JE, Xiong L, Chen H, Liu X, Wang N, Ouyang KH, Wang WJ. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohyd Polym. 2018;183:11–20.
Xu DY, Xu M, Lin L, Rao SS, Wang JP, Davey AK. The effect of isosteviol on hyperglycemia and dyslipidemia induced by lipotoxicity in rats fed with high-fat emulsion. Life Sci. 2012;90(1–2):30–8.
Guan XF, Xu QX, Zheng Y, Qian L, Lin B. Screening and characterization of lactic acid bacterial strains that produce fermented milk and reduce cholesterol levels. Braz J Microbiol. 2017;48(4):730–9.
Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48(5):712–4.
Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29(4):625–51.
Martinez-Guryn K, Hubert N, Frazier K, Urlass S, Musch MW, Ojeda P, Pierre JF, Miyoshi J, Sontag TJ, Cham CM, et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe. 2018;23(4):458.
Nguyen TDT, Kang JH, Lee MS. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol. 2007;113(3):358–61.
Xie N, Cui Y, Yin YN, Zhao X, Yang JW, Wang ZG, Fu N, Tang Y, Wang XH, Liu XW, et al. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. Bmc Complem Altern M. 2011;11:53.
Abd El-Gawad IA, El-Sayed EM, Hafez SA, El-Zeini HM, Saleh FA. The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifidobacteria in rats fed on a cholesterol-enriched diet. Int Dairy J. 2005;15(1):37–44.
Bao Y, Wang ZL, Zhang Y, Zhang JC, Wang LF, Dong XM, Su F, Yao GQ, Wang SQ, Zhang HP. Effect of Lactobacillus plantarum P-8 on lipid metabolism in hyperlipidemic rat model. Eur J Lipid Sci Tech. 2012;114(11):1230–6.
Greany KA, Bonorden MJL, Hamilton-Reeves JM, Mcmullen MH, Wangen KE, Phipps WR, Feirtag J, ., Thomas W, ., Kurzer MS: Probiotic capsules do not lower plasma lipids in young women and men. Eur J Clin Nutr 2007, 62(2):232.
Wang L, Guo MJ, Gao Q, Yang JF, Yang L, Pang XL, Jiang XJ. The effects of probiotics on total cholesterol A meta-analysis of randomized controlled trials. Medicine. 2018;97(5):e9679.
Noh DO, Kim SH, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci. 1997;80(12):3107–13.
Psomas EI, Fletouris DJ, Litopoulou-Tzanetaki E, Tzanetakis N. Assimilation of cholesterol by yeast strains isolated from infant feces and feta cheese. J Dairy Sci. 2003;86(11):3416–22.
Anandharaj M, Sivasankari B. Isolation of potential probiotic Lactobacillus oris HMI68 from mother's milk with cholesterol-reducing property. J Biosci Bioeng. 2014;118(2):153–9.
Zhang Y, Wu YT, Zheng W, Han XX, Jiang YH, Hu PL, Tang ZX, Shi LE. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J Funct Foods. 2017;38:273–9.
Zhao LY, Huang W, Yuan QX, Cheng J, Huang ZC, Ouyang LJ, Zeng FH. Hypolipidaemic effects and mechanisms of the main component of Opuntia dillenii haw. Polysaccharides in high-fat emulsion-induced hyperlipidaemic rats. Food Chem. 2012;134(2):964–71.
Wang LQ, Xu N, Zhang JJ, Zhao HJ, Lin L, Jia SH, Jia L. Antihyperlipidemic and hepatoprotective activities of residue polysaccharide from Cordyceps militaris SU-12. Carbohyd Polym. 2015;131:355–62.
Gou SH, Liu BJ, Han XF, Wang L, Zhong C, Liang S, Liu H, Qiang Y, Zhang Y, Ni JM. Anti-atherosclerotic effect of Fermentum Rubrum and Gynostemma pentaphyllum mixture in high-fat emulsion- and vitamin D-3-induced atherosclerotic rats. J Chin Med Assoc. 2018;81(5):398–408.
Acknowledgements
Not applicable.
Funding
This research work was supported by the Zheng Jiang province for Key Research & Development Projects (grant number 2019C02091), and Zhejiang YIMING food CO. LTD project (grant number 518004-I21603). The funding body has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception and design of the study; conceived and drafted the manuscript: ZYZ, FWC, JR, JXL, DXR; performed the experiments: ZYZ, WJW, JY, CC; collected the sample and data: WJW, BC, YJ; analyzed the data: ZYZ and WJW; revised the paper: ZYZ, JR and JF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Animal Care and Ethical Committee of the Zhejiang University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zheng, Zy., Cao, FW., Wang, Wj. et al. Probiotic characteristics of Lactobacillus plantarum E680 and its effect on Hypercholesterolemic mice. BMC Microbiol 20, 239 (2020). https://doi.org/10.1186/s12866-020-01922-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-020-01922-4