Abstract
Background
Colorectal cancer (CRC) is a common malignant gastrointestinal tumor. In China, CRC is the 5th most commonly diagnosed cancer. The vast majority of CRC cases are sporadic and evolve with the adenoma-carcinoma sequence. There is mounting evidence indicating that gut microbiota and inflammation play important roles in the development of CRC although study results are not entirely consistent. In the current study, we investigated the changes in the CRC-associated bacteria and plasma inflammatory factors and their relationships based on data from a case-control study of Han Chinese. We included 130 initially diagnosed CRC patients, 88 advanced colorectal adenoma patients (A-CRA), 62 patients with benign intestinal polyps and 130 controls.
Results
Fecal microbiota composition was obtained using 16S ribosomal DNA (16S rDNA) sequencing. PCOA analysis showed structural differences in microbiota among the four study groups (P = 0.001, Unweighted Unifrac). Twenty-four CRC-associated bacteria were selected by a two-step statistical method and significant correlations were observed within these microbes. CRC-associated bacteria were found to change with the degree of malignancy. Plasma C-reactive protein (CRP) and soluble tumor necrosis factor II (sTNFR-II) displayed significant differences among the four study groups and increased with adenoma-carcinoma sequence. The correlations of CRP and sTNFR-II with several CRC-associated microbes were also explored.
Conclusions
CRC-associated species and plasma inflammatory factors tended to change along the adenoma-carcinoma sequence. Several CRC-associated bacteria were correlated with CRP and sTNFR-II. It is likely that gut microbiome and inflammation gradually form a microenvironment that is associated with CRC development.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is the third common cancer worldwide causing 1.4 million newly diagnosed cases and 694,000 deaths a year [1]. In China, CRC is the 5th most commonly diagnosed cancer for both males and females and the incidence rate has been rapidly increasing recently [2].
The development of CRC is regarded as a multifactorial process involving genes mutation accumulation, inflammation and lifestyle factors, such as dietary habits and smoking [3,4,5,6]. Gut microbiome and inflammation are hypothesized to shape the tumor microenvironment and promote the tumorigenesis [7,8,9,10]. Previous observational and experimental studies have identified and suspected several microbes as potential drivers of CRC, including Fusobacterium nucleatum, Streptococcus bovis/gallolyticus, enterotoxigenic B. fragilis (ETBF), Enterococcus faecalis and colibactin-producing Escherichia coli. In recent studies, more detailed microbial profiles gained by high-throughput analyses, like metagenomic shotgun-sequencing and 16S rDNA sequencing, have revealed more bacteria that are associated with CRC [11,12,13].
Pathogenic microbes may work with inflammatory factors in CRC progression [14]. The enrichment of S. bovis is associated with an increased expression of pro-inflammatory genes [15]. ETBF can activate the signal transducer and activator of transcription 3 (STAT3) and induce Th17 cell infiltration as well as cytokines releasing in the colon of ApcMin/+ mice [5]. F. nucleatum infection can increase the expression of proinflammatory genes such as Scyb1, Interleukin-6 (IL-6), tumor necrosis factor (TNF-α), and Mmp3 [16]. However, there is a lack of consistent results for the effects of plasma inflammatory factors on CRC development from population-based studies [14, 17,18,19,20,21].
The major course of sporadic CRC progression begins with aberrant crypts, advances with early and late adenomatous polyps and finally turns into invasive carcinoma [6]. However, the changes in CRC-associated bacteria and inflammatory factors across the adenoma-carcinoma sequence and the potential correlations between them have not been clarified. We conducted a case-control study, which included CRC patients, A-CRA patients, patients with benign polyps and controls, to examine the relationships among CRC-associated microbiota, inflammatory factors and colon cancer status.
Methods
Study population and sampling
A case-control study was carried out and participants were from outpatients who received the colonoscopy at Changhai Hospital in Shanghai in 2014-2015. Persons who were capable to complete a questionnaire interview and to provide relevant biological samples were eligible for study inclusion.
Patient exclusion criteria included: (a) patients younger than 40 years of age, (b) persons not Han people, (c) patients with prior diagnoses of colorectal cancer, colorectal adenoma, inflammatory bowel disease (IBD) or other cancers, (d) patients had a family history of colorectal cancer in first- and second-degree relatives and no family history of neoplastic polyps or hereditary syndromes in first degree relatives under 60 years of age, and (e) patients had used antibiotics in last 6 months before colonoscopy. Hereditary syndromes include familial adenomatous polyposis (FAP), hereditary nonpolyposis colorectal cancer (HNPCC), Turcot syndrome, Oldfield syndrome and juvenile polyposis syndrome. Control selection was based on the same inclusion/exclusion criteria as used for case selection.
All participants had not been diagnosed or screened positive for colorectal cancer before inclusion and had no diet restrictions. Controls were outpatients who had no CRC or polyps indicated by colonoscopy and had no specific symptoms of CRC and were frequently matched with CRC patients by gender and age. Polyethylene glycol lavage solution was used for bowel preparation. Colonoscopy was performed by experienced endoscopists using a standard video colonoscope (Olympus Optical Co, Tokyo, Japan). Written informed consents were acquired from all participants.
Sample collection and laboratory testing
Before the colonoscopy, fresh stool samples (≥1 g) were collected at the hospital in a unified large centrifuge tube and stored in the − 80 °C fridge instantly. The stool samples were frozen-thawed once before an extraction of DNA. Biopsy specimens were collected during colonoscopy. After the colonoscopy, a questionnaire interview was conducted to collect basic demographic information, medical history and lifestyle factors (including smoking and alcohol drinking). Participants provided 2 ml venous blood that was preserved in − 80 °C fridge instantly for corresponding laboratory assay. Blood samples were centrifuged at 3000 rpm for 10 min to separate plasma. Plasma soluble tumor necrosis factor receptor 2 (sTNFR-II) was measured as a surrogate for TNF-α. CRP, IL-6, and sTNFR-II in plasma were measured with ELISA method (human CRP ELISA Kit 96 T, Anogen; Human IL-6 ELISA Kit 96 T, Anogen; Human sTNFR-II ELISA Kit 96 T, Raybiotech) according to standard procedures provided by the manufacturers.
Obesity/overweight status was assessed according to the criteria for Chinese adults (defines overweight as BMI ≥ 24 kg/m2 and obesity as BMI ≥ 28 kg/m2) [22]. For the patients, lesions located in the cecum, ascending colon, hepatic flexure, transverse colon and splenic flexure were considered as proximal lesions, and descending colon, sigmoid colon and rectum were deemed distal lesions [23].
DNA extraction and 16S rDNA sequencing
Bacterial genomic DNA was extracted from stool samples using OMEGA-soil DNA Kit (USA Omega Bio-Tek) and examined by 1% agarose gel electrophoresis. V3-V4 region of the 16S rDNA gene was amplified by universal primers (forward 338F 5’-ACTCCTACGGGAGGCAGCAG-3′ and reverse 806R 5’-GGACTACHVGGGTWTCTAAT-3′), while attaching Illumina adapters and sample-specific barcode sequences. The V3-V4 hypervariable region provides appropriate information for taxonomic classification of microbial analysis from specimens associated with human microbiome studies and was used by the Human Microbiome Project [24]. Polymerase chain reaction (PCR) was performed by ABI GeneAmp® 9700, with TransStart Fastpfu DNA Polymerase, 20 μl reaction systems. Each sample repeated the conduction for three times and PCR products, quantified by QuantiFluor™ -ST Fluorescence System (Promega), was pooled by AxyPrep DNA gel extraction kit (Axygen, USA) to prepare for sequencing. The Illumina MiSeq platform (Illumina, USA) was served in sequencing the amplicons.
Bioinformation analysis and statistical analysis
Paired-end reads were merged into one sequence in terms of overlapping (FLASH) and split by barcodes and primers. Reads with low quality and adaptors were removed (Trimmomatic 0.27). Dereplicated sequences (without singletons) were clustered into operational taxonomic units (OTUs), with 97% similarity. Representative sequences were picked out and classified by SINTAX (USEARCH v.10) algorithm using the RDP training set v16 with species names at a confidence threshold of 0.8 for genus and of 0.5 for species [25].
Alpha-diversity and the β-diversity of the microbiota data were computed using QIIME v.1.9.1 to assess the diversity alteration of microbiome [26]. Alpha-diversity measurements included ace, shannon index, simpson and PD whole tree. β-diversity was measured by unweighted and weighted UniFrac distances between samples considered phylogenetic information. To assess overall fecal microbiota composition discrepancies, Permutational Multivariate Analysis of Variance Using Distance Matrices (PMANOVA) was performed based on the UniFrac distances, and potential confounders (gender, age, BMI, smoking and drinking status) were taken into consideration. Principal Coordinates Analysis (PCoA) was applied to visualize similarities or dissimilarities of the microbiota of samples in different groups using the β-diversity distances mentioned previously.
CRC-associated microbes were selected by two steps. Firstly, species were applied the Zero-inflated Log-Normal mixture model in the metagenomeSeq packages and microbes with adjusted P values less than 0.05 were selected [27]. Secondly, selected microbiota was further filtered by the random forest algorithm in the Boruta package with 1000 iterations [28]. Selected species were clustered by a hierarchical ward-linkage method with Spearman correlation. P values of multiple comparisons or correlations tests were adjusted using the Benjamini-Hochberg method.
Pearson χ2 test or Fisher’s exact test was applied to analyze qualitative clinical information of patients if appropriate. One-way ANOVA followed by Tukey HSD test was used to analyze the differences in age and α-diversity among the four study groups. Kruskal-Wallis test and Dunn’s test post-hoc method was applied to assess the differences in inflammatory factors. Jonckheere-Terpstra test was used to investigate the trend for inflammatory factors and CRC-associated bacteria along with the adenoma-carcinoma sequence. Correlation networks based on Spearman’s rank correlations were performed to visualize the associations between serum factors and gut microbiota by Cytoscape 3.6.1. Statistical analyses were carried out in R v 3.4.4.
Results
Demographic and clinical information
A total of 130 CRC patients, 88 A-CRA patients, 32 patients with colorectal adenoma, 30 patients with hyperplastic polyps and 130 controls were included in our study. We combined the colorectal adenoma patients and patients with hyperplastic polyps into one group (“polyps group”). The selection of participants and collection of specimens are summarized in Fig. 1.
Flowchart for the selection of participants and collection of specimens. Gender, BMI and chronic diseases (heart disease, hypertension, and diabetes) showed no significant differences among the four study groups. Mean (SD) age of all participants was 59.1 (9.6) years. The included patients in the polyps group were younger than those with CRC (P = 0.033). The proportion of smoking in CRC (P = 0.044) and A-CRA (P = 0.002) patients was higher than controls. There were more patients with distal lesions than those with proximal lesions (Table 1). Among the CRC patients, 20.7%, 42.3% and 36.2% were in the TNM stages I, II and III, respectively
Stool microbiota sequencing results
A total of 28,800,738 high-quality reads were obtained from 410 samples (mean = 70,245.7). We subsampled 29,719 reads for each participant according to the sample with the least sequences. An OTU table with 1794 OTUs was constructed based on these sequences. Among the OTUs, 7 phyla, 22 classes, 34 orders, 70 families, 163 genera and 285 species were assigned (inclusive conditions: phylum > 0.1%, class to species > 0.001%).
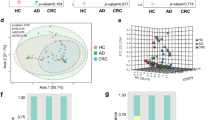
PCoA based on unweighted Unifrac distances showed a significant difference in gut microbiota among the four study groups (P = 0.001 for unweighted Unifrac distances; P = 0.320 for weighted Unifrac distances, PMANOVA, controlling for gender, age, BMI and status of smoking and drinking, Additional file 1: Figure S1). Alpha-diversity showed no significant difference among the four groups (ace: P = 0.153, shannon: P = 0.983; simpson: P = 0.814; PD whole tree: P = 0.08).
CRC-associated microbiota in CRC patients compared with controls
CRC-associated microbiota at the species level was selected. A total of 24 species were identified and filtered by the Zero-inflated Log-Normal mixture model and random forest algorithm. The CRC-associated species were divided into two clusters according to the hierarchical ward-linkage clustering in all 410 participants (Fig. 2). Pairwise correlations displayed in Fig. 3 showed that species within the cluster were positively related. Fourteen of 24 species increased in CRC patients including Peptostreptococcus stomatis, Parvimonas micra, Gemella morbillorum, Dialister pneumosintes, Porphyromonas asaccharolytica, Solobacterium moorei, Eisenbergiella tayi, Fusobacterium nucleatum, Ruminococcus torques, Eggerthella lenta, Clostridium symbiosum, Campylobacter rectus, Clostridium scindens, Clostridium lactatifermentans. The other 10 species including Eubacterium eligens, Coprococcus comes, Eubacterium hadrum, Eubacterium hallII, Fusicatenibacter saccharivorans, Blautia faecis, Roseburia faecis, Ruminococcus lactaris, Eubacterium desmolans, Streptococcus salivarius, decreased in CRC patients (Additional file 2: Table S1).
Results of inflammatory factors in plasma
CRP and sTNFR-II levels were significantly different in the four study groups. We discovered that the level of plasma CRP in CRC patients was higher compared with the A-CRA group (P < 0.001) and the control group (P = 0.002). The CRP level in the polyps group was higher than controls (P = 0.031). The plasma sTNFR-II in the CRC group (P < 0.001) and the A-CRA group (P = 0.001) was higher compared with controls.
Trend analyses of CRC-associated bacteria and inflammatory factors
We analyzed the evolution of CRC-associated microbiota as the disease progressed. The results showed that all 24 species changed with the order of control-polyps-A-CRA-CRC, but no trend was found with the TNM stage (Additional file 2: Table S1). We also observed that CRP and sTNFR-II increased with the adenoma-carcinoma sequence (Fig. 4, CRP: JT = 36,401.5, P < 0.001, sTNFR-II: JT = 37,225.5, P < 0.001).
Network analysis of CRC-associated microbiota and plasma inflammatory factors
Data from all the participants were used to analyze the correlations between the CRC-associated microbiota and inflammatory factors (Fig. 5). CRP, IL-6, and sTNFR-II were positively correlated with each other. Seven CRC-associated bacteria that increased in CRC patients including F. nucleatum, P. micra, P. stomatis, G. morbillorum, D. pneumosintes, S. moorei and C. retus displayed positive correlations with CRP. D. pneumosintes, F. nucleatum and P. stomatis showed positive correlations with sTNFR-II. Species including E. eligens, E. hadrum and R. faecis, which decreased in CRC patients, showed negative correlations with CRP. E. eligens was also negatively correlated with sTNFR-II (Additional file 3: Table S2).
Correlation network among plasma inflammatory factors and CRC-associated species. The width of each edge corresponds to the absolute values of Spearman correlation coefficients and the transparency of edge represents an adjusted P value. The line color indicates the direction of a correlation (red for positive and blue for negative). The relative size of the node was determined by the relative abundance of the microbe. Correlations with an adjusted P value less than 0.05 were displayed
Discussion
In this case-control study, we identified CRC-associated microbes in CRC patients compared with controls and divided them into two clusters according to the Spearman correlation. Among all the 410 samples from patients and controls, CRC-associated microbes and plasma inflammatory factors changed with the colorectal adenoma-carcinoma sequence. The inflammatory factors were positively correlated with CRC-associated microbes that increased in CRC patients and negatively correlated with those that decreased in CRC patients.
Our results support a previous hypothesis concerning the potent effect of oral periodontopathic bacteria in CRC carcinogenesis [29,30,31]. As expected, F. nucleatum was significantly enriched in CRC patients, which has been discussed widely in previous studies [16, 32, 33]. We also observed increased contributions of periodontal pathogens like P. micra, P. stomatis, P. oris, D. pneumosintes. and C. rectus [34,35,36,37]. The increased abundance of G. morbillorum was also observed. G. morbillorum is related with endodontic infections [38]. Flynn and his colleagues suggested a polymicrobial synergy hypothesis for the effect of oral pathogens in CRC tumorigenesis [29]. In the periodontitis, after invasive bacteria like F. nucleatum disrupt the epithelial barrier, metabolites are produced to change the microenvironment and promote inflammation for latter colonized microbiota like Porphyromonas spp. and Parvimonas spp. These pathogens can produce harmful factors that interfere with signal pathways, alter the permeability and promote periodontitis by releasing peptides and proteins. A similar mechanism may exist for oral pathogens in the colorectal tumorigenesis. We also observed co-abundance circumstance of these potential pathogens, further suggesting the possibility that they could work mutually in the development of CRC.
Mounting evidence has shown that short-chain fatty acids (SCFAs) acetate, propionate, and butyrate function as suppressors of inflammation and tumorigenesis [5, 39]. The anaerobic microbes inhabited in the large intestine can ferment the undigested dietary components to produce SCFAs. SCFAs can take anti-inflammatory and anti-apoptotic effects by the mechanism including inhibiting histone deacetylase (HDAC) activity [39, 40]. Reducing the expression of inflammatory factors by butyrate via inhibiting the activation of the NF-κB can lead to an anti-inflammatory effect and interfere pre- cancerous cells in the early stage of CRC development [41]. The relationship between changes in SCFAs-producing microbiota and CRC is elucidated in previous studies [31, 42, 43]. Consistent with this notion, our data showed that the relevant abundance of E. hall II, E. hadrum, E. desmolans, R. faecis and C. comes were depleted, which are butyrate-producing bacteria [44,45,46]. The commensal bacterium with anti-inflammatory property S. salivarius also decreased in CRC patients. As part of the intestinal commensal flora, the reduction in SCFA-producing bacteria may be caused by an increase in other pathogenic microorganisms such as F. nucleatum. It is also supported by our observation of negative correlations between potential pathogens and these commensal microbes.
It is widely accepted that most CRC cases are preceded by dysplastic adenomas, and dysplastic adenomas can progress into malignant forms following the adenoma-carcinoma sequence [47, 48]. Previous researches revealed that CRC-associated microbes altered along with the adenoma-carcinoma sequence and suggested the fecal microbiota might be useful in the early diagnosis and treatment of CRC [12, 13]. Consistent with previous studies, in the current study, the potential pathogens and SCFA-producing bacteria tended to increase or decrease with the degree of malignancy, which implied that CRC-associated microbes play an important role in the gradual formation of the tumor microenvironment.
TNF-α and IL-6 are core cytokines in the colorectal tumor promotion by activating NF-κB signaling pathways and STAT3 [5, 49, 50]. CRP can be produced by hepatocytes in response to the IL-6 activated by immune cells [11] and can also enhance resistance to apoptosis via STAT3 and NF-κB pathway [51,52,53]. Experimental studies have revealed that inflammatory factors play an important role in the relationship between pathogens like F. nucleatum and CRC [20, 54]. Elevation of related gene expression and activation of pathways are also observed in CRC patients [11, 55]. However, previous population-based studies did not support inflammatory factors (IL-6, CRP, and TNF-α) to be adequate biomarkers for CRC [14, 17]. In our study, CRP and sTNFR-II tended to increase along the adenoma-carcinoma sequence. It is intriguing that, among all the participants, CRP and sTNFR-II were significantly correlated with several CRC-associated microbes. However, no significant difference was observed in IL-6 among the four study groups and no association was observed between plasma IL-6 and bacteria. The level of IL-6 in plasma is also influenced by adiposity and inflammation of other tissues which might be the reason for the inconsistency with CRP and sTNFR-II [14]. Further human and experimental research is warranted to confirm this relationship and disentangle the complex role of immune response in CRC.
To our knowledge, this is the first case-control study to explore the associations of plasma inflammatory factors with CRC-associated microbiota. The enrolled patients were unaware of their disease status at the time of sampling and stool samples were obtained before colonoscopy, which avoided potential impacts of lifestyle and dietary changes. Strict operations conducted within one hospital reduced potential diagnostic biases.
There were some limitations in our study. Only stool samples rather than mucosal specimen were used to study the profiles of the gut microbiome. Stool samples are considered to be only partially uniform with those of the mucosal microbiota [11, 56]. Repeated measurements and external validation could improve the stability and credibility of data but were not conducted in the current study. In addition, we chose 16S rDNA amplicon sequencing considering the cost and sample size, which is less accurate compared with the shotgun sequencing method, especially at the species level.
Conclusions
The current study investigated CRC-associated microbiota and their correlations with inflammatory factors. These bacteria, as well as plasma CRP and sTNFR-II, changed along the adenoma-carcinoma sequence. Several microbes were significantly correlated with CRP and sTNFR-II. Our study results support the note that gut microbiome and inflammation may gradually form a microenvironment to promote the development of CRC.
Abbreviations
- A-CRA:
-
Advanced colorectal adenoma
- BMI:
-
Body mass index
- CRC:
-
Colorectal cancer
- CRP:
-
C-reactive protein
- ETBF:
-
Enterotoxigenic B. fragilis
- IL-6:
-
Interleukin 6
- OTU:
-
Operational taxonomic units
- PCoA:
-
Principal Coordinates Analysis
- PCR:
-
Polymerase chain reaction
- PMANOVA:
-
Permutational Multivariate Analysis of Variance Using Distance Matrices
- STAT3:
-
Signal transducer and activator of transcription 3
- sTNFR-II:
-
Soluble tumor necrosis factor II
- TNF-α:
-
Tumor necrosis factor
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. 2014;15(3):317–28.
Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016;115(3):273–80.
Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal Cancer. Annu Rev Microbiol. 2016;70:395–411.
Irrazabal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell. 2014;54(2):309–20.
Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–22.
Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–8.
Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012;10(8):575–82.
Gallimore AM, Godkin A. Epithelial barriers, microbiota, and colorectal cancer. N Engl J Med. 2013;368(3):282–4.
Flemer B, Lynch DB, Brown JM, Jeffery IB, Ryan FJ, Claesson MJ, O'Riordain M, Shanahan F, O'Toole PW. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut. 2017;66(4):633–43.
Nakatsu G, Li X, Zhou H, Sheng J, Wong SH, Wu WK, Ng SC, Tsoi H, Dong Y, Zhang N, et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat Commun. 2015;6:8727.
Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528.
Izano M, Wei EK, Tai C, Swede H, Gregorich S, Harris TB, Klepin H, Satterfield S, Murphy R, Newman AB, et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: the health, aging and body composition study. Int J Cancer. 2016;138(5):1118–28.
Abdulamir AS, Hafidh RR, Bakar FA. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. 2010;9:249.
Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14(2):207–15.
Heikkila K, Harris R, Lowe G, Rumley A, Yarnell J, Gallacher J, Ben-Shlomo Y, Ebrahim S, Lawlor DA. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20(1):15–26.
Kong SY, Tran HQ, Gewirtz AT, McKeown-Eyssen G, Fedirko V, Romieu I, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, et al. Serum endotoxins and Flagellin and risk of colorectal Cancer in the European prospective investigation into Cancer and nutrition (EPIC) cohort. Cancer Epidemiol Biomark Prev. 2016;25(2):291–301.
Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68(1):323–8.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41.
Kang M, Edmundson P, Araujo-Perez F, McCoy AN, Galanko J, Keku TO. Association of plasma endotoxin, inflammatory cytokines and risk of colorectal adenomas. BMC Cancer. 2013;13:91.
He W, Li Q, Yang M, Jiao J, Ma X, Zhou Y, Song A, Heymsfield SB, Zhang S, Zhu S. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring). 2015;23(3):684–91.
Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6(10):1117–21.
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome. 2014;2(1):6.
Edgar R. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. In: bioRxiv; 2016.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6.
Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. 2013;10(12):1200–2.
Kursa MB, Rudnicki WR. Feature selection with the Boruta package. J Stat Softw. 2010;36(11):13.
Flynn KJ, Baxter NT, Schloss PD. Metabolic and community synergy of oral Bacteria in colorectal Cancer. mSphere. 2016;1(3):e00102-16.
McCoy AN, Araujo-Perez F, Azcarate-Peril A, Yeh JJ, Sandler RS, Keku TO. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8(1):e53653.
Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66(1):70–8.
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206.
Yu J, Chen Y, Fu X, Zhou X, Peng Y, Shi L, Chen T. Wu Y: invasive fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer. 2016;139(6):1318–26.
Mansfield JM, Campbell JH, Bhandari AR, Jesionowski AM, Vickerman MM. Molecular analysis of 16S rRNA genes identifies potentially periodontal pathogenic bacteria and archaea in the plaque of partially erupted third molars. J Oral Maxillofac Surg. 2012;70(7):1507–14.
Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80(9):1421–32.
Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162(2 Pt A):22–38.
Contreras A, Doan N, Chen C, Rusitanonta T, Flynn MJ, Slots J. Importance of Dialister pneumosintes in human periodontitis. Oral Microbiol Immunol. 2000;15(4):269–72.
Gomes BP, Montagner F, Jacinto RC, Pinheiro ET, Zaia AA, Ferraz CC, Souza-Filho FJ. Gemella morbillorum in primary and secondary/persistent endodontic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(4):519–25.
Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, Yang L, Liu ZJ, Yuan YZ, Liu F, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97(5):1044–52.
Fung KY, Cosgrove L, Lockett T, Head R, Topping DL. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br J Nutr. 2012;108(5):820–31.
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72.
Gao Z, Guo B, Gao R, Zhu Q, Qin H. Microbiota disbiosis is associated with colorectal cancer. Front Microbiol. 2015;6:20.
Zeller G, Tap J, Voigt AY, Sunagawa S, Kultima JR, Costea PI, Amiot A, Bohm J, Brunetti F, Habermann N, et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol Syst Biol. 2014;10:766.
Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8.
Engels C, Ruscheweyh HJ, Beerenwinkel N, Lacroix C, Schwab C. The common gut microbe Eubacterium hallii also contributes to intestinal propionate formation. Front Microbiol. 2016;7:713.
Takada T, Watanabe K, Makino H, Kushiro A. Reclassification of Eubacterium desmolans as Butyricicoccus desmolans comb. nov., and description of Butyricicoccus faecihominis sp. nov., a butyrate-producing bacterium from human faeces. Int J Syst Evol Microbiol. 2016;66(10):4125–31.
Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89(7):845–60.
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–502.
Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–14.
Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8(9):1248–53.
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59.
West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15(10):615–29.
De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34(27):3493–503.
Arthur JC, Jobin C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes. 2013;4(3):253–8.
Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–36.
Parthasarathy G, Chen J, Chen X, Chia N, O'Connor HM, Wolf PG, Gaskins HR, Bharucha AE. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology. 2016;150(2):367–79.
Acknowledgments
The authors wish to thank the participants for providing samples. The authors also would like to thank the Epidemiology Department, Fudan University and Department of Gastroenterology, Changhai Hospital for providing necessary support. We thank reviewers who gave valuable suggestions that have helped to improve the quality of the manuscript.
Funding
Data collection and analysis was financed by the National Natural Science Foundation of China (number: 81473045). The fund from National Natural Science Foundation of China (number: 81473054) facilitated the interpretation of data and writing of the manuscript.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
ZL, QJ, QC and YC contributed to the study concept and design; YZ, EY, QS, FY directed quality assurance and control of the study; XY, YZ, NW, QC designed the analytic strategy and analyzed the actual dataset; LJ, HW, JL helped conduct the literature review; XY and YC drafted the manuscript; YC revised the manuscript critically for the interpretation of study results. All authors contributed to the revision of the manuscript, read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Shanghai Changhai Hospital Ethics Committee approved this study (CHEC2015-082.), and written informed consent was obtained from all participants. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during our research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. PCOA analysis based on unweighted and weighted Unifrac distances. Figure S1A and B are PCOA results based on the unweighted Unifrac distance. Figure S1C and D are PCOA results based on the weighted Unifrac distance. (TIF 4739 kb)
Additional file 2:
Table S1. The mean relative abundance, statistical parameter and trend analysis of CRC-associated microbes. (DOCX 20 kb)
Additional file 3:
Table S2. Correlations among CRC-associated microbes and plasma inflammatory factors. (DOCX 31 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, Y., Yu, X., Yu, E. et al. Changes in gut microbiota and plasma inflammatory factors across the stages of colorectal tumorigenesis: a case-control study. BMC Microbiol 18, 92 (2018). https://doi.org/10.1186/s12866-018-1232-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-018-1232-6