Abstract
Background
Common bean (Phaseolus vulgaris) is considered a staple food in a number of developing countries. Several diseases attack the crop leading to substantial economic losses around the globe. However, the crop has rarely been investigated for multiple disease resistance traits using Meta-analysis approach.
Results and conclusions
In this study, in order to identify the most reliable and stable quantitative trait loci (QTL) conveying disease resistance in common bean, we carried out a meta-QTL (MQTL) analysis using 152 QTLs belonging to 44 populations reported in 33 publications within the past 20 years. These QTLs were decreased into nine MQTLs and the average of confidence interval (CI) was reduced by 2.64 folds with an average of 5.12 cM in MQTLs. Uneven distribution of MQTLs across common bean genome was noted where sub-telomeric regions carry most of the corresponding genes and MQTLs. One MQTL was identified to be specifically associated with resistance to halo blight disease caused by the bacterial pathogen Pseudomonas savastanoi pv. phaseolicola, while three and one MQTLs were specifically associated with resistance to white mold and anthracnose caused by the fungal pathogens Sclerotinia sclerotiorum and Colletotrichum lindemuthianum, respectively. Furthermore, two MQTLs were detected governing resistance to halo blight and anthracnose, while two MQTLs were detected for resistance against anthracnose and white mold, suggesting putative genes governing resistance against these diseases at a shared locus. Comparative genomics and synteny analyses provide a valuable strategy to identify a number of well‑known functionally described genes as well as numerous putative novels candidate genes in common bean, Arabidopsis and soybean genomes.
Similar content being viewed by others
Introduction
Common bean (Phaseolus vulgaris) is a major source of dietary protein in many developing countries and considered the most cultivated food legume for direct consumption around the world [1]. Thanks to high diversity in common bean varieties i.e. pinto, kidney, haricot, navy and Mexican, the crop is produced in different environmental conditions ranging from sub-Saharan Africa to northern Europe and from Latin America to Canada, as well as Eastern Asia [2]. Since the beginning of the current century, there has been a fast-growing trend in the production and consumption of common bean i.e. dry bean and/or green bean around the world. According to FAOSTAT [3], global production of dry beans and green beans in 1997 was 16.29 and 8.90 million tonnes (mt), respectively. By 2021, the value was gradually increased over time and reached to 27.54 and 23.27 million tonnes (mt) for dry bean and green bean production, respectively. Such a substantial growth in the commercial production of common bean had a concomitant increase of crop loss risks due to abiotic and biotic constraints [4]. Besides the environmental factors, e.g. soil quality, precipitation, and temperature, diseases caused by bacterial, fungal and viral agents have frequently been reported to affect common bean productivity around the globe [5, 6].
Bacterial diseases of common bean include common bacterial blight caused by Xanthomonas phaseoli pv. phaseoli (formerly known as X. axonopodis pv. phaseoli), halo blight caused by Pseudomonas savastanoi pv. phaseolicola, and bacterial wilt caused by Curtobacterium flaccumfaciens pv. flaccumfaciens [5, 7, 8]. White mold caused by Sclerotinia sclerotiorum, anthracnose caused by Colletotrichum lindemuthianum, and root rot disease complex caused by a consortium of fungal species i.e. Fusarium solani f.sp. phaseoli, Fusarium oxysporum f.sp. phaseoli, Macrophomina phaseolina, and Rhizoctonia solani are considered the most important fungal diseases of common bean. As for the viral agents, Bean common mosaic virus (BCMV), Bean golden yellow mosaic virus (BGYMV), and Bean golden mosaic virus (BGMV) are considered the most important pathogens of common bean [5]. Severe epidemics of these individual diseases have frequently been reported in different corners of the globe leading to significant yield losses. However, the main economic impact of biotic constraints in common bean industry is usually due to the occurrence of a combination of bacterial, fungal and viral diseases coupled with physiological disorders and unfavourite environmental conditions [4].
The use of certified disease-free seed lots and timely application of agrochemicals e.g. fungicides are recommended to combat the risk of bacterial and fungal diseases of beans [9, 10]. However, a sustainable and durable approach should simultaneously target all biotic constraints i.e. bacterial, fungal and viral pathogens to reduce the effort and labor of disease management procedure. Development of resistant cultivars against the above-mentioned pathogens not only reduces the cost needed for disease management also increases the profitability of cropping systems within the framework of sustainable agriculture [11]. However, pathogens frequently adapt themselves to the genetic background of the host plants and could overcome host resistance particularly when it is determined by major genes or R genes (resistance genes), which also known as qualitative resistance [12]. Quantitative resistance, on the other hand, which is governed by multiple genes, has a partial effect and is difficult to be disrupted by the pathogen; thus, gained interest in recent decades to address the major challenge of genetic resistance durability. A quantitative trait locus (QTL) is a region of DNA, which is associated with a particular phenotypic trait, and identifies chromosomal regions associated with significant genetic effects for traits of interest [13, 14]. Because QTLs are important for providing broad-spectrum and long-lasting resistance to pathogens, identifying and transferring QTLs into cultivars with appropriate agronomic features is a powerful way to develop a superior cultivar with a combination of agronomically desired traits and disease resistance [15]. Identification of QTLs conferring resistance against plant diseases, validation of their chromosomal position, and characterization of the corresponding genomic markers are important for breeders to execute marker assisted selection (MAS) programs [16, 17]. However, individual QTLs reported in the literature are associated with particular genotypes and environmental conditions and in the cases of diseases; they depend on the genotype of the pathogen in different experiments [16, 17, 18, 19]. It is necessary to identify and investigate cultivars containing resistance genes conferring resistance against multiple races of pathogens [20].
When it comes to breeding crops for multiple disease resistance traits, Meta-QTL (MQTL) analysis would be the approach of choice to collect all QTLs that contribute to resistance mechanisms. MQTL analysis is a statistical method that combines results from different independent studies for removing redundant QTLs and detecting consistent QTLs [21, 22]. This method was initially proposed by Goffinet and Gerber [23], and then improved by Veyrieras et al. [24]. MQTL analysis could recommend the stable QTLs from different studies, and at the same time reduces the confidence interval (CI) of QTL [25, 26, 27]. Meta-analysis of QTLs is applied to compilation of data from independent mapping populations [27, 28]. This approach has been used to find MQTL for crop disease resistance including Fusarium head blight in wheat [29], Phytophthora-related diseases in cocoa [30], cyst nematode in soybean [31], white mold [13] and anthracnose [20] in common bean. Furthermore, there are several studies describing multiple disease resistance meta-QTLs (MDR-MQTLs) conveying resistance against different types of pathogens in different crops [32, 33, 34, 35, 36]. For instance, Pal et al. [32] recently investigated the meta-QTLs and candidate genes for multiple disease resistance against leaf rust, stem rust, and yellow rust in wheat.
No MDR-MQTLs analysis has so far been conducted to identify major QTLs contributing to disease resistance in common bean. Hence, the main objective of the present study was to evaluate 152 QTL data in common bean reported in the literature to provide a comprehensive overview on disease resistance repertories in this crop. The results obtained in this study provide a better understanding of disease resistance mechanisms in common bean and at the same time pave the way of developing novel high profitable common bean cultivars with higher level of resistance against diverse diseases.
Materials and methods
QTLs used for MQTL analysis
The QTLs for disease resistance-associated features in common bean genome published within the past 20 years (up to 2021) were collected from the literature to be used in MQTL analysis. In order to identify the most stable genomic regions governing disease resistance in common bean, the QTLs lacking proper genetic map or QTL-related information were ignored. Table 1 presents QTL data used in this study including marker type, as well as population type, size, and parents. The mapping populations ranged in size from 52 to 907 progenies of various types including two backcross (BC), seven F2, and 35 recombinant inbred lines (RIL). Eventually, based on the availability and abundance of the QTL data, two bacterial diseases i.e. common bacterial blight and halo blight, and four fungal diseases including white mold, Fusarium root rot, anthracnose, and angular leaf spot were included in MQTL analyses.
QTLs projection and meta-analysis
Based on the inclusion of different types of markers and high saturation of markers, two reference maps of common bean genome were retrieved from the the “Legume Information System (LIS)” online service https://www.legumeinfo.org/, and subjected to QTL projection [31, 32]. The reference map of Galeano et al. [69] has different marker types including AFLP, RAPD, SNP, SSR and STS. It has 1,269 markers with an average distance of 1.60 cM between markers, with the average chromosome length being 185.58 cM for a total length of 2,041.45 cM. In the MQTL analysis, the studies that were based on different types of SNP markers were projected onto the Song et al. [70] map. The latter reference map has 3,564 SNP markers with an average distance of 0.29 cM, while the average chromosome length was 94.15 cM with a total length of 1,035.68 cM [70]. Hence, among the QTL studies mentioned in Table 1, those published by Hoyos-Villegas et al. [58], Nakedde et al. [60], Wang [61], and Vasconcellos et al. [13] were projected to Song et al. [70] map, while the remaining studies were projected to the Galeano et al. [69] map.
For each of the QTLs, genetic position, proportion of phenotypic variance (R2), confidence Interval (CI) and the log of odds ratio (LOD score) were considered in the subsequent analyses. In order to incorporate the QTLs lacking the CI data, we applied the formulas CI = 530/(N*R2) for BC and F2 lines, CI = 287/(N*R2) for DH lines and CI = 163/(N*R2) for RILs lines, where N is the population size and R2 is the proportion of phenotypic variance of the QTL [71]. Correlation analysis between the number of initial QTLs and projected QTLs was conducted for all chromosomes of common bean. Further, to conduct a meta-analysis BioMercator V4.2 [72, 73] was used to integrate QTLs on the two above-mentioned reference maps. Consequently, the prevalent value among Akaike information criterion (AIC), corrected AIC (AICc), AIC 3 candidate models (AIC3), Bayesian information criterion (BIC) and average weight of evidence (AWE) models for each chromosome was chosen and considered as the number of MQTLs. Subsequently, the SOFIA package implemented in R-studio was used to show the distribution of QTLs within each MQTL, as well as the MQTL density on the reference maps [74].
Gene density and distribution of MQTLs
The data for gene density and variation distribution on common bean genome was retrieved from the EnsemblPlants (https://plants.ensembl.org/index.html) and Phytozome (https://phytozome-next.jgi.doe.gov/) databases, respectively, using RIdeogram via R-studio package [75]. Genomic position of predicted MQTLs and their distribution on each chromosome was determined using pheatmap R package and noticed on the 11 common bean chromosomes as a heatmap [76]. The position of MQTLs on common bean genome was compared to that of the gene density along each chromosome and illustrated using the pheatmap and RIdeogram R packages. Additionally, variations on the genome and the position of MQTLs among genotypes were presented using ChromoMap [77]. Sequences of flanking markers of MQTLs were obtained from the legumeinfo database (https://legumeinfo.org/search/marker), while the nearest markers to each MQTL were used to find the genomic position of MQTLs.
Comparison of MQTLs with GWAS studies
In order to improve the accuracy of our investigations, the most significant loci derived from genome-wide association studies (GWAS) were retrieved and compared to the genomic position of MQTLs in common bean for white mold- and anthracnose-related characteristics. Hence, six GWAS studies were surveyed to find the co-located significant GWAS signals and near-significant GWAS signals with MQTLs based on the common bean genome [78, 79, 80, 81, 82, 83].
Identification of Candidate Genes (CGs) in MQTL regions and KEGG enrichment analysis
The common bean genome in EnsemblPlants was used to identify disease resistance-related functional candidate genes (CGs) located at the CI of each detected MQTL. The sequences of all markers were used for BLAST analysis against common bean genome. EnsemblPlants database was used to extract gene annotations of the MQTL genomic regions (https://plants.ensembl.org/index.html). In order to improve the accuracy of our assumption on functional CGs, the orthologues of genes located at each MQTL interval were placed over the Arabidopsis (Arabidopsis thaliana) and soybean (Glycine max) genomes. This would lead to identifying more potential functional CGs based on their reported function in Arabidopsis and soybean. The Circos was used to present the gene density and orthologue genes in common bean, Arabidopsis and soybean [84]. Moreover, the KOBAS v3.0 software (http://kobas.cbi.pku.edu.cn/) was used to improve our understanding of the function of genes through KEGG pathways [85]. In order to conduct this analysis, the genes that were involved in MQTL regions were blasted against Arabidopsis genome through KOBAS v3.0 software. The ggplot2 package [86] was exploited to show the KEGG results.
Association of MQTLs with known R genes
The relationship of MQTLs with known R genes for the common bean diseases was investigated [87]. To this aim, EnsemblPlants database was used to retrieve FASTA sequences of MQTL regions, which were then BLASTed against the plant disease resistance gene database (PRGDB) to find PR-proteins and resistance gene analogs. In order to improve the accuracy, those MQTL regions less than five Mb were considered for this analysis. Genomic position of predicted PR-proteins on each chromosome was represented using Mapchart V.2.32 software [88]. Subsequently, the expression of these genes was retrieved from (https://phytozome-next.jgi.doe.gov/) for different tissues, standardized through z-scores in each tissue, and finally illustrated by pheatmap package in R.
Collection of datasets and analysis of gene expression
In order to validate the candidate genes (CGs) located on MQTL regions, four differentially expressed genes (DEGs) datasets involved in resistance to halo blight [51, 89], anthracnose [90], and white mold [91] were collected from independent studies. Venn diagram was used to compare the common bean resistance-responsive genes identified by four DEGs studies, and the genes located in MQTL regions. Candidate genes shared between MQTL regions, and DEGs data were analyzed in Venny online tool (https://bioinfogp.cnb.csic.es/tools/venny/index.html).
Results
Main features of resistance-related QTL studies in common bean
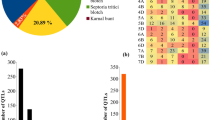
Data of 152 QTLs published between 2000 and 2021 were retrieved from the literature. These QTL studies included two BCs, seven F2s, and 35 RILs (Table 1). The number of QTLs responsible for resistance against bacterial and fungal diseases and their distribution on 11 common bean chromosomes are presented in Fig. 1. These QTLs belonged to 44 populations reported in 33 publications and contributed to common bean resistance against bacterial diseases i.e. common bacterial blight and halo blight, and fungal diseases i.e. white mold, Fusarium root rot, anthracnose and angular leaf spot. The QTLs were dispersed unevenly across the chromosomes where chromosome 11 with six QTLs and chromosome 7 with 24 QTLs had the lowest and highest number of QTLs, respectively (Fig. 1). Among the diseases investigated in this study, chromosome 7 had the highest number of resistance QTLs against fungal diseases while the highest number of QTLs conveying resistance against bacterial diseases was found on chromosome 1.
QTLs projection and detected MQTLs
In order to improve the accuracy and the number of projected QTLs, the two common bean reference maps provided by Galeano et al. [69] and Song et al. [70] were used in this study. The former map contained 1,269 markers while the latter contained 3,560 markers. To avoid any inconsistency, the case studies or reported QTLs with inverted or undetermined positions – as compared to the reference map – were excluded from the subsequent analyses. For consensus QTL prediction, the chromosomal position, LOD score, and R2 of the individual QTLs were taken into consideration. Only 57 out of 152 QTLs belonging to one bacterial disease (halo blight) and four fungal diseases (anthracnose, white mold, angular lead spot, and Fusarium root rot) were projected on the reference maps. Among the 57 projected QTLs, 32 QTLs were identified as major (PVE˃ 10%, [92, 93]), while 25 QTLs were identified as minor (Table S1). Among 57 QTLs, nine MQTLs were identified in common bean genome where three MQTLs (33%) were retrieved from at least three independent studies (Table 2). In this study, we only report MQTLs that contained more than two QTLs. As a result, MQTL analysis narrowed down the confidence Interval (CI) of genomic regions governing disease resistance compared to that of QTL studies. Moreover, a low correlation between the number of initial QTLs and projected QTLs was inferred on common bean chromosomes (r = 0.40). A MQTL with higher number of initial QTLs is the more stable one regardless of genetic background or environmental factors. MQTL7P.1 located on chromosome 7 with five QTLs had the highest number of initial QTLs generated from four separate populations followed by MQTL2P.1 and MQTL9P.2 on chromosomes 2 and 9, respectively, with three initial QTLs generated from three different populations (Table 2). These MQTLs were the most robust, viable and stable QTLs in a wide geographical and temporal span.
The MQTLs associated with resistance to each disease was irregularly scattered across common bean chromosomes where chromosomes 7 and 9 each had two MQTLs, chromosomes 1, 2, 5, 8 and 10 each had one MQTL, while no MQTL was found on the other chromosomes i.e. 3, 4, 6 and 11 (Fig. 2, Table 2). MQTL1P.1, MQTL7P.1 and MQTL9P.1 located on chromosomes 1, 7, and 9, respectively, represented the best chromosomal regions for resistance against white mold on chromosomes 1, 7 and 9, respectively. On the other hand, MQTL7P.2 on chromosome 7 had the highest number of QTLs associated with resistance against anthracnose. MQTL10P.1 on chromosome 10 showed region accommodating QTLs associated with halo blight resistance. Furthermore, four overlapping MQTLs including MQTL2P.1, MQTL5P.1, MQTL8P.1, and MQTL9P.2 located on chromosomes 2, 5, 8, and 9, respectively, that involved in resistance against at least two diseases were found. For instance, the two overlapping MQTLs i.e. MQTL5P.1 and MQTL8P.1 located on chromosomes 5 and 8, respectively, were observed to convey resistance against both bacterial (halo blight) and fungal (anthracnose) diseases of common bean on chromosomes 5 and 8, respectively. Hence, this region might contain genes controlling either types of infection or pleiotropic effects. Further details on the MQTLs flanking markers, their position, CI, and the traits which were controlled by MQTLs are reported in Table 2.
Distribution of QTLs and MQTLs on two common bean reference genome maps developed by Galeano et al. 2011 [69] (A) and Song et al. 2015 [70] (B) generated by SOFIA package in Rstudio [74]. The outermost circle shows the chromosomes’ position on the reference maps in cM. The second circle outlines the density of markers on the maps. The third circle displays the QTLs’ density (black dots) while the fourth circle displays the MQTLs density (red dots)
MQTL distribution on common bean genome
Distribution and genomic position of all MQTLs was determined on common bean genome based on the genomic location of flanking markers compared to gene density and variations existed in the genotypes. The gene density of all common bean chromosomes was retrieved from the EnsemblPlants database (Fig. 3). Gene density analyses showed that a majority of genes were located in sub-telomeric regions of the common bean chromosomes (Fig. 3). Similarly, most of the detected MQTLs were mainly located at the sub-telomeric regions where the variations were also higher for most MQTLs (Fig. 3). Comparison of resulted MQTLs with the significant loci associated with white mold and anthracnose in GWAS studies indicated that three MQTLs including MQTL2P.1, MQTL7P.2 and MQTL9P.2 respectively on chromosomes 2, 7 and 9 were in congruence with the GWAS results (Fig. 3). Additionally, three significant GWAS signals were detected near derived MQTLs (Fig. 3). This result shows the adaptability and coherence of gene density and MQTLs. Considering the synteny (physical co-localization of genetic loci on the same chromosome) among common bean, Arabidopsis and soybean genomes, the corresponding CGs of MQTLs identified in this study were investigated on Arabidopsis and soybean genomes. A schematic illustration of the CGs distribution in common bean, Arabidopsis and soybean genomes is shown in Fig. 4. This comparative genomics analysis provided a valuable approach for detecting the functional CGs and transferring information across species.
Distribution of MQTLs conveying resistance against bacterial and fungal diseases on common bean genome (95% confidence interval). Position of the MQTLs on genome is represented in Mb. In each pair of columns, gene density is shown in the inner of chromosome on the right side of the heatmap. The plot of variations is represented on the left part of the chromosome using a scatter plot in which the average of variations for each MB on each chromosome is indicated through the reference line. Additionally, the regions having variations more than average are presented with red dots. The position of MQTLs is highlighted with grid lines on each chromosome. The significant loci from genome-wide association studies (GWAS) with the genomic position in Mb are shown on the chromosomes for anthracnose and white mold
Circular map constructed using the Circos software showing the syntenic regions among common bean, soybean, and Arabidopsis genomes. A: common bean chromosomes, B: gene density on common bean chromosomes, C: position of MQTLs in common bean genome, D: gene density on soybean chromosomes, E: gene density on Arabidopsis chromosomes, F: common bean syntenic regions with soybean and Arabidopsis. The outermost circle represents the genomes in Mb
CGs for disease resistance
MQTL analysis reduced the CI value up to 2.64 folds with the average of 5.12 cM in MQTLs compared to the mean of projected QTLs. In three of the MQTLs i.e. MQTL7P.1, MQTL8P.1 and MQTL9P.1 located on chromosomes 7, 8 and 9, respectively, the CI was reduced to < 1 cM (Table 2). Hence, the probability of exploration of the functional CGs in the interval of MQTLs is higher than that in QTLs. The list of 1,891 genes underlying the CI of each MQTL region on common bean genome along with their annotations and orthologue genes in Arabidopsis and soybean is presented in Tables S2, S3 and S4, respectively. A number of functionally important genes were identified to be associated with MQTL in common bean according to their role in Arabidopsis genome. For instance, orthologue of the Arabidopsis genes RPS4 in common bean is PHAVU_002G092000g on MQTL2P.1 (chromosome 2), the orthologue of Arabidopsis gene ZAR1 in common bean is PHAVU_007G032100g on MQTL7P.1 (chromosome 7), and the orthologue of Arabidopsis gene VBF in common bean is PHAVU_007G198400g on MQTL7P.2 (chromosome 7). Further, the orthologue of Arabidopsis genes WRKY46 in common bean is PHAVU_010G111900g on MQTL10P.1 (chromosome 10). All the latter four genes are associated with defense mechanisms against plant diseases.
Subsequently, a biological pathway enrichment analysis was conducted on the CGs located at the CI of detected MQTLs. This would improve the understandings of the pathways controlling defense mechanisms of common bean against biotic stresses (Fig. 5a). One of the most noticeable mechanisms was endocytosis pathway (absorbing substances into the cell) which is involved significantly in plant defense mechanisms against different pathogens (Fig. 5b). Further details on the genes involved in the KEGG enrichment analysis are reported in Table S5.
KEGG enrichment analysis of genes underlying MQTLs associated with resistance to bacterial and fungal disease in common bean (A). The y-axis represents the enriched KEGG pathways. The color and the size of pathways descriptions represent the FDR and the count, respectively. Rich factor on the x-axis is the ratio of the gene number to the total gene number in that specific pathway. B Endocytosis pathway map which is involved significantly in plant defense mechanisms against different pathogens. Red boxes represent genes identified in our MQTLs interval. The genes in the green background represent genes that have been previously identified in Arabidopsis. For additional information on the genes for each KEGG description of MQTLs see Table S6. Permission is granted to BMC Genomics to publish under the CC BY 4.0 open access license [59]
MQTLs carrying R genes
Based on the BLAST search against PRGdb, five MQTL regions were detected carrying resistance gene homologues (Fig. 6a and Table S6). A majority of the detected genes contained kinase domain, although some genes i.e. PHAVU_005G036600g (on MQTL5P.1 located on chromosome 5); PHAVU_007G029900g, PHAVU_007G032100g, PHAVU_007g038700g, and PHAVU_007G040400g on MQTL7P.1 (chromosome 7), as well as PHAVU_009G042300g and PHAVU_009G043600g on MQTL9P.1 (chromosome 9) contained leucine-rich repeat (LRR) domain. Notably, one gene (PHAVU_008G014700g) found on MQTL8P.1 (chromosome 8) included nucleotide-binding site leucine-rich repeat (NB-LRR) domain implicated in plant defense interactions (Fig. 6a). Furthermore, the gene expression pattern in different common bean tissues revealed that PR-proteins are expressed most strongly in flower buds, young trifoliates, and leaves, respectively. The PHAVU_005G036600g gene, located on MQTL5P.1, had the greatest expression in various tissues among the 23 genes found in our analyses (Fig. 6B).
The predicted resistance genes (R gene) in MQTL regions associated with resistance to bacterial and fungal diseases in common bean. A The genomic position of predicted R genes within MQTL regions was represented on each chromosome. Different types of R genes domains were displayed by color codes in the bottom right-hand corner. B The expression of R genes identified within MQTL regions were displayed by a heat map across different tissues of the common bean
Common Candidate Genes (CGs) in MQTL regions and DEGs datasets
Resistance-responsive genes in common bean were collected from four independent studies. Based on DEGs data analysis, 1,805 DEGs were observed in response to different diseases including 293 DEGs for halo blight, 1,470 DEGs for anthracnose, and 42 DEGs for white mold. In total, 137 shared genes were detected between the DEGs studies and the genes located in MQTL regions using the Venn diagram (Figure S1, Table S7). The genes that are differentially expressed in response to diseases in common bean and located within MQTL regions can be considered as differentially expressed candidate genes (DECGs).
Discussion
Reliable management of biotic constraints in common bean production depends on proper combination of integrated pest management (IPM) strategies i.e. host resistance, cultural practices, biological control, as well as chemical applications [15, 94]. In this study, we employed a genome-wide meta-analysis approach to evaluate QTL repertories of common bean for resistance against bacterial and fungal diseases. Uneven distribution of QTLs across common bean genome was observed with the lowest number of QTLs on chromosome 11 (n = 6) and the highest number on chromosome 7 (n = 24). Chromosome 7 had the highest number of resistance QTLs against fungal disease, while the highest number of QTLs governing resistance against bacterial diseases was found on chromosome 1. Out of 152 QTLs from 44 QTL populations, 57 QTLs were successfully projected on two common bean reference maps. Although MQTL analyses on common bean has already been conducted on the amount of iron and zinc content [95], resistance to anthracnose [20], and white mold [13], to the best of our knowledge, this is the first comprehensive multiple disease resistance meta-QTLs (MDR-MQTLs) analysis to decipher disease resistance-associated repertories in the crop.
Three MQTLs were detected conferring resistance to the bacterial disease halo blight (caused by P. savastanoi pv. phaseolicola), while six MQTLs were associated with resistance against each of the fungal diseases white mold and anthracnose caused by Sclerotinia sclerotiorum and Colletotrichum lindemuthianum, respectively. We detected a low correlation between QTLs and MQTLs across the chromosomes. MQTL analysis can detect stable QTLs regardless of their genetic background, chronology and location in the corresponding original investigation [16, 26, 30, 96]. In comparison to the MQTLs reported in the previous study on white mold [13] and anthracnose [20], we identified one further overlapping MQTL named MQTL7P.1 located on chromosome 7 conveying resistance against white mold disease. Four MQTLs (MQTL1P.1, MQTL5P.1, MQTL7P.1, MQTL8P.1) in our study overlapped with five MQTLs of anthracnose disease [20], three of which located at the closest region to previous study. Furthermore, four MQTLs including MQTL2P.1, MQTL5P.1, MQTL8P.1, MQTL9P.1 located at the same regions with the hotspot of QTLs in previous study on anthracnose [20]. Five novel MQTLs were also identified in this study conveying resistance against bacterial and fungal diseases. Investigation on the distribution of MQTLs is a progressive approach in crop breeding while our study is the first one in this regard on common bean genome. Our analyses indicated that sub-telomeric regions contain most of the detected MQTLs, gene density, and variations (Fig. 3). Interestingly, previous studies in rice, maize and barley showed similar pattern where higher densities of MQTLs and genes observed at sub-telomeric regions [26, 27, 28, 96]. One of the advantages of MQTL analysis is that it significantly narrows down the CI improving the precision of finding CGs in MQTL regions [26, 27, 28, 95, 97, 98, 99, 100]. In this study, MQTL analysis reduced the average of CI up to 2.64 folds with an average of 5.12 cM in MQTLs compared to the mean of projected QTLs where 1,891 genes were detected on interval regions of studied MQTLs.
Two shared MQTLs i.e. MQTL5P.1 and MQTL8P.1 located on chromosomes 5 and 8, respectively, were detected conveying resistance against halo blight and anthracnose while the two shared MQTLs MQTL2P.1 and MQTL9P.2 on chromosomes 2 and 9, respectively, were associated with resistance against anthracnose and white mold. The corresponding genes of these MQTLs were detected on Arabidopsis genome where they are involved in a number of functionally important pathways. Hence, they could be considered in plant breeding efforts to develop new lines with resistant to fungal and bacterial diseases. Orthologue of PBL gene family was detected on MQTL2P.1. Genes of the latter family in Arabidopsis are involved in resistance against bacterial black rot of crucifers caused by Xanthomonas campestris pv. campestris in the resistant ecotype Col-0. PBL2 a serine/threonine-protein kinase involved in pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) signaling and defense responses downstream of flagellin sensing2 (FLS2) for plants. Additionally, PBL2 is the strongest interactor of XopAC which is a major avirulence gene of X. campestris pv. campestris [101, 102]. Moreover, two orthologues of WRKY3 and WRKY4 are located on MQTL2P.1. The WRKY transcription factors play a vital role in pathogen-triggered signal transduction cascades in rice during interactions with Xanthomonas oryzae [103]. Expression of WRKY3 and WRKY4 was induced by stressful conditions generated by liquid infiltration [104]. RPS4B gene on MQTL2P.1 (chromosome 5) encodes disease resistance (R) protein that specifically recognizes the type III effector AvrRps4 of P. syringae, forming a functional complex and mediating the hypersensitive response [105]. The UDP-glycosyltransferase has been shown to have a role against Fusarium head blight in wheat [106]. A cluster of these genes was found on MQTL2P.1 (Table S3).
Two MQTLs were identified governing resistance against anthracnose and halo blight diseases. We detected MQTL5P.1 on chromosome 5 of common bean containing the genes PHAVU_005G035300g and PHAVU_005G035500g, which are orthologues of the Arabidopsis gene BGLU42. BGLU42 is a novel component of the induced systemic resistance (ISR) signaling pathway that displays significantly enhanced resistance against gray mold disease caused by Botrytis cinerea. Overexpression of BGLU42 also conferred resistance against downy mildew and bacterial speck diseases caused by Hyaloperonospora arabidopsidis and P. syringae pv. tomato, respectively through ISR [107]. MQTL5P.1 hosts the orthologue of Arabidopsis ACD6 gene, which has an Ankyrin domain playing a major role in defense response against virulent bacteria through signaling of salicylic acid-dependent cell death [108, 109, 110,111]. Based on the PRGDB database, some other CGs at this MQTL were detected (Fig. 6a). Further investigations are required to validate the function of some important CGs i.e. PHAVU_008G014700g on MQTL8P.1 (chromosome 8) which contains nucleotide-binding site leucine-rich repeat (NB-LRR) domain implicated in plant defense interactions. As for the specific MQTLs, the MQTL10P.1 located on chromosome 10 was involved in resistance against halo blight disease. Three MQTLs i.e. MQTL1P.1, MQTL7P.1 and MQTL9P.1 located on chromosomes 1, 7 and 9 respectively, associated with withe mold resistance, while MQTL7P.2 located on chromosome 7 is associated with resistance to anthracnose. Hence, these MQTLs could be considered for breeding common bean towards halo blight, white mold and anthracnose resistance.
Halo blight can result in severe damages to crops when exposed to high rainfall, wind and mild temperatures [110]. In this study, MQTL10P.1 was found on chromosome 10, which contains key defensive orthologue genes like DLO family, LOX3, NUP107 and WRKY46, which could be used to breed common bean lines to be resistant against halo blight disease. The MQTL10P.1 located on chromosome 10 contained orthologue of DLO gene family, which belongs to a group of defensive negative regulators. DMR6 and DLO1 mutants showed enhanced resistant to downy mildew caused by an oomycete pathogen due to their increased level of salicylic acid [112]. Lipoxygenases (LOX) family encodes lipoxygenase, which is well known for its important role in pathogen-induced defense mechanisms in different crops including potato, tomato, and common bean [113, 114]. Common bean uses the LOX to synthesize oxylipins in response to different stresses. LOX3 is a gene on MQTL10P.1 (chromosome 10) implicated in jasmonic acid biosynthesis, which is important in inducing PR proteins that have a role in defense against plant pathogens [115]. On MQTL10P.1, orthologue of NUP107 gene was found which is required for the plant’s response to infection by virulent pseudomonads. Further, Nup107-160 members have been predicted in the Arabidopsis genome to play a role in disease resistance [116]. Another key orthologue gene on MQTL10P.1 is WRKY46, which is associated with increased basal plant defense in Arabidopsis [117].
Three MQTLs i.e. MQTL1P.1, MQTL7P.1 and MQTL9P.1 located on chromosomes 1, 7, and 9, respectively, were specifically associated with white mold resistance. The function of orthologue of Arabidopsis ZAR1 gene in common bean (PHAVU_007G032100g) on MQTL7P.1 is to mediate recognition of AvrAC, which induces effector-triggered immunity (ETI) in plant against X. campestris [118]. Furthermore, the gene ZAR1 is required for recognition of HopZ1a, a P. syringae type III secreted effector in Arabidopsis [119]. The orthologues of WRKY54, WRKY70 and WRKY55 genes located on MQTL9P.1 (chromosome 9) act as positive regulators of SA-mediated defensive signaling in Arabidopsis [120]. The MQTL7P.2 located on chromosome 7 was predicted to be specifically involved in resistance to anthracnose. Anthracnose is a major disease of common bean causing significant economic loses to the crop in tropical regions, especially when the fruiting stage is attacked [121]. The RIN13 gene on MQTL7P.2 is a plant defense gene that encodes RPM1-interacting protein 13, which is a nuclear-localized protein. Overexpression of RIN13 leads to autoimmunity with high accumulation of salicylic acid, fundamental expression of pathogenesis-related genes, and enhanced resistance to plant diseases [122]. RboHB is also related to plant’s defense against pathogens; which is encoded by At1g09090 and is involved in resistance against nematode. RBOHB gene encodes respiratory burst oxidase homolog protein B and calcium-dependent NADPH oxidase that generates superoxide [123]. RBOHB gene is on MQTL7P.2 (chromosome 7) and the role of this gene in defense against plant pathogens in common bean has been proven in the previous study [123]. RBOHB is a multigene family containing nine members (RBOHB A–I) in common bean. Several recent studies reported that RBOHs participate in signaling pathways of plant-pathogen interactions as well as plant-symbiont interactions [124, 125].
In conclusion, the data provided in this study revealed a number of genomic regions associated with resistance against bacterial and fungal diseases in common bean. The genomic positions of MQTLs are mainly located at sub-telomeric regions where higher gene density was also reported in common bean genome. Uneven distribution of QTLs across common bean genome was observed where the lowest number of QTLs was on chromosome 11 (n = 6) and the highest number of QTLs was observed on chromosome 7 (n = 24). Chromosome 7 had the highest number of resistance QTLs against fungal disease, while the highest number of QTLs governing resistance against bacterial agents was found on chromosome 1. Our results revealed that MQTL2P.1, MQTL7P.1, and MQTL9P.2 had the highest number of QTLs; thus considered the promising MQTLs for further investigations and breeding programs. These results on one hand provide a comprehensive insight into the genomic repertories of common bean to combat the risk of bacterial and fungal agents. On the other hand, pave the way of further researches in developing new common bean lines against a wider range of biotic constraints under the field conditions.
Availability of data and materials
Not applicable. There is no data in the manuscript that need to be deposited in the public databases. There is no relevant accession number.
References
Singh SP, Editor. Common bean improvement in the twenty-first century (Vol. 7). Berlin, Germany: Springer Science & Business Media. 2013. ISBN-13: 978–0792358879.
De Ron, A. et al. Common Bean. In: De Ron, A. (eds) Grain Legumes. Handbook of Plant Breeding, vol 10. Springer, New York, NY. 2015. https://doi.org/10.1007/978-1-4939-2797-5_1.
FAOSTAT. Food and Agriculture Organization of the United Nations, FAOSTAT database, available at http://faostat.fao.org/. 2022.
Osdaghi E, Young AJ, Harveson RM. Bacterial wilt of dry beans caused by Curtobacterium flaccumfaciens pv. flaccumfaciens: a new threat from an old enemy. Mol Plant Pathol. 2020;21(5):605–21.
Schwartz HF, Steadman JR, Hall R, Forster RL. Compendium of bean diseases (No. Ed. 2). Saint Paul, Minnesota, United States: American Phytopathological Society (APS Press); 2005.
Osdaghi E, Taghavi SM, Hamzehzarghani H, Fazliarab A, Harveson RM, Lamichhane JR. Occurrence and characterization of a new red-pigmented variant of Curtobacterium flaccumfaciens, the causal agent of bacterial wilt of edible dry beans in Iran. Eur J Plant Pathol. 2016;146(1):129–45.
Osdaghi E, Taghavi SM, Calamai S, Biancalani C, Cerboneschi M, Tegli S, Harveson RM. Phenotypic and Molecular-Phylogenetic Analysis Provide Novel Insights into the Diversity of Curtobacterium flaccumfaciens. Phytopathology. 2018;108:1154–64.
Chen G, Khojasteh M, Taheri-Dehkordi A, Taghavi SM, Rahimi T, Osdaghi E. Complete genome sequencing provides novel insight into the virulence repertories and phylogenetic position of dry beans pathogen Curtobacterium flaccumfaciens pv. flaccumfaciens. Phytopathology. 2021;111(2):268–80.
Lamichhane JR, Osdaghi E, Behlau F, Köhl J, Jones JB, Aubertot JN. Thirteen decades of anti-microbial copper compounds applied in agriculture. A review. Agron Sustain Dev. 2018;38:28.
Osdaghi E, Shams-Bakhsh M, Alizadeh A, Lak MR. Study on common bean seed lots for contamination with Xanthomonas axonopodis pv. phaseoli by BIO-PCR technique. J Agric Technol. 2010;6(3):503–13.
Osdaghi E, Lak MR. A source of resistance to bacterial wilt in the common bean (Phaseolus vulgaris) in Iran. Crop Prot. 2015;74:37–41.
Pilet-Nayel ML, Moury B, Caffier V, Montarry J, Kerlan MC, Fournet S, Durel CE, Delourme R. Quantitative resistance to plant pathogens in pyramiding strategies for durable crop protection. Front Plant Sci. 2017;8:1838.
Vasconcellos RC, Oraguzie OB, Soler A, Arkwazee H, Myers JR, Ferreira JJ, Song Q, McClean P, Miklas PN. Meta-QTL for resistance to white mold in common bean. PLoS One. 2017;12(2):e0171685.
Trabanco N, Asensio-Manzanera MC, Pérez-Vega E, Ibeas A, Campa A, Ferreira JJ. Identification of quantitative trait loci involved in the response of common bean to Pseudomonas syringae pv. phaseolicola. Mol Breed. 2014;33(3):577–88.
Osdaghi E, Alizadeh A, Shams-Bakhsh M, Lak MR. Evaluation of common bean lines for their reaction to the common bacterial blight pathogen. Phytopathol Mediterr. 2009;48:461–8.
Said JI, Lin Z, Zhang X, Song M, Zhang J. A comprehensive meta QTL analysis for fiber quality, yield, yield related and morphological traits, drought tolerance, and disease resistance in tetraploid cotton. BMC Genomics. 2013;14(1):1–22.
Zhang X, Shabala S, Koutoulis A, Shabala L, Zhou M. Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta. 2017;245(2):283–95.
Zhang LY, Liu DC, Guo XL, Yang WL, Sun JZ, Wang DW, Zhang A. Genomic distribution of quantitative trait loci for yield and yield-related traits in common wheat. J Integr Plant Biol. 2010;52(11):996–100.
Tyagi S, Mir RR, Balyan HS, Gupta PK. Interval mapping and meta-QTL analysis of grain traits in common wheat (Triticum aestivum L.). Euphytica. 2015;201(3):367–80.
Shafi S, Saini DK, Khan MA, Bawa V, Choudhary N, Dar WA, Mir RR. Delineating meta-quantitative trait loci for anthracnose resistance in common bean (Phaseolus vulgaris L.). Front Plant Sci. 2022;13:966339.
Hanocq E, Laperche A, Jaminon O, Lainé AL, Le Gouis J. Most significant genome regions involved in the control of earliness traits in bread wheat, as revealed by QTL meta-analysis. Theor Appl Genet. 2007;114(3):569–84.
Laperche A, Brancourt-Hulmel M, Heumez E, Gardet O, Hanocq E, Devienne-Barret F, Le Gouis J. Using genotype× nitrogen interaction variables to evaluate the QTL involved in wheat tolerance to nitrogen constraints. Theor Appl Genet. 2007;115(3):399–415.
Goffinet B, Gerber S. Quantitative trait loci: a meta-analysis. Genetics. 2000;155(1):463–73.
Veyrieras JB, Goffinet B, Charcosset A. MetaQTL: a package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinformatics. 2007;8(1):1–16.
Courtois B, Ahmadi N, Khowaja F, Price AH, Rami JF, Frouin J, Hamelin C, Ruiz M. Rice root genetic architecture: meta-analysis from a drought QTL database. Rice. 2009;2(2):115–28.
Khahani B, Tavakol E, Shariati V. Genome-wide meta-analysis on yield and yield-related QTLs in barley (Hordeum vulgare L.). Mol Breed. 2019;39(4):1–16.
Khahani B, Tavakol E, Shariati V, Fornara F. Genome wide screening and comparative genome analysis for Meta-QTLs, ortho-MQTLs and candidate genes controlling yield and yield-related traits in rice. BMC Genomics. 2020;21(1):1–24.
Khahani B, Tavakol E, Shariati V, Rossini L. Meta-QTL and ortho-MQTL analyses identified genomic regions controlling rice yield, yield-related traits and root architecture under water deficit conditions. Sci Rep. 2021;11(1):1–18.
Liu S, Hall MD, Griffey CA, McKendry AL. Meta-analysis of QTL associated with Fusarium head blight resistance in wheat. Crop Sci. 2009;49(6):1955–68.
Lanaud C, Fouet O, Clément D, Boccara M, Risterucci AM, Surujdeo-Maharaj S, Legavre T, Argout X. A meta–QTL analysis of disease resistance traits of Theobroma cacao L. Mol Breeding. 2009;24(4):361–74.
Guo B, Sleper DA, Lu P, Shannon JG, Nguyen HT, Arelli PR. QTLs associated with resistance to soybean cyst nematode in soybean: meta-analysis of QTL locations. Crop Sci. 2006;46(2):595–602.
Pal N, Jan I, Saini DK, Kumar K, Kumar A, Sharma PK, Gupta PK. Meta-QTLs for multiple disease resistance involving three rusts in common wheat (Triticum aestivum L.). Theor Appl Genet. 2022. (https://link.springer.com/article/10.1007/s00122-022-04119-7).
Saini DK, Chahal A, Pal N, Srivastava P, Gupta PK. Meta-analysis reveals consensus genomic regions associated with multiple disease resistance in wheat (Triticum aestivum L.). Mol Breed. 2022;42(3):1–23.
Ali F, Pan Q, Chen G, Zahid KR, Yan J. Evidence of multiple disease resistance (MDR) and implication of meta-analysis in marker assisted selection. PLoS One. 2013;8(7):e68150.
Schweizer P, Stein N. Large-scale data integration reveals colocalization of gene functional groups with meta-QTL for multiple disease resistance in barley. Mol Plant Microbe Interact. 2011;24(12):1492–501.
Kumar IS, Nadarajah K. A meta-analysis of quantitative trait loci associated with multiple disease resistance in rice (Oryza sativa L.). Plants. 2020;9(11):1491.
Miklas PN, Delorme R, Stone V, Daly MJ, Stavely JR, Steadman JR, Bassett MJ, Beaver JS. Bacterial, fungal, and viral disease resistance loci mapped in a recombinant inbred common bean population (Dorado’/XAN 176). J Am Soc Hortic Sci. 2000;125(4):476–81.
Tar’An B, Michaels TE, Pauls KP. Mapping genetic factors affecting the reaction to Xanthomonas axonopodis pv. phaseoli in Phaseolus vulgaris L. under field conditions. Genome. 2001;44(6):1046–56.
Yu K, Park SJ, Zhang B, Haffner M, Poysa V. An SSR marker in the nitrate reductase gene of common bean is tightly linked to a major gene conferring resistance to common bacterial blight. Euphytica. 2004;138(1):89–95.
Papa R, Acosta J, Delgado-Salinas A, Gepts P. A genome-wide analysis of differentiation between wild and domesticated Phaseolus vulgaris from Mesoamerica. Theor Appl Genet. 2005;111(6):1147–58.
Liu S, Yu K, Park SJ. Development of STS markers and QTL validation for common bacterial blight resistance in common bean. Plant Breeding. 2008;127(1):62–8.
Liu SY, Yu K, Huffner M, Park SJ, Banik M, Pauls KP, Crosby W. Construction of a BAC library and a physical map of a major QTL for CBB resistance of common bean (Phaseolus vulgaris L.). Genetica. 2010;138(7):709–16.
Durham KM. Evaluation of Common Bacterial Blight Resistance in a Resistant Inter-Cross Population of Common Bean. Guelph, Canada: M.Sc. thesis; University of Guelph. 2011.
Zhu J, Wu J, Wang L, Blair MW, Zhu Z, Wang S. QTL and candidate genes associated with common bacterial blight resistance in the common bean cultivar Longyundou 5 from China. Crop J. 2016;4(5):344–52.
Xie W, Khanal R, McClymont S, Stonehouse R, Bett K, Yu K, Pauls KP, Navabi A. Interaction of quantitative trait loci for resistance to common bacterial blight and pathogen isolates in Phaseolus vulgaris L. Mol Breeding. 2017;37(4):55.
Yaish MW, Sosa D, Vences FJ, Vaquero F. Genetic mapping of quantitative resistance to race 5 of Pseudomonas syringae pv. phaseolicola in common bean. Euphytica. 2006;152(3):397–404.
Miklas PN, Fourie D, Wagner J, Larsen RC, Mienie CM. Tagging and Mapping Pse-1 Gene for Resistance to Halo Blight in Common Bean Differential Cultivar UI-3. Crop Sci. 2009;49(1):41–8.
Miklas PN, Fourie D, Trapp J, Larsen RC, Chavarro C, Blair MW, Gepts P. Genetic characterization and molecular mapping Pse-2 gene for resistance to halo blight in common bean. Crop Sci. 2011;51(6):2439–48.
Miklas PN, Fourie D, Trapp J, Davis J, Myers JR. New loci including Pse-6 conferring resistance to halo bacterial blight on chromosome Pv04 in common bean. Crop Sci. 2014;54(5):2099–2108.
González AM, Yuste-Lisbona FJ, Godoy L, Fernández-Lozano A, Rodiño AP, De Ron AM, Lozano R, Santalla M. Exploring the quantitative resistance to Pseudomonas syringae pv. phaseolicola in common bean (Phaseolus vulgaris L.). Mol Breed. 2016;36(12):1–20.
González AM, Godoy L, Santalla M. Dissection of resistance genes to Pseudomonas syringae pv. phaseolicola in UI3 common bean cultivar. Int J Mol Sci. 2017;18(12):2503.
Kolkman JM, Kelly JD. QTL conferring resistance and avoidance to white mold in common bean. Crop Sci. 2003;43(2):539–48.
Ender M, Kelly JD. Identification of QTL associated with white mold resistance in common bean. Crop Sci. 2005;45(6):2482–90.
Miklas PN, Larsen KM, Terpstra K, Hauf DC, Grafton KF, Kelly JD. QTL analysis of ICA Bunsi-derived resistance to white mold in a pinto × navy bean cross. Crop Sci. 2007;47:174–9.
Maxwell JJ, Brick MA, Byrne PF, Schwartz HF, Shan X, Ogg JB, Hensen RA. Quantitative trait loci linked to white mold resistance in common bean. Crop Sci. 2007;47(6):2285–94.
Mkwaila WE. Quantitative Trait Loci Analysis of Resistance to White Mold (Sclerotinia sclerotiorum) in Common Bean (Phaseolus vulgaris). East Lansing, Michigan, USA: Doctoral dissertation. Michigan State University. 2013.
Pérez-Vega E, Pascual A, Campa A, Giraldez R, Miklas PN, Ferreira JJ. Mapping quantitative trait loci conferring partial physiological resistance to white mold in the common bean RIL population Xana× Cornell 49242. Mol Breeding. 2012;29(1):31–41.
Hoyos-Villegas V, Mkwaila W, Cregan PB, Kelly JD. Quantitative trait loci analysis of white mold avoidance in pinto bean. Crop Sci. 2015;55(5):2116–29.
Hagerty CH. Mapping QTL for root rot resistance, root traits, and morphological trait in a common bean recombinant inbred population. Corvallis, Oregon USA: Master of Science thesis; Oregon State University. 2013.
Nakedde T, Ibarra-Perez FJ, Mukankusi C, Waines JG, Kelly JD. Mapping of QTL associated with Fusarium root rot resistance and root architecture traits in black beans. Euphytica. 2016;212(1):51–63.
Wang W. QTL analysis and candidate genes identification associated with fusarium root rot resistance in common beans (Phaseolus vulgaris). Michigan State University. East Lansing, Michigan, USA 2016.
Geffroy V, Sévignac M, De Oliveira JC, Fouilloux G, Skroch P, Thoquet P, Gepts P, Langin T, Dron M. Inheritance of partial resistance against Colletotrichum lindemuthianum in Phaseolus vulgaris and co-localization of quantitative trait loci with genes involved in specific resistance. Mol Plant Microbe Interact. 2000;13(3):287–96.
López CE, Acosta IF, Jara C, Pedraza F, Gaitán-Solís E, Gallego G, Beebe S, Tohme J. Identifying resistance gene analogs associated with resistances to different pathogens in common bean. Phytopathology. 2003;93(1):88–95.
Oblessuc PR, Baroni RM, da Silva Pereira G, Chiorato AF, Carbonell SAM, Brinez B, Luciano Da Costa ES, Garcia AAF, Camargo LEA, Kelly JD, Benchimol-Reis LL. Quantitative analysis of race-specific resistance to Colletotrichum lindemuthianum in common bean. Mol Breed. 2014;34(3):1313–29.
Garzon LN, Blair MW. Development and mapping of SSR markers linked to resistance-gene homologue clusters in common bean. Crop J. 2014;2(4):183–94.
González AM, Yuste-Lisbona FJ, Rodiño AP, De Ron AM, Capel C, García-Alcázar M, Lozano R, Santalla M. Uncovering the genetic architecture of Colletotrichum lindemuthianum resistance through QTL mapping and epistatic interaction analysis in common bean. Front Plant Sci. 2015;6:141.
Oblessuc PR, Perseguini JMKC, Baroni RM, Chiorato AF, Carbonell SAM, Mondego JMC, Vidal RO, Camargo LEA, Benchimol-Reis LL. Increasing the density of markers around a major QTL controlling resistance to angular leaf spot in common bean. Theor Appl Genet. 2013;126(10):2451–65.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–51.
Galeano CH, Fernandez AC, Franco-Herrera N, Cichy KA, McClean PE, Vanderleyden J, Blair MW. Saturation of an intra-gene pool linkage map: towards a unified consensus linkage map for fine mapping and synteny analysis in common bean. PLoS One. 2011;6(12):e28135.
Song Q, Jia G, Hyten DL, Jenkins J, Hwang EY, Schroeder SG, Osorno JM, Schmutz J, Jackson SA, McClean PE, Cregan PB. SNP assay development for linkage map construction, anchoring whole-genome sequence, and other genetic and genomic applications in common bean. G3. 2015;5(11):2285–90.
Darvasi A, Soller M. A simple method to calculate resolving power and confidence interval of QTL map location. Behav Genet. 1997;27(2):125–32.
Arcade A, Labourdette A, Falque M, Mangin B, Chardon F, Charcosset A, Joets J. BioMercator: integrating genetic maps and QTL towards discovery of candidate genes. Bioinformatics. 2004;20(14):2324–6.
Sosnowski O, Charcosset A, Joets J. BioMercator V3: an upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics. 2012;28(15):2082–3.
Diaz-Garcia L, Covarrubias-Pazaran G, Schlautman B, Zalapa J. SOFIA: an R package for enhancing genetic visualization with Circos. J Hered. 2017;108(4):443–8.
Hao Z, Lv D, Ge Y, Shi J, Weijers D, Yu G, Chen J. RIdeogram: drawing SVG graphics to visualize and map genome-wide data on the idiograms. PeerJ Comput Sci. 2020;6:e251.
Kolde R. pheatmap: Pretty Heatmaps. R package version 0.7. 7. 2013.
Anand L, Rodriguez Lopez CM. ChromoMap: an R package for interactive visualization of multi-omics data and annotation of chromosomes. BMC Bioinformatics. 2022;23(1):1–9.
Perseguini JMKC, Oblessuc PR, Rosa JRBF, Gomes KA, Chiorato AF, Carbonell SAM, Benchimol-Reis LL. Genome-wide association studies of anthracnose and angular leaf spot resistance in common bean (Phaseolus vulgaris L.). PLoS One. 2016;11(3):e0150506.
Wu J, Zhu J, Wang L, Wang S. Genome-wide association study identifies NBS-LRR-encoding genes related with anthracnose and common bacterial blight in the common bean. Front Plant Sci. 2017;8:1398.
Fritsche-Neto R, Souza TLPOD, Pereira HS, Faria LCD, Melo LC, Novaes E, Jannink JL. Association mapping in common bean revealed regions associated with anthracnose and angular leaf spot resistance. Scientia Agricola. 2019;76:321–7.
Campa A, García-Fernández C, Ferreira JJ. Genome-wide association study (GWAS) for resistance to Sclerotinia sclerotiorum in common bean. Genes. 2020;11(12):1496.
Vaz Bisneta M, Gonçalves-Vidigal MC. Integration of anthracnose resistance loci and RLK and NBS-LRR-encoding genes in the Phaseolus vulgaris L. genome. Crop Sci. 2020;60(6):2901–18.
Vidigal Filho PS, Gonçalves-Vidigal MC, Vaz Bisneta M, Souza VB, Gilio TA, Calvi AA, Melotto M. Genome-wide association study of resistance to anthracnose and angular leaf spot in Brazilian Mesoamerican and Andean common bean cultivars. Crop Sci. 2020;60(6):2931–50.
Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–45.
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, Kong L, Gao G, Li CY, Wei L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39(suppl_2):W316–22.
Wickham H, Chang W, Wickham MH. Package ‘ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. Version. 2016;2(1):1–189.
Meziadi C, Richard MM, Derquennes A, Thareau V, Blanchet S, Gratias A, Geffroy V. Development of molecular markers linked to disease resistance genes in common bean based on whole genome sequence. Plant Sci. 2016;242:351–7.
Voorrips R. MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered. 2002;93(1):77–8.
Cooper B, Campbell KB, Beard HS, Garrett WM, Ferreira ME. The proteomics of resistance to halo blight in common bean. Mol Plant Microbe Interact. 2020;33(9):1161–75.
Padder BA, Kamfwa K, Awale HE, Kelly JD. Transcriptome profiling of the Phaseolus vulgaris-Colletotrichum lindemuthianum pathosystem. PLoS One. 2016;11(11):e0165823.
Oliveira MB, de Andrade RV, Grossi-de-Sá MF, Petrofeza S. Analysis of genes that are differentially expressed during the Sclerotinia sclerotiorum–Phaseolus vulgaris interaction. Front Microbiol. 2015;6:1162.
Wang H, Jia J, Cai Z, Duan M, Jiang Z, Xia Q, Nian H. Identification of quantitative trait loci (QTLs) and candidate genes of seed Iron and zinc content in soybean [Glycine max (L.) Merr.]. BMC Genomics. 2022;23(1):1–14.
Jin J, Liu D, Qi Y, Ma J, Zhen W. Major QTL for seven yield-related traits in common wheat (Triticum aestivum L.). Front Genet. 2020;11:1012.
Osdaghi E, Shams-Bakhsh M, Alizadeh A, Lak MR, Maleki HH. Induction of resistance in common bean by Rhizobium leguminosarum bv. phaseoli and decrease of common bacterial blight. Phytopathologia Mediterranea. 2011;50(1):45–54.
Izquierdo P, Astudillo C, Blair MW, Iqbal AM, Raatz B, Cichy KA. Meta-QTL analysis of seed iron and zinc concentration and content in common bean (Phaseolus vulgaris L.). Theor Appl Genet. 2018;131(8):1645–58.
Martinez AK, Soriano JM, Tuberosa R, Koumproglou R, Jahrmann T, Salvi S. Yield QTLome distribution correlates with gene density in maize. Plant Sci. 2016;242:300–9.
Venske E, Dos Santos RS, Farias DDR, Rother V, da Maia LC, Pegoraro C, Costa de Oliveira A. Meta-analysis of the QTLome of Fusarium head blight resistance in bread wheat: refining the current puzzle. Front Plant Sci. 2019;10:727.
Liu Y, Salsman E, Wang R, Galagedara N, Zhang Q, Fiedler JD, Liu Z, Xu S, Faris JD, Li X. Meta-QTL analysis of tan spot resistance in wheat. Theor Appl Genet. 2020;133:2363–75.
Zheng T, Hua C, Li L, Sun Z, Yuan M, Bai G, Humphreys G, Li T. Integration of meta-QTL discovery with omics: Towards a molecular breeding platform for improving wheat resistance to Fusarium head blight. Crop J. 2021;9(4):739–49.
Yang Y, Amo A, Wei D, Chai Y, Zheng J, Qiao P, et al. Large-scale integration of meta-QTL and genome-wide association study discovers the genomic regions and candidate genes for yield and yieldrelated traits in bread wheat. Theor Appl Genet. 2021;134(9):3083–3109.
Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, Mengiste T. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7(4):290–301.
Guy E, Lautier M, Chabannes M, Roux B, Lauber E, Arlat M, Noël LD. XopAC-triggered immunity against Xanthomonas depends on Arabidopsis receptor-like cytoplasmic kinase genes PBL2 and RIPK. PLoS One. 2013;8(8):e73469.
Khojasteh M, Khahani B, Taghavi M, Tavakol E. Identification and characterization of responsive genes in rice during compatible interactions with pathogenic pathovars of Xanthomonas oryzae. Eur J Plant Pathol. 2018;151(1):141–53.
Lai Z, Vinod KM, Zheng Z, Fan B, Chen Z. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens. BMC Plant Biol. 2008;8(1):1–13.
Saucet SB, Ma Y, Sarris PF, Furzer OJ, Sohn KH, Jones JD. Two linked pairs of Arabidopsis TNL resistance genes independently confer recognition of bacterial effector AvrRps4. Nat Commun. 2015;6(1):1–12.
Sharma P, Gangola MP, Huang C, Kutcher HR, Ganeshan S, Chibbar RN. Single nucleotide polymorphisms in B-genome specific UDP-glucosyl transferases associated with Fusarium Head Blight resistance and reduced deoxynivalenol accumulation in wheat grain. Phytopathology. 2018;108(1):124–32.
Zamioudis C, Hanson J, Pieterse CM. β-Glucosidase BGLU 42 is a MYB 72-dependent key regulator of rhizobacteria-induced systemic resistance and modulates iron deficiency responses in Arabidopsis roots. New Phytol. 2014;204(2):368–79.
Lu H, Rate DN, Song JT, Greenberg JT. ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell. 2003;15(10):2408–20.
Yang LP, Fang YY, An CP, Dong L, Zhang ZH, Chen H, Xie Q, Guo HS. C 2-mediated decrease in DNA methylation, accumulation of si RNAs, and increase in expression for genes involved in defense pathways in plants infected with beet severe curly top virus. Plant J. 2013;73(6):910–7.
Noble TJ, Young AJ, Douglas CA, Williams B, Mundree S. Diagnosis and management of halo blight in Australian mungbeans: a review. Crop Pasture Sci. 2019;70(3):195–203.
Zhang Z, Shrestha J, Tateda C, Greenberg JT. Salicylic acid signaling controls the maturation and localization of the Arabidopsis defense protein Accelerated Cell Death 6. Mol Plant. 2014;7(8):1365–83.
Zeilmaker T, Ludwig NR, Elberse J, Seidl MF, Berke L, Van Doorn A, Schuurink RC, Snel B, Van den Ackerveken G. Downy mildew resistant 6 and DMR 6-like oxygenase 1 are partially redundant but distinct suppressors of immunity in Arabidopsis. Plant J. 2015;81(2):210–22.
Kolomiets MV, Chen H, Gladon RJ, Braun EJ, Hannapel DJ. A leaf lipoxygenase of potato induced specifically by pathogen infection. Plant Physiol. 2000;124(3):1121–30.
Akram A, Ongena M, Duby F, Dommes J, Thonart P. Systemic resistance and lipoxygenase-related defense response induced in tomato by Pseudomonas putidastrain BTP1. BMC Plant Biol. 2008;8(1):113–24.
Porta H, Figueroa-Balderas RE, Rocha-Sosa M. Wounding and pathogen infection induce a chloroplast-targeted lipoxygenase in the common bean (Phaseolus vulgaris L.). Planta. 2008;227(2):363–73.
Wiermer M, Cheng YT, Imkampe J, Li M, Wang D, Lipka V, Li X. Putative members of the Arabidopsis Nup107-160 nuclear pore sub-complex contribute to pathogen defense. Plant J. 2012;70(5):796–808.
Sheikh AH, Eschen-Lippold L, Pecher P, Hoehenwarter W, Sinha AK, Scheel D, Lee J. Regulation of WRKY46 transcription factor function by mitogen-activated protein kinases in Arabidopsis thaliana. Front Plant Sci. 2016;7:61.
Hu M, Qi J, Bi G, Zhou JM. Bacterial effectors induce oligomerization of immune receptor ZAR1 in vivo. Mol Plant. 2020;13(5):793–801.
Lewis JD, Lee AHY, Hassan JA, Wan J, Hurley B, Jhingree JR, Wang PW, Lo T, Youn JY, Guttman DS, Desveaux D. The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the Pseudomonas syringae type III effector HopZ1a. Proc Natl Acad Sci. 2013;110(46):18722–7.
Li J, Zhong R, Palva ET. WRKY70 and its homolog WRKY54 negatively modulate the cell wall-associated defenses to necrotrophic pathogens in Arabidopsis. PLoS One. 2017;12(8):e0183731.
Melotto M, Balardin RS, Kelly JD. Host-pathogen interaction and variability of Colletotrichum lindemuthianum. Colletotrichum host specificity, pathology, host–pathogen interaction. St. Paul, MN: APS press; 2000. p. 346–61.
Liu X, Liu H, He J, Zhang S, Han H, Wang Z, Liu WC, Liang YK, Gao Z. RIN13-mediated disease resistance depends on the SNC1–EDS1/PAD4 signaling pathway in Arabidopsis. J Exp Bot. 2020;71(22):7393–404.
Hawamda AI, Zahoor A, Abbas A, Ali MA, Bohlmann H. The Arabidopsis RboHB encoded by At1g09090 is important for resistance against nematodes. Int J Mol Sci. 2020;21(15):5556.
Singh JS, Koushal S, Kumar A, Vimal SR, Gupta VK. Book review: microbial inoculants in sustainable agricultural productivity-Vol. II: functional application. Front Microbiol. 2016;7:2105.
Arthikala MK, Montiel J, Sánchez-López R, Nava N, Cárdenas L, Quinto C. Respiratory burst oxidase homolog gene is crucial for rhizobium infection and nodule maturation and function in common bean. Front Plant Sci. 2017;8:2003.
Acknowledgements
We thank the three anonymous reviewers for providing valuable comments that markedly helped to increase the quality of this manuscript.
Funding
The works of MK and EO were funded by Shiraz University and University of Tehran, respectively.
Author information
Authors and Affiliations
Contributions
MK and EO conceived and designed the work with assistance from BK and SMT. AR and BK carried out the analyses with assistance MK. AR and MK prepared the paper with assistance from BK and EO. All the authors revised the final version of the manuscript, while MK and EO acted as the corresponding authors. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors do not have financial or non-financial interests that directly or indirectly related to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Venn diagram of differential expressed genes (DEGs) derived from four independent studies and the CGs located in MQTL regions (Venn diagram was drawn using a tool in this website: http://bioinformatics.psb.ugent.be/webtools/Venn/). Detailed information is presented in the supplementary Table S5.
Additional file 2: Table S1:
The list of projected QTLs to the reference maps. QTLs related to the MQTLs were highlighted.
Additional file 3: Table S2.
The list of CGs and all annotated genes located at each MQTL interval in this study.
Additional file 4: Table S3.
The Arabidopsis orthologue genes in common bean located at each MQTL interval.
Additional file 5: Table S4.
The Arabidopsis and soybean orthologue genes of common bean located at each MQTL interval.
Additional file 6: Table S5.
List of all genes involved in the KEGG enrichment analysis.
Additional file 7: Table S6.
List of all R-genes in evaluated MQTL regions identified in this study.
Additional file 8: Table S7.
List of common genes between DEGs studies and current study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rahmanzadeh, A., Khahani, B., Taghavi, S.M. et al. Genome-wide meta-QTL analyses provide novel insight into disease resistance repertoires in common bean. BMC Genomics 23, 680 (2022). https://doi.org/10.1186/s12864-022-08914-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08914-w