Abstract
Background
Flavonols are the largest subgroup of flavonoids, possessing multiple functions in plants including protection against ultraviolet radiation, antimicrobial activities, and flower pigmentation together with anthocyanins. They are of agronomical and economical importance because the major off-taste component in rapeseed protein isolates is a flavonol derivative, which limits rapeseed protein use for human consumption. Flavonol production in Arabidopsis thaliana is mainly regulated by the subgroup 7 (SG7) R2R3-MYB transcription factors MYB11, MYB12, and MYB111. Recently, the SG19 MYBs MYB21, MYB24, and MYB57 were shown to regulate flavonol accumulation in pollen and stamens. The members of each subgroup are closely related, showing gene redundancy and tissue-specific expression in A. thaliana. However, the evolution of these flavonol regulators inside the Brassicaceae, especially inside the Brassiceae, which include the rapeseed crop species, is not fully understood.
Results
We studied the SG7 and SG19 MYBs in 44 species, including 31 species of the Brassicaceae, by phylogenetic analyses followed by synteny and gene expression analyses. Thereby we identified a deep MYB12 and MYB111 duplication inside the Brassicaceae, which likely occurred before the divergence of Brassiceae and Thelypodieae. These duplications of SG7 members were followed by the loss of MYB11 after the divergence of Eruca vesicaria from the remaining Brassiceae species. Similarly, MYB21 experienced duplication before the emergence of the Brassiceae tribe, where the gene loss of MYB24 is also proposed to have happened. The members of each subgroup revealed frequent overlapping spatio-temporal expression patterns in the Brassiceae member B. napus, which are assumed to compensate for the loss of MYB11 and MYB24 in the analysed tissues.
Conclusions
We identified a duplication of MYB12, MYB111, and MYB21 inside the Brassicaceae and MYB11 and MYB24 gene loss inside the tribe Brassiceae. We propose that polyploidization events have shaped the evolution of the flavonol regulators in the Brassicaceae, especially in the Brassiceae.
Similar content being viewed by others
Background
The mustard family (Brassicaceae) consists of 351 genera and almost 4000 species [1]. It contains the model plant Arabidopsis thaliana and several important crop plants including oilseed rape (Brassica napus) and cabbage (Brassica oleracea) domesticated for industrial use including food and biofuel production. Recent advances in Brassicaceae taxonomy revealed 51 monophyletic groups (tribes) [1,2,3,4], which can be assigned to major evolutionary lineages. Around 32 million years ago (MYA) the tribe Aethionemeae diverged from the rest of the family [5]. The diversification of the other 50 tribes began ~ 23 MYA and they are grouped into three [6, 7], four [8], or five lineages/clades [9, 10].
One whole-genome triplication (WGT), namely At-ɣ, and two whole-genome duplication (WGDs) events, called At-α and At-β have occurred in the evolution of A. thaliana and the core Brassicaceae, which are thought to increase the genetic diversity and species radiation [11,12,13]. Besides these, several meso-polyploidization events have been identified inside the Brassicaceae, e.g. in the tribe Brassiceae [14,15,16]. The whole-genome triplication (Br-α) in Brassica was shown to have occurred after At-α and before the radiation of the tribe Brassiceae [14,15,16]. Generally, polyploidization is followed by diploidization which is frequently accompanied by genome size reduction and reorganization and therefore genetic and transcriptional changes occur [17]. These changes are the basis for the “Gene Balance Hypothesis” stating that dosage-sensitive genes like transcription factors are over-retained while duplicated genes are preferentially lost after WGD events [18, 19]. It is assumed that polyploids have an adaptive advantage conferred by the availability of duplicated genes for sub- and neofunctionalization [20].
One of the largest transcription factor families in plants are MYB (myeloblastosis) transcription factors [21, 22]. They play pivotal roles in regulatory networks controlling development, metabolism and responses to biotic and abiotic stresses. MYBs are classified, based on the number of up to four imperfect amino acid sequence repeats (R) in their MYB domain, into 1R-, R2R3-, 3R-, and 4R-MYBs (summarised in Dubos et al., 2010). Each repeat forms three a–helices. While the second and third helices build a helix–turn–helix (HTH) structure [23], the third helix makes direct contact with the major groove of the DNA [24]. There are two major models describing R2R3-MYB and R1R2R3-MYB evolution: The “loss” model states that R2R3-MYB evolved from an R1R2R3 ancestral gene by the loss of the R1 repeat [25] while the “gain” model proposes that an ancestral R2R3-MYB gene gained the R1 repeat by intragenic domain duplication leading to the emergence of R1R2R3-MYBs [26]. Recent work by Du et al. suggests that the gain model provides a more parsimonious and reasonable explanation for the phylogenetic distribution of two and three repeat MYBs as both MYB classes are proposed to have coexisted in primitive eukaryotes [27]. However, Jiang et al. inferred that the gain model is unlikely, based on phylogenetic analyses [28].
R2R3-MYBs are the largest class of MYB transcription factors as they are exceptionally expanded in plant genomes [27, 28]. For example, R2R3-MYBs account for 64% and 63% of all MYB proteins in A. thaliana and B. napus, respectively [21, 22, 29]. The expansion of the R2R3-MYB family in plants resulted in a wide functional diversity of R2R3-MYBs, which regulate mainly plant-specific processes like stress responses, development and specialized metabolism [21]. R2R3-MYBs can be further classified into 23 subgroups by characteristic amino-acid motifs in the C-terminal region [22]. Several subgroups are involved in the regulation of flavonoid biosynthesis, one of the best studied plant biosynthesis pathways [30]. Flavonoids are responsible for plant pigmentation and can provide protection against biotic and abiotic stresses like UV-radiation [30]. While the subgroup 6 (SG6) family members MYB75/PAP1, MYB90/PAP2, MYB113, and MYB114 regulate anthocyanin accumulation [31, 32], the SG5 member MYB123/TT2 controls proanthocyanidin biosynthesis in A. thaliana [33].
Flavonols are one of the largest subgroup of flavonoids, and are involved in UV-protection and flower pigmentation together with anthocyanins [34, 35]. Moreover they are of agronomical and economical importance as the major off-taste component in rapeseed protein isolates is a flavonol derivative - this limits rapeseed protein palatability and human consumption [36]. The main regulators of flavonol biosynthesis in A. thaliana are the SG7 members MYB12, MYB11, and MYB111 [37, 38]. The SG7 MYBs show spatio-differential gene expression patterns in A. thaliana seedlings: MYB12 is expressed in roots, while MYB111 is expressed in cotyledons and MYB11 is marginally expressed in specific domains of the seedling including the apical meristem, the primary leaves, the apex of cotyledons, at the hypocotyl–root transition, the origin of lateral roots and the root tip as well as the vascular tissue of lateral roots [38]. However, the A. thaliana myb11/myb12/myb111 triple mutant retained flavonols in pollen grains and siliques/seeds [39]. This MYB11-, MYB12-, and MYB111-independent accumulation of flavonol glycosylates was recently addressed by the finding of a new group of flavonol regulators belonging to SG19: MYB21, MYB24, and MYB57 [40,41,42]. The three SG19 MYBs have previously been described to be involved in jasmonate-dependent regulation of stamen development and are expressed in all four whorls of the flower [43,44,45]. All SG7 MYBs can act as independent transcription factors by regulating e.g. the expression of flavonol synthase (FLS) [37, 38, 46], which produces flavonols from dihydroflavonols [47]. Studies have now shown that the SG19 MYBs can also bind and activate the FLS1 promoter [40,41,42]. Moreover, MYB99 is postulated to act in a MYB triad with MYB21 and MYB24 to regulate flavonol biosynthesis in anthers [40]. The bZIP transcription factor HY5 is required for MYB12 and MYB111 activation under UV-B and visible light in A. thaliana, while MYB24 was recently shown to regulate and bind to the HYH (HY5 ortholog) promoter in Vitis vinifera [48, 49].
In this study we used 44 species, of which 31 belong to the Brassicaceae family, to analyse the evolution of the flavonol regulators, namely the SG7 and SG19 MYBs. In total, these 31 Brassicaceae species span 17 tribes and represent all three major lineages and clades of the core Brassicaceae. By incorporating phylogenetic and synteny information, a duplication of MYB12, MYB111, and MYB21 inside the Brassicaceae and loss of MYB11 and MYB24 inside the Brassiceae was identified. Gene expression analyses revealed different spatio-temporal expression patterns of SG7 and SG19 MYBs in B. napus. Moreover, the meso-polyploidization events in the Brassicaceae likely shaped the evolution of flavonol regulators, especially in the tribe Brassiceae.
Results
Species tree and data set quality assessment
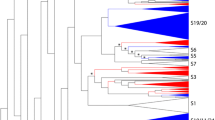
In this study we used a comprehensive data set collection derived from 44 species, including 31 Brassicaceae species spanning 17 tribes (Fig. 1, Additional file 1). The inferred species tree revealed that most of the analysed Brassicaceae tribes are monophyletic and can be assigned to the three major lineages and clades characteristic for the Brassicaceae family (Fig. 1). In this analysis the Brassiceae tribe is represented by 9 species (Brassica oleracea, Brassica cretica, Brassica rapa, Brassica napus, Raphanus sativus, Crambe hispanica, Sinapis alba, Eruca vesicaria, Cakile maritima), which has the Isatideae and Thelypodieae as sister clades.
Simplified Brassicaceae phylogeny. The phylogeny of Brassicaceae family members and outgroup species is shown. The species tree was built with OrthoFinder based on proteome data sets. The Brassicaceae family is highlighted in the beige box, while species assigned to the tribe Brassiceae are highlighted in the green box. The Brassicaceae lineages and clades [9] are coloured as followed: lineage I/clade A in blue, lineage II/clade B in red, lineage III/clade E in brown and clade C in violet. Clade D is not shown as no species was analysed from this clade. Whole genome duplication (WGD) and –triplication (WGT) events [4, 50,51,52], as well as the Brassiceae WGT event are marked with a star and named according to Barker et al., 2009. Estimated divergence times were added according to Franzke et al., 2011 and Walden et al., 2020
The quality assessment revealed that the majority of the 44 proteome data sets (Brassicaceae and non-Brassicaceae) are suitable for this analysis due to often more than 90% complete BUSCOs (Additional file 1). The 31 Brassicaceae data sets revealed 71.2% (Stanleya pinnata) to 99.3% (A. thaliana) complete BUSCOs emphasizing the overall high completeness of these data sets.
Genome-wide identification of R2R3-MYBs with focus on SG7 and SG19 R2R3-MYBs
The genome-wide identification of MYB proteins revealed different numbers of 1R-, R2R3-, 3R-MYBs and MYB-related proteins per species, ranging inside the Brassicaceae from 1 to 17 for 1R-, 90 to 442 for R2R3-, and 3 to 19 for 3R-MYBs (Additional file 2). The A. thaliana orthologues were used for classification and a phylogenetic tree of all R2R3-MYBs of A. thaliana was built to stress the phylogenetic relationship of the SG7 and SG19 R2R3-MYBs (Additional file 3). In order to analyse the SG7 and SG19 R2R3-MYBs in the Brassicaceae in detail all respective homologs per species were extracted (Additional file 4, Fig. 2) and copy number and sequence identities were identified (Additional file 5, Additional file 6). Overall, the members of each subgroup revealed a sequence identity of 33.5–96.2% (SG7) and 46.2–99.6% (SG19) for B. napus and 37.6–54.4% (SG7) and 24.5–70.7% (SG19) for A. thaliana (Additional file 6). The allotetraploid B. napus revealed one of the highest copy numbers with up to four MYB12, MYB21 and MYB57 homologs, and three MYB111 homologs (Additional file 5). However, E. vesicaria revealed five MYB111 homologs and Isatis tinctoria carries five MYB24 homologs. Up to two MYB11 homologs were identified in E. vesicaria, I. tinctoria, and Iberis amara. Next, the SG7 and SG19 homologs were used for phylogenetic analyses. In addition, all MYB123 (SG5) and MYB99 homologs were incorporated because MYB123 regulates a competing branch of the flavonoid pathway and is sister clade to SG7, and MYB99 is proposed to act in a regulatory triad with the SG19 MYBs. Interestingly, divergence into MYB11 and MYB12, as well as MYB21 and MYB24, was specifically observed for Brassicaceae members, while Cleome violacea revealed only one MYB11-MYB12 and MYB21-MYB24 homolog. Additional MYB11-MYB12 and MYB21-MYB24 homologs from several non-Brassicaceae species like tomato were identified as clusters preceding the divergence of the Brassicaceae MYB11, MYB12, MYB21 and MYB24 homologs. This suggests the emergence of separate MYB11 and MYB12 as well as MYB21 and MYB24 clades after the divergence of the Cleomaceae from its sister group the Brassicaceae (Fig. 2).
Scheme of the phylogenetic relationships of SG7 and SG19 members. The phylogenetic relationship of the SG7 (MYB11, MYB12, MYB111) and SG19 MYBs (MYB21, MYB24, MYB57) is displayed. The classification per clade is based on the respective A. thaliana homolog: the MYB12 clade is coloured in orange, MYB11 in light blue, MYB111 in violet, MYB21 in green, MYB24 in red, and MYB57 in yellow. The grey vertical bars inside the SG7 and SG19 clades mark the MYB11-MYB12 and MYB21-MYB24 sequences derived from species outside of the Brassicaceae, respectively. The MYB11-MYB12 and MYB21-MYB24 sequences of Cleome violacea, the representative species of the Cleomaceae, are highlighted with a pink rectangle. The MYB123 and MYB99 clades were collapsed and are represented by triangles as labeled. The figure is not to scale. Bootstrap values above 50% are shown and represented by circles of proportional sizes (see legend upper left corner). Please refer to Additional file 4 for the whole MYB tree and bootstrap values
Phylogeny of SG7 MYBs
The phylogenetic analysis of SG7 members MYB11, MYB12, and MYB111 revealed that at least one MYB111 homolog is present per Brassicaceae species, except for Arabis nemorensis (Fig. 3, Additional file 4, Additional file 5). Similarly, the majority of Brassicaceae members contained one MYB12 homolog. However, all Brassiceae species possess a duplication of MYB12 and MYB111 (Fig. 3). At least two MYB111 and MYB12 homologs were also identified in the closely related species Caulanthus amplexicaulis and Isatis tinctoria, while only two MYB111 and no MYB12 homolog were detected in Stanleya pinnata. However, the duplication event in I. tinctoria is likely associated with the independent meso-polyploidization event occurring in this species as shown by the close phylogenetic relationship of the respective MYB111 and MYB12 homologs (Fig. 1, Fig. 3). Even though independent meso-polyploidization events have also occurred in C. amplexicaulis and S. pinnata, the respective MYB111 homologs fall into two separate clades indicating a deeper MYB111 duplication preceding the divergence of the Brassiceae. The same applies for the MYB12 duplication of C. amplexicaulis. Interestingly, no MYB11 homolog was identified in the Brassica species, R. sativus, C. hispanica, and S. alba, indicating that MYB11 might be absent in these species (Fig. 3). As two MYB11 homologs were found in E. vesicaria and one in C. maritima, this gene loss is assumed to have occurred after the divergence of E. vesicaria. Moreover, no MYB11 homolog was detected in S. pinnata, Schrenkiella parvula, Thlaspi arvense, Malcolmia maritima, Descurainia sophioides, and Lepidium sativum.
Phylogeny of SG7 members in Brassicaceae. The phylogenies of MYB11 and MYB12 (A) and MYB111 (B) homologs of the Brassicaceae are displayed. Homologs of Brassiceae species are marked with a black rectangle. The MYB12 clade is coloured in orange, the MYB11 clade in light blue, and the MYB111 clade in violet. The classification per clade is based on the respective A. thaliana homologs. The identified SG7 homologs of Cleome violacea are displayed as C. violacea serves as representative of the Cleomaceae, which is sister group to Brassicaceae. Bootstrap values above 50% are shown and represented by circles of proportional sizes (see legend upper left corner). Please refer to Additional file 4 for the complete MYB tree and bootstrap numbers
Synteny analysis of SG7 MYBs
The potential MYB11 gene loss inside the Brassiceae was analysed in detail by examining the degree of local synteny at the MYB11 locus. In line with the phylogenetic analysis, MYB11 was absent from the genomic regions of B. napus, B. oleracea, B. rapa, R. sativus, C. hispanica, and S. alba showing the highest local synteny with the corresponding MYB11 locus from A. thaliana, while a MYB11 homolog was identified for E. vesicaria, C. maritima, I. tinctoria, and Myagrum perfoliatum (Fig. 4). Supporting these findings, no MYB11 homolog was identified via a TBLASTN search against these syntenic regions, as well as the genome sequences of the Brassica species, R. sativus, C. hispanica, and S. alba.
Synteny analysis of the MYB11 locus suggests gene loss inside the Brassiceae. The syntenic relationship at the MYB11 locus is shown for several Brassicaceae members. The position of the genomic region in the respective genome assembly is given underneath the species name in million base pairs (Mb). Grey curved beams connect the identified syntenic genes. The rectangle-shaped arrows represent annotated genes. Genes located on the forward strand are shown in grey and genes located on the reverse strand are shown in black. MYB11 homologs are marked in red and connected by light red lines
Gene expression analyses of SG7 MYBs
In order to analyse the expression patterns of SG7 members in Brassiceae and to investigate whether the duplications of MYB12 and MYB111 result in different tissue-specific expression patterns, we harnessed RNA-Seq data sets of B. napus (Table 1). In general, BnaMYB111-2_A06p030710 and BnaMYB111-1_C07p020280 show a similar expression pattern across multiple tissues (anther, petal, bud, and silique). However, BnaMYB111-2_A06p030710 revealed unique expression in developing seeds, seed coat, and sepals. BnaMYB111-3_A09p003850 was not expressed in any of the analysed tissues. While all four BnaMYB12 homologs are expressed in reproductive tissues (anthers, pistils, ovules, buds, young seeds), only three homologs (BnaMYB12-3_C04p000450, BnaMYB12-2_A03p022650, BnaMYB12-1_C03p027020) are additionally expressed in mature seeds and seed coat. Uniquely tissue-specific expression comparing all SG7 MYBs was identified for BnaMYB12-3_C04p000450 in late seed coat development (35 DAF) and BnaMYB111-2_A06p030710 is uniquely expressed in sepals and mature seeds compared to the other BnaMYB111 homologs.
Three of the four BnaMYB12 homologs (BnaMYB12-1_C03p027020, BnaMYB12-2_A03p022650, BnaMYB12-3_C04p000450) had overlapping co-expression patterns with genes related to flavonol biosynthesis, including F3’H and the flavonol glycosyltransferase UGT84A2 (Additional file 7). However, only BnaMYB12-1_C03p027020 and BnaMYB12-3_C04p000450 were additionally co-expressed with CHS, F3H, CHIL, and FLS1. Interestingly, BnaMYB12-4_A05p000540 was found to be co-expressed with MYB106, a transcription factor involved in trichome branching regulation in A. thaliana. No co-expressed genes were identified for the marginally expressed BnaMYB111-3_A09p003850. However, the other two BnaMYB111 homologs were co-expressed with genes derived from the flavonoid/flavonol biosynthesis and phenylpropanoid pathway including FLS1, F3H, flavonol glycosyltransferases, and 4CL3 (Additional file 7). Weak co-expression was identified between the B. napus SG7 and SG19 MYBs.
Phylogeny of SG19 MYBs
At least one MYB57 and one MYB21 homolog was identified in the analysed Brassicaceae species via phylogenetic analysis, except no MYB57 homolog was detected in S. pinnata (Fig. 5, Additional file 4, Additional file 5). All Brassiceae species, C. amplexicaulis and I. tinctoria revealed the presence of two MYB21 homologs, indicating a duplication event. The MYB21 duplication event in I. tinctoria is likely associated with the independent meso-polyploidization event occurring in this tribe as shown by the close phylogenetic relationship of the MYB21 homologs (Fig. 1, Fig. 5). However, the MYB21 homologs of C. amplexicaulis fall into two separate clades indicating a deeper MYB21 duplication preceding the divergence of the Brassiceae. Additionally, most Brassiceae species contained two MYB57 homologs with C. hispanica and S. alba being the exceptions with only one MYB57 homolog identified in each of them. Besides I. tinctoria none of the closest sister tribes of the Brassiceae revealed more than one MYB57 homolog. The independent meso-polyploidization event of I. tinctoria likely resulted in two MYB57 homologs from which a third MYB57 homolog likely emerged from tandem duplication. Thus, the MYB57 duplication event likely took place after the divergence of the Brassiceae and C. hispanica, and S. alba subsequently lost one MYB57 homolog.
Phylogeny of SG19 members in Brassicaceae. The phylogenies of MYB21 and MYB24 (A) and MYB57 (B) homologs of the Brassicaceae are displayed. The MYB21 clade is coloured in green, the MYB24 clade in red, and the MYB57 clade in yellow. Homologs of Brassiceae species are marked with a black rectangle. The classification per clade is based on the respective A. thaliana homologs. The identified SG19 homologs of Cleome violacea are displayed as C. violacea serves as representative of the Cleomaceae, which is sister group to Brassicaceae. Bootstrap values above 50% are shown and represented by circles of proportional sizes (see legend upper left corner). Please refer to Additional file 4 for the complete MYB tree and bootstrap numbers
No MYB24 homolog was identified in all analysed Brassiceae species, as well as S. pinnata, A. nemorensis, Capsella grandiflora, Euclidium syriacum, and Diptychocarpus strictus (Fig. 5). At least one MYB24 copy was detected in the remaining 17 Brassicaceae species. As all species of the closest Brassiceae sister tribes contain a MYB24 homolog except for S. pinnata, which has a low-quality data set, the loss of MYB24 is suggested to have occurred after the divergence of the Brassiceae tribe. Moreover, MYB24 might have been lost in the common ancestor of E. syriacum and D. strictus.
Synteny analysis of SG19 MYBs
In accordance with the phylogenetic analyses, MYB24 could not be detected via local synteny analysis in B. napus, B. oleracea, B. rapa, R. sativus, and S. alba, while the locus containing a MYB24 homolog of M. perfoliatum showed high local synteny to the MYB24 locus of A. thaliana (Fig. 6). Supporting these findings, no MYB24 homolog was identified in the syntenic regions of B. napus, B. oleracea, B. rapa, R. sativus, and S. alba via a TBLASTN search. Additionally, no MYB24 homolog was detected in all nine Brassiceae genome sequences.
Synteny analysis of the MYB24 locus suggests gene loss in the Brassiceae. The syntenic relationship at the MYB24 locus is shown for several Brassicaceae members. The position of the genomic region in the respective genome assembly is given underneath the species name in million base pairs (Mb). Grey curved beams connect the identified syntenic genes. The rectangle shaped arrows represent annotated genes. Genes located on the forward strand are shown in grey and genes located on the reverse strand are shown in black. MYB24 homologs are marked in red and connected by light red lines. The assembly continuity at the MYB24 locus was too low to analyse local synteny in C. maritima, E. vesicaria, and C. hispanica. A second S. alba locus sharing the same degree of local synteny is not shown for clarity (Additional file 8)
Gene expression analyses of SG19 MYBs
Analysis of tissue-specific expression patterns of SG19 members in B. napus revealed that all BnaMYB21 homologs are strongly expressed in stamens, pistils, sepals, and petals (Table 2). However, BnaMYB21-2_A09p002640 is expressed at higher levels in roots and seed coat 21–28 DAF compared to the other BnaMYB21 homologs. While the expression of BnaMYB57 homologs, if expressed, in stamens and sepals was lower compared to BnaMYB21 homologs, it was frequently higher in petals and pistils. Interestingly only BnaMYB57-3_C03p034120 and BnaMYB57-4_A03p028260 were expressed in all four floral tissues with BnaMYB57-3 being exceptionally strongly expressed in petals. The BnaMYB57-2_A05p000230 gene is expressed in pistils, sepals and petals but is only marginally expressed in stamens, while BnaMYB57-1_C05p047780 is only expressed in petals. Interestingly, BnaMYB57-4_A03p028260 revealed uniquely high expression in young seeds, while BnaMYB57-3_C03p034120 showed uniquely high expression in seed coat 42 DAF and endosperm. To summarize, the expression patterns of BnaMYB57-1_C05p047780 and BnaMYB57-2_A05p000230 overlap completely with the other BnaMYB57 homologs, which show as well similar expression patterns. Co-expression analysis of the majority of SG19 members in B. napus revealed a correlation level too low to be considered as strong co-expression. However, BnaMYB57-3_C03p034120 and BnaMYB57-4_A03p028260 were co-expressed with each other (Additional file 7).
Discussion
In this study we analysed flavonol regulators across 31 Brassicaceae species spanning 17 tribes. We identified a deep duplication giving rise to MYB12, MYB111 and MYB21 likely preceding the divergence of Brassiceae, which was followed by the loss of MYB11 and MYB24 after the divergence of the Brassiceae (Fig. 7).
Graphical abstract of SG7 and SG19 evolution in Brassicaceae. The proposed duplication and gene loss events inside the Brassiceae are shown. SG7 and SG19 homologs identified in Brassicaceae species are marked with different coloured circles: MYB11 in light blue, MYB12 in orange, MYB111 in violet, MYB21 in green, MYB57 in yellow, and MYB24 in red. If at least two homologs were detected in the species the circle was marked with a dark outline. The assumed loss of MYB11 is marked with a light blue cross, while the proposed loss of MYB24 is marked with a red cross. The duplication events of MYB12, MYB111 and MYB21 likely preceded the divergence of the Brassiceae tribe
Polyploidization events have shaped the evolution of the SG7 and SG19 MYBs inside the Brassicaceae
WGD events are known to influence genetic diversification and species radiation. Polyploidization events allow an adaptive advantage by providing the genetic basis for gene neo- and subfunctionalisation [20]. Additionally, affected genomes are characterized by extensive re-diploidization, typically associated with chromosomal rearrangements, genome size reduction and increased fractionation [53]. These events can lead to gene losses while duplicated genomic regions can still be identified [53, 54]. Besides the paleo-polyploidization events At-ɣ, At-β, and At-α, lineage-specific meso-polyploidization events took place during the evolution of several Brassicaceae tribes including Brassiceae, Isatideae, and Thelypodieae [50,51,52, 55]. The meso-polyploidization event of Isatis tinctoria (Isatideae) likely resulted in the duplication of all SG7 and SG19 members as inferred by the close phylogenetic relationship of the duplicated homologs (Fig. 3, Fig. 5). These duplication events are thus independent from the observed duplication events inside the Brassiceae and Thelypodieae. The duplicated MYB12, MYB111, and MYB21 homologs of the Thelypodieae fall into separate clades, thus suggesting that these duplication events might not be associated with the independent meso-polyploidization event but rather belong to a deeper duplication that took place in the common ancestor of Brassiceae and Thelypodieae. One of the most recent Brassicaceae phylogenies suggests Brassiceae and Thelypodieae to be closely related monophyletic sister clades while Isatideae is sister to both, supporting this hypothesis [4]. However, additional research including more data from Brassiceae sister tribes, e.g. the Sisymbrieae, is needed to further pin-point the time-point of the MYB12, MYB111, and MYB21 duplication events. The MYB57 duplication observed in 7/9 Brassiceae species, but not in the Thelypodieae, is likely associated with the Brassiceae-specific whole-genome triplication (WGT) dated to 7.9–14.6 my [15, 16]. This Br-α WGT event was shown to have been followed by taxon- and lineage-specific chromosome rearrangements resulting in chromosome number reductions [15, 16], which might be associated with the observed secondary loss of one MYB57 homolog in the closely related Sinapis alba and Crambe hispanica (Fig. 5).
Succeeding these duplication events we identified the loss of MYB11 after the divergence of Eruca vesicaria (Brassiceae) and the loss of MYB24 after the divergence of the Brassiceae (Fig. 3). The loss of MYB11 and MYB24 inside the Brassiceae was further supported by the absence of these homologs in the respective genomic regions showing the highest local synteny to the MYB11 and MYB24 loci in A. thaliana and other Brassicaceae species (Fig. 4, Fig. 6). Recently, Li et al. 2020 analysed the distribution of R2R3-MYBs in nine Brassicaceae (A. thaliana, Arabidopsis lyrata, Capsella rubella, Capsella grandiflora, Boechera stricta, B. napus, B. oleracea, B. rapa, Eutrema salsugineum) and seven non-Brassicaceae species (Carica papaya, Theobroma cacao, Gossypium raimondii, Citrus clementina, Citrus sinensis, Manihot esculenta, Eucalyptus grandis) [29]. In accordance with our results no MYB11 or MYB24 homolog was identified for the three analysed Brassiceae species and at least two MYB12, MYB21, MYB111, and MYB57 homologs were detected for B. rapa and B. napus. However, for B. oleracea only one MYB12, MYB111, and MYB21 homolog was identified, along with two MYB57 homologs. This difference might be explained by the use of a short-read assembly (N50 = ~ 27 kbp, 5,425 contigs) vs. a long-read assembly (N50 = ~ 9,491 kbp, 264 contigs) used in this study in which more homologs could be resolved. In summary, the duplications of MYB12, MYB111, and MYB21 identified in all Brassiceae species are derived from a deep duplication event presumably preceding the divergence of Brassiceae. The subsequent loss of MYB24 and MYB11 inside the Brassiceae might have occurred during the course of post-mesopolyploidization of the Br-α WGT event.
SG7 and SG19 MYBs reveal spatio-temporal tissue-expression patterns
Gene redundancy accompanied with differential spatial expression has been observed for the SG7 MYBs in A. thaliana seedlings: MYB12 is expressed in roots, while MYB111 is expressed in cotyledons and MYB11 is only marginally expressed in defined narrow domains of the seedling like the root tip and the apex of cotyledons [38]. Thus, MYB12 and MYB111 were designated as the main flavonol regulators in A. thaliana seedlings [38]. Moreover, Stracke et al. postulated that MYB12 and MYB111 regulate different targets involved in the production of specific flavonol derivatives because the single mutants displayed differences in the composition of flavonol derivatives. In contrast, the MYB11 single mutant revealed a flavonol composition that is comparable to the wild type [38]. Moreover, the expression pattern of SG7 members in B. napus differs from the ones described for A. thaliana seedlings: BnaMYB12 are predominantly expressed in reproductive tissues and BnaMYB111 in anthers and buds. One of the main target genes of the SG7 members, flavonol synthase (FLS), is also mainly expressed in reproductive tissues in B. napus [56] indicating the relevance of the transcriptional activation of flavonol accumulation in reproductive tissues. Reduced flavonol levels were linked with decreased pollen viability and germination, as e.g. pollen germination increased with increasing flavonol concentrations and kaempferol supplementation rescued pollen fertility [57, 58]. In general, overlapping expression patterns of BnaMYB12 and BnaMYB111 homologs were identified, accompanied by tissue-specific expression of single BnaMYB12 and BnaMYB111 homologs. The majority of BnaMYB12 and BnaMYB111 homologs were co-expressed with genes involved in or associated with flavonoid biosynthesis, indicating their proposed role in the regulation of this pathway. These findings indicate that the BnaMYB12 and BnaMYB111 homologs might be active in the same tissues, while the unique expression domains of single homologs could explain why single homologs are retained. Additionally, specific sequence features might play a role in subfamily and gene retention, as BnaR2R3-MYB subfamilies with a specific intron pattern are more likely to be retained [27, 29]. The BnaMYB21 and BnaMYB57 homologs revealed strong and overlapping expression in stamens, pistils, sepals and petals. Again tissue-specific expression of single BnaMYB21 and BnaMYB57 homologs was identified. Taken together, additional research will show if the duplicated MYB12 and MYB111 homologs and MYB21 and MYB57 homologs inside the Brassiceae can compensate for the loss of MYB11 and MYB24, respectively. Recent functional analyses of BnaWER homologs (SG15) indicate that genes derived from the same subfamily, which share high sequence similarity and similar expression patterns, frequently show functional redundancy [29]. However, further research is necessary to elucidate the biological meaning and function of the MYB12, MYB111, MYB21, and MYB57 duplications and proteins, respectively.
Lineage-specific expansion and reduction of R2R3-MYB subfamilies
One well-known example of the evolution of novel traits in the Brassicales, including Brassicaceae, is the emergence of glucosinolates (GSLs) along with the corresponding R2R3-MYB transcriptional regulators MYB28, MYB29, MYB34, MYB51, MYB76 and MYB122, which belong to subgroup 12 [22, 59]. This MYB clade is proposed to result from the At-β paleo-polyploidization event [60]. MYB28, MYB29, and MYB76 act as positive regulators of aliphatic GLSs with overlapping functions and MYB28 and MYB29 as main regulators [61]. While MYB76 is present in A. thaliana (Camelineae), no MYB76 has been identified in Brassica species (Brassiceae) [59] posing a striking example of gene loss inside specific Brassicaceae species. Interestingly, we observed that the divergence of MYB11 and MYB12, as well as MYB21 and MYB24, likely occurred after the divergence of the Cleomaceae from its sister group the Brassicaceae (Fig. 2). Previous studies included only A. thaliana as a single Brassicaceae species [27, 28], thus could not analyse Brassicaceae-specific expansion of SG7 and SG19 MYBs. However, Li et al. 2020 investigated the SG7 and SG19 homologs of nine Brassicaceae species and seven non-Brassicaceae species, thereby revealing five Brassicaceae-specific subfamilies and five subfamilies which were absent from the investigated Brassicaceae species [29]. In accordance with our hypothesis, the non-Brassicaceae SG7 and SG19 homologs did not fall into two separate MYB11 and MYB12 clades, as well as MYB21 and MYB24 clades, respectively, while the Brassicaceae homologs did [29]. Thus our study used a broad range of Brassicaceae- and related species like Cleome violacea, allowing the in-depth analysis and identification of Brassicaceae-specific expansion of SG7 and SG19 MYBs. This finding serves as an example of the adaptive evolution of the flavonol-regulating R2R3-MYB transcription factors frequently accompanied by sub- and neofunctionalization in Brassicaceae species where a MYB11 and MYB24 homolog was retained. Moreover, our results suggest that lineage-specific expansion or reduction of MYB subfamilies might have occurred frequently in the Brassicaceae, in line with the high degree of flexibility and complex evolution observed for the B. napus R2R3-MYB subfamilies.
Limitations of the study
The quality of the sequence data sets used in this study varies between species. Different degrees of completeness can influence the identification of homologs. For example, no MYB11, MYB12, MYB24, and MYB57 homolog was identified in Stanleya pinnata, probably due to the low completeness (71% complete BUSCOs) observed for this data set (Additional file 5). Additionally, Brassica cretica revealed a comparably low completeness of 74.5% and no MYB12 homolog was identified (Additional file 5). The recent release of genomic resources for several Brassicaceae members allowed us to investigate the evolution of the SG7 and SG19 MYBs in great detail. Thus, in this study we were able to cover 17 of the 51 Brassicaceae tribes with at least one representative species. However, additional genome sequences of Brassicaceae species will help to support our hypotheses and to further narrow down the time-point of the SG7 and SG19 duplication and gene loss events. The species tree revealed minor differences to the phylogeny of taxonomic studies like Huang et al. 2015 [9], Nikolov et al., 2019 [10] and Walden et al. 2020 [4]. However, the phylogenetic positions of the tribes is still not fully resolved due to different results derived from nuclear and plastid data which, among other reasons, explains the inconsistencies of Brassicaceae taxonomy studies (summarised in Walden et al., 2020).
Conclusions
In this study we unravelled the evolution of the flavonol regulators SG7 and SG19 R2R3-MYBs in the Brassicaceae with focus on the tribe Brassiceae (Fig. 7). A deep duplication of the SG7 MYBs MYB12 and MYB111, likely preceding the divergence of Brassiceae, was followed by the loss of MYB11 after the divergence of E. vesicaria. Similarly, a duplication of MYB21 likely preceding the divergence of the Brassiceae was identified along with the loss of MYB24 inside the Brassiceae. The members of each subgroup revealed frequent overlapping spatio-temporal expression patterns in the Brassiceae member B. napus, which are assumed to compensate the loss of MYB11 and MYB24 in the analysed tissues. Therefore, we propose that polyploidization events have influenced the evolution of the flavonol regulators in the Brassicaceae, especially in the tribe Brassiceae.
Methods
Data collection, quality control and species tree generation
Genomic data sets of 44 species, including 31 species of the Brassicaceae, were retrieved mainly from Phytozome, NCBI and Genoscope (Additional file 1). To assess the completeness and duplication level of all annotated polypeptide sequences BUSCO v3.0.2 was deployed using the embryophyta_odb9 lineage data set in protein mode [62]. OrthoFinder v2.5.4 [63,64,65] was used to construct a species tree using the 44 proteome data sets as input.
Genome-wide identification of MYB homologs
Genome-wide identification of MYB and MYB-like transcription factors was performed using MYB annotator v0.153 [66]. MYB annotator was run with the default bait sequences and the proteome data sets of all 44 species were subjected to this analysis. The extracted MYB polypeptide sequences per species were combined and used for the phylogenetic analysis.
Phylogenetic tree construction
For the generation of a phylogenetic tree, first the full-length polypeptide sequences of the genome-wide identified MYB homologs per species were combined into one file (Additional file 9) and then used for the construction of a MAFFT v7.475 [67] alignment. This analysis covered 44 species (Additional file 1). Next, a codon alignment was produced via pxaa2cdn [68] i.e. converting the amino acids of the alignment back to their respective codons. As no CDS file was available for Arabis nemorensis, Brassica cretica and Microthalspi erraticum, these species were not incorporated in this analysis. However, the SG7 and SG19 homologs identified in these species based on polypeptide sequences are listed in Additional file 10. Subsequently, the alignment was cleaned by removal of all columns with less than 10 percent occupancy as described before [69]. The cleaned alignment was then used for the construction of an approximately-maximum-likelihood phylogenetic tree constructed with FastTree 2 [70] using the WAG model and 10,000 bootstrap replications in addition to the following parameters to increase accuracy: -spr 4 -mlacc 2 -slownni -gamma. This phylogenetic tree covering all genome-wide MYBs from 41 species was then used for the identification of the SG7 and SG19 clade followed by the extraction of the included MYB polypeptide sequences by a customized python script (extract_red.py) [71]. The SG7 and SG19 MYBs polypeptide sequences were used for the construction of a sequence identity matrix (Additional file 6) based on MAFFT v7.475 alignments. Additionally, the SG5 and MYB99 homologs were extracted because MYB123 (SG5) regulates a competing branch of the flavonoid pathway and is sister clade to SG7 and MYB99 is involved in the regulation of SG19 MYBs. Again, an alignment of polypeptide sequences (corresponding CDS sequences are listed in Additional file 11) was constructed followed by its conversion into a codon alignment and cleaning as described above. Next, the cleaned codon alignment was used to construct a tree via RAxML-NG v.1.0.1 [72] using the GTR + GAMMA model. The best-scoring topology was inferred from 50 tree searches using 25 random and 25 parsimony-based starting trees. To infer a bootstrap tree, again the GTR + GAMMA model was used including 9800 bootstrap replicates until bootstrap convergence was reached after 8750 bootstraps (weighted Robinson-Foulds (RF) distance = 0.646, 1% cutoff). The bootstrap support values were then mapped onto the best-scoring Maximum Likelihood (ML) tree. After monophyletic tip masking, the resulting tree with bootstrap support values was visualized using FigTree v1.4.3 (Additional file 4) and iTOL v6.5.8 [73]. Please note that Fig. 2, Fig. 3, and Fig. 5 are subsets of Additional file 4, which contains the complete MYB tree. MYBs per species were classified according to their relationships with A. thaliana homologs.
Synteny and BLAST analysis
JCVI [74] was used to analyse local synteny and visualize syntenic regions. To analyse a potential gene loss event in a species in detail a TBLASTN [75] against the high local synteny regions using AthMYB11 and AthMYB24 as queries was performed with all Brassiceae members, I. tinctoria and M. perfoliatum. Moreover, TBLASTN was run against the respective assemblies of these species to search for potential gene fragments of MYB11 and MYB24 outside of the syntenic regions. For this analysis a customized python script was used (TBLASTN_check.py) [71], which identifies whether a TBLASTN hit is located inside an annotated gene or not. If several blast hits correspond to the same gene (e.g. multiple exons), the identifier of this gene will only be extracted once. If the TBLASTN hit is not located inside a gene, the start and end position on the subject sequence will be extracted and used for a web-based BLASTN search to identify potential homologs. The top five hits were then used to extract the amino acid sequence from the corresponding gene ID and then subjected to phylogenetic analysis including all 126 AthR2R3-MYBs via FastTree 2 [70]. This analysis revealed their closest AthMYB homolog for classification. If the closest homolog was not MYB11 or MYB24, this would further support the absence of these homologs in the analysed species.
Gene expression analysis
Public RNA-Seq data sets were used and retrieved from the Sequence Read Archive via fastq-dump v.2.9.64 [76] to analyze the expression of MYB genes across various tissues (Additional file 12). Transcript abundance, i.e. read counts and transcripts per millions (TPMs), was calculated via kallisto v. 0.44 [77] using default parameters and the transcript file of the B. napus cultivar Express 617 [78]. The heatmap was constructed with a customized python script calculating mean TPMs per tissue using 276 paired-end RNA-Seq data sets from B. napus as previously described [56]. Condition-independent co-expression analysis was performed as described before [56] to identify co-expressed genes using Spearman’s correlation coefficient by incorporating 696 B. napus RNA-Seq data sets.
Availability of data and materials
All data sets underlying this study are publicly available or included within the additional files. Data sets were retrieved mainly from Phytozome, NCBI and Genoscope. Please see Additional file 1 for details.
References
Koch MA, German DA, Kiefer M, Franzke A. Database Taxonomics as Key to Modern Plant Biology. Trends Plant Sci. 2018;23:4–6.
Warwick SI, Mummenhoff K, Sauder CA, Koch MA, Al-Shehbaz IA. Closing the gaps: phylogenetic relationships in the Brassicaceae based on DNA sequence data of nuclear ribosomal ITS region. Plant Syst Evol. 2010;285:209–32.
Al-Shehbaz IA. A generic and tribal synopsis of the Brassicaceae (Cruciferae). Taxon. 2012;61:931–54.
Walden N, German DA, Wolf EM, Kiefer M, Rigault P, Huang X-C, et al. Nested whole-genome duplications coincide with diversification and high morphological disparity in Brassicaceae. Nat Commun. 2020;11:3795.
Hohmann N, Wolf EM, Lysak MA, Koch MA. A Time-Calibrated Road Map of Brassicaceae Species Radiation and Evolutionary History. Plant Cell. 2015;27:2770–84.
Beilstein MA, Al-Shehbaz IA, Kellogg EA. Brassicaceae phylogeny and trichome evolution. Am J Bot. 2006;93:607–19.
Beilstein MA, Al-Shehbaz IA, Mathews S, Kellogg EA. Brassicaceae phylogeny inferred from phytochrome A and ndhF sequence data: tribes and trichomes revisited. Am J Bot. 2008;95:1307–27.
Franzke A, Lysak MA, Al-Shehbaz IA, Koch MA, Mummenhoff K. Cabbage family affairs: the evolutionary history of Brassicaceae. Trends Plant Sci. 2011;16:108–16.
Huang C-H, Sun R, Hu Y, Zeng L, Zhang N, Cai L, et al. Resolution of Brassicaceae Phylogeny Using Nuclear Genes Uncovers Nested Radiations and Supports Convergent Morphological Evolution. Mol Biol Evol. 2015;33:394–412.
Nikolov LA, Shushkov P, Nevado B, Gan X, Al-Shehbaz IA, Filatov D, et al. Resolving the backbone of the Brassicaceae phylogeny for investigating trait diversity. New Phytol. 2019;222:1638–51.
Bowers JE, Chapman BA, Rong J, Paterson AH. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 2003;422:433–8.
De Bodt S, Maere S, Van de Peer Y. Genome duplication and the origin of angiosperms. Trends Ecol Evol. 2005;20:591–7.
Barker MS, Vogel H, Schranz ME. Paleopolyploidy in the Brassicales: Analyses of the Cleome Transcriptome Elucidate the History of Genome Duplications in Arabidopsis and Other Brassicales. Genome Biol Evol. 2009;1:391–9.
Parkin IAP, Gulden SM, Sharpe AG, Lukens L, Trick M, Osborn TC, et al. Segmental Structure of the Brassica napus Genome Based on Comparative Analysis With Arabidopsis thaliana. Genetics. 2005;171:765–81.
Lysak MA, Koch MA, Pecinka A, Schubert I. Chromosome triplication found across the tribe Brassiceae. Genome Res. 2005;15:516–25.
Lysak MA, Cheung K, Kitschke M, Bureš P. Ancestral Chromosomal Blocks Are Triplicated in Brassiceae Species with Varying Chromosome Number and Genome Size. Plant Physiol. 2007;145:402–10.
Lysak MA, Koch MA, Beaulieu JM, Meister A, Leitch IJ. The Dynamic Ups and Downs of Genome Size Evolution in Brassicaceae. Mol Biol Evol. 2009;26:85–98.
Edger PP, Pires JC. Gene and genome duplications: the impact of dosage-sensitivity on the fate of nuclear genes. Chromosome Res. 2009;17:699–717.
Birchler JA, Veitia RA. The gene balance hypothesis: implications for gene regulation, quantitative traits and evolution. New Phytol. 2010;186:54–62.
Hoffmeier A, Gramzow L, Bhide AS, Kottenhagen N, Greifenstein A, Schubert O, et al. A Dead Gene Walking: Convergent Degeneration of a Clade of MADS-Box Genes in Crucifers. Mol Biol Evol. 2018;35:2618–38.
Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–81.
Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol. 2001;4:447–56.
Ogata K, Kanei-Ishii C, Sasaki M, Hatanaka H, Nagadoi A, Enari M, et al. The cavity in the hydrophobic core of Myb DNA-binding domain is reserved for DNA recognition and trans-activation. Nat Struct Mol Biol. 1996;3:178–87.
Jia L, Clegg MT, Jiang T. Evolutionary Dynamics of the DNA-Binding Domains in Putative R2R3-MYB Genes Identified from Rice Subspecies indica and japonica Genomes. Plant Physiol. 2004;134:575–85.
Rosinski JA, Atchley WR. Molecular Evolution of the Myb Family of Transcription Factors: Evidence for Polyphyletic Origin. J Mol Evol. 1998;46:74–83.
Jiang C, Gu J, Chopra S, Gu X, Peterson T. Ordered origin of the typical two- and three-repeat Myb genes. Gene. 2004;326:13–22.
Du H, Liang Z, Zhao S, Nan MG, Tran LSP, Lu K, et al. The Evolutionary History of R2R3-MYB Proteins Across 50 Eukaryotes: New Insights Into Subfamily Classification and Expansion. Sci Rep. 2015;5:1–16.
Jiang C-K, Rao G-Y. Insights into the Diversification and Evolution of R2R3-MYB Transcription Factors in Plants1. Plant Physiol. 2020;183:637–55.
Li P, Wen J, Chen P, Guo P, Ke Y, Wang M, et al. MYB Superfamily in Brassica napus: Evidence for Hormone-Mediated Expression Profiles, Large Expansion, and Functions in Root Hair Development. Biomolecules. 2020;10:E875.
Winkel-Shirley B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiology. 2001;126:485–93.
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation Tagging Identifies a Conserved MYB Regulator of Phenylpropanoid Biosynthesis. Plant Cell. 2000;12:2383–93.
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53:814–27.
Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13:2099–114.
Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504.
Zhang Q, Zhao X, Hongbin Q. Flavones and Flavonols: Phytochemistry and Biochemistry. Berlin Heidelberg: Springer; 2013. p. 1821–47.
Hald C, Dawid C, Tressel R, Hofmann T. Kaempferol 3- O-(2‴- O-Sinapoyl-β-sophoroside) Causes the Undesired Bitter Taste of Canola/Rapeseed Protein Isolates. J Agric Food Chem. 2019;67:372–8.
Mehrtens F, Kranz H, Bednarek P, Weisshaar B. The Arabidopsis Transcription Factor MYB12 Is a Flavonol-Specific Regulator of Phenylpropanoid Biosynthesis. Plant Physiol. 2005;138:1083–96.
Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, et al. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50:660–77.
Stracke R, Jahns O, Keck M, Tohge T, Niehaus K, Fernie AR, et al. Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytol. 2010;188:985–1000.
Battat M, Eitan A, Rogachev I, Hanhineva K, Fernie A, Tohge T, et al. A MYB Triad Controls Primary and Phenylpropanoid Metabolites for Pollen Coat Patterning. Plant Physiol. 2019;180:87–108.
Shan X, Li Y, Yang S, Yang Z, Qiu M, Gao R, et al. The spatio-temporal biosynthesis of floral flavonols is controlled by differential phylogenetic MYB regulators in Freesia hybrida. New Phytol. 2020;228:1864–79.
Zhang X, He Y, Li L, Liu H, Hong G. Involvement of the R2R3-MYB transcription factor MYB21 and its homologs in regulating flavonol accumulation in Arabidopsis stamen. J Exp Bot. 2021;72:4319–32.
Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, et al. Gibberellin Acts through Jasmonate to Control the Expression of MYB21, MYB24, and MYB57 to Promote Stamen Filament Growth in Arabidopsis. PLoS Genet. 2009;5: e1000440.
Mandaokar A, Browse J. MYB108 Acts Together with MYB24 to Regulate Jasmonate-Mediated Stamen Maturation in Arabidopsis. Plant Physiol. 2009;149:851–62.
Qi T, Huang H, Song S, Xie D. Regulation of Jasmonate-Mediated Stamen Development and Seed Production by a bHLH-MYB Complex in Arabidopsis. Plant Cell. 2015;27:1620–33.
Stracke R, Turgut-Kara N, Weisshaar B. The AtMYB12 activation domain maps to a short C-terminal region of the transcription factor. Z Naturforsch C J Biosci. 2017;72:251–7.
Wisman E, Hartmann U, Sagasser M, Baumann E, Palme K, Hahlbrock K, et al. Knock-out mutants from an En-1 mutagenized Arabidopsis thaliana population generate phenylpropanoid biosynthesis phenotypes. Proc Natl Acad Sci USA. 1998;95:12432–7.
Stracke R, Favory J-J, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, et al. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant, Cell Environ. 2010;33:88–103.
Chen Z, Zhanwu D, Thilia F, Luis O, Antonio S, Arnau P, et al. The grape MYB24 mediates the coordination of light-induced terpene and flavonol accumulation in response to berry anthocyanin sunscreen depletion. bioRxiv. 2021:2021.12.16.472692. https://doi.org/10.1101/2021.12.16.472692.
Kagale S, Robinson SJ, Nixon J, Xiao R, Huebert T, Condie J, et al. Polyploid Evolution of the Brassicaceae during the Cenozoic Era. Plant Cell. 2014;26:2777–91.
Mandáková T, Li Z, Barker MS, Lysak MA. Diverse genome organization following 13 independent mesopolyploid events in Brassicaceae contrasts with convergent patterns of gene retention. Plant J. 2017;91:3–21.
Kiefer C, Willing E-M, Jiao W-B, Sun H, Piednoël M, Hümann U, et al. Interspecies association mapping links reduced CG to TG substitution rates to the loss of gene-body methylation. Nat Plants. 2019;5:846–55.
Mandáková T, Lysak MA. Post-polyploid diploidization and diversification through dysploid changes. Curr Opin Plant Biol. 2018;42:55–65.
Mandáková T, Joly S, Krzywinski M, Mummenhoff K, Lysak MA. Fast Diploidization in Close Mesopolyploid Relatives of Arabidopsis. Plant Cell. 2010;22:2277–90.
Mandáková T, Hloušková P, German DA, Lysak MA. Monophyletic Origin and Evolution of the Largest Crucifer Genomes1. Plant Physiol. 2017;174:2062–71.
Schilbert HM, Schöne M, Baier T, Busche M, Viehöver P, Weisshaar B, et al. Characterization of the Brassica napus Flavonol Synthase Gene Family Reveals Bifunctional Flavonol Synthases. Front Plant Sci. 2021;12:2290.
Mo Y, Nagel C, Taylor LP. Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Natl Acad Sci U S A. 1992;89:7213–7.
Muhlemann JK, Younts TLB, Muday GK. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc Natl Acad Sci. 2018;115:E11188–97.
Seo M-S, Kim JS. Understanding of MYB Transcription Factors Involved in Glucosinolate Biosynthesis in Brassicaceae. Molecules. 2017;22:1549.
Yanhui C, Xiaoyuan Y, Kun H, Meihua L, Jigang L, Zhaofeng G, et al. The MYB transcription factor superfamily of Arabidopsis: expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol Biol. 2006;60:107–24.
Gigolashvili T, Engqvist M, Yatusevich R, Müller C, Flügge U-I. HAG2/MYB76 and HAG3/MYB29 exert a specific and coordinated control on the regulation of aliphatic glucosinolate biosynthesis in Arabidopsis thaliana. New Phytol. 2008;177:627–42.
Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–2.
Emms DM, Kelly S. STRIDE: Species Tree Root Inference from Gene Duplication Events. Mol Biol Evol. 2017;34:3267–78.
Emms DM, Kelly S. STAG: Species Tree Inference from All Genes. bioRxiv. 2018:267914. https://doi.org/10.1101/267914.
Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20:238.
Pucker B. Automatic identification and annotation of MYB gene family members in plants. BMC Genomics. 2022;23:220.
Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 2013;30:772–80.
Brown JW, Walker JF, Smith SA. Phyx: phylogenetic tools for unix. Bioinformatics. 2017;33:1886–8.
Pucker B, Reiher F, Schilbert HM. Automatic Identification of Players in the Flavonoid Biosynthesis with Application on the Biomedicinal Plant Croton tiglium. Plants. 2020;9:1103.
Price MN, Dehal PS, Arkin AP. FastTree 2 – Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE. 2010;5: e9490.
Pucker B, Iorizzo M. Apiaceae FNS I originated from F3H through tandem gene duplication. bioRxiv. 2022:2022.02.16.480750. https://doi.org/10.1101/2022.02.16.480750.
Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35:4453–5.
Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–6.
Tang H, Bowers JE, Wang X, Ming R, Alam M, Paterson AH. Synteny and Collinearity in Plant Genomes. Science. 2008. https://doi.org/10.1126/science.1153917.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Leinonen R, Sugawara H, Shumway M. International Nucleotide Sequence Database Collaboration. The sequence read archive. Nucleic Acids Res. 2011;39 Database issue:D19-21.
Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34:525–7.
Lee H, Chawla HS, Obermeier C, Dreyer F, Abbadi A, Snowdon R. Chromosome-Scale Assembly of Winter Oilseed Rape Brassica napus. Front Plant Sci. 2020;11:496.
Acknowledgements
We are grateful to all researchers who submitted the underlying sequences to the appropriate databases, and published their experimental findings. Some of the sequence data sets used were produced by the US Department of Energy Joint Genome Institute. We thank the Center for Biotechnology (CeBiTec) at Bielefeld University for providing an environment to perform the computational analyses.
Funding
Open Access funding enabled and organized by Projekt DEAL. We acknowledge support for the publication costs by the Open Access Publication Fund of Bielefeld University and the Deutsche Forschungsgemeinschaft (DFG), as well as the support of the German Academic Exchange Service.
Author information
Authors and Affiliations
Contributions
HMS and BJG designed the research. HMS performed bioinformatic analyses. HMS and BJG interpreted the results and wrote the manuscript. Both authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schilbert, H.M., Glover, B.J. Analysis of flavonol regulator evolution in the Brassicaceae reveals MYB12, MYB111 and MYB21 duplications and MYB11 and MYB24 gene loss. BMC Genomics 23, 604 (2022). https://doi.org/10.1186/s12864-022-08819-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08819-8