Abstract
Background
Drug-resistant bacteria have posed a great threat to animal breeding and human health. It is obviously urgent to develop new antibiotics that can effectively combat drug-resistant bacteria. The commensal flora inhabited in the intestines become potential candidates owing to the production of a wide range of antimicrobial substances. In addition, host genomes do not encode most of the enzymes needed to degrade dietary structural polysaccharides. The decomposition of these polysaccharides mainly depends on gut commensal-derived CAZymes.
Results
We report a novel species isolated from the chicken intestine, designated as Paenibacillus jilinensis sp. nov. and with YPG26T (= CCTCC M2020899T) as the type strain. The complete genome of P. jilinensis YPG26T is made up of a single circular chromosome measuring 3.97 Mb in length and containing 49.34% (mol%) G + C. It carries 33 rRNA genes, 89 tRNA genes, and 3871 protein-coding genes, among which abundant carbohydrate-degrading enzymes (CAZymes) are encoded. Moreover, this strain has the capability to antagonize multiple pathogens in vitro. We identified putative 6 BGCs encoding bacteriocin, NRPs, PKs, terpenes, and protcusin by genome mining. In addition, antibiotic susceptibility testing showed sensitivity to all antibiotics tested.
Conclusions
This study highlights the varieties of CAZymes genes and BGCs in the genome of Paenibacillus jilinensis. These findings confirm the beneficial function of the gut microbiota and also provide a promising candidate for the development of new carbohydrate degrading enzymes and antibacterial agents.
Similar content being viewed by others
Background
The gut microbiota plays a crucial role in the host physiology, metabolism, immunity, digestion, and nutrition uptake, but most of these microorganisms are still uncultivable due to certain limiting factors [1]. Access to these uncultivated species has the potential to significantly advance our understanding of intestinal flora. Delightfully, some recently developed approaches that isolate uncultivated microorganisms, such as situ cultivation [2] and culturomics [3], have proven successful.
Carbohydrates derived from plant polysaccharides affect the stability of the gut microbiota and restructure the composition and metabolism of the gut community, while has a profound effect on human and animal health [4]. As host genomes do not encode most enzymes needed to degrade dietary structural polysaccharides, the decomposition of these polysaccharides mainly depends on gut commensal-derived CAZymes [5]. In addition to degrading structural carbohydrates, gut commensals can also antagonize enteric pathogens by producing a wide range of antimicrobial compounds [6]. Based on their biosynthesis pathway, they are classified into three main groups: bacteriocins, nonribosomal peptides (NRPs), and polyketides (PKs) [7]. These antimicrobial substances are promising candidates for the development of new anti-infective agents.
Here, we report the isolation of an uncultured Paenibacillus species, P. jilinensis YPG26, isolated from the chicken intestine. We describe its genome characteristics and further identify novel carbohydrate degradation enzyme genes and biosynthetic gene clusters (BGCs) potentially involved in pathogen antagonism.
Results and discussion
Isolation, identification, and phylogenetic analysis
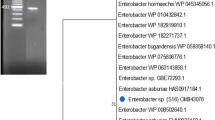
Bacteria of the genus Paenibacillus have been isolated from a variety of environments, such as humans, animals, plants, and soil [8]. The bacterial strain YPG26T was isolated from the chicken intestine. The phylogenetic tree was constructed using the neighbor-joining method and the maximum likelihood method with 1000 bootstrap replications based on the 16S rRNA gene sequence. It showed the strain YPG26T was assigned to the genus Paenibacillus and was closest related to the species Paenibacillus telluris PS38T (GenBank accession no. HQ257247) with 97.61% similarity, but the strain YPG26T formed a distinct phylogenetic branch within the genus Paenibacillus (Fig. 1A). The same relationship was also supported in trees reconstructed using the maximum likelihood method (Fig. 1B). In general, the recommended 16S rRNA sequence similarity thresholds for bacterial genus and species identification were 95% and 98.65%, respectively [9]. Therefore, based on the classification threshold, the strain YPG26T should be assigned as a novel species of Paenibacillus. The accession number for the 16S rRNA gene sequence of the strain YPG26T deposited in the GenBank database is OK324374.
Phylogenetic analysis of the strain YPG26T Neighbor-joining (A) and maximum likelihood (B) phylogenetic tree based on the 16S rRNA gene sequences of the strain YPG26T (1384 bp) showed the taxonomic position of the strain YPG26T and closely related taxa. Bootstrap values (percentages of 1000 replications) are shown at branch points. Bacillus subtilis DSM 10 T (GenBank accession no. AJ276351) was used as outgroup. The bar, 0.01 nucleotide substitutions per site
Phenotypic characteristics
Paenibacillus Species can be gram-negative, gram-positive, or gram variable, and have different growth status on the same medium (the same as P. glycanilyticus CCI5 [10], the strain YPG26T cannot grow on LB medium, while Paenibacillus alvei MP1 can grow on LB medium [11]).The strain YPG26T formed a single separated colony on TSB agar plate that was 1–1.5 mm in diameter, circular with slightly irregular edges, grayish-white, low convex, translucent, and glossy after aerobic cultivation at 37℃ for 20 h (Fig. S1A). The colony was slightly different in anaerobic culture (Fig. S1B). By transmission electron microscope observation, bacterial cells were rods approximately 0.9–1.2 μm wide and 4.0–5.0 μm long (Fig. S1C). Gram staining showed that the strain was positive (Fig. S1D). Growth was observed at pH levels ranging from 5–8, with an optimum pH at 8.0, temperatures ranging from 15–50 °C, with an optimum growth at 37 °C, and the strain tolerated NaCl concentration of up to 2.0% (Fig. S1E-G). Other physiological and biochemical characteristics are provided in Table 1, and the attributes of reference species are also described together (Table 1).
Genomic properties, ANI and DDH analyses
The generated complete genome of the strain YPG26T was composed of a 3,966,665 bp circular chromosome (Fig. 2) with a G + C content of 49.34% (mol%), which fit the range of the genome size of Paenibacillus from 3.02 Mbp (P. darwinianus Br) to 8.82 Mbp (P. mucilaginosus K02) and G + C content from 39 to 59 mol% [8]. It carried 33 rRNA genes, 89 tRNA genes, and 3871 protein-coding genes (CDSs) (Table 2). The functional gene annotation was performed by blasting predicted genes against the COG and GO databases (Fig. S2).
Circular representation of the strain YPG26T genome From the outside to the inside: circle 1, genomic position in kb; circles 2, protein-coding sequences (CDS) on the forward and the reverse strands; circles 3 and 5, COG, GO functional classification of protein-coding genes on forward and reverse strands, respectively; circles 6, non-coding RNA; circles 7, G + C content, green (outward) and red (inward) indicate higher and lower than average value of 49.34%, respectively; circles 8, GC skew ([G—C]/[G + C]), pink (outward) and light green (inward) denote positive (leading strand) and negative (lagging strand) values, respectively
Taxonomic and functional research of microorganisms has increasingly relied upon genome-based data and methods [15]. DNA-DNA hybridization (DDH) and average nucleotide identity (ANI) have become two gold standards for prokaryotic species circumscriptions at the genomic level [16]. The DDH and ANI values between the strain YPG26T and reference species of Paenibacillus ranged from 13.2 to 14.0% and 67.56% to 71.07%, respectively (Table S1), which were well below the proposed thresholds of 70% and 95% for prokaryotic species delineation[17, 18]. The results of the genome analysis were consistent with the outcome of the 16S rRNA sequence-based phylogenetic analysis. It also confirmed that the strain YPG26T was a novel Paenibacillus species at the genome level, which was suggested to be named Paenibacillus jilinensis sp. nov. (jilinensis pertaining to Jilin, a province in northeast China). The type strain is YPG26T (= CCTCC M2020899T). The whole-genome sequence of P. jilinensis YPG26T has been deposited on the GenBank database with accession number CP084530.
Identification of carbohydrate-active enzymes
Carbohydrate-active enzymes (CAZymes) are responsible for the biosynthesis, modification, and degradation of carbohydrates and glycoconjugates. They are involved in many metabolic pathways and are essential for microorganisms' survival [19]. By analyzing 41 Paenibacillus genomes comprising 25 species, Huang WC et al. found Paebacillus genomes encode a wide repertoire of CAZymes [20]. Three CAZymes classes were predicted in the genome of P. jilinensis YPG26T using the carbohydrate-active enzymes database (CAZy). Glycoside hydrolases (GHs) were the most abundant class, with 58 predicted domains, followed by 50 glycosyl transferases (GTs) and 17 carbohydrate esterases (CEs) (Fig. 3A). Moreover, a total of 52 putative carbohydrate binding modules (CBMs) were present in the genomic sequence, which are appended to CAZymes and assist in substrate binding and stimulate the catalytic efficiency of the enzymes [21]. Paenibacillus species can produce some distinct variations in the numbers and families of CAZymes. These variations also explain the good adaptability of Paenibacillus species to different circumstances [22].
CAZymes distribution and starch degrading enzyme activity of the strain YPG26T (A) Number of identified CAZymes families in the genomes of the strain YPG26T; B Starch degrading enzyme activity on starch agar plate (an area of clearance around a bacterial colony can be observed); C Phylogenetic analysis of predicted α- and β-amylases from the strain YPG26T
Thirty-eight GHs were distributed to characterize starch, chitin, and cellulose degradation based on functional categorization (Table 3). Starch degrading activity was also observed in the experiment (Fig. 3B, the chitin and cellulose degradation activity tests were not carried out). Amongst the starch degrading enzymes, 13 α-amylase from the GH13 family and 2 β-amylase from the GH14 family were represented. The phylogenetic tree of these deduced amylase showed that there were differences (Fig. 3C) and only five amylase amino acid sequences had more than 70% similarity with sequences available in the GenBank database (Table S2).
Antimicrobial activity
There is a large variety of antimicrobial substances produced by Paenibacillus species, which can target a range of human pathogens and plant pathogenic fungi [23]. Similarly, an in vitro antibacterial activity assay demonstrated some Enterococcus species are inhibited by cell-free supernatant (CFS) of P. jilinensis YPG26T, but other pathogens were not inhibited by the agar diffusion method (Table 4). We suspected that the concentration of antimicrobial substances in CFS was too low to measure. Thus, we prepared crude extract by saturated ammonium sulfate precipitation, and the antimicrobial activity was detected by growth determinations after co-culture with CFS and crude extract. The results showed that the growth of the majority of tested pathogenic bacteria was inhibited significantly after co-culturing with CFS compared to control cultures incubated without CFS, and the crude extract showed strong growth inhibition (Fig. 4), which indicated P. jilinensis YPG26T can produce broad-spectrum antimicrobial substances.
Antimicrobial activity of the strain YPG26T against different pathogens. (Antimicrobial activity of the strain YPG26T against pathogens after 6 h incubation with or without CFS and crude extract of the strain YPG26T, were determined by optical density (600 nm) measurements. Two biological replicates were set and values are expressed as mean ± SEM. Data were analyzed by student's t-test, *p<0.05, **p<0.01, ***p<0.001)
Genome mining for BGCs of antimicrobial compounds
Genomic screening for biosynthetic gene clusters (BGCs) of antibacterial substances has been increasingly utilized in natural product discovery due to the large amount of bacteria whole-genome sequencing data available [24]. The genome mining indicated the presence of BGCs coding for 6 antimicrobial substances in the genome of P. jilinensis YPG26T: NRPS, PKS, lanthipeptide, proteusin, siderophore, and terpene (Fig. 5A), which all have low similarity with the known BGCs. The total size of all BGCs was approximately 238 kb, which accounted for 6.0% of the strain YPG26T genome (Fig. 5B). Similarly, a previous study found the great number of varieties of BGCs in Paenibacillus and Bacillus strains by genome mining [25].
Experiments with proteinase processing revealed the proteinaceous nature of the antimicrobial compounds of P. jilinensis YPG26T, indicating that they could be bacteriocin-like substances, which was most likely to match the class II lanthipeptide BGCs with 3 core biosynthetic genes (named YPG26-lan A1, A2, and A3, respectively, Table S3) in the genome of P. jilinensis YPG26T (Fig. 6A). The amino acid sequences of precursor peptides encoded by the three core biosynthetic genes were compared with other known class II lanthipeptides: bacteriocin J46, bovicin HJ50, butyrivibriocin OR79A, lacticin 481, mutacin II, nukacin ISK-1, steptococcin A-FF22, and variacin. The results showed that YPG26-lan A2 and YPG26-lan A3 have similar structural features to known lanthipeptides (Fig. 6B). The antibacterial activity of P. jilinenesis YPG26T was likely conferred by YPG26-lan A2 or A3, or both. Subsequently, we will conduct further experiments to confirm the putative core biosynthetic genes of the lanthipeptide.
Putative BGCs of antimicrobial substances of the strain YPG26T (A) Putative lanthipeptide BGCs of the strain YPG26T; B Multiple sequence alignment of the precursor peptide of the strain YPG26T lanthipeptide with the precursor peptide of known class II lanthipeptide, conserved residues are highlighted in yellow
Antibiotic-resistant genes analysis
An unreasonable use of antibiotics may change the intestinal flora, as a result, some commensal strains acquire antibiotic resistant genes (ARGs) in order to survive in the intestinal tract, which may increase public health risk in the future. Therefore, evaluation and monitoring of antibiotic resistant genes is an important measure to prevent resistance transfer [26]. Due to some bacteria’s slow growth or hard-to-culture, the use of whole-genome sequencing for antibiotic susceptibility testing has gradually become a powerful alternative [27]. The results revealed that 10 putative antibiotic resistance genes in the genome of P. jilinensis YPG26T were responsible for resistance to antibiotics, including 8 efflux pump genes, 1 LlmA 23S ribosomal RNA methyltransferase gene, and 1 nucleoside resistance protein (tmrB) gene (Table S4). Culture-based antimicrobial susceptibility testing is still the primary method employed by clinical laboratories [27]. Thus, the sensitivity of P. jilinensis YPG26T to different antibiotics has also been verified experimentally using the commercial antibiotics-discs diffusion method. It is a wonder that, the results showed sensitivity to all antibiotics tested (Table 5). This indicated that the strain YPG26T has good safety.
Conclusion
In this study, we identified a novel Paenibacillus species isolated from the chicken intestine. The assembled genome analysis revealed that large numbers of carbohydrate degrading enzymes (CAZymes) genes and biosynthetic gene clusters (BGCs) of antimicrobial compounds are encoded in the genome. These genomic characteristics provid a better opportunity for understanding the intestinal niche adaption and biosynthetic potential of Paenibacillus, which will be indispensable for the direction of application in pharmaceutics, agriculture, or industry. Meanwhile, this study also contributed to the understanding of the genome features of intestinal uncultured bacteria.
Methods
Collection of microorganisms and isolation
Chicken fecal microorganisms (Changchun, Jilin Province, China) were serially diluted in sterile phosphate-buffered saline (PBS) solution and spread on yeast proteose glucose (YPG) agar plates with modifications (g/per 1000 ml: Tryptone 20.0, Yeast extract 10.0, Glucose 5.0, NaCl 0.08, L-cysteine 0.5, CaCl2 0.008, MgSO4 0.008, NaHCO3 0.4, starch 5.0, pectin 0.5, Agar 15.) [28] and cultivated in anaerobic conditions (85% N2, 10% H2, 5% CO2) for 3 days at 37℃, the strain YPG26 was purified by subculturing. After isolation and purification, unless otherwise indicated, YPG26 was routinely cultured aerobically on tryptic soy broth (TSB, Qingdao Hope Biotechnology Co., Ltd, China). The strain YPG26T was deposited in the China Center for Type Culture Collection (CCTCC) under the accession number CCTCC M2020899T.
Phylogenetic analysis
The 16S rRNA gene of the strain YPG26T was amplified and sequenced as previously described [29, 30]. Briefly, bacterial cells were dissolved in PCR lysis buffer (Takara, Japan). The genomic DNA was extracted using a commercial genomic DNA extraction kit, and the 16S rRNA gene was amplified using the universal bacterial primers 27F and 1492R [31]. The PCR product was sequenced, and the 16S rRNA gene sequence was compared with sequences available in GenBank by the nucleotide BLAST to determine an approximate phylogenetic affiliation. Bacillus subtilis DSM 10 (GenBank accession no. AJ276351) was used as an outgroup. The phylogenetic tree was set up using the neighbor-joining method and the maximum likelihood method in the MEGA 11.0 software [32], and the topologies were evaluated using the bootstrap resampling method with 1000 replications.
Phenotypic characterization
Cell growth of the strain YPG26T was monitored by measuring the optical density at 600 nm as previously described [33, 34], NaCl tolerance was measured in TSB medium supplemented with NaCl (i.e., the concentration of NaCl was 0.5–5%, w/v), and growth at different pH (2.0–10.0) and temperatures (4–50℃) was also tested. Cell morphology and the flagellum type were observed by transmission electron microscopy. The gram reaction was determined using the gram stain kit (Hangzhou Microbial Reagent Co., Ltd, China). Catalase activity was measured by the generation of bubbles in a 3% (v/v) H2O2 solution. Carbon source tests and biochemical tests were performed using the bacterial biochemical identification tube (Hangzhou Microbial Reagent Co., Ltd, China). Growth under anaerobic circumstances was measured in the anaerobic incubator (AL-B, LABIOPHY, Dalian, China) in an atmosphere of 85% N2, 10% CO2, and 5% H2 at 37 °C.
Whole-genome sequencing, assembly, and annotation
Total genomic DNA of the strain YPG26T was extracted using the bacteria DNA kit (TianGen Biotech (Beijing) Co. Ltd, China). The quality and quantity of genomic DNA were evaluated using agarose (Invitrogen, USA) gel electrophoresis and a Qubit fluorometer (Thermofisher, USA), respectively. Use the DNA library prep kit (NEB, USA) for sequencing library preparation. The whole genome of the strain YPG26T was performed using the PE150 PacBio Sequel platform and Illumina NovaSeq at the Beijing Novogene Bioinformatics Technology Co., Ltd. Assembly was completed by using SMRT link v5.0.1. Furthermore, the corrected assembly result was filtered with the base minimum mass value of 20. Finally, based on the overlap between the head and the tail, confirmed whether the genome sequence formed a circle or not and corrected the initial site by blasing with the DNA database. The annotation of the assembled genome was performed using rapid annotation subsystems technology (RAST) [35]. The tRNA and rRNA were predicted using the tRNAscan-SE [36] and rRNAmmer [37], respectively. For functional gene annotation, GO (gene ontology) [38] and COG (clusters of orthologous groups) database [39] were used.
Genome-genome distance, and average nucleotide identity
The digital DNA-DNA hybridization (dDDH) values among the strain YPG26T and other members of the Paenibacilllus were calculated using the genome-to-genome distance calculator 3.0 (GGDC) [40]. Furthermore, the pairwise genome similarity was assessed using the average nucleotide identity (ANI) calculated with the JSpeciesWS web (http://jspecies.ribohost.com/jspeciesws/#analyse) [41].
CAZymes identification and mining of starch degrading genes
The carbohydrate-active enzymes (CAZymes) of the strain YPG26T were identified and classified using the carbohydrate-active enzymes database [42]. The starch-degrading genes were further revealed by cross-checking with the annotations available in the database. In vitro determination of amylase activity was carried out according to previously described [43]. Briefly, starch agar media (g/per 1000 ml: Tryptone 10.0, Yeast extract 10.0, KH2PO4 5.0, soluble starch 3.0, Agar 15) was used, 10 μL with 108 cfu/mL of the strain YPG26T was placed on the center of the plate and incubated at 37 °C for 48 h. For visualization of the zone of clearance, the plate was flooded with 2 mL of Gram’s iodine solution.
Antimicrobial activity
To prepare cell-free supernatants (CFS), the strain YPG26T was cultured in TSB at 37℃ for 10 h with shaking at 200 rpm. After incubation, bacterial suspension was centrifuged at 8000 g for 10 min. Supernatants were collected and filter-sterilized with a 0.22 μm filter (Millipore, USA). The antimicrobial activity of CFS was evaluated initially according to the effect of CFS on the viability of the pathogenic bacteria [44] and appropriate modifications. Briefly, overnight pathogenic cultures were sub-cultured in TSB at 37℃ to logarithmic phase. Adjusting OD600 to 0.02, 50 µL of each pathogenic bacteria was added per well in a 96-well plate, followed by 50 µL CFS of the strain YPG26T or TSB medium alone (negative control). The cultures were incubated at 37℃ for 6 h, the absorbance values at 600 nm were measured using a microplate reader (Eppendorf, Germany). Relative to the absorbance value of the negative control, the absorbance value of adding CFS reflects the antimicrobial activity of the strain YPG26T. Two biological replicates were set.
To prepare crude extracts, the saturated ammonium sulfate was slowly added to the supernatant to reach 70% saturation. The precipitate was collected after standing at 4℃ overnight and 10,000 g was centrifugated for 20 min. It was then redissolved in sterile distilled water, and dialyzed extensively with sterile distilled water to remove ammonium sulfate. Finally, the crude extract was freeze-dried, then redissolved in sterile distilled water and the antibacterial activity was detected by the same method as the CFS antibacterial activity evaluation.
Genome mining for BGCs of antimicrobial compounds
Genome mining for biosynthetic gene clusters (BGCs) of antimicrobial compounds was carried out using the antiSMASH version 6.0.1 [45]. BGCs that differed from previously reported ones by less than 70% were considered novel [46]. The putative core biosynthetic genes of bacteriocin were further confirmed with NCBI protein BLAST. Multiple alignments of amino acid sequences of the deduced precursor peptide with other known lanthipeptides were performed using MEGA 7.0 software.
Antibiotic-resistant genes analysis
Antibiotic-resistant genes of the strain YPG26T were identified by comparing whole-genome sequences against the comprehensive antibiotic research database (CARD) [47]. The antibiotic susceptibility of the strain YPG26T was determined by modifying the disc diffusion test as previously described [48]. Briefly, commercial antibiotic discs (Hangzhou Microbial Reagent Co., Ltd, China) were placed on MH agar (Qingdao Hope Biotechnology Co., Ltd, China) plates inoculated with strain YPG26T (108 CFU). The plates were incubated at 37℃ for 24 h. Inhibition zone diameters were measured and referred to the Clinical and Laboratory Standards Institute (CLSI) interpretative zone diameters for disc diffusion. The results were described in terms of resistance (R), moderate resistance (MR), or susceptibility (S).
Availability of data and materials
The 16S rRNA sequence is available in GenBank with the accession OK324374 (https://www.ncbi.nlm.nih.gov/nuccore/OK324374). And the whole genome data are available at DDBJ/EMBL/GenBank under the bioproject accession PRJNA766864 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA766864).
References
Stewart EJ. Growing unculturable bacteria. J Bacteriol. 2012;194(16):4151–60.
Nichols D, Cahoon N, Trakhtenberg EM, Pham L, Mehta A, Belanger A, Kanigan T, Lewis K, Epstein SS. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl Environ Microbiol. 2010;76(8):2445–50.
Lagier JC, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203.
Cockburn DW, Koropatkin NM. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J Mol Biol. 2016;428(16):3230–52.
Klassen L, Xing X, Tingley JP, Low KE, King ML, Reintjes G, Abbott DW. Approaches to investigate selective dietary polysaccharide utilization by human gut microbiota at a functional level. Front Microbiol. 2021;12: 632684.
Garcia-Gutierrez E, Mayer MJ, Cotter PD, Narbad A. Gut microbiota as a source of novel antimicrobials. Gut Microbes. 2019;10(1):1–21.
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. Overview of the antimicrobial compounds produced by members of the bacillus subtilis group. Front Microbiol. 2019;10:302.
Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC. Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Fact. 2016;15(1):203.
Kim M, Oh HS, Park SC, Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64(Pt 2):346–51.
Akita H, Itoiri Y, Takeda N, Matsushika A, Kimura ZI. Paenibacillus glycanilyticus subsp. hiroshimensis subsp. nov., isolated from leaf soil collected in Japan. Arch Microbiol. 2021;203(4):1787–93.
Pajor M, Xiong ZR, Worobo RW, Szweda P. Paenibacillus alvei MP1 as a Producer of the proteinaceous compound with activity against important human pathogens, including staphylococcus aureus and listeria monocytogenes. Pathogens. 2020;9(5):319.
Lee JC, Kim CJ, Yoon KH. Paenibacillus telluris sp. nov., a novel phosphate-solubilizing bacterium isolated from soil. J Microbiol. 2011;49(4):617–21.
Shida OTH, Kadowaki K, Nakamura LK, Komagata K. Emended description of Paenibacillus amylolyticus and description of Paenibacillus illinoisensis sp. nov. and Paenibacillus chibensis sp. nov. Int J Syst Bacteriol. 1997;47(2):299–306.
Tonouchi A, Tazawa D, Fujita T. Paenibacillus shirakamiensis sp. nov., isolated from the trunk surface of a Japanese oak (Quercus crispula). Int J Syst Evol Microbiol. 2014;64(Pt 5):1763–9.
Shi W, Sun Q, Fan G, Hideaki S, Moriya O, Itoh T, Zhou Y, Cai M, Kim SG, Lee JS, et al. gcType: a high-quality type strain genome database for microbial phylogenetic and functional research. Nucleic Acids Res. 2021;49(D1):D694–705.
Richter M. R-MR: Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA. 2009;106(45):19126–31.
Meier-Kolthoff JP, Klenk HP, Goker M. Taxonomic use of DNA G+C content and DNA-DNA hybridization in the genomic age. Int J Syst Evol Microbiol. 2014;64(Pt 2):352–6.
Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu XW, De Meyer S, et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68(1):461–6.
Lee B. Extremophilic carbohydrate active enzymes (CAZymes). J Nutr Health Food Eng. 2017;7(1):230–7.
Huang WC, Hu Y, Zhang G, Li M. Comparative genomic analysis reveals metabolic diversity of different Paenibacillus groups. Appl Microbiol Biotechnol. 2020;104(23):10133–43.
Pasari N, Adlakha N, Gupta M, Bashir Z, Rajacharya GH, Verma G, Munde M, Bhatnagar R, Yazdani SS. Impact of module-X2 and carbohydrate binding module-3 on the catalytic activity of associated glycoside hydrolases towards plant biomass. Sci Rep. 2017;7(1):3700.
Pasari N, Gupta M, Eqbal D, Yazdani SS. Genome analysis of Paenibacillus polymyxa A18 gives insights into the features associated with its adaptation to the termite gut environment. Sci Rep. 2019;9(1):6091.
Olishevska S, Nickzad A, Deziel E. Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl Microbiol Biotechnol. 2019;103(3):1189–215.
Xu P, Xie S, Liu W, Jin P, Wei D, Yaseen DG, Wang Y, Miao W. Comparative genomics analysis provides new strategies for bacteriostatic ability of Bacillus velezensis HAB-2. Front Microbiol. 2020;11: 594079.
Zhou L, Song C, Li Z, Kuipers OP. Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential. BMC Genomics. 2021;22(1):29.
Szmolka A, Nagy B. Multidrug resistant commensal Escherichia coli in animals and its impact for public health. Front Microbiol. 2013;4:258.
Su MSS, Read TD. Genome-based prediction of bacterial antibiotic resistance. J Clin Microbiol. 2019;57(3):e01405-01418.
Ferrario C, Alessandri G, Mancabelli L, Gering E, Mangifesta M, Milani C, Lugli GA, Viappiani A, Duranti S, Turroni F, et al. Untangling the cecal microbiota of feral chickens by culturomic and metagenomic analyses. Environ Microbiol. 2017;19(11):4771–83.
Aw YK, Ong KS, Lee LH, Cheow YL, Yule CM, Lee SM. Newly Isolated Paenibacillus tyrfis sp. nov., from Malaysian tropical peat swamp soil with broad spectrum antimicrobial activity. Front Microbiol. 2016;7:219.
Akter S, Huq MA. Biological synthesis of ginsenoside rd using Paenibacillus horti sp. nov. isolated from vegetable garden. Curr Microbiol. 2018;75(12):1566–73.
Akter S, Wang X, Lee SY, Rahman MM, Park JH, Siddiqi MZ, Balusamy SR, Nam K, Rahman MS, Huq MA. Paenibacillus roseus sp. nov., a ginsenoside-transforming bacterium isolated from forest soil. Arch Microbiol. 2021;203(7):3997–4004.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–7.
Wei W, Wang K, Hu X, Yang S, Zeng C, Hou Z, Liu S, Cui H, Zhu L. Phytoactinopolyspora limicola sp. nov., an alkaliphilic actinomycete isolated from a soda alkali-saline soil. Arch Microbiol. 2021;203(4):1367–74.
Huq MA. Paenibacillus anseongense sp. nov. a silver nanoparticle producing bacterium isolated from rhizospheric soil. Curr Microbiol. 2020;77(9):2023–30.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75.
Lowe TMES. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–64.
Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35(9):3100–8.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. Gene Ontol Consortium Nat Genet. 2000;25(1):25–9.
Tatusov RLGM, Natale DA, Koonin EV. The COG database_ a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28(1):33–6.
Auch AF, Klenk HP, Goker M. Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand Genomic Sci. 2010;2(1):142–8.
Richter M, Rossello-Mora R, Oliver Glockner F, Peplies J. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics. 2016;32(6):929–31.
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The carbohydrate-active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 2009;37(Database issue):D233-238.
Latorre JD, Hernandez-Velasco X, Wolfenden RE, Vicente JL, Wolfenden AD, Menconi A, Bielke LR, Hargis BM, Tellez G. Evaluation and selection of bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front Vet Sci. 2016;3:95.
Scillato M, Spitale A, Mongelli G, Privitera GF, Mangano K, Cianci A, Stefani S, Santagati M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiologyopen. 2021;10(2): e1173.
Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021;49(W1):W29–35.
Li Z, Song C, Yi Y, Kuipers OP. Characterization of plant growth-promoting rhizobacteria from perennial ryegrass and genome mining of novel antimicrobial gene clusters. BMC Genomics. 2020;21(1):157.
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen AV, Cheng AA, Liu S, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48(D1):D517–25.
Charteris WPKP, Morelli L, Collins JK. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J Food Prot. 1998;61(12):1636–43.
Acknowledgements
Not applicable
Funding
This work was supported by National Natural Science Foundation of China [No. 32070119, 31972682].
Author information
Authors and Affiliations
Contributions
YYJ, CW and MK designed experiments. MK, YSQ, and LXQ collected samples. MK, YSQ, LXQ, LZZ, ZJB, GY performed the experiments. MK, YYJ and CW analyzed and compiled data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, K., Chen, W., Yan, SQ. et al. Genome mining reveals polysaccharide-degrading potential and new antimicrobial gene clusters of novel intestinal bacterium Paenibacillus jilinensis sp. nov.. BMC Genomics 23, 380 (2022). https://doi.org/10.1186/s12864-022-08623-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-022-08623-4