Abstract
Background
The importance of Indian germplasm as origin and primary center of diversity of cultivated melon is widely accepted. Genetic diversity of several collections from Indian has been studied previously, although an integrated analysis of these collections in a global diversity perspective has not been possible. In this study, a sample of Indian collections together with a selection of world-wide cultivars to analyze the genetic diversity structure based on Genotype by Sequence data.
Results
A set of 6158 informative Single Nucleotide Polymorphism (SNP) in 175 melon accessions was generated. Melon germplasm could be classified into six major groups, in concordance with horticultural groups. Indian group was in the center of the diversity plot, with the highest genetic diversity. No strict genetic differentiation between wild and cultivated accessions was appreciated in this group. Genomic regions likely involved in the process of diversification were also found. Interestingly, some SNPs differentiating inodorus and cantalupensis groups are linked to Quantitiative Trait Loci involved in ripening behavior (a major characteristic that differentiate those groups). Linkage disequilibrium was found to be low (17 kb), with more rapid decay in euchromatic (8 kb) than heterochromatic (30 kb) regions, demonstrating that recombination events do occur within heterochromatn, although at lower frequency than in euchromatin. Concomitantly, haplotype blocks were relatively small (59 kb). Some of those haplotype blocks were found fixed in different melon groups, being therefore candidate regions that are involved in the diversification of melon cultivars.
Conclusions
The results support the hypothesis that India is the primary center of diversity of melon, Occidental and Far-East cultivars have been developed by divergent selection. Indian germplasm is genetically distinct from African germplasm, supporting independent domestication events. The current set of traditional Indian accessions may be considered as a population rather than a standard collection of fixed landraces with high intercrossing between cultivated and wild melons.
Similar content being viewed by others
Background
Melon (Cucumis melo L.) is one of the most important fruit crop species belonging to the Cucurbitaceae family. Nowadays, melon is cultivated worldwide and extensive phenotypic variability is found among modern and traditional cultivars, landraces, feral and wild plants. Several intraspecific classifications have been proposed based on morphological traits [1, 2]. In the last proposed classification, 19 horticultural groups were defined: agrestis, kachri, chito, tibish, acidulus, momordica, conomon, makuwa, chinensis, flexuosus, chate, dudaim, chandalak, indicus, ameri, cassaba, ibericus, inodorus, and cantalupensis [3]. Concomitantly, molecular markers have been used to study the distribution of the genetic diversity in the species [4,5,6]. In general, molecular diversity analyses cluster cultivars in groups that fit the morphological ones, which indicates that the Mediterranean/Near East groups (ameri, cassaba, chandalak, ameri, ibericus, inodorus, cantalupensis) are closely related and quite differentiated from Far-East groups (conomon, makuwa, chinensis).
C. melo is native to Asia [7], although the history of domestication and diversification is not clear yet [3]. Wild melons are found in both Africa and Asia (India), what could be explained by the migration of wild melons from Asia to Africa. African and Indian cultivars/landraces are clustered with wild melons from their respective regions [6], what support at least two domestication events. Domestication of melons in Asia would have led to the diversification of most melon horticultural groups that spread rapidly westwards to the Mediterranean Basin in ancient times, as supported by C. melo seeds found which date from pre-dynastic Egypt [8,9,10]. Domestication in Africa would have had a marginal impact, as only tibish cultivars would have been generated from this event [11]. Recently, Endl et al. [12] also supported the two domestication events and the role of Asian domestication in the origin of most current melon cultivars. Therefore, Central Asia can be considered the best candidate region for the major domestication and diversification events.
Indian melon genetic diversity has been studied thoroughly with Single Sequence Repeat (SSR) markers in four collections from different regions of India: landraces North India (IC collection, [13]), East India (AM collection, [14]), South India (SM collection, [15]), and wild genotypes from North India (WM collection, [16]), together with reference accessions (from makuwa to inodorus). Most of the Indian collections showed a lateral position with the reference accession in the genetic diversity plot, except for the IC collection from North India [13]. These results suggested that the IC collection could represent a center of melon diversity, i. e., crop diversified in a divergent way from India to Mediterranean and Far East regions from North India. The rest of the collections could represent genetic diversity that would have remained mostly in India. In order to confirm this hypothesis, it is necessary to take a broader view of genetic variability by including these Indian collections together with a larger array of melon accessions. The integration of IC collection matrix data [13] with the rest of Indian collections was not possible as the SSR markers used for the experiments were different, hampering the study of the genetic relationship between the IC and AM, SM and WM collections. Other published works used different marker systems which makes it impossible for data integration.
Genotyping-by-Sequencing (GBS) approaches [17] have become popular as an efficient and affordable method to obtain a large number of marker genotypes for different genetic studies [18]. The most common approach consists of genome complexity reduction by restriction enzyme digestion and adapter ligation before sequencing. We decided to re-analyze a sample of those Indian collections with reference cultivars by GBS. Recently, Nimmakayala et al. [19] analyzed by GBS a collection of 120 melon accessions with a good representation of occidental horticultural groups (cantalupensis, inodorus,… etc.) and some Far-East accessions (makuwa and conomon horticultural groups) although with a low representation of Indian accessions. This collection perfectly complements the previously reported Indian collection. GBS library construction can produce different representation of the genome, although part of the represented genome may be common between independent experiments, making the integration possible. In the current report, we obtained GBS data from a sample of the IC-, AM-, SM- and WM- collections as well as 50 additional reference accessions and merged these with Nimmakayala et al. [19] data. Thus, a genotypic data matrix with sufficient Single Nucleotide Polymorphism (SNP) information and representing major melon horticultural groups was obtained to test the hypothesis on the origin of melon diversification in Central Asia. Furthermore, the generated data was also used to gain some insights into the evolution of the melon genome through cultivar diversification and identification of candidate genomic regions that could be involved in the process.
Results
SNP discovery by genotyping-by-sequencing
The GBS analysis of the 78 new IBMCP melon accessions (Additional file 1: Table S1) resulted in 146,367 SNPs with at least three reads of support (Additional file 2; Table S2). After filtering for Minor Allele Frequency (MAF) higher than 0.05 and frequency of missing data lower than 0.05, the retained number of SNPs was 8215, similar to the 7609 SNPs retained after filtering the WVSU SNP data set [19], which demonstrates that the quality of both data sets was similar. All the SNPs were integrated before filtering from both datasets in order to retain more SNPs., A total of 8212 common SNPs were found among data sets. The merged matrix was filtered for MAF > 0.05 and presence of SNP data in at least 100 accessions, remaining 6169 SNPs. Finally, 11 SNPs showed a heterozygosity larger than 0.7, likely an artifact due to sequencing errors or duplicated sequences, and they were also removed. Finally, 6158 SNPs were retained, with a mean of 513 SNP/chromosome, ranging from 657 SNP in chromosome 1 to 337 SNP in chromosome 10. The SNP density was 12.8 SNP/Mb.
Genetic structure and diversity
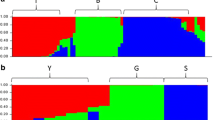
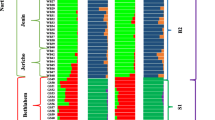
Multidimensional scaling (MDS) analysis was carried out to investigate the genetic structure of the current melon germplasm. A preliminary analysis was performed to verify the absence of bias due to GBS data merging. We found that accessions from the same horticultural group (cantalupensis, conomon, dudaim and inodorus) from the two data sets plotted in the same MDS space region, indicating that the possible bias for merging was negligible (Additional file 3: Figure S1). Furthermore, the novelty of the Indian germplasm included in the current report is also evident in this figure. Subsequent analysis were performed after pruning the genotype matrix for linkage disequilibrium, resulting in a final matrix with 4661 SNPs. The three first MDS axis explained 85% of the variance, 75, 7 and 3% the axis X, Y and Z, respectively. The MDS plot (Fig. 1a) depicted a diversity distribution through the X-axis that is concomitant with the geographic origin of the accessions: Mediterranean/Near East accessions on the left, Indian in the center towards the right and conomom (China and Far East) on the right. Integrating the X and Y MDS axis, six groups could be defined: cantaloupe (including cantalupensis cultivars), inodorus-related (flexuosus, inodurus, ameri cultivars and landraces), dudaim (dudaim landraces), Indian (acidulus, chito, snapmelons and wild Indian accessions) and conomon (makuwa and conomon cultivars and landraces) and accessions of African origin. The Z-axis also differentiated African accessions and cantaloupes from the rest (Fig. 1b) and cantaloupe. STRUCTURE analysis showed that K = 5 had the highest peak based on Delta K distribution, supporting the groups defined by MDS (Additional file 4: Figure S2), except of dudaim that appeared as a mixture population.
Multidimensional scaling (MDS) of different groups of accessions based on GBS SNPs. (a) and (b) depict the XY and XZ axis, respectively, for the whole germplasm collections, which define the groups highlighted with colors as indicated in the legend (nd indicates accessions that were not assigned to any group). (c) MDS focused only on Indian germplasm, indicating the subgroups according to the legend
Cantaloupe and inodorus-related groups were located in the left part of the MDS plot, consistent with their classification in the C. melo subsp. melo. These two groups were distinguished only in the Y-axis. STRUCTURE separated both groups at K = 4 and K = 5 (Additional file 4: Figure S2). In general, the pre-defined classification of cultivar type fitted very well with the MDS and STRUCTURE groups, except for a few cantalupensis and reticulatus types which were grouped in the inodorus-related MDS group, what could be explained as a misclassification (for example Honeydew and Ananas melons usually are classified as inodorus) as most of them are traditional cultivars with properties that may not fit exactly with the modern commercial types.
Most of the pre-defined dudaim accessions were included in a MDS group (named afterwards as dudaim group), except three of them that were ungrouped and ‘Jenny Lind’ which grouped with the cantaloupe group. This group, despite of being classified initially as C. melo subsp. melo (Additional file 1: Table S1), was located in the center of the plot with other C. melo subsp. agrestis accessions/landraces (Fig. 1a). STRUCTURE analysis did not allow a clear assignation of dudaim landraces in any subspecies. For K = 2, dudaim group seemed to be more related to Indian, conomon and African groups (all of them belonging to subsp. agrestis), however, for K > 3 the group seems to be a mixture of several populations (Additional file 4: Figure S2.). A larger sample of these landraces would be necessary to classify them firmly in any of the subspecies. Conomon and makuwa cultivars and landraces plotted together on the right part of the MDS plot, showing the highest distance from the cantaloupe and inodorus-related groups (Fig. 1a). STRUCTURE analysis also classified these groups as a single defined population for K > 3.
Indian and African groups were located in the middle of the plot, although slightly separated, and clearly differentiated from the other groups (Fig. 1b, Additional file 4: Figure S2.). STRUCTURE analysis (K = 5) also supported a separation of African and Indian accessions (Additional file 4: Figure S2.). Two African accessions (CO148 and TGR1551) gathered together with the Indian group what likely reflects migration from India. Wild melons from Africa and India were included in different STRUCTURE populations that are related with their geographical origin, with their respective cultivated accessions.
A detailed MDS analysis within the Indian group was performed (Fig. 1c). The Indian populations IC (North India), SM (North East India), AM (South India), and WM (Wild North India) were grouped according their geographic origin. Wild North Indian group (WM) was slightly separated from the cultivated North Indian group (IC), although the degree of separation was much lower than the separation of IC group from the other cultivated Indian groups (AM, SM), and one accession from North-East_India cluster with Wild_Norhth_India.
Genetic diversity
Two levels of classification were defined for the AMOVA analysis: two subspecies (C. melo subsp. melo and subsp. agrestis) and six groups (African, cantaloupe, conomon, dudaim, Indian and inodorus-related), based on MDS results. The highest percentage of variation, 58.49%, was due to the diversity within groups (Table 1a). Variation between subspecies (C. melo agrestis vs melo) was 22.29%, and the variation among groups within subspecies was 19.23% of the total variance. Variation among groups according to Fst was 41% of the total genetic variation. The AMOVA done only with the groups also reflected that around 60% of the genetic diversity was within the defined groups (Table 1b).
The average He was 0.32, with the highest value for Indian, followed by inodorus-related and cantaloupe groups (Fig. 2). The distribution of He through the genome was quite variable among them. In Inodorus-related and cantaloupe groups, genomic regions with low or no diversity (He ≃ 0) were detected to be scattered through the genome in all chromosomes (Additional file 5: Figure S3). In the case of Indian group, this low diversity was less frequent than in the previous groups. Most of the genome showed low He within the conomon group (data not shown).
Observed (Ho) and expected (He) heterozygosity fixation index (Fis) and the ratio (MAF0.4/MAF0.05) between the number of SNPs with minor allele frequency higher than 0.4 (MAF0.4, i. e., highly variable) and number of SNPs with MAF lower than 0.05 (MAF0.05 i. e. very low variability) for the MDS-defined groups. This ratio was not calculated for Dudaim and African groups due to the low representation from these groups (indicated with a star). The overall mean is indicated with a dashed line for each estimate
On the other hand, Ho was very low (average Ho = 0.08) in most groups, except for the Indian group where it was equal to He (He = Ho = 0.22) (Fig. 2). Concomitantly, the fixation index (Fis) distribution showed a reverse pattern.
The ratio between the frequency of high polymorphic SNPs (MAF > 0.4) and low polymorphic SNPs (MAF < 0.05) was also calculated (MAF0.4/MAF0.05) to obtain further details on the distribution of genetic diversity within the groups. MAF0.4/MAF0.05 ratios were low in all the groups (even nearly 0 for the conomon group), except for the Indian group where MAF0.4/MAF0.05 was high. The frequency of SNPs with different MAF also varied drastically among the groups. For the whole collection, SNP frequency at different MAF showed low variability, ranging from 0.15 to 0.22 (Additional file 6: Figure S4.). On the other hand, SNPs with MAF > 0.1 were majority in the conomon group (92%), 49 and 57% in inodorus-related and cantaloupe groups, respectively, whereas it was only 28% in Indian group. In the first three groups, the frequency of SNPs with MAF > 0.1 decreased drastically, however in the Indian group the decrease of SNP frequency was observed only from MAF > 0.3, and was very less pronounced than in the previous groups.
Finally, the Wright’s F statistics were analyzed to find candidate loci under selection during the diversification to subspecies and groups. The coefficient of genetic differentiation among subspecies (Fct) explained 22.29% of the genetic variation between melons from the two subspecies, C. melo subsp. melo and C. melo subsp. agrestis. There was a considerable variation of Fct across the genome, nevertheless, 39 SNPs showed high levels of Fct (> 0.70) with loci located throughout all the chromosomes except for chromosome12 (Fig. 3). Chromosomes 1, 6 and especially 11 showed higher numbers of SNPs with high FCT.
The pair-wise Fst statistic, that is related to genetic differentiation among groups, was calculated between groups with sufficient representation (inodorus-related, cantaloupe and Indian, Fig. 4 Additional file 7: Table S3.). In the pairwise comparison between inodorus-related and cantaloupe groups 306 SNPs with Fst > 0.70 were found to be distributed through chromosomes 2, 3, 4, 6, 7, 8, 9 10, 11 and 12 (Fig. 4a). Between inodorus-related and Indian groups (Fig. 4b), 93 SNPs showed Fst > 0.70. SNPs with the highest Fst were found in chromosomes 1, 2, 4, 5, 6, 8, and 11. The comparison between the cantaloupe and Indian groups displayed 471 SNPs with Fst > 0.70, distributed in all the chromosomes (Fig. 4c). Nevertheless, SNPs with the highest Fst were coincident with the previous pairwise comparison.
Linkage disequilibrium and haplotype blocks
LD was analyzed initially in the whole germplasm collection (Fig. 5). LD was very variable among different genomic windows. LOESS regression was applied for pairwise distances lower than 100 kb, which showed that, in general, LD was low (r2 < 0.5) even at very close distances (< 1 kb) and it decreased steadily up to 40 kb approximately. The 95th percentile of the r2 among unlinked SNP was 0.35, corresponding to a physical distance of 17 kb (Fig. 5). LD was further analyzed separately for euchromatic and heterochromatic genome regions. The LD decay in the euchromatin was sharper, with a rapid decay up to 12 Kb, and, in this case, the 95th percentile of r2 among unlinked SNP corresponded to 8 Kb. On the other hand, there was no clear LD decay with distance, except for very close distances, in the heterochromatin regions, being nearly constant around r2 = 0.35 (Additional file 8: Figure S5A). LOESS window was increased to 200 kb. LD decay with the distance was clearer in this new settings, reaching the threshold value r2 < 0.35 at 30 kb when the LD was also calculated separately for the inodorus-related and cantaloupe groups defined by the MDS. In these cases, LD was more important, with r2 > 0.7 at very short distances, and the r2 threshold (r2 = 0.2) reached 49 and 97 Kb for inodurus-related and cantaloupe groups, respectively (Additional file 8: Figure S5B).
A total of 1128 haplotype blocks were identified by SNPanalyzer. The mean haplotype size was 59 kb, although most of them were small: only 9 (0.8%) were larger than 1 Mb, 40% smaller than 1 kb, being the median 4.5 kb (Additional file 9: Table S4). A larger number of haplotypes were detected in euchromatin when compared with heterochromatin (847 vs 281, Additional file 10: Figure S6), but smaller in size (mean haplotype size 31 kb) than in the heterochromatin (mean haplotype size 147 kb). In fact, eight out of a total of nine haplotypes larger than 1 Mb were located in heterochromatin regions.
The haplotype block frequency within and between inodorus-related, cantaloupe, conomon and Indian groups and the two subspecies was studied more thoroughly. A total of 17 haplotypes were found to be fixed in one of the groups/subspecies but segregating among other groups. The size of the haplotypes was variable (from 4.7 to 875 kb) and most of them were located in euchromatic regions (Table 2) Three haplotypes were found to be fixed in inodorus-related, five in cantaloupes and nine were characteristic of C. melo subsp. melo cultivars, and therefore are candidate regions for involvement in the diversification of melon cultivars.
Discussion
SNP discovery by genotyping by sequencing and merging
In the current report, data from two independent GBS experiments were merged. The final number of common SNPs after filtering (6169 SNPs) was significantly lower than the SNPs detected in each individual experiment and in previous melon GBS reports [19,20,21]. A proportion of detected SNPs is expected to be experiment-specific, as the reduction of genome complexity based on restriction enzyme digestion and subsequent ligation and PCR-amplification for sequencing library construction may generate different genome representation between independent experiments. Nevertheless, the number of common SNPs was sufficient to perform population genetics analysis. One important concern was the possible bias of the independent experiments in classifying the accessions. Fortunately, a preliminary classification showed that cultivars belonging to known horticultural groups clustered together in the MDS plot, which confirmed that there was no bias or it was negligible. Therefore, merging GBS data from independent experiments seems to be an appropriate strategy at least for population genetic analyses.
Genetic diversity
Previous SNP-based genetic diversity analysis of melon germplasm focused mainly on occidental germplasm (mainly inodorus, cantalupensis, ameri and flexuosus) with a relatively small representation of Far-East (conomon, makuwa, chinensis) and, especially, Indian germplasm [6, 19,20,21,22,23]. The general picture of all these works is similar: Occidental germplasm appears to be closely related, although with the major groups, inodorus and cantaloupe were clearly distinct at genetic level. Far East conomon is at the other extreme of the genetic variation space, while assorted accessions with Near East to Eastern origin are dispersed in the central space. Focusing only on the accessions that belong to the same horticultural groups in the current and previous reports, the general picture is the same, what confirms the reliability of the classification with the current data set.
The main novelty of the current report is the inclusion of Indian germplasm that had not been studied previously in a global array of melon accessions. MDS analysis showed that the Indian germplasm is located in the center of the MDS space as a compact but wide group. African germplasm is closely related, but STRUCTURE analysis supports its differentiation from Indian germplasm. The fact that cultivated and wild accessions are grouped by origin (African and India) indicates at least two domestication events occurring independently in the two continents, as proposed previously [11, 12]. Indian germplasm showed the highest level of genetic diversity, which also supports this region being the primary center of diversity of Euro-Asian melon cultivars. Occidental and Oriental cultivars were derived from India through a divergent selection process. Similar divergent diversification was found previously in cucumber [24]. Interestingly, genetic diversity in inodurus-related and cantaloupe groups remains relatively high, whereas in the conomon group it is very low. Bottleneck may have occurred during the diversification of conomon group cultivars, although this fact should be verified with a larger sample. On the other hand, the bottleneck that occurred during the diversification of inodorus-related and cantaloupe group cultivars seemed to be not so strong. However, the frequency of SNPs with high MAF have indeed reduced in inodorus-related and cantaloupe groups when compared with the Indian group. This result reflects a different distribution of the genetic variability in these groups. In the Indian group, the variability is shared among a high proportion of landraces, whereas in the rest of the groups it is fixed in a low number of landraces/cultivars. This fixation could be a consequence of farmer selection and development of homogeneous cultivars. The cultivars have been maintained by different farmers communities, have adapted to their different needs, and therefore, rare alleles are not eliminated by drift, but maintained in those selected cultivars.
Another interesting result is the difference in Fis among groups. Fis is high in all the groups except in the Indian group, in which it is nearly 0. High Fis has been reported often in inodorus, cantalupensis and conomon (including chinensis and makuwa) accessions [5, 6, 13, 25]. In accordance with it, low Fis has been recently reported in landraces collected directly in fields cultivated by traditional farmers, as snapmelons from North India [13], Xinjiang landraces in China [26] and snake melons from Israel and Palestine [27]. Several reasons may explain these differences: selfing during the seed multiplication in germplasm banks, a more intensive selection pressure of traditional farmers in order to keep fruit characteristics homogeneity in the traditional inodorus, cantalupensis and conomon cultivars avoiding intercrossing when compared with the other groups, and the different sex expression, most traditional cultivars from the last groups are andromonoecious, what would facilitate self-pollinated seeds compared with monoecious cultivars as the Indian and the traditional snake melons [3].
A relatively large number of SNPs with high Fst was found in three pairwise group comparisons (inodorus-related vs cantaloupe, inodorus-related vs Indian and cantaloupe vs Indian). The largest number of SNPs with a high Fst value was found in the cantaloupe vs Indian comparison. Interestingly, the SNPs with the highest Fst value (Fst > 0.75) between inodorus-related and Indian groups also showed a high Fst between cantaloupe and Indian groups (chromosomes 1, 2, 4, 5, 6, 8, 11). These are therefore good candidates to mark genomic regions that were fixed (either by selection or by drift) during the diversification from India to the Mediterranean Basin. It is remarkable that an important proportion of SNPs with a high Fst value were found on chromosome 11. Estimation of Fst among melon groups have been investigated previously in few reports [6, 28]. Given the different germplasm studied, especially the novel Indian germplasm included in the present work, comparisons among reports are not straight forward. Fst values for SNPs between cantaloupe and inodorus collections were also reported by Esteras et al. [6]. Four of their reported SNPs (Cmpsnp1067 on chromosome 2, fr14p22 on chromosome 6, Cmpsnp590 on chromosome 9, Cmpsnp1109 on chromosome 12) are located in the same genomic regions where SNPs were found with high Fst among the groups, which supports the hypothesis that those regions may be involved in the differentiation of the groups of cultivars. Moreover, one of these SNPs (fr14p22) is located in the CmNAC-NOR gene, a member of the NAC-domain TF family, that is the gene underlying the QTL for climacteric ripening ETHQV6.3 [29, 30]. Fruits from inodorus and cantalupensis varieties have different ripening behaviors: non-climacteric and climacteric, respectively. An SNP was also found on chromosome 3 (position 28,689,022) differentiating those groups located in the region of other climacteric QTL: ETHQB3.5 [31]. These co-locations of differentiating SNPs among these groups and QTL that are involved in one of the major traits that differentiated the two groups validate the current strategy and the robustness of the other differentiating SNPs reported.
Linkage disequilibrium and haplotype blocks
Previous reports on LD in melon have shown that LD decays rapidly within a few kb [6, 20, 23]. The threshold to declare significant LD was different in those reports due probably to the different germplasm used (threshold was higher in the two first works), but the general picture is similar. In the current report, significant LD extent for the whole collection was 18 kb. LD extent was dependent on the genomic region (9 kb for euchromatin, 40 kb for heterochromatin) and groups (50 kb for inodorus-related, 100 kb for cantaloupe). These results may explain the slight differences with the previous works. Nevertheless, most genes are located in euchromatic regions, where LD is much lower than the average genome-wide LD, which indicates that a very dense SNP panel would be necessary to ensure high linkage among SNPs for performing GWAS (Genome-Wide Association Studies).
LD in heterochromatin was higher, but still, it was low. Heterochromatic regions show no recombination in melon biparental populations [32], but the current results demonstrate that recombination exists in these regions. This result contrasts with that reported in other species as barley [33], potato [34] and tomato [35, 36] where large haplotype LD blocks are found in heterochromatic regions.
Concomitantly, haplotype blocks were generally small, with a large proportion of them located in heterochromatic regions, as found previously by [19]. Nevertheless, the number of haplotype blocks in centromeres was also low and large regions of centromeres did not show any block at all. However, most of the haplotypes that were fixed in varieties that belong to one subspecies or group (Table 2) were located in euchromatic regions, where LD is much less intense than in heterochromatin. This apparent contradiction may indicate that those regions are good candidates to include genes involved in melon diversification.
Thus, LD in the melon genome can be considered as relatively low. The extent of LD depends on the genome region under study due to the recombination suppression, for instance, in the centromeric regions. Anyway, the LD in the centromeric regions can also be considered low (r2 ≈ 0.3), indicating that recombination has occurred in those regions during the history of melon cultivation, even though recombinants usually are not found in the common mapping populations [26].
Given the low LD observed in the present study (the genotyped SNPs do not cover all the genomic regions), other regions carrying genes also involved in cultivar diversification may have been missed. Higher SNP density would be necessary to obtain a full picture of the dynamics of melon genome during the diversification, which, for example, could be obtained by resequencing.
Indian germplasm in the context of melon diversity
Accessions representing several Indian collections that have been previously investigated separately have been studied to have a better picture of the genetic diversity distribution in these collections using GBS-based SNP markers. In the current report, those accessions have been integrated and contextualized using a truly representative sample of global melon germplasm. Indian germplasm appears as a group of related accessions, clearly separated from other international accessions, and it has a central position in the C. melo genetic variability space. Looking with more detail into the distribution of the genetic variability among Indian accessions, this group of accessions can be separated more clearly by geographical origin than cultivated/wild types, which indicates that probably there is no strict genetic differentiation between wild and cultivated melons in India. Indian germplasm is the group with the highest genetic diversity, indicated by high SNP variability, high proportion of SNPs with high MAF and low haplotype fixation. Its heterozygosity level was as expected under H-W equilibrium, whereas in the other groups it was very low, probably as a consequence of farmer selection to maintain cultivar homogeneity for desired traits. In fact, the low proportion of SNPs with high MAF in the other groups confirms that rare alleles are fixed in few cultivars/landraces, not shared among other cultivars, and maintained as consequence of farmer selection of specific cultivars. The situation in Indian group is radically different, the high proportion of SNPs with high MAF (in cultivated and wild melons) supports that the genetic variability is not fixed within landraces, but it is shared among them. Thus, the Indian germplasm represented here may not be a standard collection of landraces (as for inodurus-related and cantaloupe groups), but a population of plants with extensive intercrossing in the field among wild and cultivated melons that would maintain the high genetic diversity. An interesting question is why did not Indian traditional farmers caused gene fixation in landraces, whereas high values of the fixation index are relatively common in landraces from both extremes of the melon geographical distribution. One possible factor for this may be the sex determination of the female flower, being strictly female in Indian landraces (and therefore obligatory out-crossers) and hermaphrodite in the most of rest, which facilitates selfing. The co-existence of wild and cultivated melons in Indian fields would produce frequent intercrosses and making difficult the fixation of alleles in specific landraces.
Conclusions
In summary, our results confirm the crucial importance of India as a center of melon diversity. Germplasm origination in India differs from African germplasm. Mediterranean and Far-East traditional cultivars would have developed from Indian germplasm by a divergent diversification. Melons cultivated by local Indian communities might be in the early steps of domestication with genetic exchange with wild melons as there is low evidence of genetic differentiation from the wild melons and the low degree of fixation. These results suggest that the germplasm may be considered genetically as a population of plants, rather than as a standard group of landraces.
Methods
Plant material
A total of 175 melon accessions were analyzed in two independent studies at West Virginia State University (WVSU_USA, 97 accessions [19]) and at Instituto de Biología Molecular y Celular de Plantas (IBMCP, 78 accessions this report,. Additional file 1: Table S1).
All the melon accessions were classified previously into seven groups based on their origin and/or passport information: African (7 accessions), cantaloupe (51 accessions), conomon (14 accessions), dudaim (4 accessions), Indian (37 accessions), inodorus-related (48 accessions; including cultivars from the inodorus, ameri and flexuosus botanical groups), and 14 accessions without a clear category that were classified as “ungrouped” (Additional file 1: Table S1). Accessions were further classified into e two subspecies subps. Melo and subps. Agrestis according to their horticulture group: ameri, cantalupensis, dudaim, flexuosus, inodurus, reticulatus, within subps. Melo and acidulous, arya, chito, conomon, kachri, tibish, wild-India and wild-Africa.
The accessions that belonged to India included: 15 snapmelons and 7 wild accessions from North India (IC, WM, [13, 16], respectively); 3 accessions from North East India (SM, [14]), 5 accessions from South India (AM, [15]) and, finally, 3 accessions from West India (ND-, [37]). These accessions were selected based on SSR genetic diversity analysis from a total of 155 accessions [13,14,15,16, 37] in order to focus on the IC- collection and to represent the genetic diversity of the other collections. Six additional accessions from different geographical regions were also included in the Indian group.
SNP discovery by genotyping-by-sequencing
Genomic DNA from the IBMCP samples was isolated from young leaves with SpeedTools Plant DNA Extraction kit (Biotools, Spain). The GBS was performed by LGC Genomics GmbH (Germany) following the procedure reported by Elshire et al. [17]. Briefly, DNA was digested with the restriction enzyme ApeK I, barcoded libraries were prepared by accession and sequenced on an Illumina HiSeq 2000 platform.
SNPs were extracted using TASSEL-GBS Discovery/Production pipeline (https://bitbucket.org/tasseladmin/tassel-5-source/wiki/Tassel5GBSv2Pipeline). Only SNPs, not indels, with a minimum read depth of three were retained. Chromosomal assignment and position of SNPs on the physical map were deduced from the melon reference genome (version 3.5.1) at https://www.melonomics.net/ [38]. SNPs were designated by chromosome number and position. The GBS data from both analyses, WVSU and IBMCP, were merged based on the SNP genomic position. After merging, SNPs were filtered forMAF higher than 5% and present in at least 100 accessions (missing data lower than 57%). SNPs that showed a heterozygosity higher than 0.7 were also removed because they are likely are artifacts due sequencing to errors or duplicated sequences.
Genetic structure and diversity
Before structure analysis, SNP matrix was pruned for linkage disequilibrium to avoid possible bias due to the variability associated to large haplotype blocks. Tag SNPs were defined with SNPAnalyzer 2.0 [39] The genetic structure of the collection was assessed by Multidimensional Scaling (MDS) with TASSEL 5.2 (Trait Analysis by aSSociation, Evolution and Linkage, www.maizegenetics.net) [40]and STRUCTURE [41] analysis. MDS was performed with TASSEL 5.2. The three first MDS components were plotted with CurlyWhirly (Information & Computational Sciences, The James Hutton Institute. https://ics.hutton.ac.uk/curlywhirly/). For STRUCTURE analysis, 20 independent runs for each K value ranging from 2 to 10 were performed with a burn-in length of 500,000 and 1 million iterations. The optimal subpopulation was calculated from the second order rate of change of likelihood (ΔK method) [42].
The genetic diversity was investigated with Arlequin ver. 3.1 [43]. Accessions were grouped according to subspecies (C. melo subsp. agrestis or subsp. melo) and the groups were defined after MDS analysis. Distribution of genetic variability among and within groups was calculated by Analysis Molecular of Variance (AMOVA) with two classification levels: subspecies and groups and also with only the MDS grouping classification. Expected (He), observed (Ho) heterozygosity and MAF were calculated for each group, as well as the Wright’s F statistic [44] Fct (differentiation among subspecies) Fst (genetic differentiation among groups), and Fis (inbreeding coefficient). The ratio between high and low polymorphic SNPs was obtained by dividing the number of SNPs with MAF > 0.4 divided by the number of SNPS with MAF < 0.05 (MAF0.04/MAF0.05) for the whole collection and each group.
Linkage disequilibrium (LD) and haplotype analysis
LD parameter r2 was estimated with TASSEL 5.2 for each SNP pair. LD decay was drawn as a smooth line of r2 against physical distance, fitted using a second-degree locally weighted scatterplot-smoothing LOESS implemented in an excel plugin [45], https://peltiertech.com/loess-smoothing-in-excel/). The r2 statistical threshold was established as the 95th percentile of the r2 distribution for unlinked SNPs. LD was analyzed for different subdata sets: whole collection, only SNPs in euchromatin, only SNPs in heterocromatin (according to [32, 38]), inodorus-related and cantaloupe groups.
Haplotype blocks were defined with SNPAnalyzer 2.0 [39]. Haplotype frequency within cultivar groups was calculated by custom scripts and visual inspection.
Abbreviations
- AMOVA:
-
Analysis of molecular variance
- GBS:
-
Genotype-by-sequencing
- GWAS:
-
Genome-Wide Association Studies
- He :
-
Expected heterozygosity
- Ho :
-
Observed heterozygosity
- LD:
-
Linkage disequilibrium
- LOESS:
-
Local regression
- MAF:
-
Minor allele frequency
- MDS:
-
Multidimensional scaling
- QTL:
-
Quantitative trait locus
- SNP:
-
Single Nucleotide Polymorphism
- SSR:
-
Single Sequence Repeat
References
Munger HM, Robinson RW. Nomenclature of Cucumis melo L. Cucurbit Genet Coop Rep. 1991;14:43–4.
Pitrat M, Hanelt P, Hammer K. Some comments on infraspecific classification of cultivars of melon. Proc Cucurbitaceae. 2000;2000(510):29–36.
Pitrat M. Melon genetic resources: phenotypic diversity and horticultural taxonomy. In: Grumet R, Katzir N, editors. Genetics and genomics of the Cucurbitaceae. New York: J Garcia-Mas Springer; 2017. p. 25–60.
Stepansky A, Kovalski I, Perl-Treves R. Intraspecific classification of melons (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Syst Evol. 1999;217(3–4):313–32.
Monforte AJ, Garcia-Mas J, Arus P, Minch E. Genetic variability in melon based on microsatellite variation. Plant Breed. 2003;122(2):153–7.
Esteras C, Formisano G, Roig C, Diaz A, Blanca J, Garcia-Mas J, Gomez-Guillamon ML, Lopez-Sese AI, Lazaro A, Monforte AJ, et al. SNP genotyping in melons: genetic variation, population structure, and linkage disequilibrium. Theor Appl Genet. 2013;126(5):1285–303.
Sebastian P, Schaefer H, Telford IRH, Renner SS. Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc Natl Acad Sci U S A. 2010;107(32):14269–73.
Newton C. Growing, gathering and offering: predynastic plant economy at Adaïma (upper Egypt). In: R. Cappers Groningen Institute of Archaeology, editor. Fields of change: progress in African archaeobotany; 2007. p. 139–55.
Paris HS. Overview of the origins and history of the five major cucurbit crops: issues for ancient DNA analysis of archaeological specimens. Veg Hist Archaeobotany. 2016;25(4):405–14.
Sabato D, Esteras C, Oscar G, Peña-Chocarro L, Leida C, Ucchesu M, Usai A, Bacchetta G, Pico B. Molecular and morphological characterisation of the oldest Cucumis melo L. seeds found in the Western Mediterranean Basin. Archaeol. Anthropol. Sci. 2017;11(3):789–810.
Serres-Giardi L, Dogimont C. How microsatellite diversity helps to understand the domestication history of melon. In: Cucurbitaceae 2012: proceedings of the Xth Eucarpia meeting on genetics and breeding of Cucurbitaceae; 2012. p. 254–63.
Endl J, Achigan-Dako EG, Pandey AK, Monforte AJ, Pico B, Schaefer H. Repeated domestication of melon (Cucumis melo) in Africa and Asia and a new close relative from India. Am J Bot. 2018;105(10):1662–71.
Dhillon NPS, Ranjana R, Singh K, Eduardo I, Monforte AJ, Pitrat M, Dhillon NK, Singh PP. Diversity among landraces of Indian snapmelon (Cucumis melo var. momordica). Genet Resour Crop Evol. 2007;54(6):1267–83.
Dhillon NPS, Singh J, Fergany M, Monforte AJ, Sureja K. Phenotypic and molecular diversity among landraces of snapmelon (Cucumis melo var. momordica) adapted to the hot and humid tropics of eastern India. Plant Genet Resour. 2009;7(3):291–300.
Fergany M, Kaur B, Monforte AJ, Pitrat M, Rys C, Lecoq H, Dhillon NPS, Dhaliwal SS. Variation in melon (Cucumis melo) landraces adapted to the humid tropics of southern India. Genet Resour Crop Evol. 2011;58(2):225–43.
Roy A, Bal SS, Fergany M, Kaur S, Singh H, Malik AA, Singh J, Monforte AJ, Dhillon NPS. Wild melon diversity in India (Punjab state). Genet Resour Crop Evol. 2012;59(5):755–67.
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One. 2011;6(5):e19379.
Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat Rev Genet. 2011;12(7):499–510.
Nimmakayala P, Tomason YR, Abburi VL, Alvarado A, Saminathan T, Vajja VG, Salazar G, Panicker GK, Levi A, Wechter WP, et al. Genome-wide differentiation of various melon horticultural groups for use in GWAS for fruit firmness and construction of a high resolution genetic map. Front Plant Sci. 2016;7:1437.
Gur A, Tzuri G, Meir A, Sa'ar U, Portnoy V, Katzir N, Schaffer AA, Li L, Burger J, Tadmor Y. Genome-wide linkage-disequilibrium mapping to the candidate gene level in melon (Cucumis melo). Sci Rep. 2017;7:9770.
Pavan S, Marcotrigiano AR, Ciani E, Mazzeo R, Zonno V, Ruggieri V, Lotti C, Ricciardi L. Genotyping-by-sequencing of a melon (Cucumis melo L.) germplasm collection from a secondary center of diversity highlights patterns of genetic variation and genomic features of different gene pools. BMC Genomics. 2017;18:59.
Deleu W, Esteras C, Roig C, González-To M, Fernández-Silva I, Gonzalez-Ibeas D, Blanca J, Aranda MA, Arús P, Nuez F, Monforte AJ, Picó M, Garcia-Mas J. A set of EST-SNPs for map saturation and cultivar identification in melon. BMC Plant Biology. 2009;9(1):90.
Leida C, Moser C, Esteras C, Sulpice R, Lunn JE, de Langen F, Monforte AJ, Pico B. Variability of candidate genes, genetic structure and association with sugar accumulation and climacteric behavior in a broad germplasm collection of melon (Cucumis melo L.). BMC Genet. 2015;16:28.
Qi J, Liu X, Shen D, Miao H, Xie B, Li X, Zeng P, Wang S, Shang Y, Gu X, et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat Genet. 2013;45(12):1510–U1149.
Wang Y-L, Gao L-Y, Yang S-Y, Xu Y-B, Zhu H-Y, Yang L-M, Li Q, Hu J-B, Sun S-R, Ma C-S. Molecular diversity and population structure of oriental thin-skinned melons, Cucumis melo subsp agrestis, revealed by a set of core SSR markers. Sci Hortic. 2018;229:59–64.
Zhang Y, Fan X, Aierken Y, Ma X, Yi H, Wu M. Genetic diversity of melon landraces (Cucumis melo L.) in the Xinjiang Uygur autonomous region on the basis of simple sequence repeat markers. Genet Resour Crop Evol. 2017;64(5):1023–35.
Omari S, Kamenir Y, Benichou JIC, Pariente S, Sela H, Perl-Treves R. Landraces of snake melon, an ancient middle eastern crop, reveal extensive morphological and DNA diversity for potential genetic improvement. BMC Genet. 2018;19:34.
Tomason Y, Nimmakayala P, Levi A, Reddy UK. Map-based molecular diversity, linkage disequilibrium and association mapping of fruit traits in melon. Mol Breed. 2013;31(4):829–41.
Vegas J, Garcia-Mas J, Monforte AJ. Interaction between QTLs induces an advance in ethylene biosynthesis during melon fruit ripening. Theor Appl Genet. 2013;126(6):1531–44.
Rios P, Argyris J, Vegas J, Leida C, Kenigswald M, Tzuri G, Troadec C, Bendahmane A, Katzir N, Pico B, et al. ETHQV6.3 is involved in melon climacteric fruit ripening and is encoded by a NAC domain transcription factor. Plant J. 2017;91(4):671–83.
Moreno E, Obando JM, Dos-Santos N, Fernandez-Trujillo JP, Monforte AJ, Garcia-Mas J. Candidate genes and QTLs for fruit ripening and softening in melon. Theor Appl Genet. 2008;116(4):589–602.
Argyris JM, Ruiz-Herrera A, Madriz-Masis P, Sanseverino W, Morata J, Pujol M, Ramos-Onsins SE, Garcia-Mas J. Use of targeted SNP selection for an improved anchoring of the melon (Cucumis melo L.) scaffold genome assembly. BMC Genomics. 2015;16:4.
Comadran J, Ramsay L, MacKenzie K, Hayes P, Close TJ, Muehlbauer G, Stein N, Waugh R. Patterns of polymorphism and linkage disequilibrium in cultivated barley. Theor Appl Genet. 2011;122(3):523–31.
Vos PG, Paulo MJ, Voorrips RE, Visser RGF, van Eck HJ, van Eeuwijk FA. Evaluation of LD decay and various LD-decay estimators in simulated and SNP-array data of tetraploid potato. Theor Appl Genet. 2017;130(1):123–35.
Ruggieri V, Francese G, Sacco A, D'Alessandro A, Rigano MM, Parisi M, Milone M, Cardi T, Mennella G, Barone A. An association mapping approach to identify favourable alleles for tomato fruit quality breeding. BMC Plant Biol. 2014;14:337.
Bauchet G, Grenier S, Samson N, Bonnet J, Grivet L, Causse M. Use of modern tomato breeding germplasm for deciphering the genetic control of agronomical traits by genome wide association study. Theor Appl Genet. 2017;130(5):875–89.
Dhillon NPS, Monforte AJ, Pitrat M, Pandey S, Singh PK, et al. Melon landraces of India: contributions and importance. Plant Breeding Rev. 2012;35:85–150.
Garcia-Mas J, Benjak A, Sanseverino W, Bourgeois M, Mir G, Gonzalez VM, Henaff E, Camara F, Cozzuto L, Lowy E, et al. The genome of melon (Cucumis melo L.). Proc Natl Acad Sci U S A. 2012;109(29):11872–7.
Yoo J, Lee Y, Kim Y, Rha SY, Kim Y. SNP analyzer 2.0: a web-based integrated workbench for linkage disequilibrium analysis and association analysis. BMC Bioinf. 2008;9:290.
Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–5.
Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–59.
Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14(8):2611–20.
Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinforma. 2005;1:47–50.
Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population-structure. Evolution. 1984;38(6):1358–70.
Peltier J. LOESS utility for excel. Peltier Tech Blog. http://peltiertech.com/WordPress/loess-smoothing-in-excel. 2009.
Acknowledgments
Authors thank Soledad Casal for technical support, Jordi Garcia-Mas and Kostantinoss Alexiou from Centre for Research in Agricultural Genomics (CRAG) for discussions, Javier Forment from the Bioinformatics Core Service at IBMCP for support in bioinformatics analysis. We also acknowledge the support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI). NPS Dhillon was supported by long-term strategic donors to the World Vegetable Center: Republic of China (Taiwan), UK aid from the UK government, United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO)-FEDER grant AGL2015–64625-C2-R to AJM (project conception, experiments, data acquisition and analysis, manuscript writing, publication costs), AGL2017–85563-C2–1-R and the PROMETEO/2017/078 grant funded by Generalitat Valenciana (Conselleria d’Educació, Investigació, Cultura i Esport) to BP (project conception, provide samples and manuscript drafting). AD was supported by a Jae-Doc contract from CSIC (experiments and manuscript drafting).
Availability of data and materials
SNP matrix before filtering is included as Additional file 2: Table S2.
Author information
Authors and Affiliations
Contributions
AJM conceived the project, research plan and wrote the manuscript. MJG performed molecular lab work, statistical analysis and wrote the manuscript. AD performed molecular lab work and manuscript writing. NPSD provided DNA germplasm samples, contributed to the project conception and helped in manuscript writing. UKR provided genotyping data, contributed to the project conception and helped in manuscript writing. BP contributed to the project conception, provided germplasm and samples and drafted the manuscript. All authors have read and accepted the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Details of the accessions analysed in the current study. Group, subspecies and type are according passport data. MDS group is according MDS analysis (nd, ungruped). The assay indicates the origin of the data: WVSU (Nimmakayala et al. 2016), IBMCP (current work). (XLSX 20 kb)
Additional file 2:
Table S2. Single Nucleotide Polymorphsim genotypes after merging WVSU (Nimmakayala et al. 2016) and IBMCP (current work) data. (XLSX 5578 kb)
Additional file 3:
Figure S1. MDS analysis including the whole germplasm collection. The origin of the GBS data is indicated by the dot color: blue for WVSU [19] and red for IBMCP. (PPTX 102 kb)
Additional file 4:
Figure S2. STRUCTURE results for K = 2 to 5. The five groups defined by Multi Dimensional Scaling (MDS) analysis are indicated at the right with their respective colors. K = 5 showed the Delta K peak, defining five populations. Wild accessions are highlighted with a star with the color of their STRUCTURE populations. (PPTX 480 kb)
Additional file 5:
Figure S3. Expected (He) heterozygosity for SNPs across the melon genome in the groups Inodorus-related, Cantaloupe and Indian. (PPTX 765 kb)
Additional file 6:
Figure S4. Histograms of the distribution of SNPs with different Minor Allele Frequencies (MAF) among Multidimensional scaling groups and the whole collection (all). (PPTX 83 kb)
Additional file 7:
Table S3. Fst values for SNP loci for Inodurus-related-cantaloupe and Indian pairwise group comparisons (XLSX 283 kb)
Additional file 8:
Figure S5. Linkage disequilibrium (r2) versus physical distance. (A) Whole germplasm analysing euchromatin and heterochromatin independently. (B) Inodorus-related and cantaloupe groups. Dashed lines indicate the false discovery rate at p < 0.05 based on the 95th percentile of the r2 distribution of unlinked SNPs. Curves were fitted by second-degree LOESS. (PPTX 158 kb)
Additional file 9:
Table S4. Haplotypes blocks defined by SNPanalyzer 2.0 (XLSX 57 kb)
Additional file 10:
Figure S6. Genomic location of LD blocks on the melon genome. Blocks fixed in C. melo ssp. melo (M) and cantaloupe group (C) are indicated. Centromeric regions in the chromosomes are marked in black. (PPTX 164 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gonzalo, M.J., Díaz, A., Dhillon, N.P.S. et al. Re-evaluation of the role of Indian germplasm as center of melon diversification based on genotyping-by-sequencing analysis. BMC Genomics 20, 448 (2019). https://doi.org/10.1186/s12864-019-5784-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-019-5784-0