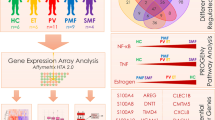
Abstract
Background
Essential thrombocythemia (ET) is one of the most common types of Ph-negative myeloproliferative neoplasms, an infrequent group of blood cancers that arise from a CD34 + hematopoietic stem cell (HSC) in the bone marrow (BM) primarily due to driver mutations in JAK2, CALR or MPL. These aberrations result in an overproduction of mature myeloid cells in peripheral blood (PB). To date, no targeted therapies have been approved for ET patients, so the study of the molecular mechanisms behind the disease and the identification of new therapeutic targets may be of interest. For this reason, in this study, we have compared the transcriptomic profile of undifferentiated CD34 + cells and mature myeloid cells from ET patients (CALR and JAK2-mutated) and healthy donors deposited in publicly available databases. The study of the similarities and differences between these samples might help to better understand the molecular mechanisms behind the disease according to the degree of maturation of the malignant clone and the type of mutation and ultimately help identify new therapeutic targets for these patients.
Results
The results show that most of the altered hallmarks in neutrophils were also found in CD34 + cells. However, only a few genes showed a similar aberrant expression pattern in both types of cells. We have identified a signature of six genes common to patients with CALR and JAK2 mutations (BPI, CRISP3, LTF, MMP8, and PTGS1 upregulated, and PBXIP1 downregulated), a different signature of seven genes for patients with CALR mutations (BMP6, CEACAM8, ITK, LCN2, and PRG2 upregulated, and MAN1A1 and MME downregulated) and a signature of 13 genes for patients with JAK2 mutations (ARG1, CAST, CD177, CLEC5A, DAPP1, EPS15, IL18RAP, OLFM4, OLR1, RIOK3, SELP, and THBS1 upregulated, and IGHM downregulated).
Conclusions
Our results highlight transcriptomic similarities and differences in ET patients according to the degree of maturation of the malignant clone and the type of mutation. The genes and processes altered in both CD34 + cells and mature neutrophils may reveal altered sustained processes that could be studied as future therapeutic targets for ET patients.
Similar content being viewed by others
Background
Ph-negative myeloproliferative neoplasms (MPNs, ET: essential thrombocythemia; PV: polycythemia vera; PMF: primary myelofibrosis) are an infrequent group of blood cancers which arise from a CD34 + hematopoietic stem cell (HSC) in the bone marrow mainly due to driver mutations in JAK2, CALR or MPL. These alterations induce the transformation of normal cells into malignant ones, finally causing an overproduction of mature myeloid cells through multiple molecular mechanisms [1, 2]. The main pathogenic mechanism seems to be the constitutive activation of JAK2-related pathways (JAK2/STAT, MAPK/ERK, and PI3K/AKT), but some other non-canonical mechanisms derived from mutant JAK2 and CALR have also been described [1]. However, there are still several issues that have not been fully resolved, such as the fact that the same alterations can lead to different, although related, clinical phenotypes.
ET is one of the most common types of Ph-negative MPNs, with an incidence of 1.2-3.0/100,000 per year [3]. This disease is characterized by excessive production of megakaryocytes in the bone marrow, which results in a high platelet count in the peripheral blood (thrombocytosis). According to a recent Mayo Clinic study, ET patients have a significantly shorter survival rate than that of age- and sex-matched controls from the general population. In some cases, this occurs because they show leukemic or fibrotic transformation [4].
At this time, the only targeted therapy approved for some Ph-negative MPN patients is ruxolitinib, a JAK1/JAK2 inhibitor. This molecule has been approved for patients with intermediate or high-risk MF and with PV resistant or intolerant to chemotherapy [5]. However, clinical trials suggest that ruxolitinib is not superior to current second line treatments for ET [6]. In this sense, the study of the molecular mechanisms behind the disease and the identification of new potential therapeutic targets for ET patients may be of special interest.
For this reason, in this work we will compare the transcriptomic profile of bone marrow (BM) CD34 + cells and peripheral blood (PB) neutrophils from ET patients and healthy donors deposited in publicly available databases. The comparison of the differentially expressed genes in these BM and PB JAK2 and CALR-mutated ET samples compared to healthy donors might help to better understand the altered processes and molecular mechanisms underlying this disease according to the degree of maturation of the malignant cell (immature CD34 + HSCs from BM vs. mature neutrophils from PB) and the type of mutation. Furthermore, the identification of equally altered hallmarks and similarly aberrantly expressed genes in both types of cells of ET patients could provide clues about the conserved processes in mature neutrophils, which may be quantitatively o functionally important in the pathogenesis of the disease for patients harboring JAK2 or CALR mutations and ultimately could help develop new therapeutic strategies.
Methods
Data source
Transcriptomic data from ET patients (GSE54644, [U133AAofAv2] Affymetrix GeneChip HT-HG_U133A Early Access Array; GSE103237, [HG-U219] Affymetrix Human Genome U219 Array) were obtained from the publicly available Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo). The GSE54644 set contained expression data from PB neutrophils obtained from 39 ET patients (14 CALR-mutated and 25 JAK2-mutated) and 11 healthy donors [7]. On the other hand, the GSE103237 dataset consisted of CD34 + cell transcriptomic data from BM obtained from 24 ET patients (7 CALR-mutated and 17 JAK2-mutated) and 15 healthy donors [8] (Table S1; Fig. 1).
For validation of the obtained results, RNA-seq experiments were analyzed in an independent cohort of patients with ET (3 CALR-mutated, 3 JAK2-mutated and 4 healthy donors) using peripheral blood mononuclear cell (PBMC) samples (GSE156336, Illumina HiSeq 2500) obtained from the GEO database (Table S1; Fig. 1).
The three datasets used in this study were the only ones found in the GEO database when searching for transcriptomic data of patients with ET with mutations in CALR and JAK2 that also contained data from healthy controls.
Data processing
The GEO2R tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/) was used to analyze microarray data (GSE54644 and GSE103237) applying lima precision weights (vooma) and the Benjamini & Hochberg adjustment. For each dataset, expression data from CALR-mutated and JAK2-mutated ET patients were independently compared with data from healthy individuals. All the transcripts with an adjusted p-value < 0.01 were considered as differentially expressed genes (DEGs) (Table S2 for CALR-mutated vs. healthy donors and Table S3 for JAK2-mutated vs. healthy donors). Then, we analyzed the genes that were similarly expressed in both PB and BM samples from CALR (Table S4) or JAK2-mutated (Table S5) patients selecting those with log2 fold change (log2FC) ≤ -1 or ≥ 1. The expression of these genes was validated by RNA-seq in independent PBMC samples from patients with ET (GSE156336) using the GEO2R tool with Benjamini & Hochberg adjusted p-values. Finally, we studied their function and relationship with MPNs (Fig. 1).
Gene Set Enrichment Analysis (GSEA) of gene expression microarray data (GSE54644 and GSE103237) was performed using the clusterProfiler R package [9] on MSigDB databases for humans obtained with the R package msigdbr [10] (Fig. 1).
Graphics were created using GraphPad Prism 8.0.2 (GraphPad Software Inc., San Diego, CA, USA).
Results
Main similarities and differences between BM CD34 + cells and PB neutrophils from JAK2 and CALR-mutated ET patients
Analysis of microarray data revealed that the transcriptomic differences between ET patients and healthy donors are greater in immature CD34 + cells from BM than in mature neutrophils from PB. In fact, the amount of DEGs (Fig. 2; Tables S2, S3) in CD34 + cells was approximately double that in neutrophils for both CALR and JAK2-mutated patients compared to healthy donors.
In general, patients with JAK2 mutations showed greater transcriptomic differences when compared to healthy donors than those with CALR mutations. Furthermore, we only found 826 common DEGs (338 equally expressed and 488 with opposite expression results) when CD34 + cells and neutrophils data from CALR-mutated patients were compared with data from healthy donors (Fig. 2a; Table S4), but there were 1983 common DEGs (919 equally expressed and 1064 with opposite expression results) when comparing data from both cell types in patients with JAK2 mutations with data from healthy donors (Fig. 2b; Table S5).
Comparison of the altered processes in BM CD34 + cells and PB neutrophils from JAK2 and CALR-mutated ET patients
Several hallmarks were exclusively altered in immature CD34 + cells or mature neutrophils. For CALR-mutated patients, 17 hallmarks involved in a wide variety of processes were found to be altered exclusively in BM CD34 + cells, but only one hallmark (mitotic spindle assembly) was exclusively altered in PB neutrophils (Fig. 3). For JAK2-mutated patients, 16 unique hallmarks were found in BM CD34 + cells, and none were found in PB neutrophils (Fig. 4). Regarding the hallmarks exclusively altered in BM CD34 + cells, it is of interest to note that some of them were shared between patients with JAK2 and CALR mutations (allograft rejection, angiogenesis, apical surface, reactive oxygen species pathway, hedgehog signaling, pancreas beta cells, fatty acid metabolism, and peroxisome), while others were only found in patients with CALR mutations (epithelial-to-mesenchymal transition, interferon alpha response, bile acid metabolism, glycolysis, IL6/JAK/STAT3, PI3K/Akt/mTOR and Notch signaling, and spermatogenesis) or JAK2 mutations (UV response, myogenesis, KRAS signaling, Wnt/β-catenin, and upregulation of MYC and E2F targets). When studying the general biological processes in which these hallmarks were involved, we observed that most of them were commonly altered in patients with mutations in JAK2 and CALR, although the mechanisms by which they are altered may differ. For example, metabolism (biological process) is altered in patients with JAK2 and CALR mutations, but the specific metabolic pathways (hallmarks) altered in patients with each mutation are different. On the other hand, only a few biological processes were exclusively altered in patients with mutations in CALR (fibrosis) and JAK2 (apoptosis and myogenesis).
Main altered processes in CD34 + cells from BM and PB neutrophils from ET patients with CALR mutations. Hallmarks found in (a) CD34 + cells from BM and (b) neutrophils from PB samples from ET patients with CALR mutations using the GSEA normalized enrichment scores. A darker red color is indicative of a lower GSEA-adjusted p-value. The uncolored hallmarks are common to both types of samples while the yellow and red colored hallmarks are exclusive to BM CD34 + cells and PB neutrophils, respectively. (c) Venn diagrams that summarize the main altered processes in BM CD34 + cells and PB neutrophils and common gene expression signature for both cell types
Main altered processes in CD34 + cells from BM and PB neutrophils from ET patients with JAK2 mutations. Hallmarks found in (a) BM CD34 + cells and (b) PB neutrophils from ET patients with JAK2 mutations using the GSEA normalized enrichment scores. A darker red color is indicative of a lower GSEA-adjusted p-value. The uncolored hallmarks are common to both types of samples while the yellow and red colored hallmarks are exclusive to BM CD34 + cells and PB neutrophils, respectively. (c) Venn diagrams that summarize the main altered processes in BM CD34 + cells and PB neutrophils and common gene expression signature for both cell types
Some hallmarks were exclusive to BM CD34 + cells or PB neutrophils, but most of the altered ones in PB neutrophils were also found altered in BM CD34 + cells (Figs. 3 and 4). This is not surprising, since MPNs originate in the BM. We focused on them since, being conserved in mature neutrophils (i.e., they remain altered throughout the entire malignant hematopoietic lineage), they might be relevant to the development or progression of the disease. In other words, the biological processes in which they are involved could be important in the pathogenesis of the disease, either quantitatively (due to the significant alteration of one of the hallmarks of the process) or functionally (because the cumulative alteration of multiple hallmarks affects a relevant biological process). Thus, the progression of the disease to MF or the severity of symptoms presented in patients could ultimately depend on these biological processes.
Potential hallmarks of interest for ET patients with CALR and JAK2 mutations
Several hallmarks shared between samples from both types of cells were common to both CALR and JAK2-mutated patients. These were related to activation of stress responses (hypoxia, UV and UPR), immunity (inflammation, interferon gamma response, TNF-α activation via NFκB, complement, and IL2/STAT5 signaling), metabolism (cholesterol homeostasis, adipogenesis, metabolism of heme and xenobiotics, and androgen and estrogen early and late responses), fibrosis (mTORC1 and TGF-β), cell adhesion disruption (apical junction), protein secretion, coagulation, and other processes typically altered in cancer, such as apoptosis (p53 pathway) and proliferation and survival (p53 pathway) (Figs. 3 and 4).
Potential hallmarks of interest for ET patients with CALR mutations
Exclusively for patients with CALR mutations, the shared hallmarks between BM CD34 + cells and PB neutrophils were involved in the activation of several processes, such as immunity (KRAS signaling), apoptosis (Wnt/β-catenin), proliferation and survival (Wnt/β-catenin and KRAS signaling), and myogenesis. Interestingly, the G2M checkpoint and MYC and E2F targets were enriched in PB neutrophils but repressed in BM CD34 + cells (Fig. 3).
Potential hallmarks of interest for ET patients with JAK2 mutations
Hallmarks of interest unique to patients with JAK2 mutations were associated with the activation of immune responses (interferon alpha response), metabolism (glycolysis and bile acid metabolism), proliferation and survival (IL6/JAK/STAT3 and PI3K/Akt/mTOR pathways), cell adhesion (epithelial-to-mesenchymal transition), mitotic spindle assembly, and the G2M checkpoint (Fig. 4).
Genes equally expressed in BM CD34 + cells and PB neutrophils from ET patients
Analysis of aberrantly and similarly expressed genes (up or downregulated) in BM and PB samples from ET patients and their function is of particular interest because they could reveal altered sustained biological processes that are triggered by CALR and JAK2 mutations. In addition to functional hallmarks, we could detect some similarly expressed genes in BM CD34 + cells and PB neutrophils of patients. These genes participate in a wide variety of processes (Tables S4, S5) and as mentioned above, we focused on the functional analysis of the genes which a log2FC ≤ -1 or ≥ 1 in both types of samples (Figs. 3, 4 and 5).
Heatmaps and tables showing data from similarly expressed genes in the BM CD34 + cells and PB neutrophils from patients with (a) CALR and (b) JAK2 mutations that show a log2FC ≤ -1 or ≥ 1 and an adjusted p-value < 0.01 compared with BM and PB samples from healthy controls. Mean fold change values are represented, ranging from shades of red (positive fold change) to shades of green (negative fold change). The common genes found in CALR and JAK2 mutated samples are identified with an asterisk
A total of 13 genes (10 upregulated and 3 downregulated) fulfilled these criteria in samples from patients with CALR mutations (Fig. 5a) and 19 genes (17 upregulated and 2 downregulated) in samples from patients harboring JAK2 mutations (Fig. 5b). Only six of these genes were common to both types of mutations (marked with an asterisk in Fig. 5).
Potential targets of interest for ET patients with CALR and JAK2 mutations
All six DEGs that were found in common in samples from patients with both types of mutations (BPI, CRISP3, LTF, MMP8, PBXIP1, and PTGS1) were upregulated except PBXIP1, which was downregulated (Fig. 5). The majority of them participate in immune processes (BPI, CRISP3, LTF), while the remaining ones are related to fibrosis (MMP8), proliferation and survival (PBXIP1) or metabolic processes involved in the biosynthesis of platelets (PTGS1) (Figs. 3 and 4; Table 1). Interestingly, aberrations in all these genes have previously been linked to blood cancers (Table 1).
The study of gene expression in PBMCs from an independent cohort of patients by RNA-seq confirmed that CRISP3, LTF, MMP8, and PTGS1 were significantly upregulated in patients with mutations in CALR, and PTGS1 in patients with mutations in JAK2. The expression of the remaining genes followed a similar trend as observed in BM CD34 + cells and PB neutrophils in the majority of cases, but the results were not statistically significant (Supplementary Figs. 1, 2).
Potential targets of interest for ET patients with mutations in CALR
In the case of CALR-mutated ET patients, seven genes were aberrantly expressed both in PB and BM samples (BMP6, CEACAM8, ITK, LCN2, MAN1A1, MME, and PRG2). All of them were upregulated except for MAN1A1 and MME, which were downregulated (Fig. 5a). Most of these genes participate in immune processes (ITK, LCN2, and PRG2) and protein synthesis, processing, and secretion (MAN1A1 and MME). The remaining ones are involved in fibrosis (BMP6) and cell adhesion (CEACAM8) (Fig. 3; Table 2).
The results obtained by RNA-seq in PBMCs confirmed that BMP6, CEACAM8, and LCN2 were upregulated in patients with mutations in CALR. No significant results were obtained for the remaining genes (Supplementary Figs. 1, 2).
Potential targets of special interest for JAK2-mutated ET patients
In samples from ET patients with JAK2 mutations, we focused on the analysis of 13 genes (ARG1, CAST, CD177, CLEC5A, DAPP1, EPS15, IGHM, IL18RAP, OLFM4, OLR1, RIOK3, SELP, and THBS1). All of them, except IGHM, were upregulated in both BM and PB-derived samples (Fig. 5b). Most of these genes participate in processes related to immune response (ARG1, CD177, CLEC5A, IGHM, IL18RAP, OLR1, and RIOK3) or cell adhesion (CD177, OLFM4, SELP, and THBS1), while some of them are involved in processes such as protein synthesis, processing, and secretion (CAST and EPS15) apoptosis (OLR1), proliferation and survival (DAPP1), or coagulation (THBS1) (Fig. 4; Table 3).
The results obtained in PBMCs confirmed that OLR1 was upregulated in patients with mutations in JAK2. No significant results were obtained for the remaining genes, although the trends were consistent with those observed in BM CD34 + cells and PB neutrophils in many cases (Supplementary Figs. 1, 2).
Discussion
The knowledge of the molecular pathogenesis of chronic MPNs has undergone considerable progress in recent decades, especially after the description of the presence of a single nucleotide mutation in JAK2 (p.V617F) in the vast majority of patients with these diseases in 2005. Subsequently, MPL mutations were found in a small proportion of patients and, finally, it was possible to determine that the third major mutated gene was CALR in 2013 thanks to the application of massive sequencing technologies. Taken together, these aberrations activate cell signaling primarily through the JAK2/STAT pathway, justifying the relationship between these diseases, but also through other related signaling pathways that lead to cell proliferation.
Apart from unveiling possible therapeutic targets and a better understanding of the mechanisms that trigger these diseases, the description of these alterations represented a considerable diagnostic revolution since it allowed the clonal nature of the disease to be demonstrated in a simple way. However, there are still several issues that have not been fully resolved, such as the fact that the same alterations can lead to different, although related, clinical phenotypes. Furthermore, in the case of ET patients no targeted therapies have been approved and there is still a certain percentage of patients with this disease that do not show any of the genetic alterations mentioned.
The present work aims to re-analyze the gene expression profiles deposited in public databases of patients with ET with JAK2 and CALR mutations and healthy donors obtained from both CD34 + cells and mature neutrophils. The study of the transcriptional alterations conserved between undifferentiated CD34 + cells and mature myeloid cells might help to discover molecular players of special importance for ET patients, pointing to new potential therapeutic targets.
The GSE103237 dataset included CD34 + cell transcriptomic data from BM obtained from 24 ET patients (7 CALR-mutated and 17 JAK2-mutated) and 15 healthy donors [8]. In the original study, the authors focused on the differences in the expression profile between CALR-mutated and JAK2-mutated CD34 + cells that supported the existence of different clinical entities for patients with both mutations. Thus, they showed that the expression of several genes involved in DNA repair, chromatin remodeling, splicing, chromatid cohesion and several genes involved in thrombin signaling and platelet activation were downregulated, which could explain the lower risk of thrombosis associated with CALR mutations.
On the other hand, the GSE54644 set contained expression data from PB neutrophils obtained from 39 ET patients (14 CALR-mutated and 25 JAK2-mutated) and 11 healthy donors [7]. In this study the authors showed that a transcriptional signature consistent with activated JAK2 signaling was present in all MPN patients regardless of clinical phenotype or mutational status. These results pointed to a shared mechanism of transformation by JAK2 and CALR mutations, demonstrating the central importance of the JAK2/STAT pathway in MPN pathogenesis. Indeed, the mutant CALR was subsequently shown to drive signaling by activating JAK2 through the thrombopoietin receptor MPL [52,53,54].
General re-analysis of both datasets showed differences and similarities between CD34 + cells and mature neutrophils from ET patients.
With respect to the differences, more DEGs were found in CD34 + samples than in mature neutrophils. In fact, immature BM CD34 + cells show more exclusive altered hallmarks (17 in CALR-mutated samples and 16 in JAK2-mutated patients) participating in a wide variety of biological processes. Some of these processes were common to JAK2 and CALR-mutated patients (allograft rejection, angiogenesis, apical surface, reactive oxygen species pathway, hedgehog signaling, pancreas beta cells, fatty acid metabolism, and peroxisome).
Focusing on the similarities, BM CD34 + cells and PB neutrophils shared many of the altered hallmarks regardless of mutation type. In fact, all but one of the hallmarks altered in neutrophils (mitotic spindle in CALR-mutated patients) were also found to be altered in immature CD34 + cells. Interestingly, the majority of these hallmarks were common to JAK2 and CALR-mutated patients, providing evidence about shared mechanisms of interest for both types of patients apart for the already described JAK2/STAT activation [7] and not only in mature neutrophils but also in immature CD34 + cells. Likewise, the differences between patients harboring JAK2 and CALR mutations support previous analyses in PB [8] but also translate these results to BM.
In addition to the hallmarks, we particularly focused on similarly expressed genes in CD34 + cells and mature neutrophils since they might be relevant molecular players in ET pathogenesis that could be targeted. In this sense, we could only observe the conservation of the expression of 338 and 919 genes between the PB neutrophils and BM CD34 + cells of CALR and JAK2-mutated patients, respectively. Among them, we have obtained an expression signature of six genes common to patients with mutations in CALR and JAK2 (BPI, CRISP3, LTF, MMP8, and PTGS1 upregulated, and PBXIP1 downregulated), and two other expression signatures of seven (BMP6, CEACAM8, ITK, LCN2, and PRG2 upregulated, and MAN1A1 and MME downregulated) and 13 genes (ARG1, CAST, CD177, CLEC5A, DAPP1, EPS15, IL18RAP, OLFM4, OLR1, RIOK3, SELP, and THBS1 upregulated, and IGHM downregulated) exclusive to patients with ET harboring mutations in CALR and JAK2, respectively. The expression of many of these genes was confirmed in PBMCs RNA-seq data from an independent cohort of ET patients, although the small number of patients and the heterogeneity among them resulted in non-significant results in many cases. Additionally, there might be some differences compared to the results obtained from PB neutrophil samples due to the analysis of different types of mature blood cells. The selected genes that were common to CALR and JAK2-mutated patients were involved in very specific biological processes that could be relevant to the pathogenesis of the disease, so it would be worthwhile to carry out further studies on these processes in both types of patients. For example, MAN1A1, one of the genes exclusively downregulated in CALR-mutated patients is involved in N-linked glycosylation. In support of our results, it has recently been described that genes in the N-glycosylation pathway are differentially depleted in mutant CALR-transformed cells. In these cells, chemical inhibition of N-glycosylation impaired their growth [55]. In addition, it is important to note that most of these genes have been previously related to MPNs or hematologic cancers.
Globally, the methodology used in this study consists of a detailed bioinformatic analysis that takes advantage of previously published data to observe them from a new perspective and has allowed the identification of some features and genes that may be relevant in the pathogenesis of ET in patients with CALR or JAK2 mutations. Strict criteria have been employed in the analysis of these data to highlight only relevant processes and genes, and the expression values of many of these highlighted genes have been validated in an independent cohort of patients using RNA-seq data. However, this study also has some limitations. Firstly, the data available for each patient are different in each of the databases, which has made it difficult to associate the results obtained with specific CALR or JAK2 mutations. Moreover, the low number and heterogeneity of the patients included in the RNA-seq dataset has not made it possible to confirm all the results obtained in the analysis of the arrays. Finally, there is no expression data for the intermediate cells between the BM CD34 + stem cells and the PB neutrophils or PBMCs, as the only available datasets in GEO that include transcriptomic data from ET patients with CALR or JAK2 mutations are those used in this study. This limitation, combined with the fact that the analyzed samples from each cell type are derived from different patients, makes it impossible to analyze the dynamics of changes in the clonogenicity of malignant cells in ET.
Conclusion
The comparison of the transcriptomic profile of bone marrow (BM) CD34 + cells and peripheral blood (PB) neutrophils from ET patients and healthy donors highlights molecular similarities and differences according to the degree of maturation of the malignant clone and the type of mutation, which enables delving into the molecular pathogenic mechanisms. Although our results show that most of the altered hallmarks in neutrophils were also found in CD34 + cells in both CALR and JAK2-mutated patients, only a few genes showed a similar expression pattern in both types of cells. These genes are of special interest since they may reveal altered sustained biological processes throughout the entire malignant hematopoietic lineage and might be relevant to the development or progression of the disease. Thus, the progression of the disease to MF or the severity of symptoms presented in ET patients could ultimately depend on these genes and processes and hence, they could be studied as future therapeutic targets for ET patients according to their driver mutation.
Data Availability
The datasets used during the current study are available in the GEO repository under accession numbers GSE103237, GSE54644, and GSE156336.
Abbreviations
- ET:
-
essential thrombocythemia
- HSC:
-
hematopoietic stem cell
- BM:
-
bone marrow
- JAK2:
-
Janus kinase 2
- CALR:
-
calreticulin
- MPL:
-
thrombopoietin receptor
- PB:
-
peripheral blood
- MPNs:
-
myeloproliferative neoplasms
- PV:
-
polycythemia vera
- PMF:
-
primary myelofibrosis
- GEO:
-
Gene Expression Omnibus
- PBMCs:
-
peripheral blood mononuclear cells
- DEGs:
-
differentially expressed genes
- GSEA:
-
Gene Set Enrichment Analysis
References
Guijarro-Hernández A, Vizmanos JL. A broad overview of signaling in Ph-Negative Classic Myeloproliferative Neoplasms. Cancers. 2021;13:984. https://doi.org/10.3390/cancers13050984.
Mead AJ, Mullally A. Myeloproliferative neoplasm stem cells. Blood. 2017;129:1607–16. https://doi.org/10.1182/blood-2016-10-696005.
Tefferi A, Pardanani A. Essential thrombocythemia. N Engl J Med. 2019;381:2135–44. https://doi.org/10.1056/NEJMcp1816082.
Szuber N, Mudireddy M, Nicolosi M, Penna D, Vallapureddy RR, Lasho TL et al. 3023 Mayo Clinic Patients With Myeloproliferative Neoplasms: Risk-Stratified Comparison of Survival and Outcomes Data Among Disease Subgroups. Mayo Clin Proc. 2019;94:599–610. https://doi.org/10.1016/j.mayocp.2018.08.022.
Li B, Rampal RK, Xiao Z. Targeted therapies for myeloproliferative neoplasms. Biomark Res. 2019;7:15. https://doi.org/10.1186/s40364-019-0166-y.
Harrison CN, Mead AJ, Panchal A, Fox S, Yap C, Gbandi E, et al. Ruxolitinib vs best available therapy for ET intolerant or resistant to hydroxycarbamide. Blood. 2017;130:1889–97. https://doi.org/10.1182/blood-2017-05-785790.
Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123:e123–33. https://doi.org/10.1182/blood-2014-02-554634.
Zini R, Guglielmelli P, Pietra D, Rumi E, Rossi C, Rontauroli S, et al. CALR mutational status identifies different disease subtypes of essential thrombocythemia showing distinct expression profiles. Blood Cancer J. 2017;12:638. https://doi.org/10.1038/s41408-017-0010-2.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innov (Camb). 2021;2:100141. https://doi.org/10.1016/j.xinn.2021.100141.
Dolgalev I. Msigdbr: MSigDB gene sets for multiple organisms in a tidy data format. R package version 7.5.1. 2022. https://igordot.github.io/msigdbr/.
Lennartsson A, Pieters K, Ullmark T, Vidovic K, Gullberg U. AML-1, PU.1, and Sp3 regulate expression of human bactericidal/permeability-increasing protein. Biochem Biophys Res Commun. 2003;311:853–63. https://doi.org/10.1016/j.bbrc.2003.10.067.
Yokota A, Huo L, Lan F, Wu J, Huang G. The clinical, molecular, and mechanistic basis of RUNX1 mutations identified in hematological malignancies. Mol Cells. 2020;43:145–52. https://doi.org/10.14348/molcells.2019.0252.
Sakurai H, Harada Y, Ogata Y, Kagiyama Y, Shingai N, Doki N, et al. Overexpression of RUNX1 short isoform has an important role in the development of myelodysplastic/myeloproliferative neoplasms. Blood Adv. 2017;1:1382–6. https://doi.org/10.1182/bloodadvances.2016002725.
Guglielmelli P, Bartalucci N, Contini E, Rotunno G, Pacilli A, Romagnoli S, et al. Involvement of RUNX1 pathway is a common event in the leukemic transformation of chronic myeloproliferative neoplasms (MPNs). Blood. 2019;134(Supplement1):2968. https://doi.org/10.1182/blood-2019-129094.
Irino T, Uemura M, Yamane H, Umemura S, Utsumi T, Kakazu N, et al. JAK2 V617F-dependent upregulation of PU.1 expression in the peripheral blood of myeloproliferative neoplasm patients. PLoS ONE. 2011;6:e22148. https://doi.org/10.1371/journal.pone.0022148.
Van Loo PF, Bouwman P, Ling KW, Middendorp S, Suske G, Grosveld F, et al. Impaired hematopoiesis in mice lacking the transcription factor Sp3. Blood. 2003;102:858–66. https://doi.org/10.1182/blood-2002-06-1848.
Schardt JA, Keller M, Seipel K, Pabst T. Functional interplay of SP family members and nuclear factor Y is essential for transcriptional activation of the human calreticulin gene. Biochim Biophys Acta. 2015;1849:1188–97. https://doi.org/10.1016/j.bbagrm.2015.07.003.
Skov V, Burton M, Thomassen M, Stauffer Larsen T, Riley CH, Brinch Madelung A, et al. A 7-gene signature depicts the biochemical profile of early prefibrotic myelofibrosis. PLoS ONE. 2016;11:e0161570. https://doi.org/10.1371/journal.pone.0161570.
Leng D, Miao R, Huang X, Wang Y. In silico analysis identifies CRISP3 as a potential peripheral blood biomarker for multiple myeloma: from data modeling to validation with RT-PCR. Oncol Lett. 2018;15:5167–74. https://doi.org/10.3892/ol.2018.7969.
Skov V, Thomassen M, Kjær L, Riley C, Stauffer Larsen T, Bjerrum OW, et al. Extracellular matrix-related genes are deregulated in peripheral blood from patients with myelofibrosis and related neoplasms. Blood. 2018;132:5491. https://doi.org/10.1182/blood-2018-99-117122.
Steinl C, Essl M, Schreiber TD, Geiger K, Prokop L, Stevanović S, et al. Release of matrix metalloproteinase-8 during physiological trafficking and induced mobilization of human hematopoietic stem cells. Stem Cells Dev. 2013;22:1307–18. https://doi.org/10.1089/scd.2012.0063.
Yamada Y, Amagasaki T, Jacobsen DW, Green R. Lactoferrin binding by leukemia cell lines. Blood. 1987;70:264–70.
Butler TW, Heck LW, Huster WJ, Grossi CE, Barton JC. Assessment of total immunoreactive lactoferrin in hematopoietic cells using flow cytometry. J Immunol Methods. 1988;108:159–70. https://doi.org/10.1016/0022-1759(88)90415-2.
Brown RD, Rickard KA, Kronenberg H. Lactoferrin in the myeloproliferative disorders: a search for granulopoietic regulator defects. Br J Haematol. 1985;59:617–26. https://doi.org/10.1111/j.1365-2141.1985.tb07356.x.
Müller CI, Luong QT, Shih LY, Jones LC, Desmond JC, Kawamata N, et al. Identification of marker genes including RUNX3 (AML2) that discriminate between different myeloproliferative neoplasms and normal individuals. Leukemia. 2008;22:1773–8. https://doi.org/10.1038/leu.2008.41.
Muggeo S, Crisafulli L, Uva P, Fontana E, Ubezio M, Morenghi E, et al. PBX1-directed stem cell transcriptional program drives tumor progression in myeloproliferative neoplasm. Stem Cell Reports. 2021;16:2607–16. https://doi.org/10.1016/j.stemcr.2021.09.016.
Hasselbalch HC, Thomassen M, Riley CH, Kjær L, Larsen TS, Jensen MK, et al. Whole blood transcriptional profiling reveals deregulation of oxidative and antioxidative defence genes in myelofibrosis and related neoplasms. Potential implications of downregulation of Nrf2 for genomic instability and disease progression. PLoS ONE. 2014;9:e112786. https://doi.org/10.1371/journal.pone.0112786.
Palma-Barqueros V, Bohdan N, Revilla N, Vicente V, Bastida JM, Rivera J. PTGS1 gene variations associated with bleeding and platelet dysfunction. Platelets. 2021;32:710–6. https://doi.org/10.1080/09537104.2020.1782370.
Camaschella C. BMP6 orchestrates iron metabolism. Nat Genet. 2009;41:386–8. https://doi.org/10.1038/ng0409-386.
Bock O, Höftmann J, Theophile K, Hussein K, Wiese B, Schlué J, et al. Bone morphogenetic proteins are overexpressed in the bone marrow of primary myelofibrosis and are apparently induced by fibrogenic cytokines. Am J Pathol. 2008;172:951–60. https://doi.org/10.2353/ajpath.2008.071030.
Topić I, Ikić M, Ivčević S, Kovačić N, Marušić A, Kušec R, et al. Bone morphogenetic proteins regulate differentiation of human promyelocytic leukemia cells. Leuk Res. 2013;37:705–12. https://doi.org/10.1016/j.leukres.2013.03.002.
Taniguchi A, Nemoto Y, Yokoyama A, Kotani N, Imai S, Shuin T, et al. Promoter methylation of the bone morphogenetic protein-6 gene in association with adult T-cell leukemia. Int J Cancer. 2008;123:1824–31. https://doi.org/10.1002/ijc.23749.
Hasselbalch HC, Skov V, Larsen TS, Thomassen M, Riley CH, Jensen MK, et al. High expression of carcinoembryonic antigen-related cell adhesion molecule (CEACAM) 6 and 8 in primary myelofibrosis. Leuk Res. 2011;35:1330–4. https://doi.org/10.1016/j.leukres.2011.03.013.
Tillmann S, Olschok K, Schröder SK, Bütow M, Baumeister J, Kalmer M, et al. The unfolded protein response is a major driver of LCN2 expression in BCR-ABL- and JAK2V617F-positive MPN. Cancers. 2021;13:4210. https://doi.org/10.3390/cancers13164210.
Lu M, Xia L, Liu YC, Hochman T, Bizzari L, Aruch D, et al. Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood. 2015;126:972–82. https://doi.org/10.1182/blood-2014-12-618595.
Kagoya Y, Yoshimi A, Tsuruta-Kishino T, Arai S, Satoh T, Akira S, et al. JAK2V617F + myeloproliferative neoplasm clones evoke paracrine DNA damage to adjacent normal cells through secretion of lipocalin-2. Blood. 2014;124:2996–3006. https://doi.org/10.1182/blood-2014-04-570572.
Zhong Y, Johnson AJ, Byrd JC, Dubovsky JA. Targeting Interleukin-2-Inducible T-cell kinase (ITK) in T-Cell related Diseases. Postdoc J. 2014;2:1–11. https://doi.org/10.14304/surya.jpr.v2n6.1.
Legler K, Rosprim R, Karius T, Eylmann K, Rossberg M, Wirtz RM, et al. Reduced mannosidase MAN1A1 expression leads to aberrant N-glycosylation and impaired survival in breast cancer. Br J Cancer. 2018;118:847–56. https://doi.org/10.1038/bjc.2017.472.
Spivak JL, Considine M, Williams DM, Talbot CC Jr, Rogers O, Moliterno AR, et al. Two clinical phenotypes in polycythemia vera. N Engl J Med. 2014;371:808–17. https://doi.org/10.1056/NEJMoa1403141.
Wang JC, Kundra A, Andrei M, Baptiste S, Chen C, Wong C, et al. Myeloid-derived suppressor cells in patients with myeloproliferative neoplasm. Leuk Res. 2016;43:39–43. https://doi.org/10.1016/j.leukres.2016.02.004.
Niapour M, Farr C, Minden M, Berger SA. Elevated calpain activity in acute myelogenous leukemia correlates with decreased calpastatin expression. Blood Cancer J. 2012;2:e51. https://doi.org/10.1038/bcj.2011.50.
Passamonti F, Pietra D, Malabarba L, Rumi E, Della Porta MG, Malcovati L, et al. Clinical significance of neutrophil CD177 mRNA expression in Ph-negative chronic myeloproliferative disorders. Br J Haematol. 2004;126:650–6. https://doi.org/10.1111/j.1365-2141.2004.05098.x.
Inoue D, Kitaura J, Togami K, Nishimura K, Enomoto Y, Uchida T, et al. Myelodysplastic syndromes are induced by histone methylation–altering ASXL1 mutations. J Clin Invest. 2013;123:4627–40. https://doi.org/10.1172/JCI70739.
Naudin C, Chevalier C, Roche S. The role of small adaptor proteins in the control of oncogenic signaling driven by tyrosine kinases in human cancer. Oncotarget. 2016;7:11033–55. https://doi.org/10.18632/oncotarget.6929.
De Braekeleer E, Meyer C, Douet-Guilbert N, Basinko A, Le Bris MJ, Morel F, et al. Identification of MLL partner genes in 27 patients with acute leukemia from a single cytogenetic laboratory. Mol Oncol. 2011;5:555–63. https://doi.org/10.1016/j.molonc.2011.08.003.
Hasselbalch HC, Skov V, Stauffer Larsen T, Thomassen M, Hasselbalch Riley C, Jensen MK, et al. Transcriptional profiling of whole blood identifies a unique 5-gene signature for myelofibrosis and imminent myelofibrosis transformation. PLoS ONE. 2014;9:e85567. https://doi.org/10.1371/journal.pone.0085567.
Suknuntha K, Ishii Y, Hu K, Brayan M, Yang DT, Swanson S, et al. Induced pluripotent stem cell model of chronic myeloid leukemia revealed Olfactomedin 4 as a novel survival factor for primitive leukemia cells. Blood. 2014;124:397. https://doi.org/10.1182/blood.V124.21.397.397.
Franzini A, Pomicter AD, Yan D, Khorashad JS, Tantravahi SK, Than H, et al. The transcriptome of CMML monocytes is highly inflammatory and reflects leukemia-specific and age-related alterations. Blood Adv. 2019;3:2949–61. https://doi.org/10.1182/bloodadvances.2019000585.
Guy A, Gourdou-Latyszenok V, Le Lay N, Peghaire C, Kilani B, Dias JV, et al. Vascular endothelial cell expression of JAK2V617F is sufficient to promote a pro-thrombotic state due to increased P-selectin expression. Haematologica. 2019;104:70–81. https://doi.org/10.3324/haematol.2018.195321.
Gangaraju R, Song J, Kim SJ, Tashi T, Reeves BN, Sundar KM, et al. Thrombotic, inflammatory, and HIF-regulated genes and thrombosis risk in polycythemia vera and essential thrombocythemia. Blood Adv. 2020;4:1115–30. https://doi.org/10.1182/bloodadvances.2019001379.
Mondet J, Hussein K, Mossuz P. Circulating cytokine levels as markers of inflammation in Philadelphia negative myeloproliferative neoplasms: diagnostic and prognostic interest. Mediators Inflamm. 2015;670580. https://doi.org/10.1155/2015/670580.
Araki M, Yang Y, Masubuchi N, Hironaka Y, Takei H, Morishita S, et al. Activation of the thrombopoietin receptor by mutant calreticulin in CALR-mutant myeloproliferative neoplasms. Blood. 2016;127:1307–16. https://doi.org/10.1182/blood-2015-09-671172.
Chachoua I, Pecquet C, El-Khoury M, Nivarthi H, Albu RI, Marty C, et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127:1325–35. https://doi.org/10.1182/blood-2015-11-681932.
Elf S, Abdelfattah NS, Chen E, Perales-Patón J, Rosen EA, Ko A, et al. Mutant calreticulin requires both its mutant C-terminus and the thrombopoietin receptor for oncogenic transformation. Cancer Discov. 2016;6:368–81. https://doi.org/10.1158/2159-8290.
Jutzi JS, Marneth AE, Ciboddo M, Guerra-Moreno A, Jiménez-Santos MJ, Kosmidou A, et al. Whole-genome CRISPR screening identifies N-glycosylation as a genetic and therapeutic vulnerability in CALR-mutant MPN. Blood. 2022;140:1291–304. https://doi.org/10.1182/blood.2022015629.
Acknowledgements
The authors acknowledge Cristina Hurtado by her technical support.
Funding
This study was supported by the PIUNA 2020 program of the University of Navarra (Code 150582003).
Author information
Authors and Affiliations
Contributions
Conceptualization, J.L.V. and A.G-H.; methodology, J.L.V. and A.G-H.; investigation and formal analysis, A.G-H.; writing—original draft preparation, J.L.V. and A.G-H.; writing—review and editing, J.L.V. and A.G-H.; supervision, J.L.V. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable, since this is a re-analysis of data previously published and deposited in the publicly available GEO database. These previous studies were conducted in accordance with the Declaration of Helsinki and performed under the local Institutional Review Board’s approved protocol. According to published articles, all subjects provided informed written consent.
Consent for publication
Not applicable, since this is a re-analysis of data previously published and deposited in the publicly available GEO database.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12863_2023_1142_MOESM2_ESM.xlsx
Supplementary Material 2: Table S2. Results of microarray analysis when comparing CALR-mutated ET patients vs. healthy donors. Non-differentially expressed genes are shaded in red (adjusted p-value > 0.01).
12863_2023_1142_MOESM3_ESM.xlsx
Supplementary Material 3: Table S3. Results of microarray analysis when comparing JAK2-mutated ET patients vs. healthy donors. Non-differentially expressed genes are shaded in red (adjusted p-value > 0.01).
12863_2023_1142_MOESM6_ESM.tif
Supplementary Material 6: Supplementary Figure 1. Results of RNA-seq analysis of samples from PBMCs for the gene signature found in this study when comparing (a) CALR-mutated or (b) JAK2-mutated ET patients vs. healthy donors. Individual transcript per million (TPM) values and means are represented.
12863_2023_1142_MOESM7_ESM.tif
Supplementary Material 7: Supplementary Figure 2. Tables showing data from RNA-seq analysis of samples from PBMCs for the gene signature found in this study when comparing (a) CALR-mutated or (b) JAK2-mutated ET patients vs. healthy donors. Mean log2FC values and adjusted p-values are represented.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guijarro-Hernández, A., Vizmanos, J.L. Transcriptomic comparison of bone marrow CD34 + cells and peripheral blood neutrophils from ET patients with JAK2 or CALR mutations. BMC Genom Data 24, 40 (2023). https://doi.org/10.1186/s12863-023-01142-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-023-01142-5