Abstract
Background
This study explored the key genes related to immune cell infiltration in endometriosis.
Results
The Gene Expression Omnibus (GEO) datasets (GSE7305, GSE7307, and GSE11691), containing a total of 37 endometriosis and 42 normal tissues, were retrieved and analyzed to determine the differentially expressed genes (DEGs). Gene ontology (GO) annotations and Kyoto Encyclopedia of Genes (KEGG) analysis were performed to identify the pathways that were significantly enriched. The xCell software was used to analyze immune cell infiltration and correlation analyses were performed to uncover the relationship between key genes and immune cells. The analysis identified 1031 DEGs (581 upregulated and 450 downregulated DEGs), while GO analysis revealed altered extracellular matrix organization, collagen-containing extracellular matrix, and glycosaminoglycan binding and KEGG enrichment showed genes related to metabolic pathways, pathways in cancer, phosphatidylinositol 3-kinase-protein kinase B (PI3K-Akt) signaling, proteoglycans in cancer, and the mitogen-activated protein kinase (MAPK) signaling pathway. Furthermore, the protein–protein interaction network revealed 10 hub genes, i.e., IL6, FN1, CDH1, CXCL8, IGF1, CDK1, PTPRC, CCNB1, MKI67, and ESR1. The xCell analysis identified immune cells with significant changes in all three datasets, including CD4+ and CD8+ T cells, CD8+ Tem, eosinophils, monocytes, Th1 cells, memory B-cells, activated dendritic cells (aDCs), and plasmacytoid dendritic cells (pDCs). These 10 hub genes were significantly associated with at least three types of immune cells.
Conclusions
Aberrant gene expression was related to abnormal infiltration of different immune cells in endometriosis and was associated with endometriosis development by affecting the tissue microenvironment and growth of ectopic endometrial cells.
Similar content being viewed by others
Background
Endometriosis is a benign gynecological condition characterized by the abnormal presence and growth of endometrial tissue outside the uterus. The disease most frequently occurs in the ovaries, fossa ovarica, uterosacral ligaments, and posterior cul-de-sac [1] or in rare cases, in the diaphragm, pleura, and pericardium [2]. Approximately 10% of childbearing-age women may be subject to endometriosis [3]. The main clinical symptoms of endometriosis include pelvic pain, dysmenorrhea, sexual difficulty, dysuria, and infertility [4]. However, to date, endometriosis pathogenesis remains to be defined, although the underlying molecular mechanism could be genetic, environmental, or immune-related [5]. Endometriosis was first discovered microscopically by Karl von Rokitansky in 1860 [5]. Sampson JA proposed the endometrial implantation theory (or the retrograde menstruation theory) for the development of endometriosis in 1927 [6], i.e., during menstruation, endometrial epithelium and stromal cells mixed in the menstrual blood could flow backward through the Fallopian tubes into the abdominal cavity and implant in the ovary and pelvic peritoneum, some of which could proliferate and spread to form endometriosis. Normally, the immune defense system in the peritoneum can suppress such a situation, like attachment and growth of refluxed cells. Indeed, although menstrual reflux occurs in more than 90% of women, only 6%-10% develop the disease [7]. Therefore, this theory alone may not fully explain endometriosis development, and other factors, including genetic, immunological, stem cell migration-related factors, could also play a role in endometriosis development [8,9,10].
To date, a great number of studies have shown that abnormal immunity could play an important role in endometriosis development; for example, the immune cells in the abdominal cavity are the first line of the body’s defense system against novel antigens entering the abdominal cavity. Changes in these immune cells, including monocytes, macrophages, natural killer (NK) cells, or other cytotoxic lymphocytes in the abdominal cavity, occur in endometriosis patients and the subsequent defense could be aberrant [11, 12], resulting in the transformation and growth of ectopic endometrial cells and endometriosis development. Moreover, these ectopic endometrial cells can release cytokines and inflammatory mediators and change the local peritoneum microenvironment to further promote endometriosis development. Since endometriosis development is a tissue-specific phenomenon, the local microenvironment obviously plays a role in endometriosis formation, in addition to the abdominal environment and body defense system, e.g., the ovary, which has high hormone levels, is an ideal site for a high frequency of endometriosis [13]. Secretion of immune-related cytokines and immune cell infiltration are also important to promote ectopic endometrial adhesion, angiogenesis, and matrix remodeling during endometriosis development [14,15,16]. In this regard, aberrant presence of immune cells, types, and functions was reported to be associated with endometriosis pathogenesis [17] and the affected cells included lymphocytes, macrophages, dendritic cells, NK cells, neutrophils, and eosinophils [18,19,20].
In this study, we utilized the online xCell tool to analyze the infiltration of 22 different immune cell subtypes between endometriosis and normal tissues [21]. After obtained the HUB gene associated with endometriosis with the R software, we then analyzed the association between HUB gene and immune cells with significant difference. Because endometriosis is a chronic inflammatory disease and lacks the effective diagnostic markers, we tried to provide the related genes for early and non-invasive diagnosis of endometriosis in future and for further study of the possible immune mechanism in endometriosis development.
Results
Identification of infiltrating immune cell subtypes in endometriosis
In this study, we included 37 cases of endometriosis and 42 cases of normal endometrium obtained from the GSE7305, GSE7307, and GSE11691 datasets. The diseased samples consisted of 28 cases of ovarian endometrioma and 9 cases of peritoneal endometriosis. All surgical samples were taken before any medications, such as hormone therapy. We first determined the cell types potentially involved in endometriosis in the three GEO datasets (GSE7305, GSE7307, and GSE11691) using the xCell tool analysis with the “Charoentong signatures (N = 22)” selected as the gene signatures [21]. We then plotted the split violin diagrams to visualize differences in immune cell infiltration using the cut-off value of p < 0.05 (Fig. 1). Our data showed nine significantly different immune cell types in the GSE7305, GSE7307, and GSE11691 datasets. The xCell scores for these nine different immune cell subtypes in endometriosis were significantly higher than those of the normal endometrium (Fig. 1).
Profiling of differentially expressed genes in endometriosis
After downloading the gene chip analytic data, we normalized the gene expression and the data are shown in Fig. 2. We then utilized the limma R package to screen and identify the DEGs using the criteria of adjusted p < 0.05 and |log fold change (FC)|> 1. The GSE7305 dataset contained 1,446 DEGs (813 upregulated and 633 downregulated DEGs), GSE7307 consisted of 1,782 DEGs (934 upregulated and 848 downregulated DEGs), and GSE11691 profiled a total of 367 DEGs (265 upregulated and 102 downregulated DEGs). The volcano map for the DEGs in these three dataset is shown in Fig. 3 and the cluster heat maps of the top 100 DEGs in each dataset are presented in Fig. 4.
The volcano map of the DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets. A GSE7305; (B) GSE7307; (C) GSE11691 datasets. The red dots represent the upregulated DEGs using the cut-off values of adjusted p < 0.05 and |log fold change|> 1, whereas the green dots show the downregulated DEGs using the cut-off values of adjusted p < 0.05 and -|log fold change|< -1. The black spots represent genes with no significant difference in expression
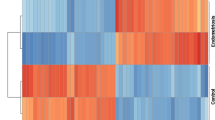
The cluster heatmaps of the top 100 DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets. A GSE7305, (B) GSE7307, and (C) GSE11691 datasets. The red color indicates relative upregulated DEGs, whereas the blue color shows the relative downregulated DEGs. The white color indicates no significant change in gene expression
We utilized the Robust Rank Aggregation method (RRA) according to a previous study [22] to analyze the DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets. RRA analysis theoretically assumes that each gene in each dataset is randomly arranged (expressed), but if a given gene ranks high in all datasets, the associated p value will be lower, indicating that the potential for the expression of this DEG is greater. After RRA ranking analysis with a corrected p < 0.05 and logFC > 1 or − logFC < − 1, we identified 1031 integrated DEGs (including 581 upregulated and 450 downregulated genes). The top 20 upregulated and downregulated genes are shown in Fig. 5.
Gene ontology (GO) terms for the DEGs
Next, we performed GO term analysis of the DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets in endometriosis using the “clusterProfiler” package. The GO analysis data could be grouped into three categories, i.e., molecular functions, cellular components, and biological processes. Table 1 lists the top 10 GO terms for the DEGs. Using the cutoff criteria of p < 0.05, the three categories of GO terms are shown in Fig. 6. The molecular functions of the DEGs were mainly enriched in glycosaminoglycan binding, receptor ligand activity, and signaling receptor activator activity. The GO terms in the cellular components category were mainly involved in the collagen-containing extracellular matrix, cell–cell junction, and apical part of cells. The GO terms in the biological processes category were mainly involved in extracellular matrix organization, extracellular structure organization, and reproductive structure development.
KEGG pathway enrichment of the DEGs
To further evaluate the DEG-related gene pathways, we performed KEGG [23,24,25] pathway enrichment of the DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets in endometriosis using the KOBAS software. The top 20 KEGG enriched gene pathways are shown in Fig. 7, while the top 10 KEGG enriched gene pathways are listed in Table 2. The DEGs were mostly enriched in metabolic pathways, pathways in cancer, the phosphatidylinositol 3‑kinase-protein kinase B (PI3K-Akt) signaling pathway, proteoglycans in cancer, mitogen-activated protein kinase (MAPK) signaling pathway, cell adhesion molecules (CAMs), and human papillomavirus infection. Overall, the GO term and KEGG pathway analyses suggested that immunity and inflammation were involved in the pathophysiological process of endometriosis.
Top 20 KEGG enriched gene pathways for DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets. The horizontal axis is the ratio of the number of target proteins enriched in the pathway to the total number of proteins in the pathway, and the vertical axis represents the pathway. The size of the dot represents the number of genes enriched in the pathway. Different colors represent different correction p values; a color change from red to green indicates a change in the correction p values from large to small values and an increase in the statistical significance of enrichment
Protein–protein interaction (PPI) network of the DEGs
We constructed the PPI network for the DEGs in the GEO GSE7305, GSE7307, and GSE11691 datasets using the online STRING database and analyzed the data using the Cytoscape software. Thereafter, we further screened the top 10 hub genes using the cytoHubba tool in the Cytoscape software and identified the hub genes as IL6, Fibronectin 1 (FN1), CDH1, CXCL8, IGF1, CDK1, PTPRC, CCNB1, MKI67, and ESR1. We also performed MCODE analysis in the Cytoscape software with the default parameters to analyze the functional modules of the PPI network. Figure 8 shows the two most important modules. The 10 hub genes were mainly involved in pathways in cancer, cellular senescence, the PI3K-Akt signaling pathway, the p53 signaling pathway, and the AGE-RAGE signaling pathway in diabetic complications. The genes in Module 1 were mainly enriched in the cell cycle and oocyte meiosis while the genes in Module 2 were mainly enriched in neuroactive ligand-receptor interactions and complement and coagulation cascades.
Association of the hub genes with immune cells
Finally, we assessed the association of the 10 hub genes with the infiltration of immune cells. The expression of these 10 hub genes was associated with the scores of nine significantly different immune cell subtypes after Pearson correlation analysis (p < 0.05; Table 3). These 10 hub genes were significantly associated with at least three immune cells and the most significant gene was associated with eight kinds of immune cells. Th1 cells and memory B-cells were the top two cell types associated with the highest number of hub genes. The correlation index of FN1 vs. five kinds of immune cells was greater than 0.5 and the correlation coefficient between aDCs and CXCL8 was the highest (Fig. 9), indicating a close interplay between the immune/inflammatory response and endometriosis development and progression.
Discussion
Our current study showed significant differences in levels of CD4+ and CD8+ T cells, CD8+ Tem cells, eosinophils, monocytes, Th1 cells, memory B cells, aDCs, and pDCs in endometriosis tissue samples. The key DEGs were IL6, FN1, CDH1, CXCL8, IGF1, CDK1, PTPRC, CCNB1, MKI67, and ESR1, while the 10 hub genes were associated with nine kinds of immune cells, among which FN1 was associated with eight kinds of immune cells. The correlation of IL-8 to aDCs was the strongest, with a correlation coefficient score of 0.71. Our current study revealed that DEGs were associated with abnormal immune cell infiltration in endometriosis as well as the development of endometriosis by affecting the tissue microenvironment and the growth of ectopic endometrial cells. Poli-Neto et al. [26] also performed bioinformatical analysis and revealed differences in immune cell expression profiles among different stages of endometriosis, which were independent of the hormonal milieu; for example, they showed a high expression rate of NKT cells in endometriosis, independently of the cycle phase or disease stages, therefore, suggested a sustained stress or damage of the eutopic endometrium. Based on the analysis of immune expression profile, our current study provided the correlation between differentially expressed genes and differential immune cells as a novel strategy for further study of immune mechanism of endometriosis.
Indeed, a recent study of the GEO GSE11691, GSE23339, GSE25628 and GSE78851 datasets showed that the DEGs were closely associated with cell migration, adherens junction signaling, and hypoxia-inducible factor signaling [27]. Another recent study of the GEO GSE25628, GSE5108, and GSE7305 datasets showed that the DEGs and hub genes included genes involved in DNA strand separation, cellular proliferation, degradation of the extracellular matrix, encoding of smooth muscle myosin as a major contractile protein, exiting the proliferative cycle and entering quiescence, and growth regulation and were implicated in a wide variety of biological processes [28]. Nanda et al. [29] speculated that degradation of the extracellular matrix (ECM) in endometriosis was generally induced and the release of VEGF from the ECM promoted the angiogenesis of endometrial tissue in endometriosis patients. Thus, the combination of excessive ECM degradation and damage of cellular functions might induce the growth of ectopic endometrium and the development of endometriosis. Their pathway enrichment analysis showed the involvement of PI3K-Akt signaling, MAPK signaling, and CAMs. Honda et al. [30] reported that the PI3K-Akt and MAPK signaling pathways were activated in endometriosis. The PI3K-Akt pathway enhances cell survival, proliferation, and migration and the upregulated MAPK subfamily promotes the growth and maintenance of ectopic endometrial tissues by affecting the functions of various cytokines (such as IL-6, COX-2, and IL-8) [31]. Another study [32] revealed that specific CAMs were involved in the development of early endometriosis lesions and the unique CAM expression in endometriosis might contribute to the persistence of ectopic endometrium. In our current study, the GO terms of the DEGs were mainly enriched in extracellular matrix organization, collagen-containing extracellular matrix, and glycosaminoglycan binding, while the KEGG analysis of the DEGs were mainly enriched in PI3K-Akt signaling pathway, MAPK signaling pathway and CAMs. Our current data are consistent with the above reported research results [29,30,31,32,33]. However, although these studies, including our current study, were conducted using different datasets from the GEO database, the data could have identified different DEGs in endometriosis and gene pathways, indicating that further in vitro and in vivo studies are needed to confirm our data and determine the true associations or causes of endometriosis development.
Furthermore, we analyzed immune cell infiltration in endometriosis using the xCell tool and found significant differences in and high levels of CD4+ and CD8+ T cells, CD8+ Tem cells, eosinophils, monocytes, Th1 cells, memory B cells, aDCs, and pDCs in endometriosis vs. normal endometrial tissue samples. Endometriosis is considered a chronic inflammatory disease with known immune disorders. Growing evidence suggests that almost all subtypes of immune cells and functions are abnormal in endometriosis; for example, reduced T cell responsiveness and NK cytotoxicity, but increased B cell polyclonal activation and antibody production and peritoneal macrophages as well as changes in various inflammatory mediators and cytokines in endometriosis [33]. The ectopic endometrium contains significantly more scattered stromal CD4, CD8, and activated T cells than does the proliferative and secretory eutopic endometrium [34] and produces more cytokines, with specific immune processes to induce growth and differentiation of the ectopic endometrium. The increase of the CD4+/CD8+ T cell ratio and decrease of anti-inflammatory IL-10 could be involved in the pathogenesis of endometriosis and may secondarily affect the functions of monocytes and macrophages [35]. Immature dendritic cells (DCs) are increased in endometriosis and the surrounding peritoneum in endometriosis, but the number of mature DCs in the endometrium of patients with endometriosis is significantly lower than that in healthy endometrium, indicating that the functions of DCs in endometriosis are impaired [36]. However, in our current study, we found that level of pDC cells was increased in endometriosis. To date, only peripheral blood pDC has been studied in endometriosis samples [37] vs. the samples without endometriosis and the data showed that the number of pDC was reduced throughout the menstrual cycle. In contrast, in women with endometriosis, pDC increased as the cycle progresses, although the clinical significance of pDC dynamics throughout the menstrual cycle remains to be determined. This disorder of DC in patients with endometriosis may lead to immune escape or abnormal immune targeting of endometrial fragments that fall off during menstruation, and promote the survival of ectopic endometrium and the formation of endometriosis. Eosinophil is thought to be the most significant mammalian immune and inflammatory cells and possesses various receptors for inflammatory mediators in addition to producing a variety of pro-inflammatory and homeostatic mediators [38]. The level of CD69+ eosinophil occurred to be high in the peritoneal fluid of endometriosis patients, indicating that activated eosinophils accumulated in the early stages of endometriosis and played an important role in endometriosis pathogenesis [39]. Our current study further confirmed the difference in the infiltration of immune cells in endometriosis.
In addition, our current study using prospective bioinformatics analysis identified IL6, FN1, CDH1, CXCL8, IGF1, CDK1, PTPRC, CCNB1, MKI67, and ESR1 as key DEGs in endometriosis. These 10 hub genes are associated with nine subtypes of immune cells in endometriosis; for example, the upregulated FN1 expression was associated with eight subtypes of immune cells, i.e., monocytes, CD8+ Tem cells, Th1 cells, memory B cells and eosinophils. The correlation of aDCs with CXCL8 was the highest, suggesting that FN1 and CXCL8 (IL-8) may promote the infiltration of immune cells and change the local immune microenvironment during the development of endometriosis. Efthymiou et al. [40] speculated that FN could help to shape the tumor microenvironment as the central position for the "vascular group" to not only play a key role in angiogenesis, but also enhance vascular recruitment through integrin-dependent binding of endothelial cells. FN mediates the release of inflammatory cytokines through Toll-like receptor 4 (TLR4) and the ECM to transport, mature, and activate immune cells, but prevents CD8+ T cells from reaching tumor cells; thereby preventing tumor cells from being destroyed by immune cells. Another study [41] showed that NKp46, the receptor on NK cells, mediated the production of IFN-γ and the latter induced FN1 expression in tumor lesions to induce tumor metastasis. Furthermore, reduced NK cell cytotoxicity in endometriosis was not due to a decrease in their number but rather to defects in their functions [42]; therefore, there was no difference in NK cell infiltration between normal endometrium and endometriosis endometrium. However, the interaction mechanism between FN1 and immune cells in endometriosis needs further study. CXCL8 (IL-8), one of the first and most studied chemokines [43], acts on CXCR1 and CXCR2 receptors and is an effective neutrophil chemotactic factor to promote inflammation and angiogenesis [43]. In the current study, we found that CXCL8 expression was higher in endometriosis than in normal endometrium. Previous studies also reported that CXCL8 expression was significantly higher in the peritoneal fluid of endometriosis patients than that of patients without endometriosis [44, 45]. The concentration of CXCL8 in the peritoneal fluid of patients with moderate/severe endometriosis was also higher than that of patients with mild endometriosis [46], indicating that CXCL8 might be important in endometriosis development [47]. As a pro-inflammation chemokine, CXCL8 participates in the development of many diseases; for example, CXCL8 induces PD-L1 expression in macrophages to inhibit the functions of CD8+ T cells and promote an immunosuppressive microenvironment in gastric cancer [48]. Additionally, the expression of CXCL8 and its receptors was found to enhance the angiogenesis, proliferation, migration, invasion, and survival of colorectal cancer cells [49] and induce the EMT and metastasis of colorectal cancer cells. Similarly, Singh et al. [50] demonstrated that a low level of CXCL8/IL-8 expression led to a decrease in neutrophil exudation in macular patients, suggesting that CXCL8/IL-8 and related signaling affected disease development. Burke et al. [51] reported that human cord blood-derived mast cells (CBMCs) produced significant amounts of CXCL8 after the response to low levels of reovirus infection. Additionally, CBMC supernatants infected with reovirus induced substantial NK cell chemotaxis that was highly dependent on CXCL8 and CXCR1 expression, indicating CXCL8 played a role in the recruitment of human NK cells by mast cells. Vujanovic et al. [52] demonstrated that CXCL8/IL-8 was a key chemokine for DCs to recruit NK cells. CXCL8 was reported to be involved in all processes in the development of endometriosis, including adhesion, invasion, and implantation of the ectopic tissues [53]. However, whether endometriosis depends on CXCL8 to regulate the immune microenvironment in the development of endometriosis requires further study.
However, our current study did possess some limitations; for example, it is merely a proof of principle and further experimental investigation is needed to confirm our bioinformatics data. Moreover, this study was based on the gene expression profile provided by the Affymetrix platform to identify some important genes that can be investigated in our subsequent study to experimentally verify their roles in endometriosis development. In addition, due to the lack of detailed clinical data on GSE7307, we are unable to identify more associations between genotypes and phenotypes and to analyze the interference of the menstrual cycle stages with disease stages. Again, it is necessary to remove the batch effects to minimize such a batch effect through the PCA analysis and standardization, but the batch effect between different data sets can not be completely eliminated; in addition, although additional filters are used to eliminate samples that may be contaminated, but tissue pollution is still unavoidable. The current study, similar to previous studies [54, 55], compared very different tissues, i.e., eutopic endometrium from healthy patients, ectopic endometrium in the ovary, and ectopic endometrium in the peritoneum, which should have had very different adjacent tissues. To resolve this issue, the authors of one of the previous studies [54] first identified the probe sets that were significantly up-regulated in endometriosis compared to the control endometrium and then applied an additional set of the filters for it. This was necessary because many probe sets were the result of tissue contamination in endometriosis samples. Thus, finding a probe set that was differentially expressed between the normal ovary and normal endometrium indicated that there was a non-disease-related difference in gene expression and the probe set was removed from the endometriosis vs. the control endometrium for the differentially regulated list. Consequently, gene expression in the different normal tissues could be resolved.
Conclusions
In the current study, we performed various bioinformatics analyses to explore the key DEGS associated with immune infiltrating cells in endometriosis. We found different levels of immune cell infiltration and a high level in endometriosis vs. normal endometrial tissues, including CD4+ and CD8+ T cells, CD8 + Tem cells, eosinophils, monocytes, Th1 cells, memory B cells, aDCs, and pDCs. The top 10 hubs were IL6, FN1, CDH1, CXCL8, IGF1, CDK1, PTPRC, CCNB1, MKI67, and ESR1. Among them, FN1 was associated with eight subtypes of immune cells and the correlation co-efficiency between CXCL8 and aDCs was the highest, with a value of 0.71.
Methods
Search and download of Gene Expression Omnibus (GEO) datasets
In this study, we first searched the GEO database (https://www.ncbi.nlm.nih.gov/geo/) using the keyword “endometriosis” and used the “GEOquery” package of the R software (version 4.0.4) to download the gene expression profiles for endometriosis (GSE7305, GSE7307, and GSE11691). The GSE7305 dataset [54] was based on the GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array and included 10 samples each of ovarian endometriosis and normal endometrium. The surgical samples were taken before any medications, such as hormone therapy. GSE7305 had often been used to identify differentially expressed genes (DEGs) and analyze endometriosis in biological research related to endometriosis. A previous study showed that epithelial-mesenchymal transformation (EMT) may be induced by inflammatory cytokines and is related to smooth muscle metaplasia and fibrosis [56]. The molecular markers that regulate the development and progression of endometriosis and potential therapeutic drugs have also been identified [57]. Based on the same GSE7305 platform, GSE7307 was selected to increase the sample size for ovarian endometriosis [58], which included 18 endometriosis and 23 normal endometrial tissues. In addition, GSE11691 was added to increase the number of peritoneal endometriosis samples to enrich the data; the platform of GSE11691 [55] was the GPL96 [HG-U133A] Affymetrix Human Genome U133A Array and included 9 endometriosis tissues and 9 normal endometrial tissue samples, resulting in a total of 37 endometriosis (including 28 cases of ovarian endometriosis and 9 cases of peritoneal endometriosis as seven proliferative phase, 12 secretory phase, and 18 unknown phase samples) and 42 normal endometrial tissues (including seven proliferative phase, 12 secretory phase, and 23 unknown phase samples) in this study.
Analyses of different immune cells in endometriosis vs. normal tissues
xCell (https://xcell.ucsf.edu/) is an online tool that can enrich gene expression in specific cell types, including 64 types of immune and stromal cells [21]. Specifically, xCell is a gene signature-based methodology that deals with thousands of pure cell types from various sources and applies a novel technique to differentiate between closely related cell types. In this study, we identified xCell signatures in three GEO datasets on endometriosis using extensive in-silico simulations and cytometric immunophenotypes. The gene signatures we selected in this study were the “Charoentong signatures (N = 22)” and the cut-off point was p < 0.05. Thereafter, we utilized the “ggplot2” package to draw split violin diagrams to visualize the differences in immune cell infiltrations in endometriosis.
Data preprocessing and identification of differentially expressed genes
Each dataset, GSE7305, GSE7307, and GSE11691, was first normalized using the limma R package (http://www.bioconductor.org/) using the normalizing array functions. All gene expression data were then subjected to log2 transformation. Afterward, we obtained DEGs between endometriosis and normal endometrial tissues using the R package with the limma function [p value < 0.05 and the log fold change (FC)|> 1). The volcano map of these identified DEGs and the heatmap of the top 100 DEGs in each microarray datasets were obtained using R package.
Furthermore, we used the RRA R package to integrate the expression matrix of these three datasets and further screened the integrated DEGs (corrected p < 0.05, logFC > 1 or − logFC < − 1).
Gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analysis of gene pathways
The “clusterProfiler” package was used to further explore the biological significance of the DEGs, including the GO biological process, cellular components, and molecular function terms. We next used the online KOBAS software (https://david.ncifcrf.gov/) to perform KEGG pathway enrichment analysis. A p value < 0.05 was used as the cutoff criterion for statistical significance.
Construction and analysis of protein–protein interaction (PPI) network
To construct the PPI network for the DEGs in the three GEO datasets, GSE7305, GSE7307, and GSE11691, we used the Search Tool for the Retrieval of Interacting Genes Database (STRING; https://www.string-db.org/) to explore the relationship among the DEGs. Afterward, we utilized the Cytoscape software to convert the resulting data visually and screen for hub genes according to the degree of connectivity. In addition, we analyzed the functional modules in the PPI network using the Molecular Complex Detection (MCODE) plug-in in the Cytoscape software with the default parameters.
Association of the hub genes with infiltrating immune cells
The Pearson correlation test was employed to analyze the hub genes in the infiltrating immune cells in endometriosis using the R package and the resulting data were visualized using the "ggplot" package.
Statistical analysis
The data were summarized as mean ± SD and individual GSE7305, GSE7307, and GSE11691 data on endometriosis were analyzed for DEGs using the cut-off values of p < 0.05 and log fold change (FC)|> 1. The integrated DEGs of the three datasets were obtained by rank sum analysis with a corrected p < 0.05 and log FC > 1 or –log FC < -1 and Pearson’s rank test was used to analyze the correlation between key genes and immune cells. All statistical analyses were executed using the statistical programming language R for windows and a p value < 0.05 was considered statistically significant.
Availability of data and materials
Data is available at NCBI GEO, accession numbers: GSE7305: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7305. GSE7307: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7307. GSE11691: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11691.
Abbreviations
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differentially Expressed Genes
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes
- PI3K-Akt:
-
Phosphatidylinositol 3-Kinase-Protein Kinase B
- MAPK:
-
Mitogen-Activated Protein Kinase
- CAMs:
-
Cell Adhesion Molecules
- FN1:
-
Fibronectin 1
- DCs::
-
Dendritic Cells
- aDCs:
-
Activated Dendritic Cells
- pDCs:
-
Plasmacytoid Dendritic Cells
- TNF-α:
-
Tumor Necrosis Factor-α
- VEGFR:
-
Vascular Endothelial Growth Factor Receptor
- PDGF:
-
Platelet-Derived Growth Factor
- FGF:
-
Fibroblast Growth Factor
- MMPs:
-
Matrix Metalloproteinases
- STAT4:
-
Signal Transducer and Activator of Transcription 4
- STING:
-
Stimulator of Interferon Genes
- PPI:
-
Protein-Protein Interaction
- RRA:
-
Robust Rank Aggregation
- MCODE:
-
Molecular Complex Detection
- ECM:
-
Extracellular Matrix
- TLR4:
-
Toll-Like Receptor 4
- EMT:
-
Epithelial-Mesenchymal Transformation
- CBMCs:
-
Cord Blood-Derived Mast Cells
References
Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7:349–57.
Giudice LC. Clinical practice. Endometriosis N Engl J Med. 2010;362:2389–98.
Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15.
Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. 2020;382:1244–56.
Batt RE. A history of endometriosis. Springer. London, UK: Springer; 2011. p. 13–38.
Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3:93-110.43.
Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA. Endometriosis: where are we and where are we going? Reproduction. 2016;152:R63-78.
Koninckx PR, Barlow D, Kennedy S. Implantation versus infiltration: the Sampson versus the endometriotic disease theory. Gynecol Obstet Invest. 1999;47(Suppl 1):3–9 discussion -10.
Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis. Semin Reprod Med. 2015;33:333–40.
van der Linden PJ. Theories on the pathogenesis of endometriosis. Hum Reprod. 1996;11(Suppl 3):53–65.
Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. 2019;25:564–91.
Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann N Y Acad Sci. 2002;955:183–98 discussion 19–200, 396–406.
Koninckx PR, Kennedy SH, Barlow DH. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update. 1998;4:741–51.
Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59.
Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–9.
Barbe AM, Berbets AM, Davydenko IS, Koval HD, Yuzko VO, Yuzko OM. Expression and Significance of Matrix Metalloproteinase-2 and Matrix Metalloproteinas-9 in Endometriosis. J Med Life. 2020;13:314–20.
Dmowski WP, Steele RW, Baker GF. Deficient cellular immunity in endometriosis. Am J Obstet Gynecol. 1981;141:377–83.
Agostinis C, Balduit A, Mangogna A, Zito G, Romano F, Ricci G, et al. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front Immunol. 2020;11.
Izumi G, Koga K, Takamura M, Makabe T, Satake E, Takeuchi A, et al. Involvement of immune cells in the pathogenesis of endometriosis. J Obstet Gynaecol Res. 2018;44:191–8.
Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. 2009;24:325–32.
Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18:220.
Yan S, Wang W, Gao G, Cheng M, Wang X, Wang Z, et al. Key genes and functional coexpression modules involved in the pathogenesis of systemic lupus erythematosus. J Cell Physiol. 2018;233:8815–25.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–51.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–51.
Poli-Neto OB, Meola J, Rosa ESJC, Tiezzi D. Transcriptome meta-analysis reveals differences of immune profile between eutopic endometrium from stage I-II and III-IV endometriosis independently of hormonal milieu. Sci Rep. 2020;10:313.
Dai FF, Bao AY, Luo B, Zeng ZH, Pu XL, Wang YQ, et al. Identification of differentially expressed genes and signaling pathways involved in endometriosis by integrated bioinformatics analysis. Exp Ther Med. 2020;19:264–72.
Wang T, Jiang R, Yao Y, Qian L, Zhao Y, Huang X. Identification of endometriosis-associated genes and pathways based on bioinformatic analysis. Medicine (Baltimore). 2021;100.
Nanda A K T, Banerjee P, Dutta M, Wangdi T, Sharma P, et al. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Ann Lab Med. 2020;40:390–7.
Honda H, Barrueto FF, Gogusev J, Im DD, Morin PJ. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod Biol Endocrinol. 2008;6:59.
Makker A, Goel MM, Das V, Agarwal A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol Endocrinol. 2012;28:175–81.
Witz CA. Cell adhesion molecules and endometriosis. Semin Reprod Med. 2003;21:173–82.
Riccio L, Santulli P, Marcellin L, Abrão MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. 2018;50:39–49.
Witz CA, Montoya IA, Dey TD, Schenken RS. Characterization of lymphocyte subpopulations and T cell activation in endometriosis. Am J Reprod Immunol. 1994;32:173–9.
Szyllo K, Tchorzewski H, Banasik M, Glowacka E, Lewkowicz P, Kamer-Bartosinska A. The involvement of T lymphocytes in the pathogenesis of endometriotic tissues overgrowth in women with endometriosis. Mediators Inflamm. 2003;12:131–8.
Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24:1695–703.
Maridas DE, Hey-Cunningham AJ, Ng CHM, Markham R, Fraser IS, Berbic M. Peripheral and endometrial dendritic cell populations during the normal cycle and in the presence of endometriosis. J Endometr Pelvic Pain Disord. 2014;6:67–119.
Chusid MJ. Eosinophils: Friends or Foes? J Allergy Clin Immunol Pract. 2018;6:1439–44.
Eidukaite A, Tamosiunas V. Activity of eosinophils and immunoglobulin E concentration in the peritoneal fluid of women with endometriosis. Clin Chem Lab Med. 2004;42:590–4.
Efthymiou G, Saint A, Ruff M, Rekad Z, Ciais D, Van Obberghen-Schilling E. Shaping Up the Tumor Microenvironment With Cellular Fibronectin. Front Oncol. 2020;10:641.
Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, et al. NKp46 Receptor-Mediated Interferon-γ Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity. 2018;48:107-19.e4.
Ho HN, Chao KH, Chen HF, Wu MY, Yang YS, Lee TY. Peritoneal natural killer cytotoxicity and CD25+ CD3+ lymphocyte subpopulation are decreased in women with stage III-IV endometriosis. Hum Reprod. 1995;10:2671–5.
Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics. 2017;7:1543–88.
Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996;2:40–5.
Gazvani MR, Christmas S, Quenby S, Kirwan J, Johnson PM, Kingsland CR. Peritoneal fluid concentrations of interleukin-8 in women with endometriosis: relationship to stage of disease. Hum Reprod. 1998;13:1957–61.
Calhaz-Jorge C, Costa AP, Santos MC, Palma-Carlos ML. Peritoneal fluid concentrations of interleukin-8 in patients with endometriosis depend on the severity of the disorder and are higher in the luteal phase. Hum Reprod. 2003;18:593–7.
Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101–9 discussion 18, 396–406.
Lin C, He H, Liu H, Li R, Chen Y, Qi Y, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. 2019;68:1764–73.
Bie Y, Ge W, Yang Z, Cheng X, Zhao Z, Li S, et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis Markers. 2019;2019:8023460.
Singh AK, Das VNR, Amit A, Dikhit MR, Mahantesh V, Singh SK, et al. Cytokines and chemokines differentially regulate innate immune cell trafficking during post kala-azar dermal leishmaniasis. J Cell Biochem. 2018;119:7406–18.
Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–76.
Vujanovic L, Ballard W, Thorne SH, Vujanovic NL, Butterfield LH. Adenovirus-engineered human dendritic cells induce natural killer cell chemotaxis via CXCL8/IL-8 and CXCL10/IP-10. Oncoimmunology. 2012;1:448–57.
Sikora J, Smycz-Kubańska M, Mielczarek-Palacz A, Kondera-Anasz Z. Abnormal peritoneal regulation of chemokine activation-The role of IL-8 in pathogenesis of endometriosis. Am J Reprod Immunol. 2017;77.
Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci U S A. 2007;104:12451–6.
Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC, et al. Endometrial-peritoneal interactions during endometriotic lesion establishment. Am J Pathol. 2008;173:700–15.
Chen M, Zhou Y, Xu H, Hill C, Ewing RM, He D, et al. Bioinformatic analysis reveals the importance of epithelial-mesenchymal transition in the development of endometriosis. Sci Rep. 2020;10:8442.
Peng Y, Peng C, Fang Z, Chen G. Bioinformatics Analysis Identifies Molecular Markers Regulating Development and Progression of Endometriosis and Potential Therapeutic Drugs. Front Genet. 2021;12.
Hu W, Xie Q, Xu Y, Tang X, Zhao H. Integrated Bioinformatics Analysis Reveals Function and Regulatory Network of miR-200b-3p in Endometriosis. Biomed Res Int. 2020;2020:3962953.
Acknowledgements
None.
Funding
This study was supported in part by a grant from the National Natural Science Foundation of China (#81873826).
Author information
Authors and Affiliations
Contributions
SN.C conceived and designed the experiments; SN.C and XQ.W prepared the manuscript; SN.C, XQ.W and XS.C performed the experiments; SN.C and XQ.W analyzed the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consenst to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, S., Chai, X. & Wu, X. Bioinformatical analysis of the key differentially expressed genes and associations with immune cell infiltration in development of endometriosis. BMC Genom Data 23, 20 (2022). https://doi.org/10.1186/s12863-022-01036-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-022-01036-y