Abstract
Background
The immense geologic and ecological complexity of the Caribbean has created a natural laboratory for interpreting when and how organisms disperse through time and space. However, competing hypotheses compounded with this complexity have resulted in a lack of unifying principles of biogeography for the region. Though new data concerning the timing of geologic events and dispersal events are emerging, powerful new analytical tools now allow for explicit hypothesis testing. Arthropods, with varying dispersal ability and high levels of endemism in the Caribbean, are an important, albeit understudied, biogeographic model system. Herein, we include a comprehensive analysis of every publicly available genetic dataset (at the time of writing) of terrestrial Caribbean arthropod groups using a statistically robust pipeline to explicitly test the current extent of biogeographic hypotheses for the region.
Results
Our findings indicate several important biogeographic generalizations for the region: the South American continent is the predominant origin of Caribbean arthropod fauna; GAARlandia played a role for some taxa in aiding dispersal from South America to the Greater Antilles; founder event dispersal explains the majority of dispersal events by terrestrial arthropods, and distance between landmasses is important for dispersal; most dispersal events occurred via island hopping; there is evidence of ‘reverse’ dispersal from islands to the mainland; dispersal across the present-day Isthmus of Panama generally occurred prior to 3 mya; the Greater Antilles harbor more lineage diversity than the Lesser Antilles, and the larger Greater Antilles typically have greater lineage diversity than the smaller islands; basal Caribbean taxa are primarily distributed in the Greater Antilles, the basal-most being from Cuba, and derived taxa are mostly distributed in the Lesser Antilles; Jamaican taxa are usually endemic and monophyletic.
Conclusions
Given the diversity and deep history of terrestrial arthropods, incongruence of biogeographic patterns is expected, but focusing on both similarities and differences among divergent taxa with disparate life histories emphasizes the importance of particular qualities responsible for resulting diversification patterns. Furthermore, this study provides an analytical toolkit that can be used to guide researchers interested in answering questions pertaining to Caribbean biogeography using explicit hypothesis testing.
Similar content being viewed by others
Background
The Caribbean region (Fig. 1) holds a long and rich history of entomological research and discovery. After nearly two centuries of entomological studies [1], we are still collecting and describing many new species of terrestrial arthropods (e.g., [2], and references therein, [3,4,5,6,7]). The > 700 islets and islands of the Caribbean (~ 240,000 km2), their dramatic elevational gradients (− 39 m to + 3098 m), and their proximity to two continents (North and South America) have resulted in a hyperdiversity of arthropods that can be both a boon and a pitfall for research. Unlike island systems such as Hawaii and French Polynesia where the terrestrial arthropod fauna is well-known [8], the Caribbean fauna is more diverse and less well-known, hampering areas of research such as biogeography.
Map of the study region. The Caribbean Basin. Colors correspond to those used in subsequent figures of ancestral range estimations from BioGeoBEARS analyses. For information about geographic areas and geologic dates, see Additional file 1: Table S1. Base map created in ArcGIS v9.2 and areas shaded in Adobe Illustrator Creative Cloud. Red = South America (SA); Orange = Central America (CA); Yellow-Green = North America (NA); Purple = Florida (FL); Bright Pink = Bahamas and Turks and Caicos Islands (BA, TCI); Green = Greater Antilles (GA); Yellow = Jamaica (JA); Light Blue = Northern Lesser Antilles (NLA); Dark Blue = Southern Lesser Antilles (SLA); Blue-Green = Barbados (BAR)
The Caribbean Basin is a geologically complex region, bounded on the north, west, and south by continental landmasses. The Antillean Archipelago within the basin comprises islands with broad ages, size ranges, and elevational gradients. To the east is a volcanic arc comprising older volcanics as well as active volcanoes, and to the north are the Greater Antilles and the Bahamas Bank. While land may have been continuously available in the Greater Antilles for nearly 40 million years (my), islands such as those on the Bahama Bank are just over 100 thousand years old (kyo). Additionally, some islands, such as the Greater Antilles, may have been connected to one another and to the continental landmasses by land bridges (Wallacean islands), whereas the Lesser Antilles have never been connected to one another or the continents (de novo islands). For reviews of Caribbean geology, see [9,10,11,12,13] and references therein.
The primary biogeographic questions concerning Caribbean biota are how it arrived to the islands and where it is derived from. The ages of the present-day island biota have also been debated. For example, Hedges et al. [14] suggested that the bolide impact at the K-T boundary would have wiped out all organisms present on the islands, whereas Crother and Guyer [15] presented evidence to the contrary. However, geologic data [11, 12, 16] indicate that continuous land in the Greater Antilles was unavailable until after ~ 40 mya. More recent evidence about the region’s geology has shifted the discussion from whether organisms became established on islands via vicariance or dispersal to when organisms arrived and what happened after [17].
Competing hypotheses
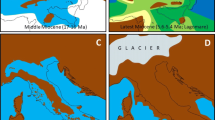
In the last 25 years, new geologic and biologic evidence has allowed researchers to propound two somewhat controversial hypotheses (Fig. 2). The first being the GAARlandia (Greater Antilles-Aves Ridge) hypothesis of Iturralde-Vinent and MacPhee [11]. According to geologic and paleobiogeographic data, a landspan may have connected northern South America with the Greater Antilles 35–32 mya. This hypothesis has been invoked to explain the distribution of mammals [11], including bats [22, 23], as well as toads [24], spiders [25, 26], butterflies [27], fish [28], and plants [29, 30], although only two of these studies [25, 27] explicitly tested the GAARlandia hypothesis using statistical methods. Contrarily, some researchers (e.g., [31]) remain unconvinced that the Aves Ridge was subaerial based on the available geologic data and suggest the need for more sea-floor drilling to elucidate the extent of the land bridge or island chain.
Historical Caribbean landmasses and major competing hypotheses for dispersal. Schematic representations of the Caribbean land areas hypothesized to be available at certain time periods (see Additional file 1: Table S1 for geologic references) and the major competing hypotheses examined herein (see Table 1). Arrows indicate dispersal, dates next to the images depicting the Isthmus of Panama relate to the hypothesized Central American Seaway closure(s)
A second equally controversial hypothesis that could greatly impact the origins of Caribbean fauna is the timing of the Central American Seaway (CAS) closure. The prevailing paradigm from both biologic and geologic data has been that the Isthmus of Panama (IoP) was completely formed (the CAS was completely closed) by the Late Pliocene, approximately 3 mya, but that the entire process occurred gradually over tens of millions of years. O’Dea et al. [18, 32] and Leigh et al. [33] and references therein review the geologic and biologic evidence for CAS closure at various times. In general, collision between the southern tip of Central America and the South American landmass began 24–23 mya ([19] and references therein), and it is thought that most of the volcanic arc was submerged; however, there is evidence that indicates significant land may have been above sea level during the Mid Miocene (~ 15–13 mya) in the form of an archipelago or a peninsula. Coates and Stallard [20] suggest a closure at 15 mya, and Osborne et al. [21] suggest the CAS closed between 12 and 7 mya. Leigh et al. [33] suggest that by 10 mya the land bridge was nearly complete, allowing some biotic interchange, and by 7 mya, most deep water populations were separated. After O’Dea et al. [18] exhaustively reviewed all available data, they concluded final closure nearer to the Late Pliocene. Bacon et al. [34, 35] used biologic data to infer geologic hypotheses, concluding an older age for IoP formation/CAS closure.
Terrestrial arthropods in biogeographic modeling
The first study of Caribbean biogeography focused on vertebrates [36], though soon after, studies including terrestrial arthropods followed suit [37]. Arthropods are excellent study organisms for biogeography because they have relatively large populations and resilient life stages [38, 39]. Their resilience additionally means that there are often older lineages which can help clarify the effects of older geologic events [40]. Also, because similar patterns are often found between vertebrate and invertebrate taxa (e.g., [25, 41, 42]), such congruence only serves to reinforce the significance of the results (e.g., [43, 44]).
Great advances in our understanding of Caribbean arthropods have been made in the last 30 years. Liebherr [2] edited the first synthesis of Caribbean insect biogeography. Soon after, the first Caribbean biogeography studies using molecular data from insects began to emerge [45,46,47,48]. Over the past 10 years, numerous papers featuring terrestrial arthropods and focusing on historical biogeography of the Caribbean have been published (e.g., [25,26,27, 49,50,51,52]) as well as several phylogeographic studies (e.g., [41, 53, 54]). However, as noted by Rosen [55] and emphasized by Slater [56], “a stupendous multidisciplinary effort to resolve decisive patterns for the [Caribbean] region” is needed.
In this study we have examined all publicly available datasets of terrestrial arthropod taxa that met our criteria for selection: a taxon with a distribution across a significant part of the Caribbean Basin (> 75% land areas) with some endemic species or populations; mostly complete sampling of known lineages and distributions (> 75% across the distribution); genetic differentiation sufficient for phylogenetic analysis. Our analyses consist of ten taxa: Platythyrea ants, Heterotermes and Nasutitermes termites, butterflies from the genera Calisto and Papilio, Drosophila flies, centruroidine scorpions, and Micrathena, Spintharus, and Selenops spiders. These taxa provide a good representation of sedentary and more vagile taxa with various life histories. Our strategy was to maximize the sampling effort, including geographic, taxonomic, and phylogenetic coverage in order to test all of the prevailing hypotheses regarding the timing and route of dispersal into the Caribbean Archipelago. We time-calibrated phylogenies to explicitly test up to 252 biogeographic models per taxon in order to elucidate the overarching patterns of Caribbean biogeography for terrestrial arthropods.
All models are wrong, but some are useful
We would like to make a call to biologists to interact more with geologists when studying biogeography so that the maximum information may be gleaned from the geological evidence and unlikely or impossible conclusions aren’t invoked to explain the results. Using biology to infer specific geologic events should only be done with extreme caution (e.g. – [43, 44, 57]) as the work of researchers outside of a specific discipline can be easily misinterpreted and subsequently mis-cited repeatedly in the literature. Most Caribbean biogeographic research has tried to invoke support for or against the GAARlandia land bridge (35–32 mya) using diversification rates coupled with the timing of diversification events. While the authors acknowledge GAARlandia is a controversial hypothesis, in this study the models assume that GAARlandia existed as a geologic entity, and we have evaluated the extent to which it would have played a role in dispersal into the Caribbean from South America.
There is also ongoing debate [58] about whether there are fundamental problems with biogeographic models including the ‘jump dispersal’ parameter. However, for island taxa, jump dispersal is known to be very important [59]. Portions of the Caribbean archipelago were never connected or proximal to other islands (Darwinian islands); therefore, the only route to these islands is via jump dispersal. Following this logic, jump dispersal is expected to be common in island taxa, especially those that fly or balloon, and any potential bias introduced is insignificant compared to the bias introduced by removing the ‘jump’ parameter. To fully examine this effect, we have tested 54–252 models per dataset. Similarly, we have incorporated our current understanding of fossils, molecular clocks, and geology to obtain dated phylogenies for hypothesis testing considering the current ongoing debate [60].
A note about the term ‘colonization’
We would like to add a note about our omission of the term ‘colonization’ in this paper and make a plea to the biogeography community that this terminology be used with caution. The term ‘colonization’ has been used outside of the biology literature to refer to the arrival of European culture outside of Europe. The process of ‘colonization’ of the Americas (and many other parts of the world) by Europeans resulted in the death and enslavement of millions of people. This is particularly true in the Caribbean region. In an acknowledgement of the negative connotations associated with this term, and because none of the taxa we examined are invasive, we have chosen to omit it and instead discuss the process by which populations arrive to, and become established in, a new area by simply using the term ‘dispersal’.
Results
For each arthropod taxon (Platythyrea ants, Heterotermes termites, Nasutitermes termites, Calisto butterflies, Papilio butterflies, Drosophila fruit flies, centruroidine scorpions, Micrathena spiders, Spintharus spiders, and Selenops spiders), we followed a pipeline of analyses, and the detailed results of all analyses can be found in Additional file 1. Briefly, for each, a phylogeny was constructed in MrBayes, and if the resulting topology was congruent with a published phylogeny for that taxon, then it was used as the constraint for dating in BEAST 2 and subsequent analyses in BioGeoBEARS (Papilio, centruroidine scorpions, Heterotermes). If the resulting topology was incongruent with the published phylogeny, both a topology constrained to the previously published topology and a topology constrained to our Bayesian consensus tree were used for all downstream dating and analyses (Platythyrea, Nasutitermes). In some cases, there was incongruence among published phylogenies (Drosophila), or irreconcilable differences between the published phylogeny and our own topology (Micrathena, Spintharus). For the former, we analyzed multiple phylogenies with various constraints based on previous studies, and for the latter, we used our Bayesian consensus tree for downstream analysis. Finally, some taxa had no published topology for which we could compare our results due to the addition of data, so the Bayesian consensus tree was used for all downstream dating and analysis (Selenops, Calisto).
Dating analyses for each taxon established minimum ages for the group. For those taxa that were younger than the proposed date of GAARlandia, the set of models which allow for the putative landspan were excluded from testing (Platythyrea, Nasutitermes, Papilio, Drosophila, Selenops – younger dated tree, see Table 2 and Additional file 1). Those taxa that were old enough to show any potential effect of GAARlandia were tested for the full suite of 252 models (Heterotermes, Centruroidinae, Micrathena, Spintharus, Selenops – older dated tree, see Table 2 and Additional file 1). Finally, Calisto is endemic to the Greater Antilles, so could only be analyzed under 18 models.
A total of 2282 biogeographic models were tested across all 10 taxa in BioGeoBEARS, and the results of biogeographic model testing for all taxa are summarized in Table 2. Resulting phylograms are shown in Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 and 14, and phylograms representing probabilities as pie charts can be found in Additional file 1: Figures S1–S5 and S7–S13 along with tables listing included taxa and respective GenBank reference numbers for samples (Additional file 1: Tables S5, S7–S15). All input and output files for all analyses can be found on figshare. Results from individual taxa are detailed in Additional file 1. In a few instances, the BioGeoBEARS analyses for certain models simply would not run, even when we changed starting parameters, probably because the likelihoods were too low for that particular model (Additional file 1: Table S3 and Table S4).
Phylogeny and ancestral range estimation for Platythyrea ants. BioGeoBEARS phylogram corresponding to the DIVALIKE C1a model (Table 1). The tree shows the single most probable geographic range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; BA = Bahamas; FL = Florida. Ant photo by Judy Gallagher
Phylogeny and ancestral range estimation for Heterotermes termites. BioGeoBEARS phylogram corresponding to the DIVALIKE A1a model (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; TCI = Turks and Caicos Islands; FL = Florida. Heterotermes photo provided by Rudolf Scheffrahn
Phylogeny and ancestral range estimation for Nasutitermes termites. BioGeoBEARS phylogram corresponding to the DEC D1b model (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; BA = Bahamas. Nasutitermes photo by Bernard Dupont
Phylogeny and ancestral range estimation for Calisto butterflies (Euptychia as the outgroup). BioGeoBEARS phylogram corresponding to the DEC + J A1b model (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Additional file 1: Figure S6. SA = South America; CU = Cuba; JA = Jamaica; HI = Hispaniola; PR = Puerto Rico; BA = Bahamas. Photo by S. Crews
Phylogeny and ancestral range estimation for Calisto butterflies (Euptychia is not the sister taxon). BioGeoBEARS phylogram corresponding to the DEC + J A2a model (B2a, D2a, E2a, and G2a being equiprobable as the best models based on the AICc weights) (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Additional file 1: Figure S6. SA = South America; NA = North America; CU = Cuba; JA = Jamaica; HI = Hispaniola; PR = Puerto Rico; BA = Bahamas. Photo by S. Crews
Phylogeny and ancestral range estimation for Papilio butterflies. BioGeoBEARS phylogram corresponding to the BAYAREALIKE + J C1a model (D1a is equally likely) (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; FL = Florida; BA = Bahamas. Papilio photo by Eduardo Lopez
Phylogeny and ancestral range estimation for Drosophila flies. BioGeoBEARS phylogram corresponding to the BAYAREALIKE + J D1a model (Table 1) for the tree with the relationship of D. belladunni and D. acutilabella constrained (see Additional file 1). Asterisks indicate differences between the constrained and unconstrained trees in the ancestral range estimation. The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; FL = Florida; BAR = Barbados. Drosophila photo by Mark Yokoyama
Phylogeny and ancestral range estimation from BioGeoBEARS for Drosophila flies without constraining the relationship of D. belladunni and D. acutilabella. The best model based on AICc weights is BAYAREALIKE + J D1a (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; FL = Florida; BAR = Barbados. Drosophila photo by Mark Yokoyama
Phylogeny and ancestral range estimation for centruroidine scorpions. BioGeoBEARS phylogram corresponding to the BAYAREALIKE + J B1a and B2a models (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; FL = Florida; BA = Bahamas. Photo by S. Crews
Phylogeny and ancestral range estimation for Micrathena spiders. BioGeoBEARS phylogram corresponding to the DIVALIKE + J B2a model (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles. Photo by S. Crews
Discussion
Our analyses indicate some shared patterns and processes of dispersal to and subsequent diversification on islands in the Caribbean biodiversity hotspot, including dispersal across the IoP/CAS (Table 2). Below we discuss some of the major themes surrounding the evolution and biogeography in the Caribbean. These data provide a baseline for indicating the types of questions that can be confidently answered given the available data and highlight where knowledge is lacking and can be improved upon in future research. We base our discussion on the phylogeny that shows the single most probable ancestral range for each taxon; for trees that show the probabilities for each possible range, see Additional file 1.
Origins of Caribbean taxa
South America is the most common place of origin for the Caribbean fauna examined, followed by Central America and North America (Table 2). This is congruent with the generalized track analysis and cladistic biogeography of Morrone [61] and Echeverry and Morrone [62] for many arthropod (and other) taxa. There does not seem to be a shared evolutionary history, ecology, or life history among groups that share a point of origin, and this does not appear to coincide with the timing of dispersal or age of the group in question. Some taxa appear to have dispersed to the islands from more than one area of the mainland, including a relatively young group of good dispersers (Drosophila) [63], and a relatively old group of poor dispersers (Micrathena) [26] (Figs. 9, 10, 12). These results are congruent with other literature on Caribbean arthropods; for example, beetles are also thought to have dispersed to the islands from both Central America and South America [40, 64], and ants [65] and auchenorrhynchous homoptera [66] are thought to have dispersed to the Caribbean from both Central America and North America.
Number of dispersal events to Caribbean islands
We find that all but the poorest dispersers have arrived in the Caribbean multiple times (Table 2). The primary predictor of the number of dispersal events into the Caribbean appears to be dispersal ability, including mode of locomotion, as well as adaptability and ecological plasticity, which are important for establishment after dispersal. However, extinction events could also produce patterns of multiple dispersal events. It should be noted that the plot of most-probable areas is not the same as event counts [67]. Simple counts give some idea of event number but do not consider events occurring along a branch; for better estimates, biogeographic stochastic mapping is required.
Among flying insects, there is a general pattern of multiple dispersal events. Drosophila fruit flies, Papilio butterflies, and Nasutitermes termites each dispersed at least 4 times (Figs. 5, 8, 9 and 10), and Heterotermes termites and Calisto butterflies dispersed twice (Figs. 4, 6, 7). Drosophila is thought to be a good disperser [63], and the groups examined here are thought to have dispersed relatively recently (~ 4 my) to the Caribbean, perhaps indicating that dispersal ability has played a role in the number of dispersal events to the islands. Papilio’s dispersal abilities have been noted by Lewis et al. [52] who also found several separate dispersal events rather than a single dispersal followed by diversification (Fig. 8). In contrast, Calisto butterfly species have only dispersed one or two times (depending on the outgroup used) to the Greater Antilles from South America (Figs. 6, 7). Among the termites, Heterotermes dispersed at least 3 times from South America and North America (Fig. 4). However, according to our results, Nasutitermes is relatively new in the Caribbean (~ 15 my) but appears to be a good disperser (Fig. 5). In at least one species (N. corniger), Scheffrahn et al. [68] noted that kings and queens are quite good flyers and can adapt to many different types of habitats, from urban to more natural, and are good at taking over and defending territories.
Selenops spiders have dispersed to Caribbean islands only twice, from South America to the Lesser Antilles for one event, and to the Greater Antilles for the second event, although the point of origin remains unclear (Fig. 14). Within Centruroides scorpions, there are also two dispersal events, one from South America and one from North America (Fig. 11). Both Selenops spiders and Centruroides scorpions likely have a similar ecology as nocturnal somewhat stationary sit-and-wait predatory arachnids. Spintharus spiders have seemingly only dispersed to the Caribbean once, and although this species is thought to be a poor disperser [51], it does appear capable of dispersal to proximal islands (Fig. 13).
Phylogeny and ancestral range estimation for Spintharus spiders. BioGeoBEARS phylogram corresponding to the DIVALIKE + J A1b model (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; CA = Central America; NA = North America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles. Photo by Judy Gallagher
Somewhat puzzling are Micrathena spiders, considered to be a poor dispersersing taxon [26], they have dispersed to the Caribbean multiple times (6 in our analyses) (Fig. 12). One possible explanation is, as our analyses indicate, that they are a rather old group which would allow for more time in which dispersal events could occur. Missing taxa from the mainland could also produce a pattern of multiple dispersal events. Nevertheless, other studies with more taxa sampled also indicated multiple dispersal events [69], leading us to conclude that this taxon is in fact a rather good disperser like many other orb-weaving spiders.
Szalanski et al. [48] suggested that the present distribution of Heterotermes in the Caribbean is likely due to overwater dispersal, however he emphasized that anthropogenic introductions couldn’t be ruled out. In our analyses, it does appear that most species lineages are at least a few million years old (Fig. 4), but that some of the intraspecific distributions may be anthropogenically caused. Human-mediated dispersal, even intraspecific dispersal within the native range, could have a significant impact on biogeographic results. In some cases, this may be a very evident pattern which emerges from the data, however, there may be examples such as this where it is cryptic and confounds the conclusions that can be drawn about the taxon.
Islands as sources of diversity
Islands are not usually thought of as sources for diversity, but rather sinks [70, 71]. Recently, however, more examples of dispersal from an island source in the Caribbean has become more well-known, particularly in Anolis lizards, including from a large island to smaller islands or even from islands to the mainland [72, 73]. Dispersal from the islands to the mainland is also seen in weevils [74] and orioles [75]. One reason we may not be as aware of this phenomenon is because mainland sampling from Mexico, Central America, and South America is often poor and therefore unable to provide the phylogenetic resolution required to detect such events; even excluding a single species could change the results.
There are many examples from our analyses of dispersal from Cuba to the Bahamas, perhaps due to these land areas being particularly close to one another during times of low sea level. Although we only used a single specimen of Nasutitermes ripperti from the Bahamas in our analyses, this species is also found in Cuba, so perhaps dispersed from Cuba to the Bahamas. In Calisto butterflies, dispersal from Cuba to the Bahamas has occurred at least twice (Fig. 13). In Selenops spiders and Centruroides scorpions, taxa are shared between Cuba and the Bahamas and presumably dispersed from Cuba to the Bahamas (Figs. 11, 13, 14). Similarly, the large island of Hispaniola is a source of fauna for the small Turks and Caicos Islands in one Heterotermes termite lineage, Selenops spiders, and Centruroides scorpions (Figs. 4, 11, 13, 14).
Phylogeny and ancestral range estimation for Selenops spiders. BioGeoBEARS phylogram corresponding to the DIVALIKE + J B1a model (Table 1). The tree shows the single most probable geographical range at each node pre- and post-split. Colors correspond to Fig. 1. SA = South America; NA = North America; CA = Central America; GA = Greater Antilles; JA = Jamaica; SLA = Southern Lesser Antilles; NLA = Northern Lesser Antilles; FL = Florida; TCI = Turks and Caicos Islands; BA = Bahamas. Photo by S. Crews
Contrarily, there are instances of smaller islands as sources of taxa for larger islands, or even the mainland. Drosophila is unique in that there seems to have been a small species radiation in the Southern Lesser Antilles (Figs. 9, 10), and from this, two dispersal events occurred to the Greater Antilles (D. ornatipennis and D. dunni). Additionally, it appears that one lineage (D. acutilabella) emerged from a dispersal event to the Greater Antilles from Jamaica. Our analysis of Heterotermes indicates that one species (H. tenuis) from Guadeloupe island is basal to the South American lineage, with the caveats that sampling across the range of the species is poor, and these data are from a single gene (Fig. 4). In Platythyrea ants, there have been at least 2 dispersal events from the Greater Antilles to North America (Fig. 3).
Notably, the two butterfly species we examined show contrasting patterns of dispersal. Papilio are good dispersers and don’t seem to be affected by allopatric speciation or within-island vicariance, so whereas there are many species on the islands, ranges are broad and large species radiations do not appear to have occurred (Fig. 8). Calisto butterflies, however, are small and poor fliers. These butterflies show no evidence of dispersal from islands to the mainland and very few examples of dispersal from one island to the next; however, they are very diverse within islands likely due to being habitat specialists as well as poor dispersers (Figs. 6, 7) [27]. For Neotropical butterflies, Condamine et al. [76] emphasized the importance of islands as sources of diversity for the mainland. In Papilio butterflies there have been multiple dispersal events from the Greater Antilles to North America or to South America. This result differs from those of Lewis et al. [52] who found several “reverse” dispersal events, or events where the taxon is returning to the ancestral range of origin (dispersal from islands to the mainland); they found the entire clade of Heraclides to have a Caribbean origin whereas our analyses reveal a South American origin. Nevertheless, both analyses found that islands can be a source of diversity for other islands or even the mainland. Lewis et al. [52] attributed this in part to Papilio being big, strong fliers that favor edge habitats.
Founder event dispersal
The ability of BioGeoBEARS to incorporate “jump” dispersal into models has been advantageous in biogeographic studies (but see [58]). The program allows for models that emphasize the importance of founder event dispersal in island taxa [59]. In our analyses, models incorporating jump dispersal were favored in 8/12 analyses and were more common in taxa with older origins in the Caribbean (pre-35 my) (Table 2). However, in selenopid spiders, it was favored in the analysis with the younger tree, but not the older tree (see Additional file 1). The only analyses where a model incorporating jump dispersal was not favored were of ants and termites—organisms that only fly for a part of their adult life. Our results indicate that jump dispersal plays a role for both good dispersers, like Papilio, and poor dispersers, like spiders and scorpions. Founder event dispersal was always the favored model of dispersal for spiders and animals that fly during their entire adult life stage (Calisto, Papilio, and Drosophila). This finding corroborates results from Matos-Maraví et al. [27] for Calisto butterflies and Zhang et al. [74] for weevils.
Distance between areas of dispersal
To understand the influence of distance between neighboring landmasses (islands or continents) on dispersal probability we used manual dispersal multiplier (mdm) matrices in BioGeoBEARS analyses. Despite incorporating jump dispersal into our models, a model in which dispersal was very difficult (mdm values set to 0.01) was never favored (Table 2). We did find that in most taxa, distance-dependent models were favored (Platythyrea ants, Heterotermes termites, Papilio butterflies, centruroidine scorpions, Micrathena and Selenops spiders). For Calisto butterflies, distance did not affect dispersal when Euptychia was used as the sister taxon but did affect dispersal when other taxa were used. In Drosophila, two models were favored, and in one, distance affected dispersal, but in the other it did not.
Geologically, the Lesser Antilles islands can be divided into volcanic islands and two limestone volcanic arcs with the Southern Lesser Antilles older than the Northern Lesser Antilles (Additional file 1: Table S1). There were many episodes of gradual emergence, even within single islands, so a strict northern vs. southern boundary is elusive. These islands are younger and smaller than the Greater Antilles and have never been connected to the mainland, lowering overall dispersal probability. Previous biogeographic studies have recovered a clear pattern of a genetic split between organisms, congruent with Northern/Southern Lesser Antilles geology. This faunal-island split isn’t always between sister taxa, such as in Anolis lizards, Selenops spiders, and Centruroides scorpions where it appears there were two dispersal events: one from the Greater Antilles south to the Northern Lesser Antilles and one from South America north to the Southern Lesser Antilles, but without any discreet faunal break between islands. In this and a previous analysis [41], selenopid spider distribution agrees with the geologic data in that dispersal from South America to the older islands occurred sometime after 15 mya, whereas taxa didn’t disperse to the Northern Lesser Antilles until after 8 mya and are instead derived from the Greater Antillean fauna. The precise location of the split is also highly variable; for example, in Anolis lizards [77], it occurs between Martinique and Dominica, however it occurs further south between St. Vincent and St. Lucia [46] in lygaeid bugs [56], carabid beetles [40], Eleutherodactylus frogs [78], and bananaquits [79]. The location of the split recovered in Selenops spiders occurs between Dominica and Les Saintes, Guadeloupe. In the termite Heterotermes tenuis, we recovered some population structure between northern (Guadeloupe) and more southern islands (St. Lucia, St. Vincent, Grenada, Trinidad) [48]. For Dryas iulia butterflies [46] there have been multiple splits inferred, between both Martinique/Dominica and St. Vincent/St. Lucia. Multiple splits were also recovered in this study in Spintharus spiders: one between St. Kitts and Nevis, and another between Dominica and more southern islands.
Dispersal across the Isthmus of Panama/Central American Seaway
It should be noted that we are not aiming to disprove or provide evidence for a geologic hypothesis using biologic data, nor are we suggesting that our results indicate that the Central American Seaway had completely receded > 3 mya. The taxa we have examined do not require a land connection to be able to disperse and diversify across aquatic barriers. Most of our results indicate that the favored biogeographic model includes a single dispersal event between North and South America that occurred prior to 3 mya (Table 2). This result was also found for Hercules beetles [80], where it was suggested that the beetles could fly over water for short distances or that larvae could raft in downed wood. Taxa in which older dispersals (23 mya) were favored are centruroidine scorpions (Fig. 11), Micrathena spiders (Fig. 12), and Selenops spiders (Fig. 14). Selenops spiders and centruroidine scorpions are known from Chiapas and Dominican amber, indicating they have been present in southern North America and the Caribbean for at least 20 my.
The only taxon with a favored model of earliest dispersal at 15 my is Platythyrea ants (Table 2; Fig. 3). Seal et al. [53] mention that the ants probably disperse by walking over land, so perhaps they dispersed via a land connection or floated on flotsam. For some taxa, the models couldn’t be differentiated to determine a favored one. This was true in the analysis of Calisto butterflies, where 5 models were equiprobable (Table 2). In Nasutitermes and Papilio, both 15 and 8 my were favored (Table 2, Figs. 5, 8).
Several taxa were recovered with a favored model of 8 my or younger: Drosophila fruit flies, Spintharus spiders, and Heterotermes termites (Figs. 4, 9, 10, 13). Other studies have determined dispersal across the IoP/CAS to have occurred prior to 3 my, including those of butterflies [76] and Loxosceles spiders [81], although in the latter, the authors suggest dispersal occurred via a land bridge, which according to geologic data is not possible [18]. Bagley and Johnson [17] suggest that earlier dispersals were bidirectional and could have occurred via island hopping. Having better Central American sampling where possible, especially in taxa where we know there are species missing from the analyses, would certainly improve the level of uncertainty.
The role of GAARlandia in shaping Caribbean arthropod biodiversity
Several of the datasets we analyzed were younger than 32 mya, precluding GAARlandia as a transport path from South America to the Greater Antilles for these taxa. GAARlandia also did not play a role in the dispersal of Heterotermes termites, Selenops spiders, or Spintharus spiders (Figs. 4, 13, 14). In many cases our findings contrast those of previous research, although these studies lacked thorough sampling for parts of the distribution [25, 51] or hypothesis testing was not conducted [51]. For example, Dziki et al. [51] suggested that GAARlandia played a role in the dispersal of Spintharus from South America to the Greater Antilles. However, this was based on the coincidental timing of the diversification of a Caribbean clade rather than hypothesis testing. Our results, based on the relative AICc weights of the BioGeoBEARS analyses, favored a dispersal model without GAARlandia and also recovered a later date of dispersal into the Caribbean (~ 20 my). The results we obtained for Selenops spiders contradict those of Crews and Gillespie [25] in which similar hypothesis testing methods using Lagrange [82, 83] (which only uses the DEC model) found that models including GAARlandia were supported. Ancestral range estimation (Fig. 3) suggests that Selenops was in the Greater Antilles shortly after 40 my, before GAARlandia is hypothesized to have existed, and the model favored includes the jump dispersal parameter (Fig. 14, Table 2). This difference could be due to better dating methods now available, the use of different geologic dates/time slices, differences in the programs Lagrange and BioGeoBEARS, including the incorporation of jump dispersal, or the addition of more taxa/data, indicating how missing taxa affect biogeographic conclusions. For Micrathena spiders, the favored model indicated that GAARlandia played a role in dispersal to the Greater Antilles (Fig. 12). This is slightly confounding given the ancestral range estimation. Although there was dispersal to the Greater Antilles around 35–32 mya, these taxa came from North American ancestors. These findings contrast the results of McHugh et al. [26], and more sampling is required to explain these results. In centruroidine scorpions, two models had equal relative probabilities, one that included GAARlandia and another that did not (Fig. 11, Table 2). Esposito and Prendini [84], using Lagrange, previously found that the best fitting model was a distance-dependent dispersal model that included GAARlandia. According to the ancestral range estimation (Fig. 11), the timing of Heteroctenus scorpions entering the Caribbean is nearly simultaneous with the proposed timing of GAARlandia; however, the ancestral range estimation indicates a North American origin (although with some uncertainty), contradicting the results of Esposito and Prendini [84], who recovered a South American + Greater Antilles ancestral range using RASP [85, 86]. In Calisto butterflies, the results depend on whether Euptychia is the sister taxon or if other outgroups are used, emphasizing the importance of outgroup selection. For the former, distance-independent dispersal without GAARlandia was chosen as the favored model (Fig. 6). For the latter, distance-dependent dispersal with GAARlandia was the favored model (Fig. 7).
Species-area relationships and endemism
MacArthur and Wilson [70] suggested that island area may be responsible for lineage diversity or species numbers on islands. We know that the way in which island area is measured, habitat diversity, island age, and distance from the mainland are also important determiners [8, 87,88,89,90]. Generally, for all taxa examined, the Greater Antilles harbor more species than the Lesser Antilles, and the larger Greater Antilles islands, like Cuba and Hispaniola, typically have more species than the smaller islands of Puerto Rico and Jamaica. This expected pattern of Cuba > Hispaniola > Jamaica > Puerto Rico generally holds for the bug family Lyagaeidae [56], pseudoscorpions [91], scaritine beetles [92], Centruroides scorpions (Fig. 11), Micrathena spiders (Fig. 12), and Papilio butterflies (Fig. 8). However, in many taxa there are more species in Hispaniola than Cuba, such as in Platynus carabid beetles and Amphiacusta crickets [40, 49], the latter with 18 species in the Lesser Antilles. In Calisto butterflies, Hispaniola has nearly double the species of Cuba, and there are 2 species in the Bahamas but only 1 each in Puerto Rico and Jamaica. Grimaldi [63] suggested that there may be less endemism in fruit flies because they are good dispersers whose ranges usually cover large areas. This appears to be true in the cardini and dunni groups examined here, which have only a few species in the Greater Antilles and few single island endemics in the Lesser Antilles (Figs. 9, 10). For selenopid spiders, there are more endemics in Hispaniola than in Cuba (Fig. 12), although both islands harbor the same total number of species. Reasons for discrepancies from the typical pattern are worth a second look, and in the case of Cuba vs. Hispaniola could relate to differences in the toplogical complexity.
Lineage age and lineage radiations
Older lineages found on the Greater Antilles are generally ancestral to those of the Lesser Antilles; and Cuba, Hispaniola, or Puerto Rico lineages diverge earlier than Jamaican lineages. Species occurring in Cuba are the most basal for Selenops and the Caribbean endemic clade of Centruroides scorpions (Figs. 11, 14). Hispaniolan lineages are basal in Platythyrea ants and Calisto butterflies when Euptychia is used as a sister group; alternatively, Puerto Rican lineages are basal, which is also found in weevils [74] (Figs. 3, 6). Jamaican lineages are basal in Argiope spiders [93], however, it is possible that Argiope butchko is not a valid species but another lineage of Argiope argentata, in which case Cuban lineages would be basal. If we accept the proposal of Agnarsson et al. [93], then A. argentata has managed to disperse throughout the Caribbean to the exception of Cuba. Although competition could perhaps prevent A. argentata from becoming established in Cuba, multiple species of Argiope can be found in sympatry all over the world (e.g., [94]). Argiope butchko was described based solely on a few Cox1 differences and appears to show no morphological differences from A. argentata).
Lineage radiations have occurred in Cuba and Hispaniola in the butterfly species Calisto and Heteroctenus scorpion species, and all are single island endemics (Figs. 6, 7, 11). In species from the Caribbean clade of Centruroides scorpions, most exhibit patterns of within-island diversification or are single island endemics (Fig. 11). Considering that Calisto and scorpions are poor dispersers, the pattern of a single dispersal event followed by within-island radiation is expected. Conversely, Papilio, a good disperser, has not diversified on islands. Selenops spiders have undergone multiple lineage radiations in Hispaniola and Jamaica, and likely in Cuba, but without improved sampling it is unclear how many.
Island monophyly
Some groups exhibit patterns of dispersing to an island only once, whereas others have dispersed to a single island multiple times. Sampling is obviously important when examining island monophyly because missing species could cause false negatives as well as false positives. There does not appear to be island monophyly for the majority of taxa, including Papilio butterflies (Fig. 8), Drosophila flies (Figs. 9 and 10), Micrathena (Fig. 12) and Selenops spiders (Fig. 14), Platythyrea ants (Fig. 3), termites (Figs. 4 and 5), or weevils [74]. In the analysis of Calisto with Euptychia as the sister taxon, the butterflies have dispersed to Cuba at least 3 times from Hispaniola, and to Hispaniola at least twice, but to Jamaica and Puerto Rico only once (Fig. 6). However, in the analysis without Euptychia as the sister taxon, the butterflies dispersed to Cuba and Hispaniola only once each (Fig. 7). In Spintharus spiders there appears to be 1 Jamaican lineage, 1 Hispaniolan lineage, and 1 Puerto Rican lineage, but 2 Cuban lineages (Fig. 13). In Argiope argentata, lineages on Hispaniola and Jamaica are monophyletic [93]. Among scorpions, taxa are almost always monophyletic on islands, with the exception of 2 Cuban lineages of Centruroides.
Although Jamaica is considered one of the Greater Antilles, it has a rather different geologic history than Cuba, Hispaniola, and Puerto Rico. The island began emerging 40–30 mya and is thought to have had continuous available land since 15 mya, whereas the other Greater Antilles may have had continuous available land for much longer (~ 40 my). It is plausible that the Blue Mountains Block of eastern Jamaica has been above sea level for much longer and/or the part of western Jamaica that was attached to the Nicaraguan Rise of Central America was never submerged. Jamaica appears to have had a unique biogeographic history compared to the other Greater Antilles [95]. Of taxa included in this study, there are Jamaican endemic lineages in Calisto (1 species), Papilio (2 species), fruit flies (2 species), Centruroides scorpions (clade of 2 endemic species), Micrathena (1 species), Argiope (clade), Spintharus (clade), and Selenopid spiders (clade of 4 endemic species).
Conclusions
Terrestrial arthropods represent an immense wealth of biodiversity, dispersal abilities, life history traits, and ecologies. Their species assemblages in the Caribbean region underpin the importance of the Caribbean as both a major gateway for faunal exchange between the continents of the Western Hemisphere as well as a hotspot of endemic biodiversity and diversification. Our results indicate several biogeographic generalizations for terrestrial arthropods: South America is the predominant origin of the Caribbean arthropod fauna, and GAARlandia may have played an important role in aiding dispersal from South America to the Greater Antilles for some taxa but not universally. Founder (“jump” dispersal) events explain the majority of dispersal into the Caribbean, and most dispersal events occurred from the mainland to the islands and subsequent island hopping; this holds true for both good and poor dispersers. Distance is an important predictor of dispersal between landmasses, and there is evidence of islands as sources of diversity for the mainland in some taxa. Dispersal across what is now the Isthmus of Panama generally occurred prior to closure of the Central American Seaway 3 mya. The Greater Antilles harbor more species than the Lesser Antilles, and the larger Greater Antilles islands, Cuba and Hispaniola, typically have more species than the smaller islands of Puerto Rico and Jamaica. Among the Caribbean endemic taxa, basal taxa are most often distributed in the Greater Antilles, with the basal-most being Cuba, and derived taxa are most often distributed in the Lesser Antilles. Jamaican taxa are usually younger, endemic, and monophyletic for the island, whereas this is not the case for the other Greater Antillean islands.
The data presented herein provide a good baseline for understanding the biogeography of terrestrial arthropods in the Caribbean through explicit hypothesis testing. These methods allow for reproducible science and an improvement of our understanding of geologically complex regions. As genomic data continue to become more commonplace, genetic data from older museum specimens can be obtained, filling in gaps and helping resolve phylogenies and improve dating inferences. We hope that this paper will serve as a guide to help researchers determine what sorts of questions they can ask and answer with the data available to them.
Methods
Selection of datasets
We selected datasets using the following criteria: 1) Availability of a phylogenetic hypothesis. Because we are not attempting to represent ourselves as taxonomic experts for all terrestrial arthropods, we required a published phylogeny, corresponding datasets, and information that could be used to build time calibrated trees. Sometimes the datasets and calibration information were available in the publications’ supplementary materials, but sometimes only parts of datasets were available, or datasets did not correspond to the published trees, in which case we began the analyses from scratch. 2) Mostly complete sampling of species and distributions. As discussed in the introduction, the number of species of certain taxa and their distributions are often unknown for terrestrial arthropods. However, in some taxa, we know the true diversity, or at least that there are many unsampled lineages. We chose to omit these datasets from our analyses at this time and hope that they will be subjected to explicit hypothesis testing when more data become available. Datasets we were unable to use because of too little Caribbean or mainland sampling or too many missing taxa were Newportia centipedes [96], onychophora [97], Loxosceles spiders [81], and Amphiacusta crickets [49]. 3) Genetic differentiation sufficient for phylogenetic analysis. In the case of Argiope argentata spiders [93] and Dryas iulia and Heliconius charithonia butterflies [46], the taxa lacked enough genetic differentiation to get at the questions we are asking.
Using these criteria, we were left with 10 datasets, some better than others for addressing the hypotheses we wished to test. Our analyses consist of Platythyrea ants, Heterotermes and Nasutitermes termites, butterflies from the genera Calisto and Papilio, Drosophila flies, centruroidine scorpions, and Micrathena, Spintharus, and Selenops spiders. These taxa provide a good representation of sedentary and more vagile taxa with various life histories. When possible, molecular data were supplemented with new data from GenBank (Additional file 1: Tables S5–S15), including both markers and species not available in previous publications. In some cases, the addition of taxa for better calibration precluded the use of certain markers – our strategy was to maximize the sampling effort, including geographic, taxonomic, and phylogenetic coverage. New molecular sequence data were collected only in the case of Selenops spiders.
Phylogenetic and dating analysis
For each dataset (Platythyrea ants, Heterotermes termites, Nasutitermes termites, Calisto butterflies, Papilio butterflies, Drosophila fruit flies, centruroidine scorpions, Micrathena spiders, Spintharus spiders, and Selenops spiders) a phylogeny was estimated in MrBayes [98] (details of each analysis can be found in Additional file 1). Missing data were treated as “?”, and the BIC was used to select the best partitioning scheme and models in PartitionFinder2 [99] and jModelTest2 [100, 101]. Additionally, all data blocks were separated by gene (for non-coding genes) and by codon (for coding genes) for the input files. Further information about each dataset (e.g., genes used, partitioning schemes, models of molecular evolution, generations) can be found Additional file 1. Analyses were run with the default setting of 2 analyses with 4 chains, sampling every 1000 generations. Clades with support values ≥0.95 are considered well-supported. Tracer v. 1.6 [102] was used to examine ESS values to ensure they were > 200 and to examine stationarity plots. All trees were viewed in FigTree v1.4.3 [103]. Each phylogeny was compared to the published phylogeny, then used as a topological constraint for the dating analysis in BEAST 2 [104]. If our results were inconsistent with those previously published or certain relationships were unresolvable, multiple possibilities were tested.
BEAST 2 input files were constructed using BEAUti. Constrained MrBayes molecular phylogenies were dated in BEAST 2 using either fossil calibrations or a molecular clock rate. For information on the dating parameters, please refer to the detailed methods for each taxon in Additional file 1. Burn-in for Tracer 1.6 analyses was 10%, and for trees in TreeAnnotator, it was set to 25% unless otherwise noted. All MrBayes and BEAST 2 analyses were run using XSEDE [105] on the CIPRES Science Gateway [106]. The .xml files and output trees used for subsequent BioGeoBEARS analyses are available on figshare.
Biogeographic model testing
We conducted biogeographic hypothesis testing and ancestral range reconstruction using the BEAST 2 time-calibrated phylogenies in BioGeoBEARS [107]. The models used (Table 1) to test the biogeographic hypotheses generally follow those of Crews and Gillespie [25] and consider GAARlandia, the various proposed closure dates of the IoP/CAS, and various multipliers of “dispersal ability” in shaping biogeographic histories. The most basic model considered the probability of dispersal without GAARlandia vs. a model that considers GAARlandia. Manual dispersal multiplier matrices were employed to further model the effect of distance and dispersal ability [42] as follows:
-
a)
Distance dependent dispersal. The probability of reaching a land area decreased as distance between land areas increased. If there was a land connection available during a particular time period, the manual dispersal multiplier (mdm) value was increased (Note: mdm values were only set to 1 in very closely adjacent areas).
-
b)
Distance independent dispersal. Distance did not play a role in dispersal and establishment probability (presence absence of landmasses only). If there was a hypothesized or known land connection, mdm values were set to 1 (land) and with no connection, set to 0.5. During the proposed timing of GAARlandia, the probability of dispersal from South America to the Greater Antilles increased from 0.5 to 1.
-
c)
Vicariance only model. A very low probability of dispersal. Land connections remained set to 1, but overwater dispersal was lowered to 0.01. The mdm values from South America to the Greater Antilles during the proposed timing of GAARlandia were increased from 0.01 to 1. When there was no land available for a designated landmass, probabilities were set to 0.0000001 [67].
Finally, we considered various geologic times of taxa moving across the IoP. (See Table 1 and Fig. 2 for details and citations.) All data were analyzed using all models available in the BioGeoBEARS package (DEC, DEC + J, DIVALIKE, DIVALIKE+J, BAYAREALIKE, BAYAREALIKE+J) [107,108,109]. This resulted in a maximum of 252 models per dataset. The best model was evaluated using the AICc weights [59].
Model limitations
The Caribbean islands have moved and increased in area and connectedness through time. We acknowledge that these processes are gradual, and geologic dates may not be exact. We’ve therefore elected to examine dispersal vs. vicariance using large generalized areas for both geologically-grouped islands and continents (e.g., “Northern Lesser Antilles” vs. each individual island, “Greater Antilles” vs. each individual island) in spite of potential criticisms of oversimplification (e.g., [110] of [25]). We’ve opted to employ a strategy that avoids over-parameterization while utilizing the data available. We encourage other authors to use the generalized biogeoraphic models we have provided as input files on figshare as a baseline – using different dispersal probabilities, different time periods, different areas, etc. – to test their own hypotheses.
For the time periods and geographic areas used for each analysis, see Tables 1 and 2. We relied on authors and experts for taxa distribution information. While it is possible that taxa may have broader or even narrower distributions, this potentially would not have a large effect on the results because we used broader land areas as our geographic areas for analyses. Geologic dates based on the current published literature for the first appearance of geographic areas guided our manual dispersal multiplier values and are provided in Table 1 and Additional file 1: Table S1.
Availability of data and materials
All data generated or analyzed during this study are included in the additional files, are available on GenBank, or are available as a downloadable package at https://ndownloader.figshare.com/files/18548144.
Abbreviations
- BA:
-
Bahamas and/or Turks and Caicos Islands
- BAR:
-
Barbados
- CA:
-
Central America
- CAS:
-
Central American Seaway
- CU:
-
Cuba
- FL:
-
Florida
- GA:
-
Greater Antilles
- GAARlandia:
-
Greater Antilles Aves Ridge
- HI:
-
Hispaniola
- IoP:
-
Isthmus of Panama
- JA:
-
Jamaica
- my:
-
Million years
- NA:
-
Non-applicable
- NA:
-
North America
- NLA:
-
Northern Lesser Antilles
- PR:
-
Puerto Rico
- SA:
-
South America
- SLA:
-
Southern Lesser Antilles
References
Poey F. Memorias sobre la historia natural de la isla de Cuba, acompañadas de sumarios latinos y extractos en frances, por Felipe Poey Habana. Cuba: Impr De Barcina; 1861. p. 1851–8.
Liebherr JK. Zoogeography of Caribbean insects. 1st ed. Ithaca: Cornell University Press; 1988.
Alayón G. La familia Selenopidae (Arachnida: Araneae) en Cuba. Solenodon. 2005;5:10–52.
Scheffrahn RH, Křeček J, Chase JA, Maharajh B, Mangold JR. Taxonomy, biogeography, and notes on termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands. Ann Entomol Soc Amer. 2006;99:464–86. https://doi.org/10.1603/0013-8746(2006)99%5B463:TBANOT%5D2.0.CO.2.
Crews SC. A revision of the spider genus Selenops Latreille, 1819 (Arachnida, Araneae, Selenopidae) in North America, Central America and the Caribbean. ZooKeys. 2011:1–82. https://doi.org/10.3897/zookeys.105.724.
Bloom T, Binford G, Alayón G, Esposito L, Peterson I, Nishida A, et al. Discovery of two new species of eyeless spiders within a single Hispaniola cave. J Arachnol. 2014:148–54. https://doi.org/10.1636/K13-84.1.
Crews SC, Esposito LA, Cala-Riquelme F. The arachnids of Hellshire Hills, Jamaica. Caribbean Nat. 2015;28:1–14.
Gillespie RG, Roderick GK. Arthropods on islands: colonization, speciation, and conservation. Annu Rev Entomol. 2002:595–632. https://doi.org/10.1146/annurev.ento.47.091201.145244.
Donnelly TW. In: Liebherr JK, editor. Geologic constraints on Caribbean biogeography. Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 15–37.
Dengo G, Case JE. The geology of North America, vol. H, the Caribbean region (a decade of north American geology) Boulder. Colorado: Geologic Society of America; 1990.
Iturralde-Vinent MA, MacPhee RDE. Paleogeography of the Caribbean region: implications for Cenozoic biogeography. Bull Amer Mus Nat Hist. 1999:238;1–95.
Iturralde-Vinent MA. Meso-Cenozoic Caribbean paleogeography: implications for the historical biogeography of the region. Int Geol Rev. 2006:791–827. https://doi.org/10.2747/0020-6814.48.9.791.
James KH. In situ origin of the Caribbean: discussion of data. Geol Soc, London, Special Publications. 2009:77–125. https://doi.org/10.1144/SP328.3.
Hedges SB, Hass CA, Maxson LR. Caribbean biogeography: molecular evidence for dispersal in West Indian terrestrial vertebrates. P Natl Acad Sci USA. 1992:1909–13. https://doi.org/10.1073/pnas.89.5.1909.
Crother BI, Guyer C. Caribbean historical biogeography: was the dispersal vicariance debate eliminated by an extraterrestrial bolide? Herpetologica. 1996:52;440–65.
MacPhee RDE, Iturralde-Vinent MA. The interpretation of Caribbean paleogeography: reply to Hedges. Proc Int Symposium on Insular Vert Evol: the paleontological approach; 2005.
Bagley JC, Johnson JB. Phylogeography and biogeography of the lower central American Neotropics: diversification between two continents and between two seas. Biol Rev. 2014:767–90. https://doi.org/10.1111/brv.12076.
O'Dea A, Lessios HA, Coates AG, Eytan RI, Restrepo-Moreno SA, Cione AL, et al. Formation of the Isthmus of Panama. Sci Adv. 2016;2:e1600883. https://doi.org/10.1126/sciadv.1600883.
Montes C, Cardona A, McFadden R, Morón SE, Silva CA, Restrepo-Moreno S, et al. Evidence for middle Eocene and younger land emergence in Central Panama: implications for isthmus closure. Geol Soc Amer Bull. 2012;124:780–99. https://doi.org/10.1130/B30528.1.
Coates AG, Stallard RF. How old is the isthmus of Panama? B Mar Sci. 2013;89:801–13. https://doi.org/10.5343/bms.2012.1076.
Osborne AH, Newkirk DR, Groeneveld J, Martin EE, Tiedemann R, Frank M. The seawater neodymium and lead isotope record of the final stages of central American seaway closure. Paleoceanography. 2014;29:715–29.
Tavares VC, Simmons NB. Phylogenetic relationships and biogeography of Short-faced stenodermatine bats: preliminary results and hypotheses. Bat Res News. 2000;41:143.
Dávalos LM. Phylogeny and biogeography of Caribbean mammals. Biol J Linn Soc. 2004:373–94. https://doi.org/10.1111/j.1095-8312.2003.00302.x.
Alonso R, Crawford AJ, Bermingham E. Molecular phylogeny of an endemic radiation of Cuban toads (Bufonidae: Peltophryne) based on mitochondrial and nuclear genes. J Biogeogr. 2011:434–51. https://doi.org/10.1111/j.1365-2699.2011.02594.x.
Crews SC, Gillespie RG. Molecular systematics of Selenops spiders (Araneae: Selenopidae) from north and Central America: implications for Caribbean biogeography. Biol J Linn Soc. 2010:288–322. https://doi.org/10.1111/j.1095-8312.2010.01494.x.
McHugh A, Yablonsky C, Binford G, Agnarsson I. Molecular phylogenetics of Caribbean Micrathena (Araneae: Araneidae) suggests multiple colonisation events and single island endemism. Invertebr Syst. 2014:337–49. https://doi.org/10.1071/IS13051.
Matos-Maraví P, Águila RN, Peña C, Miller JY, Sourakov A, Wahlberg N. Causes of endemic radiation in the Caribbean: evidence from the historical biogeography and diversification of the butterfly genus Calisto (Nymphalidae: Satyrinae: Satyrini). BMC Evol Biol. 2014:199. https://doi.org/10.1186/s12862-014-0199-7.
Říčan O, Piálek L, Zardoya R, Doadrio I, Zrzavý J. Biogeography of the Mesoamerican Cichlidae (Teleostei: Heroini): colonization through the GAARlandia land bridge and early diversification. J Biogeogr. 2013:579–93. https://doi.org/10.1111/jbi.12023.
Fritsch PW. Multiple geographic origins of Antillean Styrax. Syst Bot. 2003:28;421–30.
van Ee B, Berry PE, Riina R, Gutiérrez Amaro JE. Molecular phylogenetics and biogeography of the Caribbean-centered Croton subgenus Moacroton (Euphorbiaceae s.s.). Bot Rev. 2008;74:132–65. https://doi.org/10.1007/s12229-008-9003-y.
Ali JR. Colonizing the Caribbean: is the GAARlandia land-bridge hypothesis gaining a foothold? J Biogeogr. 2011;39:431–3. https://doi.org/10.1111/j.1365-2699.2011.02674.x.
O'Dea A, Hoyos N, Rodríguez F, Degarcia B, De Gracia C. History of upwelling in the tropical eastern Pacific and the paleogeography of the isthmus of Panama. Palaeogeogr Palaeocl. 2012;348:59–66. https://doi.org/10.1016/j.palaeo.2012.06.007.
Leigh EG, O’Dea A, Vermeij GJ. Historical biogeography of the isthmus of Panama. Biol Rev. 2014;89:148–72. https://doi.org/10.1111/brv.12048.
Bacon CD, Mora A, Wagner WL, Jaramillo CA. Testing geologic models of evolution of the isthmus of Panama in a phylogenetic framework. Bot J Linn Soc. 2013:287–300. https://doi.org/10.1111/j.1095-8339.2012.01281.x.
Bacon CD, Silvestro D, Jaramillo C, Smith BT, Chakrabarty P, Antonelli A. Biologic evidence supports an early and complex emergence of the isthmus of Panama. P Natl Acad Sci USA. 2015;112:6110–5. https://doi.org/10.1073/pnas.1423853112.
Barbour T. A contribution to the zoogeography of the West Indies, with special reference to amphibians and reptiles. Mem Mus Comp Zool Harvard College. 1914:44;205–359.
Darlington PJ. The origin of the fauna of the greater Antilles, with discussion of dispersal of animals over water and through the air. Q Rev Biol. 1938;13:274–300.
Ricklefs RE, Lovette IJ. The roles of island area per se and habitat diversity in the species-area relationships of four lesser Antillean faunal groups. J Anim Ecol. 1999;68:1142–60.
Penney D, Wheater CP, Selden PA. Resistance of spiders to cretaceous–tertiary extinction events. Evolution. 2003;57:2599–607. https://doi.org/10.1554/03-024.
Liebherr JK. In: Liebherr JK, editor. Biogeographic patterns of West Indian Platynus carabid beetles (Coleoptera). Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 121–52.
Crews SC, Puente-Rolón AR, Rutstein E, Gillespie RG. A comparison of populations of island and adjacent mainland species of Caribbean Selenops (Araneae: Selenopidae) spiders. Mol Phylogenet Evol. 2010;54:970–83. https://doi.org/10.1016/j.ympev.2009.10.012.
Van Dam MH, Matzke NJ. Evaluating the influence of connectivity and distance on biogeographic patterns in the south-western deserts of North America. J Biogeogr. 2016;43:1514–32. https://doi.org/10.1111/jbi.12727.
Crews SC, Hedin MC. Studies of morphological and molecular phylogenetic divergence in spiders (Araneae: Homalonychus) from the American southwest, including divergence along the Baja California peninsula. Mol Phylogenet Evol. 2006:470–87. https://doi.org/10.1016/j.ympev.2005.11.010.
Leaché AD, Crews SC, Hickerson MJ. Two waves of diversification in mammals and reptiles of Baja California revealed by hierarchical Bayesian analysis. Biol Lett. 2007:646–50. https://doi.org/10.1098/rsbl.2007.0368.
Hollocher H, Williamson M. Island hopping in Drosophila: patterns and processes [and discussion]. Phil Trans R Soc London B: Biol Sci. 1996;351:735–43. https://doi.org/10.1098/rstb.1996.0068.
Davies N, Bermingham E. The historical biogeography of two Caribbean butterflies (Lepidoptera: Heliconiidae) as inferred from genetic variation at multiple loci. Evolution. 2002;56:573–89. https://doi.org/10.1111/j.0014-3820.2002.tb01368.x.
Wilder JA, Hollocher H. Recent radiation of endemic Caribbean Drosophila of the dunni subgroup inferred from multilocus DNA sequence variation. Evolution. 2003;57:2566–79. https://doi.org/10.1554/02-696.
Szalanski AL, Scheffrahn RH, Austin JW, Křeček J, Su N-Y. Molecular phylogeny and biogeography of Heterotermes (Isoptera: Rhinotermitidae) in the West Indies. Ann Entomol Soc Amer. 2004;97:556–66. https://doi.org/10.1603/0013-8746(2004)097[0556:MPABOH]2.0.CO;2.
Oneal E, Otte D, Knowles LL. Testing for biogeographic mechanisms promoting divergence in Caribbean crickets (genus Amphiacusta). J Biogeogr. 2010;37:530–40. https://doi.org/10.1111/j.1365-2699.2009.02231.x.
Sourakov A, Zakharov EV. “Darwin’s butterflies”? DNA barcoding and the radiation of the endemic Caribbean butterfly genus Calisto (Lepidoptera, Nymphalidae, Satyrinae). Comp Cytogenet. 2011;5:191–210. https://doi.org/10.3897/CompCytogen.v5i3.1730.
Dziki A, Binford GJ, Coddington JA, Agnarsson I. Spintharus flavidus in the Caribbean—a 30 million year biogeographical history and radiation of a ‘widespread species’. PeerJ. 2015;3:e1422. https://doi.org/10.7717/peerj.1422.
Lewis DS, Sperling FA, Nakahara S, Cotton AM, Kawahara AY, Condamine FL. Role of Caribbean Islands in the diversification and biogeography of Neotropical Heraclides swallowtails. Cladistics. 2015;31:291–314. https://doi.org/10.1111/cla.12092.
Seal JN, Kellner K, Trindl A, Heinze J. Phylogeography of the parthenogenic ant Platythyrea punctata: highly successful colonization of the West Indies by a poor disperser. J Biogeogr. 2011;38:868–82. https://doi.org/10.1111/j.1365-2699.2010.02447.x.
Esposito LA, Bloom T, Caicedo-Quiroga L, Alicea-Serrano AM, Sánchz-Ruíz JA, May-Collado LJ, et al. Islands within islands: diversification of tailless whip spiders (Amblypygi, Phrynus) in Caribbean caves. Mol Phylogenet Evol. 2015;93:107–17. https://doi.org/10.1016/j.ympev.2015.07.005.
Rosen DE. Geologic hierarchies and biogeographic congruence in the Caribbean. Ann Mo Bot Gard. 1985:636–59. https://doi.org/10.2307/2399218.
Slater J. In: Liebherr JK, editor. Zoogeography of West Indian Lygaeidae (Hemiptera). Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 38–60.
Riddle BR, Hafner DJ, Alexander LF, Jaeger JR. Cryptic vicariance in the historical assembly of a Baja California Peninsular Desert biota. P Natl Acad Sci USA. 2000:14438–43. https://doi.org/10.1073/pnas.250413397.
Ree R, Sanmartín I. Conceptual and statistical problems with the DEC+J model of founder-event speciation and its comparison with DEC via model selection. J Biogeogr. 2018;45(4):741–9. https://doi.org/10.1111/jbi.13173.
Matzke NJ. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst Biol. 2014;63:951–70. https://doi.org/10.1093/sysbio/syu056.
Lieberman BS. Phylogenetic biogeography with and without the fossil record: gauging the effects of extinction and paleontological incompleteness. Palaeogeogr Palaeoclimatol Palaeoecol. 2002;178:39–52. https://doi.org/10.1016/S0031-0182(01)00367-4.
Morrone JJ. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol. 2006;51:467–94. https://doi.org/10.1146/annurev.ento.50.071803.130447.
Echeverry A, Morrone JJ. Generalized tracks, area cladograms and tectonics in the Caribbean. J Biogeogr. 2013;40:1619–37. https://doi.org/10.1111/jbi.12117.
Grimaldi DA. In: Liebherr JK, editor. Relicts in the Drosophilidae (Diptera). Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 183–213.
Browne DJ, Peck SB, Ivie MA. The longhorn beetles (Coleoptera Cerambycidae) of the Bahama Islands with an analysis of species-area relationships, distribution patterns, origin of the fauna and an annotated species list. Trop Zool. 1993;6:27–53. https://doi.org/10.1080/03946975.1993.10539207.
Wilson EO. In: Liebherr JK, editor. The biogeography of the West Indian ants (Hymenoptera: Formicidae). Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 214–30.
Ramos JA. In: Liebherr JK, editor. Zoogeography of the auchenorrhynchous Homoptera of the Greater Antilles. Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 61–70.
Matzke NJ. http://phylo.wikidot.com/biogeobears-mistakes-to-avoid; Accessed 31 Aug 2017.
Scheffrahn RH, Krecek J, Szalanski AL, Austin JW, Roisin Y. Synonymy of two arboreal termites (Isoptera: Termitidae: Nasutitermitinae): Nasutitermes corniger from the Neotropics and N. polygynus from New Guinea. Fla Entomol. 2005:28–33. https://doi.org/10.1653/0015-4040(2005)088%5B0028:SOTATI%5D2.0.CO;2.
Magalhães ILF, Santos AJ. Phylogenetic analysis of Micrathena and Chaetacis spiders (Araneae: Araneidae) reveals multiple origins of extreme sexual size dimorphism and long abdominal spines. Zool J Linn Soc-Lond. 2012:14–53. https://doi.org/10.1111/j.1096-3642.2012.00831.x.
MacArthur RH, Wilson EO. An equilibrium theory of insular zoogeography. Evolution. 1963:373–87. https://doi.org/10.1111/j.1558-5646.1963.tb03295.x.
MacArthur RH, Wilson EO. The theory of island biogeography. 1st ed. Princeton: Princeton University Press; 1967.
Nicholson KE, Glor RE, Kolbe JJ, Larson A, Hedges SB, Losos JB. Mainland colonization by island lizards. J Biogeogr. 2005;32:929–38. https://doi.org/10.1111/j.1365-2699.2004.01222.x.
Glor RE, Losos JB, Larson A. Out of Cuba: overwater dispersal and speciation among lizards in the Anolis carolinensis subgroup. Mol Ecol. 2005:2419–32. https://doi.org/10.1111/j.1365-294X.2005.02550.x.
Zhang G, Basharat U, Matzke N, Franz NM. Model selection in statistical historical biogeography of Neotropical insects—the Exophthalmus genus complex (Curculionidae: Entiminae). Mol Phylogenet Evol. 2016:226–39. https://doi.org/10.1016/j.ympev.2016.12.039.
Sturge RJ, Jacobsen F, Rosensteel BB, Neale RJ, Omland KE. Colonization of South America from Caribbean Islands confirmed by molecular phylogeny with increased taxon sampling. Condor. 2009;111:575–9. https://doi.org/10.1525/cond.2009.080048.
Condamine FL, Silva-Brandao KL, Kergoat GJ, Sperling FA. Biogeographic and diversification patterns of Neotropical Troidini butterflies (Papilionidae) support a museum model of diversity dynamics for Amazonia. BMC Evol Biol. 2012;12:82. https://doi.org/10.1186/1471-2148-12-82.
Losos JB, Thorpe RS.Evolutionary Diversification of Caribbean Anolis Lizards. In: Adaptive Speciation, eds. Dieckmann U, Doebeli M, Metz JAJ & Tautz D. International Institute for Applied Systems Analysis. Cambridge: Cambridge University Press.; 2004:322–344.
Kaiser H, Hardy JD Jr, Green DM. Taxonomic status of Caribbean and south American frogs currently ascribed to Eleutherodactylus urichi (Anura: Leptodactylidae). Copeia. 1994. https://doi.org/10.2307/1447195.
Seutin G, Klein NK, Ricklefs RE, Bermingham E. Historical biogeography of the bananaquit (Coereba flaveola) in the Caribbean region: a mitochondrial DNA assessment. Evolution. 1994:1041–61. https://doi.org/10.1111/j.1558-5646.1994.tb05292.x.
Huang J-P. The great American biotic interchange and diversification history in Dynastes beetles (Scarabaeidae; Dynastinae). Zool J Linnean Soc. 2016:88–96. https://doi.org/10.1111/zoj.12393.
Binford GJ, Callahan MS, Bodner MR, Rynerson M, Núñez PB, Ellison CE, et al. Phylogenetic relationships of Loxosceles and Sicarius spiders are consistent with Western Gondwanan vicariance. Mol Phylogenet Evol. 2008:538–53. https://doi.org/10.1111/brv.12076.
Ree RH, Moore BR, Webb CO, Donoghue MJ. A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution. 2005:2299–311. https://doi.org/10.1111/j.0014-3820.2005.tb00940.x.
Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst Biol. 2008:4–14. https://doi.org/10.1080/10635150701883881.
Esposito LA, Prendini L. Island ancestors and New World biogeography: a Case study from the scorpions (Buthidae: Rhopalurusinae). Sci Rep. 2019:1–11. https://doi.org/10.1038/s41598-018-33754-8.
Yu Y, Harris AJ, He XJ. RASP (reconstruct ancestral state in phylogenies): a tool for historical biogeography. Mol Phylogenet Evol. 2010:848–50. https://doi.org/10.1016/j.ympev.2010.04.011.
Yu Y, Harris AJ, Blair C, He XJ. S-DIVA (statistical dispersal-Vicariance analysis): a tool for inferring biogeographic histories. Mol Phylogenet Evol. 2015:46–9. https://doi.org/10.1016/j.ympev.2015.03.008.
Losos JB. Phylogenetic perspectives on community ecology. Ecology. 1996:1344–54. https://doi.org/10.2307/2265532.
Ricklefs RE, Bermingham E. The concept of the taxon cycle in biogeography. Global Ecol and Biogeogr. 2002:353–61. https://doi.org/10.1046/j.1466-822x.2002.00300.x.
Ricklefs R, Bermingham E. The West Indies as a laboratory of biogeography and evolution. Rev Zool Bot Afr. 2008:2393–413. https://doi.org/10.1098/rstb.2007.2068.
Triantis KA, Nogués-Bravo D, Hortal J, Borges PA, Andersen H, Fernández-Palacios J. Measurements of area and the (island) species–area relationship: new directions for an old pattern. Oikos. 2008:1555–9. https://doi.org/10.1111/j.0030-1299.2008.16808.x.
Cosgrove JG, Agnarsson I, Harvey MS, Binford GJ. Pseudoscorpion diversity and distribution in the West Indies: sequence data confirm single island endemism for some clades, but not others. J Arachnol. 2016:257–71. https://doi.org/10.1636/R15-80.1.
Cenzi De Ré F, Loreto EL, Robe LJ. Gene and species trees reveal mitochondrial and nuclear discordance in the Drosophila cardini group (Diptera: Drosophilidae). Invertebr Biol. 2010; 353–367. doi: https://doi.org/10.1111/j.1744-7410.2010.00207.x
Agnarsson I, LeQuier SM, Kuntner M, Cheng R-C, Coddington JA, Binford G. Phylogeography of a good Caribbean disperser: Argiope argentata (Araneae, Araneidae) and a new ‘cryptic’ species from Cuba. ZooKeys. 2016:25–44. https://doi.org/10.3897/zookeys.625.8729.
Levi HW. The spider genera Gea and Argiope in America (Araneae: Araneidae). Bull Mus Comp Zool. 1968:136;319–52.
Buskirk RE. Zoogeographic patterns and tectonic history of Jamaica and the northern Caribbean. J Biogeogr. 1985. https://doi.org/10.2307/2844953.
Edgecombe GD, Vahtera V, Giribet G, Kaunisto P. Species limits and phylogeography of Newportia (Scolopendromorpha) and implications for widespread morphospecies. ZooKeys. 2015:65–77. https://doi.org/10.3897/zookeys.510.8573.
Murienne J, Daniels SR, Buckley TR, Mayer G, Giribet G. A living fossil tale of Pangaean biogeography. P Roy Soc Lond B Bio. 2014;281:20132648. https://doi.org/10.1098/rspb.2013.2648.
Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4. https://doi.org/10.1093/bioinformatics/btg180.
Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 2016;34:772–3. https://doi.org/10.1093/molbev/msw260.
Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–794. https://doi.org/10.1080/10635150390235520.
Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. https://doi.org/10.1038/nmeth.2109.
Rambaut A, Suchard MA, Xie D, Drummond AJ. Tracer v1.6. 2014; http://beast.bio.ed.ac.uk/Tracer
Rambaut A. FigTree. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 2017.
Boukaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Suchard MA, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2014;10:e1003537. https://doi.org/10.1371/journal.pcbi.1003537.
Towns J, Cockerill T, Dahan M, Foster I, Gaither K, Grimshaw A, et al. XSEDE: accelerating scientific discovery. Comput Sci Eng. 2014;16:62–74. https://doi.org/10.1109/MCSE.2014.80.
Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees, Proc of the Gateway Computing Env Workshop (GCE); 2010. p. 1–8.
Matzke NJ. BioGeoBEARS: BioGeography with Bayesian (and Likelihood) Evolutionary Analysis in R Scripts. R package, version 0.2.1, published July 27, 2013 at: http://CRAN.R-project.org/package=BioGeoBEARS http://CRAN.R-project.org/package=BioBeoBEARS 2013.
Matzke NJ. rexpokit: R wrappers for EXPOKIT. R package, version 0.24.2, published July 15, 2013 at: http://CRAN.R-project.org/package=rexpokit http://CRAN.R-project.org/package=cladoRcpp 2013.
Matzke NJ, Sidje RB. cladoRcpp: C++ implementations of phylogenetic calculations. R package, version 0.14.2, published July 15, 2013 at: http://CRAN.R-project.org/package=cladoRcpp http://CRAN.R-project.org/package=rexpokit 2013.
Santamaria CA, Mateos M, Hurtado LA. Diversification at the narrow sea-land interface in the Caribbean: phylogeography of endemic supralittoral Ligia isopods. Front Ecol and Evol. 2014;2:42. https://doi.org/10.3389/fevo.2014.00042.
Nichols SW. In: Liebherr JK, editor. Kaleidoscopic biogeography of West Indian Scaratinae (Coleoptera: Carabidae). Ithaca: Comstock Publishing Associates, Cornell University Press; 1988. p. 71–121.
Matzke NJ. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front Biogeogr. 2013:8;242–8.
Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 3.2. 2017. http://mesquiteproject.org.
Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013:2869–76. https://doi.org/10.1093/bioinformatics/btt499.
Schmidt C. Molecular phylogenetics of ponerine ants (Hymenoptera: Formicidae: Ponerinae). Zootaxa. 2013:201–50. https://doi.org/10.11646/zootaxa.3647.2.1.
Brady SG, Larkin L, Danfort BN. In: Hedges SB, Kumar S, editors. Bees, ants, and stinging wasps (Aculeata). Oxford: Oxford University Press; 2009.
Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002:3059–66. https://doi.org/10.1093/nar/gkf436.
Ware JL, Grimaldi DA, Engel MS. The effects of fossil placement and calibration on divergence times and rates: an example from the termites (Insecta: Isoptera). Arthropod Struct Dev. 2010:204–19. https://doi.org/10.1016/j.asd.2009.11.003.
Vaidya G, Lohman DJ, Meier R. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 2011:171–80. https://doi.org/10.1111/j.1096-0031.2010.00329.x.
Roy V, Constantino R, Chassany V, Giusti-Miller S, Diouf M, Mora P, et al. Species delimitation and phylogeny in the genus Nasutitermes (Termitidae: Nasutitermitinae) in French Guiana. Mol Ecol. 2014:902–20. https://doi.org/10.1111/mec.12641.
Sukumaran J, Holder MT. DendroPy: a Python library for phylogenetic computing. Bioinformatics. 2010:1569–71. https://doi.org/10.1093/bioinformatics/btq228.
Sukumaran J, Holder MT. SumTrees: Phylogenetic Tree Summarization. 2015; https://github.com/jeetsukumaran/DendroPy
Peña C, Nylin S, Wahlberg N. The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods. Zool J Linnean Soc. 2011:64–87. https://doi.org/10.1111/j.1096-3642.2009.00627.x.
Lotts K, Naberhaus T. Butterflies and moths of North America. 2017. https://www.butterfliesandmoths.org.
Obbard DJ, Maclennan J, Kim KW, Rambaut A, O'Grady PM, Jiggins FM. Estimating divergence dates and substitution rates in the Drosophila phylogeny. Mol Biol Evol. 2012:3459–73. https://doi.org/10.1093/molbev/mss150.
Brisson JA, Wilder J, Hollocher H. Phylogenetic analysis of the cardini group of Drosophila with respect to changes in pigmentation. Evolution. 2006:1228–41. https://doi.org/10.1554/05-552.1.
Viera J. Molecular evolution group: Drosophila distributions. 2016. http://evolution.ibmc.up.pt/node/35.
Drosophila Species Stock Center Geography. 2017. https://stockcenter.ucsd.edu/info/geography.php. Accessed 2017.
Ashburner M, Carson HL, Thompson JN. The Genetics and Biology of Drosophila, vol. Volume 3a. London: Academic Press, London; 1981.
Dimitrov D, Lopardo L, Girbet G, Arnedo MA, Álvarez-Padilla F, Hormiga G. Tangled in a sparse spider web: single origin of orb weavers and their spinning work unravelled by denser taxonomic sampling. P Roy Soc Lond B Bio. 2011. https://doi.org/10.1098/rspb.2011.2011.
Wheeler WC, Coddington JA, Crowley LM, Dimitrov D, Goloboff P, Griswold C, et al. The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics. 2016:1–43. https://doi.org/10.1111/cla.12182.
Kallal RJ, Hormiga G. Systematics, phylogeny and biogeography of the Australasian leaf-curling orb-weaving spiders (Araneae: Araneidae: Zygiellinae), with a comparative analysis of retreat evolution. Zool J Linn Soc-Lond In press. https://doi.org/10.1093/zoolinnean/zly014.
Gregorič M, Agnarsson I, Blackledge TA, Kuntner M. Phylogenetic position and composition of Zygiellinae and Caerostris, with new insight into orb-web evolution and gigantism. Zool J Linn Soc-Lond. 2015:225–43. https://doi.org/10.1111/zoj.12281.
Stucky BJ. SeqTrace: a graphical tool for rapidly processing DNA sequencing chromatograms. J Biomol Tech. 2012;23:90. https://doi.org/10.7171/jbt.12-2303-004.
Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014:1312–3. https://doi.org/10.1093/bioinformatics/btu033.
Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006:1658–9. https://doi.org/10.1093/bioinformatics/btl158.
Huang Y, Niu B, Gao Y, Fu L, Li W. CD-HIT suite: a web server for clustering and comparing biologic sequences. Bioinformatics. 2010:680–2. https://doi.org/10.1093/bioinformatics/btq003.
Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012:3150–2. https://doi.org/10.1093/bioinformatics/bts565.
Polotow D, Carmichael A, Griswold CE. Total evidence analysis of the phylogenetic relationships of Lycosoidea spiders (Araneae, Entelegynae). Invertebr Syst. 2015:124–63. https://doi.org/10.1071/IS14041.
Ramírez MJ. The morphology and phylogeny of dionychan spiders (Araneae: Araneomorphae). Bull Amer Mus Nat Hist. 2014:1–374. https://doi.org/10.1206/821.1.
Bidegaray-Batista L, Arnedo MA. Gone with the plate: the opening of the Western Mediterranean basin drove the diversification of ground-dweller spiders. BMC Evol Biol. 2011;317. https://doi.org/10.1186/1471-2148-11-317.
Selden PA, Wang Y. Fossil spiders (Araneae) from the Eocene Green River formation of Colorado. Arthropoda Selecta. 2014:23;207–19.
Fouke BW. Quaternary geology and depositional history of Provodenciales, Turks and Caicos Islands, British West Indies. M.Sc. Thesis; 1984.
Cuezzo C, Carrijo TF, Cancello EM. Transfer of two species from Nasutitermes Dudley to Cortaritermes Mathews (Isoptera: Termitidae: Nasutitermitinae). Austral Entomol. 2015:172–9. https://doi.org/10.1111/aen.12107.
Futch DG. Hybridization tests within the cardini species group of the genus Drosophila. Univ Texas Publ. 1962;6205:539–54.
Heed WB. Genetic characteristics of island populations. Univ Texas Publ. 1962;6205:502–18.
Garrison NL, Rodriguez J, Agnarsson I, Coddington JA, Griswold CE, Hamilton CA, et al. Spider phylogenomics: untangling the spider tree of life. PeerJ. 2016;4:e1719. https://doi.org/10.7717/peerj.1719.
Degnan JH, Salter LA. Gene tree distributions under the coalescent process. Evolution. 2005:24–37. https://doi.org/10.1554/04-385.
Kubatko LS, Degnan JH. Inconsistency of phylogenetic estimates from concatenated data under coalescence. Syst Biol. 2007:17–24. https://doi.org/10.1080/10635150601146041.
Iturralde-Vinent MA. Aspectos geológicos de la biogeografía de Cuba. Ciencias de la Tierra y el Espacio. 1982:5;85–100.
Bouysse P, Westercamp D. Subduction of Atlantic aseismic ridges and late Cenozoic evolution of the Lesser Antilles island arc. Tectonophysics. 1990:349357–55380. https://doi.org/10.1016/0040-1951(90)90180-G.
Maury RC, Westbrook GK, Baker PE, Bouysse Ph, Westercamp D. Geology of the Lesser Antilles. In: Dengo G and Case JE, editors. Boulder. Geologic Society of America. 1990.
Stephan J-F, Mercier-de-Lepinay B, Calais E, Tardy M, Beck C, Carfantan J-C, et al. Paleogeodynamic maps of the Caribbean: 14 steps from Lias to present. Bull Soc Géol Fr. 1990;6:6–919.
Mann P, Schubert C, Burke K. In: Dengo G, Case JE, editors. Review of Caribbean neotectonics. Boulder: Geologic Society of America; 1990.
Randazzo AF, Jones DS. The geology of Florida; 1997.
Hine AC. Geologic history of Florida: major events that formed the sunshine state; 2013.
Speed RC, Larue DK. Barbados: Architecture and implications for accretion. J Geophys Res-Sol Ea. 1982;87(B5):3633–43.
Fouke BW. Quaternary geology and depositional history of Providenciales, Turks and Caicos Islands, British West Indies. M.Sc. Thesis; 1984.
Wanless HR, Dravis JJ. Carbonate environments and sequences of Caicos platform. 1989; 28th International Geologic Congress, Fieldtrip Guidebook T-374.
Morgan WA, Harris PM. Developing models and analogs for isolated carbonate platforms - Holocene and Pleistocene carbonates of Caicos Platform, British West Indies. 2008; SEPM Core Workshop 22.
Smith AM, Fisher BL, Hebert PD. DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: the ants of Madagascar. Phil Trans R Soc London B: Biol Sci. 2005;360:1825–34. https://doi.org/10.1098/rstb.2005.1714.
Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE. Phylogeny of the ants: diversification in the age of angiosperms. Science. 2006:101–4. https://doi.org/10.1126/science.1124891.
Spagna JC, Vakis AI, Schmidt CA, Patek SN, Zhang X, Tsutsui N, et al. Phylogeny, scaling, and the generation of extreme forces in trap-jaw ants. J Exp Biol. 2008:2358–68. https://doi.org/10.1242/jeb.015263.
Marino A, Sabatini AG, Carpana E, Lodesani M, Mantovani B. ND2 and CO1 mitochondrial genes in Apis mellifera L.: A Molecular Approach to Mediterranean Population Monitoring. Proc 6th Euro Bee Conf. 2002:4;157.
Field J, Ohl M, Kennedy M. A molecular phylogeny for digger wasps in the tribe Ammophilini (Hymenoptera, Apoidea, Sphecidae). Syst Entomol. 2011;36:732–40.
Heraty J, Ronquist F, Carpenter JM, Hawks D, Schulmeister S, Dowling AP, et al. Evolution of the hymenopteran megaradiation. Mol Phylogenet Evol. 2011:73–88. https://doi.org/10.1016/j.ympev.2011.04.003.
Eaton TD, Jones SC, Jenkins TM. Species diversity of Puerto Rican Heterotermes (Dictyoptera: Rhinotermitidae) revealed by phylogenetic analyses of two mitochondrial genes. J Insect Sci. 2016;111. https://doi.org/10.1093/jisesa/iew099.
Saldarriaga JF, Gile GH, James ER, Horak A, Scheffrahn RH, Keeling PJ. Morphology and molecular phylogeny of Pseudotrichonympha hertwigi and Pseudotrichonympha Paulistana (Trichonymphea, Parabasalia) from neotropical rhinotermitids. J Eukaryot Microbiol. 2011:487–96. https://doi.org/10.1111/j.1550-7408.2011.00575.x.
Lo N, Kitade O, Miura T, Constantino R, Matsumoto T. Molecular phylogeny of the Rhinotermitidae. Insect Soc. 2004:365–71. https://doi.org/10.1007/s00040-004-0759-8.
Casalla R, Scheffrahn R, Korb J. Cryptotermes colombianus a new drywood termite and distribution record of Cryptotermes in Colombia. ZooKeys. 2016:39–52. https://doi.org/10.3897/zookeys.596.9080.
Buijang NS, Harrison NA, Su NY. A phylogenetic study of endo-beta-1, 4-glucanase in higher termites. Insect Soc. 2014:29–40. https://doi.org/10.1007/s00040-013-0321-7.
Dejernaes M, Klass KD, Picker MD, Damgaard J. Phylogeny of cockroaches (Insecta, Dictyoptera, Blattodea), with placement of aberrant taxa and exploration of out-group sampling. Syst Entomol. 2012:65–83. https://doi.org/10.1111/j.1365-3113.2011.00598.x.
Legendre F, Whiting MF, Bordereau C, Cancello EM, Evans TA, Grandcolas P. The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: implications for the evolution of the worker and pseudergate castes, and foraging behaviors. Mol Phylogenet Evol. 2008:615–27. https://doi.org/10.1016/j.ympev.2008.04.017.
Reid NM, Addison SL, West MA, Lloyd-Jones G. The bacterial microbiota of Stolotermes ruficeps (Stolotermitidae), a phylogenetically basal termite endemic to New Zealand. FEMS Microbiol Ecol. 2014:678–88. https://doi.org/10.1111/1574-6941.12424.
Kambhampati S. A phylogeny of cockroaches and related insects based on DNA sequence of mitochondrial ribosomal RNA genes. Proc Natl Acad Sci. 1995:2017–20. https://doi.org/10.1073/pnas.92.6.2017.
Liu C. Molecular phylogeny of an enigmatic Heterochaeta Wood-Mason, 1889 (Mantodea: Toxoderidae: Oxyothespinae), with description of one new species from China. unpubl. data 2015; GenBank.
Atkinson L, Adams ES, Crozier RH. Microsatellite markers for the polygamous termite Nasutitermes corniger (Isoptera: Termitidae). Mol Ecol Resour. 2007:299–301. https://doi.org/10.1111/j.1471-8286.2006.01586.x.
Miura T, Roisin Y, Matsumoto T. Molecular phylogeny and biogeography of the nasute termite genus Nasutitermes (Isoptera: Termitidae) in the Pacific tropics. Mol Phylogenet Evol. 2000:1–10. https://doi.org/10.1006/mpev.2000.0790.
Akoth P. Genetic characterization and distribution of termites in Taita Taveta County, Kenya. Ph.D. dissertation, College of Pure Science, Jomo Kenyatta University of Agriculture and Technology. Available from: http://ir.jkuat.ac.ke:8080/handle/123456789/3291. Accessed 2017.
Svenson GJ, Whiting MF. Reconstructing the origins of praying mantises (Dictyoptera, Mantodea): the roles of Gondwanan vicariance and morphological convergence. Cladistics. 2009;25:468–514. https://doi.org/10.1111/j.1096-0031.2009.00263.
Pellens R, D'Haese CA, Bellés X, Piulachs MD, Legendre F, Wheeler WC, et al. The evolutionary transition from subsocial to eusocial behaviour in Dictyoptera: phylogenetic evidence for modification of the “shift-in-dependent-care” hypothesis with a new subsocial cockroach. Mol Phylogenet Evol. 2007;43:616–26. https://doi.org/10.1016/j.ympev.2006.12.017.
Inward D, Beccaloni G, Eggleton P. Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol Lett. 2007:331–5. https://doi.org/10.1098/rsbl.2007.0102.
Fukuda H, Koshikawa S, Kusaka Y, Toda M, Katoh T. Molecular phylogeny of the immigrans-tripuncata lineage (Diptera: Drosophilidae). unpubl. data 2014; GenBank.
Hollocher H, Hatcher JL, Dyreson EG. Evolution of abdominal pigmentation differences across species in the Drosophila dunni subgroup. Evolution. 2000:2046–56. https://doi.org/10.1111/j.0014-3820.2000.tb01248.x.
Colón-Parilla WV, Pérez-Chiesa I. Partial characterization and evolution of Adh-Adhr in Drosophila dunni. Biochem Genet. 2007:225–38. https://doi.org/10.1007/s10528-006-9067-5.
Robe LJ, Loreto EL, Valente VL. Radiation of the, Drosophila subgenus (Drosophilidae, Diptera) in the Neotropics. J Zool Syst Evol Res. 2010:310–21. https://doi.org/10.1111/j.1439-0469.2009.00563.x.
O'Grady P, DeSalle R. Out of Hawaii: the origin and biogeography of the genus Scaptomyza (Diptera: Drosophilidae). Biol Lett. 2008:195–9. https://doi.org/10.1098/rsbl.2007.0575.
Hatadani LM, McInerney JO, Fonseca de Medeiros H, ACM J, de Azeredo-Espin AM, Klaczko LB. Molecular phylogeny of the Drosophila tripunctata and closely related species groups (Diptera: Drosophilidae). Mol Phylogenet Evol. 2009:595–600. https://doi.org/10.1016/j.ympev.2009.02.022.
Chaumot A, Da Lage JL, Maestro O, Martin D, Iwema T, Brunet F, et al. Molecular adaptation and resilience of the insect’s nuclear receptor USP. BMC Evol Biol. 2012;12:199. https://doi.org/10.1186/1471-2148-12-199.
Dyer KA, Jaenike J. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila. Genetics. 2004:1443–55. https://doi.org/10.1534/genetics.104.027854.
Remsen J, O'Grady P. Phylogeny of Drosophilinae (Diptera: Drosophilidae), with comments on combined analysis and character support. Mol Phylogenet Evol. 2002:249–64. https://doi.org/10.1016/S1055-7903(02)00226-9.
Katoh TK, Komoda M, Watada M. A survey of the drosophilid fauna and genetic diversity of two dominant species of Drosophila in Ehime prefecture, Japan. Biogeogr. 2016;18:35–46.
Albalat R, Gonzàlez-Duarte R. Adh and Adh-dup sequences of Drosophila lebanonensis and D. immigrans: interspecies comparisons. Gene. 1993;126:171–8.
Wolff JN, Camus MF, Clancy DJ, Dowling DK. Complete mitochondrial genome sequences of thirteen globally sourced strains of fruit fly (Drosophila melanogaster) form a powerful model for mitochondrial research. Mitochondr DNA Part A. 2016:4672–2674. https://doi.org/10.3109/19401736.2015.1106496.
Kellerman V, van Heerwaarden B, Sgrò CM, Hoffmann AA. Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science. 2009:1244–6. https://doi.org/10.1126/science.1175443.
Li F-B. The complete mitochondrial genome of Drosophila melanogaster unpubl. data 2014; GenBank.
Yeh S-D, von Grotthuss M, Gandasetiawan KA, Jayasekera S, Xia XQ, Chan C, et al. Functional divergence of the miRNA transcriptome at the onset of Drosophila metamorphosis. Mol Biol Evol. 2014:2557–72. https://doi.org/10.1093/molbev/msu195.
Gramates LS, Marygold SJ, dos Santos G, Urbano J-M, Antonazzo G, Matthews BB, et al. FlyBase at 25: looking to the future. Nucleic Acids Res. 2017:D663–71. https://doi.org/10.1093/nar/gkw1016 http://flybase.org/cgi-bin/getseq.html?source=dmel&id=FBgn0000075&chr=2L&dump=PrecompiledFasta&targetset=CDS.
Yotoko KS, Medeiros HF, Solferini VN, Klaczko LB. A molecular study of the systematics of the Drosophila tripunctata group and the tripunctata radiation. Mol Phylogenet Evol. 2003:614–9. https://doi.org/10.1016/S1055-7903(03)00218-5.
Jaenike J, Dyer KA, Cornish C, Minhas MS. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biol. 2006:e325. https://doi.org/10.1371/journal.pbio.0040325.
Satta Y, Takahata N. Evolution of Drosophila mitochondrial DNA and the history of the melanogaster subgroup. Proc Natl Acad Sci. 1990:9558–62. https://doi.org/10.1073/pnas.87.24.9558.
Meiklejohn CD, Holmbeck MA, Siddiq MA, Abt DN, Rand DM, Montooth KL. An incompatibility between a mitochondrial tRNA and its nuclear-encoded tRNA synthetase compromises development and fitness in Drosophila. PLoS Genet. 2013;9:e1003238. https://doi.org/10.1371/journal.pgen.1003238.
Junqueira ACM, Azeredo-Espin AML, Paulo DF, Marinho MAT, Tomsho LP, Drautz-Moses DI, et al. Large-scale mitogenomics enables insights into Schizophora (Diptera) radiation and population diversity. Sci Rep-UK. 2016;6:21762. https://doi.org/10.1371/journal.pgen.1003238.
Clary DO, Wahleithner JA, Wolstenholme DR. Transfer RNA genes in Drosophila mitochondrial DNA: related 5’flanking sequences and comparisons to mammalian mitochondrial tRNA genes. Nucleic Acids Res. 1983;11:2411–25.
Llopart A, Herrig D, Brud E, Stecklein Z. Sequential adaptive introgression of the mitochondrial genome in Drosophila yakuba and Drosophila santomea. Mol Ecol. 2014:1124–36. https://doi.org/10.1111/mec.12678.
Cheng R-C, Kuntner M. Phylogeny suggests nondirectional and isometric evolution of sexual size dimorphism in argiopine spiders. Evolution. 2014:2861–72. https://doi.org/10.1111/evo.12504.
Agnarsson I, Blackledge TA. Can a spider web be too sticky? Tensile mechanics constrains the evolution of capture spiral stickiness in orb-weaving spiders. J Zool. 2009:134–40. https://doi.org/10.1111/j.1469-7998.2009.00558.x.
Álvarez-Padilla F, Dimitrov D, Giribet G, Hormiga G. Phylogenetic relationships of the spider family Tetragnathidae (Araneae, Araneoidea) based on morphological and DNA sequence data. Cladistics. 2009:109–46. https://doi.org/10.1111/j.1096-0031.2008.00242.x.
Blackledge TA, Scharff N, Coddington JA, Szuts T, Wenzel JW, Hayashi CY, Agnarsson I. Reconstructing web evolution and spider diversification in the molecular era. Proc Natl Acad Sci. 2009:106;5229–34.
Coddington JA, Agnarsson I, Cheng R-C, Čandek K, Driskell A, Frick H, et al. DNA barcode data accurately identify higher taxa. PeerJ. 2016. https://doi.org/10.7717/peerj.2201.
Arnedo MA, Coddington J, Agnarsson I, Gillespie RG. From a comb to a tree: phylogenetic relationships of the comb-footed spiders (Araneae, Theridiidae) inferred from nuclear and mitochondrial genes. Mol Phylogenet Evol. 2004:225–45. https://doi.org/10.1016/S1055-7903(03)00261-6.
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Amer. 1994:651–701. https://doi.org/10.1093/aesa/87.6.651.
Colgan DJ, McLauchlan A, Wilson GDF, Livingston SP, Edgecombe GD, Macaranas J, et al. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust J Zool. 1998:419–37. https://doi.org/10.1071/ZO98048.
Acknowledgements
We would like to acknowledge Peter Roopnarine for helpful suggestions that improved the manuscript. We thank Matt Van Dam for answering an endless stream of questions about BioGeoBEARS analyses, and Delano Lewis for providing us with BEAST input files. We are grateful to Bob Kallal for his advice regarding araneid outgroups and BEAST calibration points. We acknowledge Corrie Moreau, Barbara Thorne, Rudolf Scheffrahn, and Michele Esposito for answering questions about specimen localities and distributions. We thank Boni Cruz and Anna Sellas for help with lab work and acknowledge the CarBio project and National Museum of Natural History for providing selenopid extractions. We are grateful to Paul Mann, Bruce Fouke, and René Maury for answering lots of questions about geology. Finally, we are grateful to Judy Gallagher, Mark Yokoyama, Eduardo Lopez, Bernard Dupont and Rudolf Scheffrahn for providing images and allowing us to alter them for some of our figures.
Funding
NA
Author information
Authors and Affiliations
Contributions
SCC and LAE contributed to the conception and design of the study; SCC acquired the data and conducted analyses; SCC and LAE contributed to interpreting the data and drafting and revising the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
NA
Consent for publication
NA
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Geologic dates used in BioGeoBEARS analyses. Table S2. Partitions and models used for each dataset. Table S3. Summary of untested models for datasets from older taxa. Table S4. Summary of untested models for datasets from younger taxa. Figure S1. Ancestral range estimation for Platythyrea ants. Table S5. Sequences for Platythyrea analyses. Table S6. Bayes Factors from Platythyrea analysis. Figure S2. Phylogeny and ancestral range estimation for Heterotermes termites. Table S7. Sequences for Heterotermes analyses. Figure S3. Phylogeny and ancestral range estimation for Nasutitermes termites. Table S8. Sequences for Nasutitermes analyses. Figure S4. Phylogeny and ancestral range estimation for Calisto butterflies (Euptychia as the outgroup). Figure S5. Ancestral range estimation for Calisto butterflies (Euptychia is not the sister taxon). Figure S6. Map of the geographical areas used in the Calisto BioGeoBEARS analyses. Table S9. Calisto butterfly sample information. Figure S7. Ancestral range estimation for Papilio butterflies. Table S10. Papilio butterfly sample information Figure S8. Ancestral range estimation for Drosophila flies. Table S11. Sequences for Drosophila analyses. Figure S9. Ancestral range estimation for centruroidine scorpions. Table S12. Sequences for centruroidine scorpion analyses. Figure S10. Phylogeny and ancestral range estimation for Micrathena spiders. Table S13. Sequences for Micrathena analyses. Figure S11. Ancestral range estimation for Spintharus spiders. Table S14. Sequences for Spintharus analyses. Figure S12. Ancestral range estimation for Selenops spiders using more recent dated phylogenies. Figure S13. Ancestral range estimation for Selenops spiders using more recent dated phylogenies. Figure S14. Selenopidae tree from the Bayesian analysis using no redundant haplotypes. Figure S15. Selenopidae tree from the RAxML analysis using no redundant haplotypes. Table S15. Sequences for Selenops analyses. Table S16. Primers and PCR protocols used for amplification of DNA from Selenops species [111,112,113,114,115,116,117,118,119,120,121,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,213].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Crews, S.C., Esposito, L.A. Towards a synthesis of the Caribbean biogeography of terrestrial arthropods. BMC Evol Biol 20, 12 (2020). https://doi.org/10.1186/s12862-019-1576-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-019-1576-z