Abstract
Background
Genes of the Major Histocompatibility Complex (MHC) are essential for adaptive immune response in vertebrates, as they encode receptors that recognize peptides derived from the processing of intracellular (MHC class I) and extracellular (MHC class II) pathogens. High MHC diversity in natural populations is primarily generated and maintained by pathogen-mediated diversifying and balancing selection. It is, however, debated whether selection at the MHC can counterbalance the effects of drift in bottlenecked populations. The aim of this study was to assess allelic diversity of MHC genes in a recently bottlenecked bird of prey, the White-tailed Eagle Haliaeetus albicilla, as well as to compare mechanisms that shaped the evolution of MHC class I and class II in this species.
Results
We showed that significant levels of MHC diversity were retained in the core Central European (Polish) population of White-tailed Eagles. Ten MHC class I and 17 MHC class II alleles were recovered in total and individual birds showed high average MHC diversity (3.80 and 6.48 MHC class I and class II alleles per individual, respectively). Distribution of alleles within individuals provided evidence for the presence of at least three class I and five class II loci the White-tailed Eagle, which suggests recent duplication events. MHC class II showed greater sequence polymorphism than MHC class I and there was much stronger signature of diversifying selection acting on MHC class II than class I. Phylogenetic analysis provided evidence for trans-species similarity of class II, but not class I, sequences, which is likely consistent with stronger balancing selection at MHC class II.
Conclusions
Relatively high MHC diversity retained in the White-tailed Eagles from northern Poland reinforces high conservation value of local eagle populations. At the same time, our study is the first to demonstrate contrasting patterns of allelic diversity and selection at MHC class I and class II in an accipitrid species, supporting the hypothesis that different mechanisms can shape evolutionary trajectories of MHC class I and class II genes.
Similar content being viewed by others
Background
The Major Histocompatibility Complex (MHC) multigene family constitutes an essential part of vertebrate immune system, as MHC receptors bind and recognize peptides derived from pathogen processing [1]. Each MHC molecule has a peptide-binding groove which binds to a limited range of foreign peptides and, thus, the number of different MHC molecules expressed within an organism determines the spectrum of pathogens that can be recognized and killed via adaptive immune defences. Within-individual diversity of MHC alleles is primarily limited by the number of duplicated MHC loci [2]. In birds, the number of MHC loci is relatively low in ancestral lineages, but extensive MHC duplication seems to have occurred in more recently evolved taxa [3]. A single dominantly expressed MHC gene at both class I and class II has been recorded in some old non-passerine lineages such as galliforms or penguins (so called ‘minimal essential‘ MHC [4]), whereas tens of putatively transcribed loci have been described for some passerine species [5,6,7]. The apparent fitness advantage of MHC heterozygosity at the individual level is often reflected by an astonishing allelic diversity maintained in natural populations of vertebrates. For example, over 3500 MHC class I alleles were found within a Central European population of sedge warblers Acrocephalus schoenobaenus [7] and nearly a thousand MHC class II alleles were recorded in the common yellowthroat Geothlypis trichas [5]. This extreme MHC polymorphism is thought to be generated and maintained via pathogen-mediated diversifying and balancing selection [8], acting through the mechanisms of overdominance [9], negative frequency-dependence [10], and fluctuating selection [11]. Strong balancing selection can maintain alleles for long evolutionary periods, often beyond neutral expectations. Persistence of adaptive MHC alleles beyond species diversification due to balancing selection produces a pattern where some alleles are more similar between species than within species, which is referred to as trans-species polymorphism [12].
There are two major types of transmembrane MHC receptors: class I and class II. Class I receptors are expressed on nearly all nucleated vertebrate cells and are responsible for the presentation of antigens from within a cell to cytotoxic T-cells [1]. In contrast, class II receptors are expressed on specific antigen-presenting cells, such as dendrintic cells, monocytes or B cells, which phagocytose extracellular antigens and present them to T helper cells [1]. Since MHC class I and class II initiate adaptive immune response against intra- and extracellular pathogens, respectively (but see [13] for antigen cross-presentation mechanisms), there might be differences in the strength of pathogen-mediated selection acting on these genes, resulting in different evolutionary trajectories and contrasting patterns of polymorphism between class I and class II genes. In birds, the relative exposure rate to intra- and extracellular pathogens can vary with morphology, habitat, general ecology, and life-history strategies [14], and the same set of traits is expected to govern selection patterns at the MHC. For example, it has been shown that migratory and colonial avian species show stronger signature of diversifying selection acting on MHC class II genes, likely because of elevated transmission rates of pathogens in socially breeding species and exposure to more diverse fauna of pathogens during migration [15]. Selection patterns at the MHC can also differ between phylogenetic lineages or taxa that show strong ecological divergence. A recent analysis of selection across the avian tree of life provided evidence for striking differences in the strength of diversifying selection acting on MHC class I and class II in passerine and non-passerine birds [16]. Specifically, non-passerines showed stronger selection at MHC class II (presentation of extracellular antigens), while passerines had stronger signature of selection at MHC class I (presentation of intracellular antigens) [16]. It was concluded that non-passerine birds are primarily selected to recognize a broader spectrum of extracellular versus intracellular pathogens, which could be attributed to their larger body sizes (body size is thought to determine extracellular pathogen richness [17]) and stronger preferences for freshwater aquatic habitats (aquatic environments are thought to accommodate more diverse faunas of extracellular, but not necessarily intracellular pathogens [18, 19]). In spite of these general findings, most molecular research on MHC in non-model avian taxa focus on a single MHC class, while signatures of pathogen-mediated selection have rarely been compared between MHC class I and class II within a single population or species [20,21,22].
A robust methodological pipeline to genotype MHC class I and class II in diurnal birds of prey from three distinct orders of Accipitriformes, Cathartiformes and Falconiformes has been developed as a part of pioneer research by Alcaide et al. [23, 24]. Since then, an extensive scientific effort has been devoted to examine the patterns of MHC polymorphism and its consequences in natural populations of several falcon species [25,26,27,28]. Thus, it may seem surprising that only few accipitrids had their MHC characterized in detail up to date [29,30,31], especially considering that the order of Accipitriformes includes most of diurnal raptors, such as hawks, eagles, and Old World vultures. Here, we used Next-Generation Sequencing methods (Illumina MiSeq) to genotype MHC class I and class II in a recently bottlenecked accipitrid species, the White-tailed Eagle Haliaeetus albicilla.
The genus of Haliaeetus contains eight species of large diurnal raptors, often referred to as sea eagles, that have wide distribution around the globe [32]. They are widely considered as flagship species for conservation and often serve as umbrella species to protect animal communities or entire ecosystems. White-tailed Eagle has a large breeding range spanning from Central and Northern Europe (including Iceland and SW costs of Greenland) through the Middle East and Central Siberia to the Pacific coast of Asia [32]. A rough estimate of the global population size is 25–50 thousand mature individuals, including 9–12 thousand pairs breeding in Europe [33]. Nowadays, Norway and Russia are the major strongholds of the species in Europe, as they hold over half of the European population [33]. The White-tailed Eagle was formerly a common species across Europe, but in nineteenth century the population started to decline following rapid landscape changes (intensification of agriculture) and direct persecution. The species went extinct in the Western Europe (including Great Britain) by the early 1900s. High environmental pollution resulting in low reproductive success led to further extinctions of many local Central European populations (e.g. in Austria, Czech Republic, and Slovakia) in the middle of twentieth century. By the 1970s, central and northern European population reached the most severe demographic bottleneck, reaching approximately one thousand of pairs, 700–800 of which bred at the Atlantic coast of Norway [34]. With the prohibition of chemical pesticides (DDT and PCBs) in 1970s and strict legal protection of the species, population started to recover and many previously abandoned areas become recolonized, occasionally helped with reintroduction programs [35]. In Poland, the population size of White-tailed Eagles was estimated at 80–90 breeding pairs during 1970s and it has recently increased to 1100–1200 pairs, as estimated in 2017 (Eagle Conservation Committee, unpublished data). Despite this severe population bottleneck, the species seems to have retained relatively high genetic variation, as assessed with neutral genetic markers [34, 36]. However, as far as we aware, polymorphism of innate or adaptive immune genes has never been examined in this species. The main goals of this study were to: i) assess diversity of MHC genes within a recovered Central European population of the White-tailed Eagle; ii) examine historical patterns of diversifying selection acting on these genes; and iii) compare evolutionary mechanisms responsible for maintaining polymorphism at MHC class I and class II in this species.
Methods
Sample collection
Samples for this study were collected in 2017 from the core population of White-tailed Eagles in the northern Poland. In total, 67 White-tailed Eagle nestlings from 44 broods (1.52 ± 0.08 [SE] nestling per brood) were sampled. Between one and three growing (in shafts) feathers lost or broken during standard ringing procedures were collected from each bird into 96% ethanol. All samples were collected between 13 May and 03 June. All experiments complied with the current laws of Poland (Act on Nature Conservation from 16 April 2004, Journal of Laws from 2004, No. 92, item 880) and were approved by the Local Bioethical Commission for Animal Welfare in Łódź and the General Environmental Protection Directorate in Poland.
Amplification and Illumina sequencing
Nuclear DNA was extracted from feathers using Bio-Trace DNA Purification Kit (EURx, Gdańsk, Poland). For this purpose, the tip of feather shaft filled with blood was cut with a sterile blade and DNA isolation followed the manufacturer’s protocol. The methods yielded an average DNA concentration of 189.0 ± 9.5 [SE] ng/μl. To genotype MHC in the White-tailed Eagle we used degenerate primers previously developed for other accipitrid species: MHCI-int2F and MHCI-ex4R for class I [24] and Acc2FC and Acc2RC [23] for class II. The first pair of primers amplifies MHC class I exon 3 by binding to the flanking region of intron 2 and conserved region of exon 4, while the second pair of primers amplifies MHC class II exon 2 by binding to the flanking regions of introns 1 and 3. Previously, both pairs of primers were successfully tested for MHC cross-amplification in a large spectrum of accipitrid species from different genera [23, 24]. We genotyped MHC class I exon 3 and MHC class II exon 2 because they form a peptide-binding groove of MHC molecules and most MHC research in non-model avian species has focused exclusively on these regions [16]. For example, polymorphism of class I exon 2 was examined only for a handful of species, mostly domestic ones [e.g. 37, 38], and consequently almost no information on the allelic diversity and selection signature at this exonic region exist for wild birds, including accipitrids.
PCR amplifications were conducted in a final volume of 20 μl containing 10 μl of 2X HotStarTaq Plus Master Mix Kit (Qiagen, Venlo, Netherlands), 50–150 ng of genomic DNA and 0.2 μM of each primer. Amplifications of MHC followed the steps of: (i) initial denaturation at 94 °C for 5 min; (ii) 25 (class I) or 30 (class II) cycles of denaturation at 94 °C for 40 s followed by 40 s at 56 °C annealing temperature and elongation at 72 °C for 40 s; (iii) final elongation at 72 °C for 10 min. Relatively low number of amplification cycles (25–30) was used to minimize the risk of chimera formation. All PCR products were evaluated by electrophoresis on 2% agarose gel to detect positive amplifications. Amplifications were completed using fusion primers containing Illumina adapter sequences, a 7-bp barcode that indicated sample identity, and a pair of either the MHC class I or II primers. Fourteen samples were amplified in two independent PCRs to obtain technical replicates. All PCR products were purified and their concentrations were determined with Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). Equimolar quantities of PCR products were pooled to form a library using NEBNext DNA Library Prep Master Mix Set for Illumina (New England Biolabs, Ipswich, MA, USA). The library was sequenced using Illumina v2 Kit at a 2 × 250 bp paired-end Illumina MiSeq platform.
Processing of Illumina data and MHC allele validation
Processing of raw Illumina MiSeq data was conducted using Amplicon Sequencing Analysis Tools (AmlpiSAT) web server developed by Sebastian et al. [39] and followed the algorithm recommended by Biedrzycka et al. [7]. First, pair-ended reads were merged with AmpliMERGE tool that is based on FLASH algorithm with optimum overlapping parameters [40]. Then, AmpliCHECK tool with default settings (minimum per amplicon frequency of 1%) was used for the preliminary exploration of the data set. There was a clear predominance (88.8%, n = 711) of 412-bp sequences at the MHC class I. Twelve sequences of similar lengths (± 2 bp) were retrieved, but they showed minor frequencies (1.11–2.04%) and each variant was recorded in a single individual. Thus, we considered these sequences as originating from genotyping errors. Also, 67 short-length (67–298 bp) sequences were considered as genotyping artefacts. Only 269-bp sequences were retrieved at the MHC class II. Based on AmpliCHECK results, the exact read length of 412 bp and 269 bp for MHC class I and class II, respectively, was set in further data processing. AmpliSAS tool was used for the final de-multiplexing, clustering, and filtering of Illumina reads. Default parameters for Illumina data were used for clustering (1% substitution errors, 0.001% indel errors, and 25% minimum dominant frequency). In the filtering step, we discarded chimeras and sequences that had less than 3% frequency, as well as those that were recorded in a single sample. Minimum amplicon depth was set to 100 reads, while maximum amplicon depth was set to 5000 reads because of AmpliSAS performance reasons. After processing, the average number of reads per sample was 2402 ± 164 [SE]. Reproducibility of alleles between technical replicates (amplicon depth > 1000) was 90.1%, which is consistent with an expected repeatability of MHC allele calling for the AmpliSAS software [41]. MHC class I and class II alleles were aligned separately in Geneious v10.0.5 (Biomatters Ltd., Auckland, New Zealand). Intron regions were removed from the alignments, retaining a 273-bp fragment of MHC class I exon 3 (total exon length: 276 bp) and 258-bp fragment of MHC class II exon 2 (total exon length: 270 bp).
Sequence polymorphism and selection
Sequence polymorphism was assessed as the number of polymorphic sites, total number of mutations, average nucleotide diversity, and average number of nucleotide differences using DnaSP v.6.10.3 [42]. To infer selection acting on MHC class I exon 3 and MHC class II exon 2 we inferred relative rates of nonsynonymous (amino acid altering) and synonymous (silent) nucleotide substitutions. Under positive (diversifying) selection, nonsynonymous substitutions are expected to accumulate faster than synonymous substitutions (new allelic variants are promoted), while the opposite pattern occurs under negative (purifying) selection, which purges most allelic variants that arise through point mutations. Thus, the relative rate of nonsynonymous substitutions per nonsynonymous site (dN) to synonymous substitutions per synonymous site (dS) is commonly used a measure of selection acting on the sequence over evolutionary time, where dN/dS < 1 indicates negative selection, dN/dS > 1 indicates positive selection, and dN/dS ≈ 1 indicates neutral evolution [43]. We assessed codon-specific signatures of positive (pervasive diversifying) and negative (pervasive purifying) selection using two different (Bayesian and maximum-likelihood) approaches implemented in HyPhy software [44], which was run through the Datamonkey Adaptive Evolution Server [45, 46]. First, we used Fast Unconstrained Bayesian AppRoximation (FUBAR) method, which uses a Markov Chain Monte Carlo (MCMC) routine that ensures robustness against model misspecifications and leaves the distribution of selection parameters essentially unconstrained [47]. Second, we used Fixed Effects Likelihood (FEL) method, which shows intermediate conservatisms in term of type I error when compared with other maximum-likelihood approaches implemented in Hyphy [48]. We also used Mixed Effect Model of Evolution (MEME) [49] to identify sites under episodic diversifying selection, i.e. evolving under positive selection at a proportion of branches. All analyses were run with default settings and input trees built from the sequence alignments. Amino acid residues with > 0.95 Bayesian posterior probabilities (FUBAR) or p < 0.05 (FEL and MEME) were considered to have enough support for positive or negative selection. We also used Genetic Algorithm for Recombination Detection (GARD) implemented in HyPhy to search for evidence of recombination breakpoints and identify putative recombinant fragments (partitions) in MHC class I and class II sequences [50]. Significance threshold of p = 0.1 was used to identify recombination breakpoints. Since no unique tree topology can describe the evolutionary history of recombinant sequences, selection inference on recombinant data may produce false positives [51]. Thus, we re-run all codon-specific selection models using a unique phylogenetic history for each detected recombination block, as identified with GARD. The dN/dS ratio was measured for three different subsets of amino acid residues: i) all residues, (ii) human peptide-binding residues (PBRs), as identified based on the crystallography models of human MHC molecules [52, 53], and iii) putative PBRs of non-passerine birds, as identified based on the global analysis of selection at the avian MHC [16] (Fig. 1).
Alignments of amino acid sequences of MHC class I exon 3 and MHC class II exon 2 in the White-tailed Eagle. Dots in the alignments indicate the same amino acid as the top consensus sequence. Amino acid residues under positive selection are marked with red, under negative selection are marked with blue. Positively and negatively selected residues inferred for non-recombinant fragments (sequence partitions identified with GARD analysis) are marked with stars. Large dots (●) above the alignment indicate peptide-binding residues (PBRs) of humans (according to Saper et al. [46] for class I and Brown et al. [47] for class II) and putative PBRs of non-passerine birds (according to Minias et al. [14]). Spatial variation in the selection parameter (dN – dS) is shown at the top of each alignment
Phylogenetic clustering
To assess phylogenetic relationships between MHC sequences of the White-tailed Eagle and other accipitrid species, we used BLAST search (as implemented in Geneious v10.0.5) for the two query White-tailed Eagle alleles that showed lowest pairwise identity within each MHC class. In each search we retrieved eight sequences most similar to the query sequence (no more than two sequences per species). Phylogenetic relationships were inferred using approximately-maximum-likelihood approach, as implemented in FastTree v2.1.5 [54]. FastTree software constructs an initial tree with neighbour-joining and then refines its topology with subtree-pruning-regrafting (SPRs), minimum-evolution nearest-neighbour interchanges (NNIs), and maximum-likelihood NNIs. Tree topologies produced with this approach show similar accuracy to topologies produced with standard maximum likelihood methods [55]. General time-reversible (GTR) model of nucleotide substitution with a discrete Gamma distribution was used to account for different rates of evolution at different sites and for uncertainty in these rates [56]. Local support values were computed based on the Shimodaira-Hasegawa test [57]. Sequences from the domestic chicken Gallus gallus (GenBank accession nos.: AM419160 for class I, AM489767 for class II) were used as outgroups.
Results
We found that White-tailed Eagle nestlings had lower allelic diversity at MHC class I than class II. After clustering and filtering of Illumina reads, 10 MHC class I and 17 MHC class II alleles were retained (Fig. 1). These alleles corresponded to 9 and 15 unique amino acid sequences, respectively, all of which were putatively functional. One MHC class I allele was found in > 90% of individuals, whereas three MHC class II alleles were found in all genotyped birds (Fig. 2). The mean number of alleles per individual was 3.80 ± 0.20 and 6.48 ± 0.20 for MHC class I and class II, respectively. A maximum number of MHC class I and class II alleles per individual was six (n = 7 individuals) and nine (n = 11 individuals), respectively, which indicated for the presence of at least three class I and five class II loci in the White-tailed Eagle. MHC class II sequences had higher proportion of polymorphic sites (50 polymorphic sites per 258 bp) and higher total number of mutations (η = 60) than MHC class I sequences (27 polymorphic sites per 273 bp and η = 29 mutations). The average nucleotide diversity was 0.043 ± 0.010 [SD] for MHC class I and 0.087 ± 0.007 [SD] for MHC class II. Consistently, the average number of nucleotide differences was 11.9 and 22.4 for MHC class I and class II, respectively.
We found evidence for much stronger positive (diversifying) selection acting on MHC class II than class I in the White-tailed Eagle. Bayesian methods (FUBAR) identified seven sites under positive selection within MHC class II exon 2, while only two sites were recognized as under positive selection within MHC class I exon 3 (Fig. 1). Similarly, maximum-likelihood approach (FEL) recognized four sites under positive selection at MHC class II, whereas none of the sites were recognized as positively selected at MHC class I. Finally, we found evidence for episodic diversifying selection (as assessed with MEME) acting on five MHC class II sites and only one MHC class I site. All three measures of dN/dS ratio (calculated for the entire sequence or exclusively for codons identified as putative PBRs in birds or humans) were higher at MHC class II than class I (Table 1). The greatest difference was observed at the putative avian PBRs (Fig. 1), where strong diversifying selection was observed for MHC class II (dN/dS = 3.64), while signature of diversifying selection at MHC class I was very weak (dN/dS = 1.11). GARD analysis identified a single recombination breakpoint in both class I (at 140 bp) and class II (at 123 bp) alignments (Fig. 3). Re-running codon-specific selection models for non-recombinant fragments of sequences did not qualitatively change the results and provided further support for stronger positive (diversifying) selection at MHC class II than class I, as assessed with the number of positively selected sites and dN/dS ratios (Table 1).
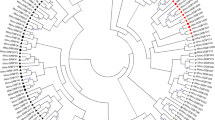
Both MHC class I and class II sequences of the White-tailed Eagle showed high pairwise identity with sequences of a congeneric species, the Bald Eagle (90.8–100% pairwise identity for MHC class I; 85.3–99.6% pairwise identity for MHC class II). All MHC class I sequences of both Haliaeetus species clustered closely in a phylogenetic analysis and showed little evidence of trans-species polymorphism with sequences of accipitrids from other genera (Fig. 4). In contrast, MHC class II sequences of Haliaeetus formed three distinct clusters that were separated with sequences of other accipitrids, producing a pattern of trans-specific similarity (Fig. 4).
Consensus maximum likelihood topology for MHC class I exon 3 (a) and MHC class II exon 2 (b) in the White-tailed Eagle and other Accipitridae species. Local bootstrap support is provided at each node. Scale bar indicates genetic distance in units of nucleotide substitutions per site. Domestic chicken was used as an outgroup
Discussion
In this study we used Illumina MiSeq sequencing to genotype MHC class I and class II in White-tailed Eagle nestlings from the core Central European (Polish) population. Despite recent demographic bottleneck, we found moderate level of MHC diversity in our sample and found evidence for the presence of at least three class I and five class II loci in the White-tailed Eagle, as estimated with the maximum number of MHC alleles recorded per individual. MHC class II showed higher allelic diversity than MHC class I and there was much stronger signature of diversifying selection acting on MHC class II than class I. Finally, phylogenetic analysis of MHC sequences in White-tailed Eagle and other accipitrid species provided support for trans-specific similarity of class II, but not class I sequences.
Despite a severe demographic decline in the second half of twentieth century, European populations of the White-tailed Eagle have apparently retained significant level of genetic diversity. Analysis of neutral (both nuclear and mitochondrial) genetic markers in the extant Central European populations revealed no signs of recent genetic bottleneck and indicated that sustained genetic diversity had a predominantly local origin with little contribution of effective dispersal [34,35,36]. It has been argued that long generation time of the White-tailed Eagle (average and maximum lifespan in the wild was estimated at 17 and 26 years, respectively [32]) might have minimized genetic drift and acted as an intrinsic buffer against rapid loss of genetic diversity, especially in the case of relatively short bottleneck episodes [34]. This scenario was consistent with theoretical simulations showing that < 5% of the original heterozygosity is expected to be lost during a 30-year bottleneck which reduced the effective population size of White-tailed Eagles from 300 to 30 reproducing pairs [34]. Our results on MHC allelic diversity seem to support these conclusions, as we found moderately high variation at both MHC class I and class II in White-tailed Eagles from northern Poland. Although the total number of MHC amino acid variants recorded in our study population was moderate (9 MHC class I and 15 MHC class II variants recorded in 67 nestling from 44 families), individuals showed high average MHC diversity (3.80 and 6.48 MHC class I and class II alleles per individual, respectively). The level of MHC polymorphism in the White-tailed Eagle, as revealed in our study, was comparable to other accipitrid species that have broad distribution ranges and large population sizes. For example, 20 MHC class II alleles were isolated from Swainson’s Hawks Buteo swainsoni (n = 20) wintering in Argentina [31], while 13 MHC class II alleles were detected in eight Black Kites Milvus migrans from Spain [29]. Seventeen MHC class II alleles were identified within continental (Iberian) population of the Egyptian Vulture Neophron percnopterus (n = 96 individuals), while ten and nine alleles were found in the islandic populations from Canaries (n = 236 individuals) and Baleares (n = 36 individuals), respectively [30]. Only three MHC class II alleles were recovered from 32 individuals of an island endemic species, the Galápagos Hawk Buteo galapagoensis [31], indicating that viable accipitrid populations can persist with extremely low MHC variation. We are aware of no previous research on MHC class I diversity in accipitrid birds of prey.
Our study provided support for the presence of at least three class I and five class II loci in the White-tailed Eagle, which is unusual when compared with other birds of prey. In general, non-passerine birds have low number of MHC loci, which is thought to be an ancestral state in avian evolution [3]. Two MHC class II loci were recorded in most accipitrid species studied in detail up to date [29,30,31], while the presence of three loci was suggested for the Red Kite Milvus milvus [58]. Four MHC class II loci were found in non-passerines from several other taxonomic orders, e.g. in Anseriformes, Pelecaniformes, or Procellariiformes [58,59,60]. So far, Blakiston’s Eagle-owl Bubo blakistoni yielded the highest estimate of MHC class II loci among non-passerines, as up to 16 alleles were detected per individual, indicating for the presence of eight class II loci in this species [61]. Surprisingly, much fewer (1–3) MHC class II loci have been reported for other owl species, although these estimates were based on considerably smaller sample sizes [23, 62]. Similar pattern has been found in Galliformes, as most phasianid species tend to have 2–3 MHC class II loci [22, 63,64,65,66], while seven loci were identified in the Japanese Quail Coturnix japonica [67]. This suggests that, as in the case of White-tailed Eagle and other accipitrids, closely related species can show high divergence in the number of MHC loci and some non-passerines may have relatively high number of MHC loci, probably as a result of recent duplications.
There was much stronger signature of diversifying selection at the MHC class II than class I in the White-tailed Eagle. Seven MHC class II amino acid residues (in contrast to only two class I residues) showed evidence for pervasive diversifying selection, and the dN/dS ratio estimates were higher for MHC class II sequences, when calculated either for all residues or putative PBRs. Stronger signature of diversifying selection at MHC class II was consistent with higher sequence polymorphism of class II genes, as assessed with the proportion of polymorphic sites, total number of mutations, and nucleotide diversity. This pattern is consistent with a recent global analysis of selection across the avian tree of life, showing stronger signature of diversifying selection at MHC class II versus class I in non-passerine birds [16]. Similar conclusions have been reached by few available direct comparisons of nucleotide substitution rates (dN/dS ratios) between MHC class I and class II genes in non-passerine birds. Higher dN/dS ratios at MHC class II versus class I have been reported for the Blue Petrel Halobaena caerulea (Procellariiformes) [20], six species from flamingo complex (Phoenicopteriformes) [21], Centrocercus and Tympanuchus grouse (Galliformes) [22], as well as in the Domestic Chicken Gallus gallus [68]. Genus Falco seems to constitute an exception to this rule, as both MHC classes in falcons were shown to accumulate synonymous and non-synonymous mutations at similar rates [28]. However, many falcon species show remarkably low MHC variation at both intra- and inter-specific level and, thus, it has been suggested that falcons may primarily depend on other immune mechanisms, e.g. powerful innate defences that override the need to trigger a costly adaptive response via MHC [28].
On the other hand, we cannot exclude that divergent MHC class II alleles of the White-tailed Eagle were adaptively retained through the bottleneck to preserve high capacity of extracellular pathogen recognition, while the loss of variation at class I could be primarily driven by stochastic processes, such as drift, rather than purifying selection. There is compelling empirical evidence for the role of genetic drift in causing rapid loss of MHC variation in bottlenecked populations of birds [69,70,71] and other vertebrates [72, 73]. This scenario is also consistent with the results of theoretical and meta-analysis approaches, showing that the effect of drift combined with selection acting prior to a bottleneck event should cause greater loss of MHC polymorphism than the loss of neutral genetic diversity [74, 75]. However, although we acknowledge that drift could have caused some loss of MHC diversity in our population of White-tailed Eagles, it is unlikely to solely explain marked differences in dN/dS ratios and sequence polymorphism between MHC class I and class II genes. Consequently, we conclude that these differences should, at least to some extent, reflect different selective pressures at the two MHC classes.
Although our results provided a clear support for higher allelic diversity and stronger diversifying selection at the MHC class II exon 2 than MHC class I exon 3, we have to acknowledge several methodological limitations of our study. First, our conclusions were based on the analysis of a single exon per each MHC class, while peptide-binding grooves of MHC molecules are formed by two domains (α1 and α2 at class I; α1 and β1 at class II), each coded by one exon. Although genotyping both domains per each class would produce much more robust results, it was methodologically not feasible for our study species, as there are no available primers for amplifications of α1 class I and class II domains in accipitrids and other closely related clades. Second, using a single pair of primers per class for PCR amplifications may have an excluding effect on some gene sequences or loci [2]. While the exclusion of certain loci could produce a significant bias in the analyses, we consider this bias rather unlikely in the case our study, as we recovered sequences from the highest number of MHC loci ever reported in accipitrids. Third, we had no information on the expression profiles of genotyped MHC loci and, thus, we could not exclude that our sequences originated from both classical (high variability and strong expression) and non-classical (low variability and reduced or tissue-specific expression) loci, which may have different evolutionary histories and be under different selective pressures [3, 76]. Although within our dataset we detected no non-functional alleles (having stop codons or frame-shift mutations), which could be characteristic for pseudogenes, a presence of non-classical sequences could not be excluded using our methodology. Taking all this into account, we recommend development of locus-specific primers for MHC class I and class II amplifications and assessing tissue-specific expression of MHC alleles using complementary DNA (cDNA) as crucial next steps for a better understanding of MHC evolution in the White-tailed Eagle. Having said that, it must be acknowledged that sample collection for examination of tissue-specific expression profiles of the MHC is highly invasive and must require a careful consideration when applied to an endangered species, such as the White-tailed Eagle.
Finally, phylogenetic clustering of White-tailed Eagle alleles with sequences recovered from other accipitrid species produced contrasting patterns at MHC class I and class II. In general, both MHC class I and class II sequences of White-tailed Eagle clustered together with sequences of the congeneric species, the Bald Eagle. However, all Haliaeetus class I sequences formed a single cluster, while class II sequences formed three distinct clusters separated with the sequences of other accipitrids. Higher similarity of sequences between rather than within species (trans-specific similarity) is a common characteristic of MHC genes and it is usually explained with trans-species polymorphism (TSP), which arises from the passage of alleles from ancestral species to descendant species through incomplete lineage sorting [13]. While neutral (transient) TSP maintains ancestral allelic variants in both descendant species for a short period of time after speciation (according to neutral expectation) and gradually disappears, strong balancing selection can maintain orthologous MHC lineages for much longer evolutionary times (so called balanced TSP) [13]. In fact, there are cases of MHC allele sharing between avian genera that are estimated to have diverged 20–30 mln years ago and, within Accipitridae, identical MHC alleles were recovered from Aegypius and Gyps genera, which diverged 12.7 mln year ago [16] (divergence time estimates according to Jetz et al. [77]). Although trans-specific similarity can be also explained with convergent evolution resulting from adaptation to similar selective pressures, molecular studies indicate that balanced TSP is a predominant mechanism responsible for trans-specific allele clustering at the avian MHC [78]. Considering stronger signature of diversifying selection at MHC class II versus class I in the White-tailed Eagle, we find it likely that trans-specific similarity of class II sequences in our study may also reflect balanced TSP rather than convergence.
Conclusions
Our study shows that recently bottlenecked Central European population of White-tailed Eagles has retained significant levels of MHC diversity, which reinforces high conservation value of local White-tailed Eagle populations, as previously postulated by Hailer et al. [34]. At the same time, our study is the first to demonstrate contrasting patterns of allelic diversity and selection at MHC class I and class II in an accipitrid species, supporting the hypothesis that different mechanisms can shape evolutionary trajectories of MHC class I and class II genes.
Abbreviations
- DDT:
-
Dichlorodiphenyltrichloroethane
- dN:
-
Nonsynonymous substitution rate per nonsynonymous site
- dS:
-
Synonymous substitution rate per synonymous site
- FEL:
-
Fixed Effects Likelihood
- FUBAR:
-
Fast Unconstrained Bayesian AppRoximation
- GARD:
-
Genetic Algorithm for Recombination Detection
- GTR:
-
General time-reversible
- MCMC:
-
Markov Chain Monte Carlo
- MEME:
-
Mixed Effect Model of Evolution
- MHC:
-
Major Histocompatibility Complex
- NNI:
-
Nearest-neighbour interchanges
- PBR:
-
Peptide-binding residue
- PCB:
-
Polychlorinated biphenyl
- PCR:
-
Polymerase chain reaction
- SPR:
-
Subtree-pruning-regrafting
References
Klein J. The natural history of the major histocompatibility complex. New York: John Wiley & Sons; 1986.
O'Connor EA, Strandh M, Hasselquist D, Nilsson JÅ, Westerdahl H. The evolution of highly variable immunity genes across a passerine bird radiation. Mol Ecol. 2016;25:977–89.
Bollmer JL, Dunn PO, Whittingham LA, Wimpee C. Extensive MHC class II B gene duplication in a passerine, the common yellowthroat. J Hered. 2010;101:448–60.
Kaufman J, Milne S, Göbel TW, Walker BA, Jacob JP, Auffray C, et al. The chicken B locus is a minimal essential major histocompatibility complex. Nature. 1999;401:923–5.
Bollmer JL, Dunn PO, Freeman-Gallant CR, Whittingham LA. Social and extra-pair mating in relation to major histocompatibility complex variation in common yellowthroat. Proc R Soc B. 2012;279:4478–785.
Sepil I, Moghadam HK, Hucard E, Sheldon BC. Characterization and 454 pyrosequencing of major histocompatibility complex class I genes in the great tit reveal complexity in a passerine system. BMC Evol Biol. 2012;12:68.
Biedrzycka A, O’Connor E, Sebastian A, Migalska M, Radwan J, Zając T, et al. Extreme MHC class I diversity in the sedge warbler (Acrocephalus schoenobaenus); selection patterns and allelic divergence suggest that different genes have different functions. BMC Evol Biol. 2017;17:159.
Spurgin LG, Richardson DS. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc R Soc B. 2010;277:979–88.
Hughes AL, Nei M. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature. 1988;335:167–70.
Takahata N, Nei M. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 1990;124:967–78.
Hedrick PW. Pathogen resistance and genetic variation at MHC loci. Evolution. 2002;56:1902–8.
Těšický M, Vinkler M. Trans-species polymorphism in immune genes: general pattern or MHC-restricted phenomenon? J Immunol Res. 2015;2015:838035.
Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557.
Benskin CMH, Wilson K, Jones K, Hartley IR. Bacterial pathogens in wild birds: a review of the frequency and effects of infection. Biol Rev. 2009;84:349–73.
Minias P, Whittingham LA, Dunn PO. Coloniality and migration are related to selection on MHC genes in birds. Evolution. 2017;71:432–41.
Minias P, Pikus E, Whittingham LA, Dunn PO. A global analysis of selection at the avian MHC. Evolution. 2018;72:1278–93.
Morand S, Poulin R. Density, body mass and parasite species richness of terrestrial mammals. Evol Ecol. 1998;12:717–27.
Bush AO, Aho JM, Kenndy CR. Ecological versus phylogenetic determinants of helminth parasite community richness. Evol Ecol. 1990;4:1–20.
Lutz HL, Hochachka WM, Engel JI, Bell JA, Tkach VV, Bates JM, et al. Parasite prevalence corresponds to host life history in a diverse assemblage of Afrotropical birds and haemosporidian parasites. PLoS One. 2015;10:e0121254.
Strandh M, Lannefors M, Bonadonna F, Westerdahl H. Characterization of MHC class I and II genes in a subantarctic seabird, the blue petrel, Halobaena caerulea (Procellariformes). Immunogenetics. 2011;63:653–66.
Gillingham MA, Courtiol A, Teixeira M, Galan M, Bechet A, Cezilly F. Evidence of gene orthology and trans-species polymorphism, but not of parallel evolution, despite high levels of concerted evolution in the major histocompatibility complex of flamingo species. J Evol Biol. 2016;29:438–54.
Minias P, Bateson Z, Whittingham LA, Johnson JA, Oyler-McCance S, Dunn PO. Contrasting evolutionary histories of MHC class I and class II in grouse – effects of selection and gene conversion. Heredity. 2016;116:466–76.
Alcaide M, Edwards SV, Negro JJ. Characterization, polymorphism, and evolution of MHC class II B genes in birds of prey. J Mol Evol. 2007;65:541–54.
Alcaide M, Edwards SV, Cadahía L, Negro JJ. MHC class I genes of birds of prey: isolation, polymorphism and diversifying selection. Conserv Genet. 2009;10:1349.
Alcaide M, Edwards SV, Negro JJ, Serrano D, Tella JL. Extensive polymorphism and geographical variation at a positively selected MHC class II B gene of the lesser kestrel (Falco naumanni). Mol Ecol. 2008;17:2652–65.
Rodríguez A, Alcaide M, Negro JJ, Pilard P. Using major histocompatibility complex markers to assign the geographic origin of migratory birds: examples from the threatened lesser kestrel. Anim Conserv. 2011;14:306–13.
Alcaide M, Rodríguez A, Negro JJ, Serrano D. Male transmission ratio distortion supports MHC-linked cryptic female choice in the lesser kestrel (Aves: Falconidae). Behav Ecol Sociobiol. 2012;66:1467–73.
Gangoso L, Alcaide M, Grande JM, Muñoz J, Talbot SL, Sonsthagen SA, et al. Colonizing the world in spite of reduced MHC variation. J Evol Biol. 2012;25:1438–47.
Alcaide M, López L, Tanferna A, Blas J, Sergio F, Hiraldo F. Simultaneous analysis of multiple PCR amplicons enhances capillary SSCP discrimination of MHC alleles. Electrophoresis. 2010;31:1353–6.
Agudo R, Alcaide M, Rico C, Lemus JA, Blanco G, Hiraldo F, Donázar JA. Major histocompatibility complex variation in insular populations of the Egyptian vulture: inferences about the roles of genetic drift and selection. Mol Ecol. 2011;20:2329–40.
Bollmer JL, Hull JM, Ernest HB, Sarasola JH, Parker PG. Reduced MHC and neutral variation in the Galápagos hawk, an island endemic. BMC Evol Biol. 2011;11:143.
del Hoyo J, Elliott A, Sargatal J. (eds.) Handbook of the birds of the world. Vol. 2. New World Vultures to Guineafowl. Lynx Edicions, Barcelona; 1994.
BirdLife International. IUCN Red List for birds; 2018. Downloaded from http://www.birdlife.org on 06/07/2018.
Hailer F, Helander B, Folkestad AO, Ganusevich SA, Garstad S, Hauff P, et al. Bottlenecked but long-lived: high genetic diversity retained in white-tailed eagles upon recovery from population decline. Biol Lett. 2006;2:316–9.
Nemesházi E, Kövér S, Zachos FE, Horváth Z, Tihanyi G, Mórocz A, et al. Natural and anthropogenic influences on the population structure of white-tailed eagles in the Carpathian Basin and Central Europe. J Avian Biol. 2016;47:795–805.
Honnen AC, Hailer F, Kenntner N, Literak I, Dubska L, Zachos FE. Mitochondrial DNA and nuclear microsatellites reveal high diversity and genetic structure in an avian top predator, the white-tailed sea eagle, in Central Europe. Biol J Linn Soc. 2010;99:727–37.
Moon DA, Veniamin SM, Parks-Dely JA, Magor KE. The MHC of the duck (Anas platyrhynchos) contains five differentially expressed class I genes. J Immunol. 2005;175:6702–12.
Wang Y, Qiu M, Yang J, Zhao X, Wang Y, Zhu Q, Liu Y. Sequence variations of the MHC class I gene exon 2 and exon 3 between infected and uninfected chickens challenged with Marek’s disease virus. Infect Genet Evol. 2014;21:103–9.
Sebastian A, Herdegen M, Migalska M, Radwan J. AMPLISAS: a web server for multilocus genotyping using next-generation amplicon sequencing data. Mol Ecol Res. 2016;16:498–510.
Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–63.
Biedrzycka A, Sebastian A, Migalska M, Westerdahl H, Radwan J. Testing genotyping strategies for ultra-deep sequencing of a co-amplifying gene family: MHC class I in a passerine bird. Mol Ecol Res. 2017;17:642–55.
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–302.
Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–26.
Kosakovsky Pond SL, Frost SD, Muse SV. HyPhy: hypothesis testing using phylogenies. Bioinformatics. 2005;21:676–9.
Delport W, Poon AF, Frost SD, Kosakovsky Pond SL. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26:2455–7.
Weaver S, Shank SD, Spielman SJ, Li M, Muse SV, Kosakovsky Pond SL. Datamonkey 2.0: a modern web application for characterizing selective and other evolutionary processes. Mol Biol Evol. 2018;35:773–7.
Murrell B, Moola S, Mabona A, Weighill T, Sheward D, Kosakovsky Pond SL, Scheffler K. FUBAR: a fast, unconstrained bayesian approximation for inferring selection. Mol Biol Evol. 2013;30:1196–205.
Kosakovsky Pond SL, Frost SD. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol. 2005;22:1208–22.
Murrell B, Wertheim JO, Moola S, Weighill T, Scheffler K, Kosakovsky Pond SL. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 2012;8:e1002764.
Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SD. GARD: a genetic algorithm for recombination detection. Bioinformatics. 2006;22:3096–8.
Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SD. Automated phylogenetic detection of recombination using a genetic algorithm. Mol Biol Evol. 2006;23:1891–901.
Saper MA, Bjorkman PJ, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2·6 °A resolution. J Mol Biol. 1991;219:277–319.
Brown JH, Jardetzky T, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–9.
Price MN, Dehal PS, Arkin AP. FastTree2 – approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490.
Liu K, Linder R, Warnow T. RAxML and FastTree: comparing two methods for large-scale maximum likelihood phylogeny estimation. PLoS One. 2011;6:e27731.
Yang Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol. 1994;39:306–14.
Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–6.
Burri R, Promerová M, Goebel J, Fumagalli L. PCR-based isolation of multigene families: lessons from the avian MHC class IIB. Mol Ecol Res. 2014;14:778–88.
Zhou X, Li C, Yi K, Liang H, Chen L, Sun Y, et al. Patterns of variation of the major histocompatibility complex class IIB loci in Chinese goose (Anser cygnoides). Immunogenetics. 2009;61:443–50.
Wang Z, Zhou X, Lin Q, Fang W, Chen X. Characterization, polymorphism and selection of major histocompatibility complex (MHC) DAB genes in vulnerable Chinese egret (Egretta eulophotes). PLoS One. 2013;8:e74185.
Kohyama TI, Omote K, Nishida C, Takenaka T, Saito K, Fujimoto S, Masuda R. Spatial and temporal variation at major histocompatibility complex class IIB genes in the endangered Blakiston’s fish owl. Zool Lett. 2015;1:13.
Burri R, Hirzel HN, Salamin N, Roulin A, Fumagalli L. Evolutionary patterns of MHC class II B in owls and their implications for the understanding of avian MHC evolution. Mol Biol Evol. 2008;25:1180–91.
Hale ML, Verduijn MH, Møller AP, Wolff K, Petrie M. Is the peacock’s train an honest signal of genetic quality at the major histocompatibility complex? J Evol Biol. 2009;22:1284–94.
Strand TM, Höglund J. Genotyping of black grouse MHC class II B using reference Strand-mediated conformational analysis (RSCA). BMC Res Notes. 2011;4:183.
Chen W, Bei Y, Li H. Genetic variation of the major histocompatibility complex (MHC class II B gene) in the threatened Hume’s pheasant, Syrmaticus humiae. PLoS One. 2015;10:e0116499.
Meyer-Lucht Y, Mulder KP, James MC, McMahon BJ, Buckley K, Piertney SB, Höglund J. Adaptive and neutral genetic differentiation among Scottish and endangered Irish red grouse (Lagopus lagopus scotica). Conserv Genet. 2016;17:615–30.
Shiina T, Shimizu S, Hosomichi K, Kohara S, Watanabe S, Hanzawa K, et al. Comparative genomic analysis of two avian (quail and chicken) MHC regions. J Immunol. 2004;172:6751–63.
Hosomichi K, Miller MM, Goto RM, Wang Y, Suzuki S, Kulski JK, et al. Contribution of mutation, recombination, and gene conversion to chicken MHC-B haplotype diversity. J Immunol. 2008;181:3393–9.
Miller HC, Lambert DM. Genetic drift outweighs balancing selection in shaping post-bottleneck major histocompatibility complex variation in New Zealand robins (Petroicidae). Mol Ecol. 2004;13:3709–21.
Strand TM, Segelbacher G, Quintela M, Xiao L, Axelsson T, Höglund J. Can balancing selection on MHC loci counteract genetic drift in small fragmented populations of black grouse? Ecol Evol. 2012;2:341–53.
Eimes JA, Bollmer JL, Whittingham LA, Johnson JA, van Oosterhout C, Dunn PO. Rapid loss of MHC class II variation in a bottlenecked population is explained by drift and loss of copy number variation. J Evol Biol. 2011;24:1847–56.
Biedrzycka A, Radwan J. Population fragmentation and major histocompatibility complex variation in the spotted suslik, Spermophilus suslicus. Mol Ecol. 2008;17:4801–11.
Miller HC, Allendorf F, Daugherty CH. Genetic diversity and differentiation at MHC genes in island populations of tuatara (Sphenodon spp.). Mol Ecol. 2010;19:3894–908.
Ejsmond MJ, Radwan J. MHC diversity in bottlenecked populations: a simulation model. Conserv Genet. 2011;12:129–37.
Sutton JT, Nakagawa S, Robertson BC, Jamieson IG. Disentangling the roles of natural selection and genetic drift in shaping variation at MHC immunity genes. Mol Ecol. 2011;20:4408–20.
Cloutier A, Mills JA, Baker AJ. Characterization and locus-specific typing of MHC class I genes in the red-billed gull (Larus scopulinus) provides evidence for major, minor, and nonclassical loci. Immunogenetics. 2011;63:377–94.
Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. The global diversity of birds in space and time. Nature. 2012;491:444–8.
Eimes JA, Townsend AK, Sepil I, Nishiumi I, Satta Y. Patterns of evolution of MHC class II genes of crows (Corvus) suggest trans-species polymorphism. PeerJ. 2015;3:e853.
Acknowledgments
We thank all colleagues who contributed to sample collection: Leszek Damps, Tomasz Janiszewski, Jacek Jezierski, Cezary Korkosz, Stefan Lewandowski, and Mariusz Urban. We also thank two anonymous reviewers and the Editor, Helmut Schaschl, for constructive comments on the earlier draft of the manuscript.
Funding
The study was financially supported by the research grant of the National Science Centre in Poland (2015/19/D/NZ8/01310). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and/or analysed during the current study are available as the Additional file 1. All sequences were deposited in GenBank (accession nos: MK186004-MK186030).
Author information
Authors and Affiliations
Contributions
PM and DA designed the study. DA collected field samples. EP conducted laboratory analyses. PM analysed data. PM, EP, and DA wrote and revised manuscript for intellectual content. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted in accordance with the current laws of Poland, where it was performed under the permission of the Local Bioethical Commission for Animal Welfare in Łódź and the General Environmental Protection Directorate in Łódź.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
White-tailed Eagle MHC seqences. (FASTA 9 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Minias, P., Pikus, E. & Anderwald, D. Allelic diversity and selection at the MHC class I and class II in a bottlenecked bird of prey, the White-tailed Eagle. BMC Evol Biol 19, 2 (2019). https://doi.org/10.1186/s12862-018-1338-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12862-018-1338-3