Abstract
Objective
This study aims to explore the predictive value of SLC25A17 in the prognosis and tumor microenvironment (TME) of patients with head and neck squamous cell carcinoma (HNSCC) and to provide ideas for individual clinical treatment.
Methods
A pancancer analysis of the differential expression of SLC25A17 among different tumors was first conducted via the TIMER 2.0 database. Subsequently, the expression of SLC25A17 and related clinical information of HNSCC patients were obtained from the TCGA database, and patients were divided into two groups according to the median value of SLC25A17 expression. K‒M survival analysis was conducted to compare the overall survival (OS) and progression-free survival (PFS) between the groups. The Wilcoxon test was used to compare the distribution of SLC25A17 in different clinical characteristics, and univariate Cox and multivariate Cox analyses were performed to analyze independent prognostic factors to establish a predictive nomogram. Calibration curves were generated to verify the reliability of predicting 1-year, 3-year and 5-year survival rates and another cohort (GSE65858) was used for external validation. Gene set enrichment analysis was conducted to compare the enriched pathways, and the immune microenvironment was assessed using the CIBERSORT and estimate packages. Furthermore, the expression levels of SLC25A17 in immune cells were also analyzed with single-cell RNA-seq via the TISCH. Moreover, the immunotherapeutic response and chemotherapy drug sensitivity were compared between the two groups to guide precise treatment. The TIDE database was applied to predict the possibility of immune escape in the TCGA-HNSC cohort.
Results
Compared with normal samples, the expression of SLC25A17 was much higher in HNSCC tumor samples. For patients with high SLC25A17 expression, the OS and PFS were shorter than those with low SLC25A17 expression, indicating a worse prognosis. The expression of SLC25A17 varied in different clinical features. Univariate Cox and multivariate COX analyses showed that SLC25A17, age, and lymph node metastasis are independent prognostic risk factors for HNSCC, and the survival prediction model based on these factors had reliable predictive value. Patients in the low-expression group exhibited more immune cell infiltration, higher TME scores, higher IPS scores and lower TIDE scores than those in the high-expression groups, suggesting better immunotherapeutic response with lower SLC25A17 expression. Moreover, patients in the high-expression group were more sensitive to chemotherapy.
Conclusions
SLC25A17 can effectively predict the prognosis of HNSCC patients and could be a precise individual-targeted indicator for the treatment of HNSCC patients.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a common cancer in humans, accounting for approximately 3.5% of all new cancer cases and 2.5% of all cancer deaths worldwide [1]. Although the current treatments for HNSCC patients include surgery, radiotherapy, chemotherapy, immunotherapy, targeted therapy, and combined therapy, the survival rate of HNSCC patients is still not high, and the 5-year survival rate is approximately 50% [2]. More than 65% of patients develop recurrent and/or metastatic (R/M) HNSCC, and the majority are considered uncurable by palliative chemotherapy [3]. Immune checkpoint inhibitors have revolutionized the therapy of all cancers including HNSCC. However, approximately 70–90% of patients with R/M HNSCC have no response to it [4]. Many novel immunotherapies including immunotherapy combinations, adaptive cellular therapy and therapeutic vaccines are underway. In recent years, a large number of biomarkers for the diagnosis, treatment and prognosis of HNSCC have been reported. We also published a paper about the reliability and acceptability of necroptosis-related lncRNA (nrlncRNA) as a potential biomarker of HNSCC [5], but it still has some limitations because of the complex tumor mechanism. It is still necessary to explore more precise biomarkers to indicate the prognosis of HNSCC patients or as therapeutic targets to improve the survival rate of HNSCC patients.
Human solute carrier family 25 (SLC25) is the largest transporter family in the human body. It consists of 53 members, named SLC25A1-SLC25A53 [6]. Members of this family transport various solutes, such as amino acids, nucleotides, dinucleotides, carboxylic acids and ketoacids, across the mitochondrial membrane, thus participating in various physiological processes of cells [7]. DNA mutation or abnormal expression of SLC25 can cause cancer and noncancer diseases through abnormal metabolism [8]. Some members of this family play a role in the occurrence and development of tumors by regulating cell metabolism, death and proliferation [9]. For example, SLC25A1 can promote colorectal tumor growth and survival by reprogramming energy metabolism [10], and it can also be used as a therapeutic target to provide new treatment prospects for drug-resistant advanced non-small cell lung cancer [11]. The absence or low expression of SLC25A43 is closely related to poor prognosis and shortened survival of breast cancer patients [12]. SLC25A8 expression can significantly increase the risk of prognosis in patients with cervical squamous cell carcinoma, but it may prolong the survival of patients with acute myeloid leukemia [13]. Taken together, SLC25 members may be potential biomarkers for various cancers.
SLC25A17 is a mitochondrial carrier located in the peroxisome membrane, which is distributed in all tissues of the human body, and its main function may be to transport free CoA, FAD and NAD+ to peroxisomes to replace PAP, FMN and AMP produced in peroxisomes [14]. SLC25A17 has been predicted to play a critical role in the functions of the peroxisome, but the in vivo role of SLC25A17 has not been demonstrated to date [15]. A study showed that SLC25A17 may be involved in the HPV infection pathway, while HPV infection is a cause of HNSCC [16]. It has been reported that SLC25A17 may play an important role in the formation of esophageal squamous cell carcinoma [17]. In addition, it has been reported that the absence of SLC25A17 leads to lower overall and recurrence-free survival rates in patients with neuroblastoma [18]. Another report shows that SLC25A17 plays an essential role in the development of enzalutamide resistance which is used for the treatment of advanced-stage prostate cancer, and SLC25A17 may be identified as a therapeutic target to circumvent drug resistance [19]. SLC25A17 may play a role in the tumorigenesis, development and efficacy of cancer; however, the application value of SLC25A17 as a biomarker in head and neck squamous cell carcinoma has not been explored. Therefore, this paper will analyze the relevant data of HNSCC patients in online databases by bioinformatics methods and explore the role of the SLC25A17 gene in the survival prediction and treatment of HNSCC patients.
Materials and methods
Downloading gene expression and clinical data
A pancancer analysis of SLC25A17 in different tumors was performed using the TIMER2.0 database (http://timer.cistrome.org/). Differential expression of SLC25A17 was analyzed in 504 tumor samples and 44 normal para-carcinoma tissue samples of the HNSC cohort from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). Clinical information of the patients, including age, gender, grade, stage, T stage (T), lymph node metastasis (N), and overall survival status, was obtained. IHC slides from The Human Protein Atlas (THPA) database (https://www.proteinatlas.org/) were used to verify the different expression of SLC25A17 in HNSCC and normal tissue. According to the median value of SLC25A17 expression, the tumor samples in this cohort were divided into a high-expression group and a low-expression group, Kaplan‒Meier analysis was performed by using the survival and survminer R software packages to evaluate the differences in overall prognosis (OS) and progression-free survival (PFS) between the SLC25A17 high-expression and low-expression groups.
Establishment and validation of the prognostic model
Differences in clinical characteristics between the high- and low-expression groups were compared. Univariate and multivariate Cox regression were used to analyze SLC25A17 expression and clinical characteristics. When the p value of a factor was less than 0.05 in both analyses, the characteristic was considered to be an independent prognostic factor. The nomogram for survival prediction was constructed with the independent prognostic factors. Calibration curves were used to verify the reliability of predicting 1-, 3-, and 5-year survival rates. To further test the predictive effects of the nomogram, another cohort with 270 HNSCC cohorts named GSE65858 from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) was used for external validation. In addition, the concordance index (C-index) was used to compare the effects of the nomogram with other clinical features in both the TCGA-HNSCC and GSE65858 cohorts.
Gene set enrichment analysis revealing the signaling pathways
The differentially expressed genes between the two groups were identified with the use of “limma” R packages. Subsequently, gene set enrichment analysis (GSEA) (http://www.gseamsigdb.org/gs-ea/index.jsp) was used to explore the signaling pathways enriched in the SLC25A17 high- and low-expression groups to reveal their potential functional differences.
Analysis of tumor microenvironment (TME) and tumor mutation burden (TMB)
The tumor immune single-cell hub (TISCH) (http://tisch.comp-genomics.org/home/) was used to analyze single-cell RNA-seq data derived from HNSCC-GSE103322 (a dataset of the single-cell RNA-seq of HNSCC from GEO database) and to explore the specific cell types expressing SLC25A17 in the TME. The estimate package was used to score the TME, including immune scores, stromal scores and ESTIMATE scores, between the two groups. The immune cell infiltration status was assessed with CIBERSORT, and the relationship between immune cell infiltration and SLC25A17 expression was determined by the Spearman correlation test. Similarly, the correlations between SLC25A17 and immune checkpoint gene expression and TMB were also compared.
Efficacy analysis of immunotherapy and chemotherapy
Data from The Cancer Immunome Atlas (TCIA) were used to evaluate the immunotherapy efficacy of PD-1 and CTLA4 inhibitors in the SLC25A17 high- and low-expression groups. In addition, the Tumor Immune Dysfunction and Exclusion (TIDE) database was applied to predict the possibility of immune escape for the TCGA-HNSC cohort as a validation. Through the “pRRophetic” package, the half maximal inhibitory concentration (IC50) of four common HNSCC chemotherapeutic drugs (cisplatin, docetaxel, gemcitabine and paclitaxel) was calculated to evaluate the sensitivity of the two groups to chemotherapy drugs.
Results
SLC25A17 has higher expression in HNSCC tissues than in normal para-carcinoma tissues and patients with high-expression of SLC25A17 have worse survival
Information from the TIMER 2.0 database showed that the expression of SLC25A17 in tumor tissues and normal para-carcinoma tissues was significantly different in most cancers, and SLC25A17 had a higher expression level in HNSCC tissues than in normal para-carcinoma tissues as shown in Fig. 1A. Detailed data on SLC25A17 expression and clinical characteristics of patients with HNSCC from TCGA were presented in the Additional file 1: Table S1. In the TCGA-HNSC cohort of 504 tumor tissues and 44 normal para-carcinoma tissues, the expression of SLC25A17 in tumor tissues was significantly higher than that in normal para-carcinoma tissues (Fig. 1B, C), and IHC slides from THPA also supported the result (Fig. 1D). The overall survival and progression-free survival in the SLC25A17 high-expression group were significantly lower than those in the SLC25A17 low-expression group (Fig. 1E, F).
SLC25A17 expression and clinical prognosis of HNSCC based on TIMER2.0 and TCGA databases. A Expression of SLC25A17 in different tumors and normal para-carcinoma tissues; B expression of SLC25A17 in HNSCC tumors and normal tissues in all included patients; C expression of SLC25A17 in HNSCC tumors and normal para-carcinoma tissues in the same individual; D IHC slides show that expression of SLC25A17 in HNSCC tissues was higher than that in normal tissues; E overall survival K‒M survival curve; F progression-free survival K‒M survival curve. (**P < 0.01; ***P < 0.001)
SLC25A17 expression, age and lymph node metastasis were used to construct a survival prediction nomogram and verify the reliable predictive value of the model
The clinical information of the SLC25A17 high- and low-expression groups in the TCGA-HNSC cohort was compared, including age, gender, grade, stage, T stage, and lymph node metastasis. The results showed that there were significant differences in SLC25A17 expression in different genders, grade, and lymph node metastases (Fig. 2A–G). Univariate COX and multivariate COX analyses were performed for SLC25A17 expression level, age, gender, grade, stage, T stage and lymph node metastasis (Fig. 3A, B). The results showed that the P values of SLC25A17 expression level (HR uni-Cox: 1.602, HR multi-Cox: 1.520), age (HR uni-Cox: 1.020, HR multi-Cox: 1.024) and lymph node metastasis (HR uni-Cox: 1.553, HR multi-Cox: 1.374) were all less than 0.05 and could be used as independent prognostic factors. Based on this, a prognostic nomogram model was constructed (Fig. 3C). It shows that different SLC25A17 expression, age, and lymph node metastasis have a corresponding score, and the total score they add up corresponds to the 1-, 3-, and 5-year survival rates of HNSCC patients below. Calibration curves showed that there was a high degree of consistency between the actual observations and nomogram predictions (Fig. 3D). The survival prediction nomogram based on SLC25A17 expression, age and lymph node metastasis from GSE65858 was constructed (Fig. 3F), and it also shows that there is a certain consistency between the actual observations and nomogram predictions (Fig. 3G). The comparisons of the C-index indicated that the nomogram had better predictive effects than other clinical features in both the TCGA-HNSC and GSE65858 cohorts (Fig. 3E, H).
Analysis of independent prognostic factors and establishment and validation of the prediction model. A Univariate COX shows that SLC25A17 expression, age, stage, T stage and lymph node metastasis were prognostic risk factors; B multivariate COX shows that SLC25A17 expression, age, and lymph node metastasis were prognostic risk factors; C, D survival prediction nomogram based on TCGA-HNSC cohorts and 1-, 3-, and 5-year survival calibration curves. E The C-index of the nomogram and other clinical features in TCGA-HNSC cohorts. F, G Survival prediction nomogram based on the GSE65858 cohort and 1-, 3-, and 5-year survival calibration curves. H The C-index of the nomogram and other clinical features in the GSE65858 cohort
Five differential signaling pathways were screened by analyzing the differential gene expression between the high and low SLC25A17 expression groups
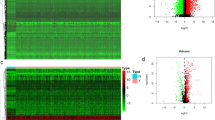
The top 40 differentially expressed genes between the high and low SLC25A17 expression groups was shown in Fig. 4A. As indicated by the results of GSEA, antigen processing and presentation, autoimmune thyroid disease, graft versus host disease, and natural killer cell-mediated cytotoxicity pathways were relatively active in the low-expression group, and the neuroactive ligand receptor interaction signal pathway was more active in the high-expression group (Fig. 4B).
Signaling pathways and immune infiltration. A Heatmap of the top 40 differentially expressed genes in the low- and high- SCL25A17 groups; B Gene Set Enrichment Analysis of differently enriched pathways between the SCL25A17 low- and high- expression groups; C–E The t-SNE projection of all cells and SLC25A17 expression from HNSCC-GSE103322; F violin plots present the differential TME score between the SLC25A17 low-and high-expression groups; G heatmap for gene correlation analysis of immune detection sites; H correlation analysis chart of immune cell infiltration; I scatter plot shows the correlation between SLC25A17 and tumor somatic mutations (**P < 0.01; ***P < 0.001)
The low SLC25A17 expression group obtained higher TME scores, CD244 may be a key immune checkpoint gene, and TMB is unrelated to SLC25A17 expression
The result of t-distributed stochastic neighbor embedding (t-SNE) presented that 11 clusters were identified in HNSCC-GSE103322. Data showed that SLC25A17 is expressed in malignant tumor cells, immune cells, and stromal cells (such as endothelial cells, myofibroblasts, etc.) but not in muscle cells (Fig. 4C). The distribution of the specific type cells in the TME and the expression of corresponding SLC25A17 were shown in Fig. 4D, E. In the TME score, there were significant differences in the stromal cell score, immune cell score and comprehensive estimation score between the high and low SLC25A17 expression groups, and the low-expression group obtained higher scores (Fig. 4F). SLC25A17 expression was negatively correlated with CD244 expression (Fig. 4G). The infiltration of memory resting CD4 T cells and M1 macrophages was positively correlated with the expression of SLC25A17, while the infiltration of CD8 T cells, regulatory T cells and neutrophils was negatively correlated with the expression of SLC25A17 (Fig. 4H). In addition, the expression of SLC25A17 was not significantly correlated with TMB (Fig. 4I).
Patients with low SLC25A17 expression are more sensitive to immunotherapy while patients with high SLC25A17 expression are more suitable for chemotherapy
Comparing the efficacy of ctla4 and pd1 immunotherapy in the high- and low-expression groups, we found that there was no significant difference in the efficacy of ctla4 alone between the two groups. For pd1 or pd1 combined with ctla4 treatment, the IPS score of the low-expression group was higher, and the immunotherapy effect was better (Fig. 5A–C). Wilcoxon's test indicated that the SLC25A17 high-expression group exhibited a higher TIDE score, suggesting that the increased expression of SLC25A17 might promote immune escape in HNSCC (Fig. 5D). Analysis of sensitivity differences of commonly used chemotherapy drugs showed that the IC50 value of the SLC25A17 high-expression group was lower and the treatment response was more sensitive to cisplatin, docetaxel, gemcitabine and paclitaxel (Fig. 5E–H), and the difference between the two groups was statistically significant.
Discussion
The pathogenesis of HNSCC is complex. Although there are many treatment methods, the 5-year survival rate of patients is still not high. It is still necessary to explore more effective biomarkers in multiple directions to provide the basis for clinical treatment. The SLC25 family plays a huge role in the transport of solutes and energy metabolism, and members of the family have been identified as potential biomarkers of various cancers [9]. SLC25A17 may also play a role in the tumorigenesis, development and efficacy of cancer. Therefore, this study explored the application value of SLC25A17 as a potential biomarker of HNSCC.
This study showed that the expression level of SLC25A17 in HNSCC tumor samples was higher than that in normal tissues. Patients with higher expression levels had a worse prognosis and shorter overall survival and progression-free survival. The expression level of SLC25A17, age, and lymph node metastasis were three independent risk factors, in which the SLC25A17 expression level had the highest risk ratio. The nomogram for survival prediction was developed, and the 1-, 3-, and 5-year calibration plots showed high consistency with the survival model. Notably, the survival nomogram based on SLC25A17, age, and lymph node metastasis could better predict the prognosis of patients with HNSCC. Therefore, it is believed that SLC25A17 has good application value in predicting the prognosis of HNSCC patients.
The expression of SLC25A17 was negatively correlated with the expression of CD244 (natural killer cell receptor 2B4), which weakened the natural killing effect of T cells in the high-expression group. This was a preliminary inquiry and the correlation between SLC25A17 and NK cell functional gene expression may be verified through experiments in the future. According to GSEA results, the cytotoxic signaling pathway mediated by natural killer cells was more active in the low-expression group. The cytotoxic signaling pathway plays a crucial role in the body's antitumor activity [20], which may also be the reason for the better prognosis of the low-expression group. The neuroactive ligand receptor interaction signaling pathway was more active in the high-expression group, in which neuroactive steroid production played an important role. Neuroactive steroids affect the regulation of GABA receptors, which regulate cell proliferation and are closely related to the growth and progression of tumor cells [21]. SLC25A17 may promote tumor cell growth by regulating the neural active ligand receptor interaction signaling pathway.
In recent years, an increasing number of immunotherapies have been carried out in the clinical treatment of HNSCC patients, which has improved the survival and prognosis of some patients. Studies have shown that the TME plays an important role in immunotherapy [22]. In addition to tumor cell carcinoma, the TME also includes stromal cells and immune cells that infiltrate tumors. Our data showed that SLC25A17 was expressed in malignant tumor cells, immune cells and stromal cells but not in myocytes, and this difference might be caused by the small number of muscle cells in the sample tissue. The TME matrix is involved in the regulation of immune cell infiltration and the antitumor immune response and is closely related to the generation, progression, metastasis and drug resistance of tumor cells [23]. Immune cells may exert antitumor effects through various mechanisms [24]. For example, CD8+ T cells can kill cancer cells, destroy immune tolerance and enhance immunotherapy through the PD-1/PD-L1 immunosuppressive axis [25]. Regulatory T cells (Tregs) play an important role in maintaining self-tolerance and immune homeostasis. They can inhibit the antitumor immune response by secreting inhibitory cytokines, killing effector cells or affecting the function of effector cells, which leads to tumor progression in various types of cancers [26]. The classic function of neutrophils is to defend against infection. They contain antibacterial and cytotoxic compounds to destroy malignant cells and can also induce cytotoxic cells to kill tumor cells by secreting many cytokines and chemokines [27, 28]. Tumor tissues with low SLC25A17 expression showed more significant infiltration of CD8+ T cells, regulatory T cells, and PMNs. Therefore, HNSCC patients with low SLC25A17 expression are more sensitive to immunotherapy. This is consistent with the TIDE score in the two groups.
In addition, the sensitivity of four common chemotherapeutic agents was compared by IC50 values in the two groups. The results showed that the high-expression group showed higher sensitivity to cisplatin, docetaxel, gemcitabine and paclitaxel. This might be closely related to the transport function of SLC25A17 on peroxisomes and mitochondrial activity. It is believed that high expression of SLC25A17 may be a potential predictor for HNSCC patients receiving chemotherapy.
Our new survival prediction model has certain reliability and application value, but it also has some limitations. The main database is relatively single, and the data volume is limited, so more database information is needed for verification. In addition, our study lacks external experimental verification. Prospective studies are needed to verify the analysis results through laboratory analysis combined with long-term follow-up. The efficacy of SLC25A17 as a biomarker in immunotherapy and chemotherapy needs to be further verified by long-term clinical practice.
In conclusion, SLC25A17 is closely related to the prognosis of HNSCC patients and the survival prediction model based on SLC25A17 can effectively predict the survival rate of HNSCC patients. SLC25A17 can help guide immunotherapy and chemotherapy and has good guiding value for the individualized and precise treatment of HNSCC patients.
Availability of data and materials
The datasets generated and analyzed during the current study are all from public databases, and the main data come from the TCGA database (https://portal.gdc.cancer.gov/). The dataset supporting the conclusions, R code and R data files for analysis are also available if required (https://www.jianguoyun.com/p/DXXjKN0Q7OShCxjP3-0E).
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Canning M, Guo G, Yu M, Myint C, Groves MW, Byrd JK, et al. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front Cell Dev Biol. 2019;7:52.
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–709.
Muzaffar J, Bari S, Kirtane K, Chung CH. Recent advances and future directions in clinical management of head and neck squamous cell carcinoma. Cancers (Basel). 2021;13(2):338.
Huang J, Xu Z, Teh BM, Zhou C, Yuan Z, Shi Y, et al. Construction of a necroptosis-related lncRNA signature to predict the prognosis and immune microenvironment of head and neck squamous cell carcinoma. J Clin Lab Anal. 2022;36(6): e24480.
Ruprecht JJ, Kunji ERS. The SLC25 mitochondrial carrier family: structure and mechanism. Trends Biochem Sci. 2020;45(3):244–58.
Palmieri F. The mitochondrial transporter family SLC25: identification, properties and physiopathology. Mol Asp Med. 2013;34(2–3):465–84.
Kunji ERS, King MS, Ruprecht JJ, Thangaratnarajah C. The SLC25 carrier family: important transport proteins in mitochondrial physiology and pathology. Physiology (Bethesda). 2020;35(5):302–27.
Rochette L, Meloux A, Zeller M, Malka G, Cottin Y, Vergely C. Mitochondrial SLC25 carriers: novel targets for cancer therapy. Molecules. 2020;25(10):2417.
Yang Y, He J, Zhang B, Zhang Z, Jia G, Liu S, et al. SLC25A1 promotes tumor growth and survival by reprogramming energy metabolism in colorectal cancer. Cell Death Dis. 2021;12(12):1108.
Fernandez HR, Gadre SM, Tan M, Graham GT, Mosaoa R, Ongkeko MS, et al. The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ. 2018;25(7):1239–58.
Tina E, Lindqvist BM, Gabrielson M, Lubovac Z, Wegman P, Wingren S. The mitochondrial transporter SLC25A43 is frequently deleted and may influence cell proliferation in HER2-positive breast tumors. BMC Cancer. 2012;12:350.
Liu AR, Liu YN, Shen SX, Yan LR, Lv Z, Ding HX, et al. Comprehensive analysis and validation of solute carrier family 25 (SLC25) and its correlation with immune infiltration in pan-cancer. Biomed Res Int. 2022;2022:4009354.
Agrimi G, Russo A, Scarcia P, Palmieri F. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem J. 2012;443(1):241–7.
Kim YI, Nam IK, Lee DK, Bhandari S, Charton L, Kwak S, et al. Slc25a17 acts as a peroxisomal coenzyme A transporter and regulates multiorgan development in zebrafish. J Cell Physiol. 2020;235(1):151–65.
Ito R, Kitamura K, Inohara H, Yusa K, Kaneda Y, Nimura K. Peroxisomal membrane protein PMP34 is involved in the human papillomavirus infection pathway. Front Virol. 2022;2:870922.
Chen SW, Zhou HF, Zhang HJ, He RQ, Huang ZG, Dang YW, et al. The clinical significance and potential molecular mechanism of PTTG1 in esophageal squamous cell carcinoma. Front Genet. 2020;11: 583085.
Khan FH, Pandian V, Ramraj S, Natarajan M, Aravindan S, Herman TS, et al. Acquired genetic alterations in tumor cells dictate the development of high-risk neuroblastoma and clinical outcomes. BMC Cancer. 2015;15:514.
Kushwaha PP, Verma SS, Shankar E, Lin S, Gupta S. Role of solute carrier transporters SLC25A17 and SLC27A6 in acquired resistance to enzalutamide in castration-resistant prostate cancer. Mol Carcinog. 2022;61(4):397–407.
Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. 2017;31:37–54.
Watanabe M, Maemura K, Oki K, Shiraishi N, Shibayama Y, Katsu K. Gamma-aminobutyric acid (GABA) and cell proliferation: focus on cancer cells. Histol Histopathol. 2006;21(10):1135–41.
Frankel T, Lanfranca MP, Zou W. The role of tumor microenvironment in cancer immunotherapy. Adv Exp Med Biol. 2017;1036:51–64.
Turley SJ, Cremasco V, Astarita JL. Immunological hallmarks of stromal cells in the tumor microenvironment. Nat Rev Immunol. 2015;15(11):669–82.
Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment. Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–33.
Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenviron-ment. Trends Cancer. 2020;6(7):605–18.
Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. 2021;9(7):e002591.
Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. 2015;2015: 983698.
Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97(2):339–45.
Acknowledgements
Thanks to the individuals and institutions that contributed to the creation of common databases and shared resources. Thanks to all the authors for their hard work in completing this article.
Funding
This study was supported by Ningbo Public Science Research Foundation (Grant/Award Number: 2021S170); Ningbo Natural Science Foundation (Grant/Award Number: 2022J260); Zhejiang Provincial Natural Science Foundation (Grant/Award Number: LY23H130001); Zhejiang Provincial Medical and Health Science Research Foundation (Grant/Award Number: 2020KY274 and 2022KY1086) and National Natural Science Foundation of China (Grant/Award Number: 81670920).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of the study. YS wrote the manuscript, JH and YH were responsible for data collection and analysis, and YS was responsible for the verification of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The data analyzed in this study were from public databases, so ethical approval and consent were not needed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The clinicopathlogical characteristics of TCGA-HNSC cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, Y., Huang, J., Hu, Y. et al. Multiomics data analyses to identify SLC25A17 as a novel biomarker to predict the prognosis and immune microenvironment in head and neck squamous cell carcinoma. BMC Bioinformatics 24, 269 (2023). https://doi.org/10.1186/s12859-023-05399-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12859-023-05399-6