Abstract
Background
Eurasian perch (Perca fluviatilis) is an ecologically significant fish species in the Baltic Sea and has been recognized as a suitable organism to measure concentrations of mercury (Hg) contamination. The adult species occupy a high trophic position; therefore, significant levels of the hazardous substances tend to bioaccumulate in their tissues. However, the ability of the species to inhabit a wide range of feeding ground raises concerns about the adequacy of monitoring data in relation to the representativeness of measured levels of Hg at specific locations. Accounting for the migratory characteristics of this species can shed light on the origin of the analyzed specimens and thus trace Hg uptake chain. Perch samples and potential perch prey were collected at three remote stations in a fully interlinked system river–lake–coastal/transitional waters of the Gulf of Riga. Total mercury (THg) concentration and stable isotope ratios were measured in each sampled item. The perch data were divided into three subgroups associated with specific feeding grounds and one mixed group. A Bayesian mixing model was implemented to quantify the feeding preferences of each group, and based on the results, influence of each food source on Hg uptake by perch was modeled by means of Gaussian GAM model.
Results
Calculated carbon and nitrogen stable isotope values demonstrated clear evidence of perch specimens migrating between the sampling stations. Substantial proportion of specimens sampled in river and lake stations had isotopic signals consistent with feeding in the gulf. The group of perch associated with feeding in the river grounds exhibited the highest THg concentrations with mean value of 209 µg kg−1 wet weight. The food items C. harengus membras and Crustacean showed significant mitigating effects on THg concentration. The rest of the food items showed a secondary influence on the variation of THg concentration.
Conclusions
The study clearly showed that the high mobility of perch along associated aquatic systems has a noticeable effect on Hg concentrations measured in the fish. Therefore, trophic position and isotopic signatures, along with identification of the food sources, can serve as important supplementary tools for more accurate data interpretation of Hg accumulation.
Similar content being viewed by others
Background
The global mercury (Hg) cycle is dominated by anthropogenic and natural emissions of gaseous substances of Hg to the atmosphere [1]. Simultaneously, the wide use of Hg and its containing products [2] has resulted in more than 3000 localized Hg-contaminated sites worldwide [3]. Furthermore, the improperly disposed industrial, household and medical products as well as pesticides used in the past have created legacy Hg contamination sources. Although the sources are not classifiable as Hg-contaminated sites, they are impacting environmental quality of geographically localized water basins they are discharging into.
In order to address local Hg sources and to improve environmental quality, various programs, like river basin management plans in European Union, are being developed and implemented. To assess the effectiveness of implemented measures, Hg levels and their trends are usually analyzed in the frame of environmental monitoring programs. Consequently, environmental research employs a variety of scientific methods [4, 5] in quantitative and qualitative analyses of the Hg in aquatic systems, including selection of a matrix for analysis such as water, sediments and biological material. Eurasian perch (Perca fluviatilis) is one of the ecologically significant fish species, along with Baltic herring (Clupea harengus membras), cod (Gadus morhua) and eelpout (Zoarces viviparus), proposed by a regional platform for environmental policy-making—Baltic Marine Environment Protection Commission (HELCOM) as a biological matrix for environmental studies in the Baltic Sea, which reflects local environmental concentrations of hazardous substances [6]. Particularly, a dorsal muscle (fillet) was suggested as an appropriate matrix to measure levels of organic Hg accumulated in fish [6], moreover the tissues were proposed by ‘EC regulation 1881/2006’ [7] in order to control contamination in fishery products. A fish takes up Hg by absorbing it through the body surface and gills, but a primary source is the diet [8]. Perch is omnivorous in the first years of life, although the adults mostly follow a piscivorous diet [9]. The species occupy a high trophic position; therefore, high concentrations of Hg [10] are commonly found in their tissues. The perch is widespread in freshwater and brackish water ecosystems, but usually are not considered to be an anadromous fish. At the same time, it has been put forward by Järv [11] that the home-range migration (average 20 km, maximum observed 180 km) is a common behavioral feature for perch. The salt tolerance of perch and relatively low water salinity of the Baltic Sea and the Gulf of Riga allows this species to move from inland lakes and rivers to coastal waters. Generally, it has been assumed that once the feeding grounds in the coastal waters have been reached the specimens become reasonably stationary [12], consequently they can be used as a representative organism to characterize concentrations of hazardous substances, such as Hg, providing biologically relevant context for local pollution exposure. However, the high mobility of perch, the ability of the species to inhabit a wide range of feeding ground [9, 11], in addition to relatively high variability of measured concentrations raises concerns about adequacy of monitoring data regarding to the representability of measured levels of hazardous substances, including Hg, at specific locations. These concerns are most pronounced in the cases where different water bodies form an interlinked water system which is fully within the range of perch migration distance, like the river–lake–marine waters system, which is highly common at coastal regions of Latvia due to a number of lagoon type lakes. Consideration of migratory characteristics of the species can shed light on the origin of the analyzed samples, thus filling knowledge gaps on Hg distribution in the interconnected aquatic systems.
Stable isotopes of nitrogen (N) and carbon (C) in soft tissues of fish are commonly used to study food webs and migration in aquatic ecosystems [13–15]. Carbon stable isotope ratios 13C/12C in marine and fresh water systems have been used to determine the movements of migratory species between coastal and pelagic ecosystems based on changes in dietary preferences during the migration [16, 17], which is possible due to clear isotopic differences between 13C-depleted freshwater and 13C-enriched marine food webs. Changes in nitrogen isotope ratios 15N/14N have been used to distinguish between trophic levels in freshwater and marine environments [18, 19].
Stomach content analysis provides important information regarding the recently consumed prey and helps to describe feeding habits of fish. The aim of the analysis is to determinate the most frequently consumed prey and indicate general food categories occurring in the stomach [20].
By employing analysis of C and N isotopes in combination with perch stomach content analysis, the aim of this study was to determine how the fundamentally different food bases affect the uptake of Hg from food chains, as well as whether the perch, caught at a particular location, are representative of specific location, or rather of the entire interconnected coastal–freshwater aquatic system.
Materials and methods
Study site description
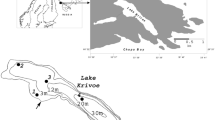
For this study the fully interlinked system, river Daugava–lake Ķīšezers–coastal area of the Gulf of Riga (Baltic Sea) was chosen (Fig. 1). Sampling station 1, the river Daugava, was selected on the last section of the river between Riga Hydroelectric Power Plant (HEPP) and the estuary Daugavgriva, circa 5 km downstream to the HEPP and 14 and 19 km upstream of the channels connecting lake Ķīšezers and river Daugava. The site can be characterized by rocky sediment type and rapid stream velocity. Sampling station 2, a lagoon type lake Ķīšezers, is connected to river Daugava by two natural channels. It is rich with aquatic vegetation and represents a stagnant water pool. Sampling station 3, the Gulf of Riga, was selected near the mouth of river Daugava. The location represents a brackish coastal ecosystem with a significant amount of detritus originating from adjacent rivers.
Sampling and pre-treatment
The sampling campaigns took place in April and August 2017. Perch and other fish species were collected by means of scientific Coastal Survey multimesh gillnets (Nippon Verkko Oy, Finland) with nine 5-m-long panels of different mesh size (mesh size ranging between 10 and 60 mm). Benthic organisms were sampled by Petite Ponar Grab (Wildco, USA), while zooplankton and fish juveniles were collected by means of beach seine (hand-made: the wing length 10 m with the mesh size of 10 mm knot to knot, the depth of 1.5 m, the mesh size of the cod end was of 5 mm). Crayfish were caught by two-ring drop net with the diameter of 80 cm and the mesh size 20 mm. For suspended particulate matter (SPM) surface water samples (up to 5 L) were taken from each sampling site by pre-cleaned plastic bottles. Collected surface water samples were vacuum filtered for at least 30 min on pre-combusted (at 450 °C for 2 h) 24 mm diameter microfiber glass filters (Whatman grade GF/F, pore size 0.7 µm) to collect a sufficient amount of the material. Only Hg concentration and stable isotope analyses were performed for SPM samples. The data were not incorporated into any of the statistical analyses or the conducted models, instead were later used as a reference point or a proxy of phytoplankton.
Length and weight of the whole fish was determined immediately after sampling by measuring board (accuracy ± 0.1 cm) and technical scale KERN FCE3K1N (KERN & SOHN GmbH, Germany; accuracy ± 1 g), thereafter, dorsal muscles were extracted. The dorsal muscles were placed into a polyethylene container and frozen at temperature − 18 °C. The samples of muscle tissues, zooplankton and benthic organisms were dried in vacuum freeze dryer (LYOVAC GT 2-E, STERIS GmbH, Germany) until sample weight loss stopped and then homogenized by knife mill (IKA A11 basic, IKA—WERKE GmbH &CO.KG, Germany) or agate pestle. Plastic containers with dried tissue samples were stored in desiccator in dark at room temperature (+ 20 °C) until further analyses.
Analytical methods
The concentration of total mercury (THg) in the dorsal muscles of fish, other organisms and SPM was determined in laboratory at Latvian Institute of Aquatic Ecology (Daugavpils University) using direct combustion Hg analyser (Teledyne Leeman labs, “Hydra IIc”, (Mason, Ohio, USA) following US EPA Method 7473 [21, 22]. The analyses of reference material of mussel tissue ERM-CE278k and aquatic plant ERM-BCR060 (both certified by Institute for Reference Materials and Measurements at Joint Research Centre, European Commission, Geel, Belgium) were performed for calibration verification at the start and to prove accuracy of determination at the end of every batch of 20 samples (Additional file 1: Table S1). Our results were in good agreement with the certified value given for the reference materials—recovery range 100–126% (n = 24). Method blank (50 µL of deionized water) and random sample duplicates were also run during each batch. Method blank was less than 25% of the lowest detected THg content in the sample (0.20 ng) and was considered acceptable. Relative percent difference between sample duplicate analyses was 1.5 ± 2% (n = 22).
Stomach content analysis
Stomach content of the perch was assessed by means of stomach content analysis, using the dissection method suggested by Manko [20]. Full length of uncoiled extracted gastrointestinal tract from esophagus to anus and its parts (stomach and intestine) were measured on a measuring board to the nearest mm, the stomach and intestine was separated, blotted between tissue paper sheets for 1 min to remove excess water, and weighed on electronic balance (LIBROR AEU-210, Shimadzu Corporation, Kyoto, Japan; accuracy ± 0.0001 g) to the nearest 0.001 g. Stomach was opened by making a shallow cut with a scalpel to avoid damage to contents, and washed in a Petri dish with a small amount of water. Intestine contents were removed into a Petri dish by sliding a blunt probe along the length of the segment. Stomach fullness was assessed visually using a scoring system of 1 to 7 (empty to distended/bursting, respectively). Stomach and intestine contents were inspected under a stereomicroscope (Leica MEB126, Leica Microsystems, Singapore). The digestion state of food items in the stomach was visually assessed by a scoring system of 1 to 6 (empty stomach to intact condition, respectively). Food items were identified to species level where possible, and separated into identified taxonomic groups or species, blotted for 1 min between tissue paper sheets, and weighed to the nearest 0.001 g. Intestinal contents were used for identification of undigested food items to the lowest possible taxonomic level and for counting and identification of intestinal parasites.
Analysis of stable isotopes
Analysis of stable isotopes and calculation of C and N isotope ratios was performed for all the caught organisms and SPM. Prior to the stable isotope analyses 2 mg per sample of dried tissues were wrapped in a tin cup and analyzed in the Laboratory of Analytical Chemistry at Faculty of Chemistry, University of Latvia by elemental analyser (EuroEA-3024, EuroVector S.p.A, Italy) coupled with continuous flow stable isotope ratio mass spectrometer (Nu-HORIZON, Nu Instruments Ltd., UK). Isotope values were reported relative to Vienna Pee Dee Belemnite with a lithium carbonate anchor (VPDB-LSVEC) for carbon isotope value δ13C and to atmospheric nitrogen (air) for nitrogen isotope value δ15N. Stable isotope values were denoted as parts per thousand (‰) deviation from the standard, as follows:
where δX is δ13C or δ15N and the R ratio is 13C/12C or 15N/14N.
An internal standard sample (glutamic acid) was used to check reproducibility of the stable isotope ratio determination. The pooled standard deviations were 0.14‰ (n = 121) for δ13C and 0.21‰ (n = 121) for δ15N. Reference material l-glutamic acid USGS-40 (Reston Stable Isotope Laboratory of the US Geological Survey, Reston, Virginia, NIST®RM 8573) was used to check accuracy of the stable isotope ratio determination where stable carbon isotopic and nitrogen isotopic compositions with combined uncertainties are δ13CVPDB-LSVEC = − 26.39 ± 0.04‰ and δ15NAIR = − 4.52 ± 0.06‰ [23]. Our results for reference standard USGS-40 were δ13C = − 26.38, (SD = ± 0.03‰, n = 20) and δ15N = − 4.54 (SD = ± 0.08‰, n = 20).
Data analysis and statistical assessment
Ward’s minimum variance Clustering analysis was performed for identification of perch subgroups with agglomeration of objects based on variables δ13C, δ15N and sampling location. The aim of the analysis was to split spatially subgroups of perch with consideration of sampling location and geographical markers provided by the signals of stable isotope values. Optimal number of clusters was selected according to the Silhouette Widths method [24].
Bayesian mixing model SIAR was chosen for quantification of common diet in the computed subgroups of perch: group 1 (representatives of the station 1), group 2 (representatives of the station 2), group 3 (representatives of the station 3) and group 4 (a mixed group including the specimens with the isotopic values which cannot be attributed to any of the station) [25]. Trophic discrimination factor was approximated for each station separately, based on δ13C and δ15N values and trophic levels found in the literature (Additional file 1: Table S2) of every organism sampled. The estimated trophic discrimination factors were 3.37 ± 1.27‰ (δ15N) and 0.36 ± 1.00‰ (δ13C) for station 1, 3.92 ± 1.31‰ (δ15N) and 1.04 ± 1.52‰ (δ13C) for station 2, 3.32 ± 1.14‰ (δ15N) and 0.74 ± 1.21‰ (δ13C) for station 3. Sources for the model were selected based on results of the stomach content analysis and the list of sampled organisms at the study areas. The food items’ contribution ratios were extracted from the model and used for the further analysis. Because the system had two isotopes and more than three sources, unique solutions for each fish were replaced by averaged ranges of food items’ contribution ratios. The data tables are available in Additional file 1: Table S3.
Trophic magnification factor (TMF) was calculated based on the species included into the station-specific diet of perch and perch itself. The food web TMF was computed from parameter b or slope of the following equation [26, 27]:
where TMF = 10b.
Analysis of covariance (ANCOVA) was implemented to compare obtained trophic magnification regression curves. During the analysis interactions between assigned groups (from cluster analysis) and δ15N values were evaluated to understand whether the focus on specific diet shows significant difference in slopes of Hg trophic magnification. Two regression models were compared: M1 − LOG THg concentration estimated from the independent variable “δ15N” and independent factor “Group”; M2 − LOG THg concentration estimated from the interrelated variable “δ15N” and factor “Group”. “Group” (the dataset was divided into subgroups, based on results of the clustering analysis mentioned above) as an independent factor was considered for identification of differences between intercepts of the trophic magnification regressions.
Smoothing function of Generalized Additive Models (GAM) was used to cover a slightly non-linear relationship of LOG-transformed THg concentration in perch dorsal muscles and length of specimens, thus allowing more sensitive evaluation of effect of dietary preferences. Data exploration protocol recommended by Zuur et al. [28] was applied before the modeling process. The obtained models were validated according to the guide suggested by Zuur & Ieno [29], including check of homogeneity, independence, influential observations, normality and fit of estimated values. Akaike Information Criterion (AIC) [30] was applied to compare the obtained GAMs and determine the best fit for the data, thus identifying feeding sources and other concomitant factors impacting Hg accumulation in consumer tissues. Due to collinearity of some variables, such as Crustacean and Neogobius melanostomus (correlation coefficient − 0.8), Crustacean and Neomysis integer (correlation coefficient − 0.7), Gymnocephalus cernua and N. integer (correlation coefficient − 0.7), G. cernua and N. melanostomus (correlation coefficient − 0.7), N. melanostomus and N. integer (correlation coefficient − 0.7), three different models (A, B and C) were performed. Each of the models includes a combination of non-collinear variables, and the three models together contain all the food items selected as sources for the SIAR model mentioned above. Ammodytes tobianus was excluded from the models because of high covariance with C. harengus membras (correlation coefficient 1.0), thus further it may be considered that the species have similar effect on Hg uptake. The following three models were selected:
Model A:
Model B:
Model C:
The Model C, with the most negative slope coefficient demonstrated by significant food item C. harengus membras, was selected as an example for visualization of modeling results. Wilcoxon rank sum exact test was implemented to examine differences in THg concentrations estimated from the Gaussian GAM model. The model was simulated for the scenarios with the maximum and minimum contribution ratio of C. harengus membras and continuously ranged from the maximum to minimum consumption (contribution) ratios of the other food items. The range limits at specific sampling stations were the same as computed by the SIAR model mentioned above. The visualization example can be found in Additional file 2.
Relationships tested were considered to be statistically significant for p < 0.05, except for GAM outputs, because p-values of estimates included produced by the model are not defining for model interpretation, but indicate secondarily variable. Data exploration, artworks, and statistical analyses were performed using R software for Windows, release 4.0.3 [31].
Results
Hg concentrations and stable isotope analysis
The full list of THg concentrations and stable isotope values measured in the SPM and all collected organisms can be found in Additional file 1: Table S4. THg concentrations measured in the dorsal muscles of perch varied notably in all three stations, thus mean concentrations and standard deviations (± SD) were 188.2 ± 42.0, 154.2 ± 71.3 and 110.8 ± 65.1 µg kg−1 of wet weight in station 1, 2 and 3, respectively. The ranges of δ15N values measured in perch were similar at all three sites (between approximately 14 and 18‰). At the same time, δ13C values showed wide ranges (approximately 12 ‰) for perch individuals caught in stations 1 and 2, covering also isotopic signals associated with the coastal sampling station, and relatively narrow ranges (approximately 4‰) for the individuals from station 3. Similarly, to perch, also ruffe (Gymnocephalus cernua) and roach (Rutilus rutilus) exhibited high variations of THg concentration and stable isotope values.
Stomach content analysis
The analysis of stomach content showed that stomach composition of perch significantly differs between freshwater and brackish water habitats (Fig. 2). At sampling stations 1 and 2, the Crustacean (found in 56% and 42% of the analyzed stomachs, respectively) was the predominant prey. Juvenile perch (22% at station 1 and 25% at station 2) and Chironomidae larvae (11% at station 1 and 21% at station 2) were the second most frequently consumed prey organisms while O. limosus and G. cernua were found mainly only in the digestive tract of perch from station 1. At the same time, N. integer was the most frequent prey in station 3 (found in 78% of stomachs). N. integer was also found in 25% of perch stomachs from freshwater station 2. The N. melanostomus was the second most common prey in station 3, where it was found in 29% of perch stomachs. From station 3, the A. tobianus and C. harengus membras were represented only in 12% and 6% of stomachs, respectively.
Cluster analysis
Scatterplot of the calculated stable isotope values δ13C and δ15N demonstrated clear evidence that perch specimens migrate between the sampling stations (Fig. 3). Substantial proportion of specimens sampled in stations 1 and 2 had isotopic signals consistent with feeding in the station 3 (Fig. 3A). Consequently, we divided the dataset into four subgroups, according to the three characteristics: sampling place, stable isotope values δ13C, and stable isotope values δ15N related to the trophic position of an organism (Fig. 3B and C).
Scatterplots of the measured of stable isotope values (δ13C and δ15N): A distribution between the sampling stations; B distribution between the groups assigned by means of the clustering analysis; C cluster dendrogram indicating new grouping of the sampled perch, where blue—group 1, green—group 2, red—group 3, purple—the mixed group 4 (potential recent arrivals, isotope values cannot be attributed to any of sampling stations)
The division was done as a cluster analysis based on the linear model criterion of least squares. Three of the subgroups were clearly representing respective sampling stations, while the fourth subgroup was well positioned as the mixed group (group 4) with overlapping isotope values, which cannot be associated with any of the three sampling stations. The mixed group was then considered as recent arrivals exhibiting different isotopic values of the station they were collected. The group of recent arrivals provided key information on gradual change of the modeled dietary composition explored by means of the following Bayesian mixing model, thus connecting lake, river and gulf into one ecosystem.
Exploration of the identified groups
The data were re-examined comparing THg concentrations and distribution of individual’s length among the new groups designated via cluster analysis. Group 1 exhibited the highest THg concentrations (Fig. 4A) while the lowest mean concentration of THg was found in group 3. Opposite to concentration levels, the highest mean length of perch was found in group 3 while the lowest one in group 2. The groups 1 and 4 exhibited the middle values (Fig. 4B). Although the calculated bioaccumulation slopes were quite similar among the groups (coefficient values from 0.015 to 0.029), the intercepts differed noticeably (coefficient values from 1.4 for group 3 to 2.1 for group 1), thus indicating high variation of background Hg concentrations (Fig. 4C).
Quantification of feeding ecology
Feeding ecology of the assigned subgroups were defined by means of Bayesian mixing model SIAR (Fig. 5) based on results of the stomach content analysis of every perch individual representing the corresponding subgroup. The different types of crustaceans, G. cernua, Chironomidae larvae and O. limosus were defined as the main food sources for perch in station 1. The feeding base in station 2 mainly consisted of crustaceans, G. cernua, Chironomidae larvae and juvenile perch while the N. integer, N. melanostomus, A. tobianus, C. harengus membras and crustaceans were main food items of perch in station 3. In order to reflect the geographical distribution of the available diet in the interconnected system, as food items representative of each sampling stations were selected: O. limosus from station 1, juvenile perch from station 2, and N. integer and N. melanostomus from station 3. The four sources formed an appropriate mixing polygon which covered the vast majority of data points of the mixed group.
TMF
TMF was calculated for specific food chains, reflecting the localized diet of perch for each identified subgroup. The absolute values of the calculated TMFs were quite similar for the station-assigned groups (1.45, 1.40, 1.46, respectively), but substantially higher for the mixed group (1.76).
The trophic magnification curve of the mixed group (group 4) was significantly different from the others by a steeper slope (Table 1, M2) and by higher intercept compared to group 3 (Table 1, M1). Meanwhile, groups 1 to 3 had statistically similar slopes, thus indicating similar trophic magnification patterns (Table 1, M2). At the same time, significantly lower intercept of group 3, compared to groups 1 and 2 (Table 1, M1) probably denotes lower Hg background concentrations found at the station.
Influence of dietary preferences
Generalized Additive Modeling was implemented to understand how dietary preferences of perch in different feeding grounds affect the Hg uptake. To avoid covariance of food source variables, three validated models with different combinations of food items were selected and interpreted (Table 2). The obtained results indicated seasonality (spring and autumn sampling) as a significant factor affecting measured THg LOG-concentrations, for example, samples collected in spring had higher THg concentrations than the autumn samples (demonstrated by a positive intercept correction for the spring season from 0.106 up to 0.120). The δ15N values showed a significant relationship with the THg concentration; however, the positive slope coefficient was only 0.09 in the all three models.
The models let us establish that the food item C. harengus membras had the most significant mitigating effect on THg concentration, with negative slopes ranging from -0.349 to -0.501. Another food item with significant negative slope coefficient (-0.460) was Crustacean. The rest of the food items were secondarily significant for the model (α > 0.05), although they had different directions of the influence and slope values. A highly positive effect was observed for Chironomidae larvae (slope values from 0.233 to 0.460). O. limosus (slope values from 0.143 to 0.184), perch juvenile (slope values from 0.010 to 0.186) and G. cernua (slope value 0.120) were other food items that contributed to the uptake of Hg by perch. N. melanostomus and N. integer exhibited a neutral influence on THg concentration measured in consumer perch, indicating slightly positive slope coefficients of 0.080 and 0.023, respectively.
Discussion
The combination of stomach content analysis, as a sort of “snap-shot” of the recently consumed prey, with metabolically active tissues (such as muscles) that provide dietary and source information for up to several weeks [32] were instrumental in sorting out to which geographically distinct sampling area each perch specimen should be assigned. Since perch in the Gulf of Riga (station 3 area) do not have suitable spawning and nursing grounds, the specimen group assigned to that area has probably migrated to the Gulf of Riga from freshwater similarly to that observed elsewhere by Järv [11]. This agrees with behavioral features of perch, like seasonal patterns in their distribution and movement between habitats [33]. The distinct stomach content and characteristic of isotopic signals for this group suggests that once migrated to the coastal waters the perch specimens stay there whether as stable kin-related groups as suggested by Gerlach et al. [12] and Semeniuk et al. [33] or as separate individuals. The approach applied in this study enabled us also to identify recent arrivals, e.g., specimens that have been feeding and accumulating Hg in areas different from where they were caught.
As we successfully demonstrated, the perch specimens in freshwater ecosystems (river and lake stations) have substantially higher THg concentrations. So, with some degree of certainty we can speculate that observed inter-annual differences, from 30 μg kg−1 ww in 2019 to 103 μg kg−1 ww in 2015, of THg values obtained within national monitoring program (LIAE database) in coastal waters represented by station 3, can mostly be explained by different proportions between recent arrivals from adjacent freshwater basins and specimens that have been feeding in an area for more extended periods of time. Furthermore, the seasonal factor produced by all three GAM models, e.g., higher THg concentrations were associated with spring sampling, and can be clearly related to recent migration from inland waters to the coastal.
Although the concentration of THg in specimens representing freshwater ecosystems is substantially higher than in specimens representing marine coastal waters, the subtraction of values measured in recent arrivals resulted in a slight increase of mean concentration in the coastal group. Most likely, the observed phenomenon is related to significant upward change of median size of perch, and not to the Hg concentration itself. Therefore, it can be argued that comparison of concentration means alone is a poor approach for assessment of Hg contamination.
At the same time, the Hg bioaccumulation curves in relation to the length of individual gave more detailed information about the specific uptake tendencies. The results indicate that functional processes responsible for Hg accumulation (for example fish biometrics), Hg bioavailability and chemical composition of Hg substances [34] are quite similar, regardless of the specimen origin or local feeding base. So, the geographical differences in THg concentration were mainly observed because background concentrations of Hg are substantially higher in the inland water bodies than in the Gulf of Riga. This conclusion is supported by notably higher THg levels in SPM, used as a proxy of phytoplankton, collected in river and lake stations (Additional file 1: Table S4). And, as stated by Kehrig [35], Hg enters the food web at phytoplankton level and is transferred to higher organisms via trophic transfer.
The general structure of the perch diet was quite similar among the studied areas, e.g., mostly several types of crustaceans, Chironomidae larvae and small fish. However, C. harengus membras present only in the gulf station exhibited noticeable reduction properties of Hg uptake, which explains the substantial differences in the levels of THg measured in the station-associated groups indicated by the clustering analysis. Moreover, according to the study, the trophic position of prey alone (in our case, δ15N values) cannot be associated with the intensity of Hg uptake by consumers. For example, Chironomidae larvae (δ15N 8.3 to 13.4 ‰) and Crustacean (δ15N 7.0–12.9‰) within the comparable maximum consumption ratio exhibited the opposite modeled effects on the estimated THg concentration in perch tissues, and N. integer (δ15N 11.4‰) despite the twofold maximum consumption ratio showed a neutral impact. Similarly, higher THg concentration rates cannot be associated with the trophic position of prey within the same feeding ground, which was well demonstrated by Chironomidae larvae and G. cernua (δ15N 15.7 to 18.5‰), where the prey with lower δ15N values had stronger correlation with high THg concentrations estimated from the model. In the case discussed above Chironomidae larvae showed substantially higher THg concentration values than Crustacean (Amphipoda) and N. integer, therefore we suggest that consumption ratio has to be discussed in conjunction with Hg concentrations measured in prey. Thus, besides precise determination of the food sources for better tracing of metal accumulation suggested by Le Croizier et al. [36], information on background concentrations at the site is important in our study as well. In addition, Jones et al. [37] suggests that, to avoid misinterpretation of spatial and temporal trends, fish biometrics modeling is of high significance when designing any monitoring program focused on seafood safety.
We propose that for future perspectives, besides Hg, other trace metals such as arsenic (As), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), nickel (Ni) and zinc (Zn) can be investigated with the supplementary tool presented in this article. Although due to limits of models’ sensitivity the effectiveness may vary depending on intensity of trophic magnification and/or biodilution slopes [38, 39], we assume that if TMF is close to 1, the tool may not be able to distinguish the difference in concentration of the contaminant between stations. The recent trophic magnification and biomagnification studies in the Baltic Sea indicated that Ni, Zn, Pb, Cd tend to be effectively biodiluted with increasing trophic level (TMF < 1), As, Cr and Cu showed no significant relationship with trophic levels (TMF = 1), while Hg trophically magnified (TMF > 1) [38]. In contrast to this, the global meta-analytical study conducted by Sun et al. [39] suggests that in other regions Pb and Zn also show trophic magnification tendencies [39].
The limitation of this study is that we presented a complete picture only from a single year perspective. We can of course speculate that the site-specific food items defined in this study will influence perch THg concentrations at an equal level also during following years. However, the well-known opportunistic feeding behavior of perch [33] suggests that they will inevitably switch to other taxa if availability of previously consumed taxa becomes limited, or if a more profitable source of energy appears, similarly as round goby (Neogobius melanostomus, invasive in the Baltic Sea) became a highly consumed prey for perch in recent years [40, 41]. Another weak point to be considered is that the isotopic signal changes faster than the level of accumulated Hg [24, 42]. So, the specimens, that at the onset of a feeding period have spent sufficient time in one area to equilibrate Hg concentration with the level characteristic for that area and then migrates to another area and have time to change isotopic signals before they are caught, might not have sufficient time to adjust also Hg concentrations. This could be improved by more regular sampling, which would give more precise information about influence of perch mobility on measured Hg concentrations. Also, comparison with another distant Gulf of Riga station which is insignificantly affected by the large freshwater ecosystems, could be a useful adjunct for the further studies.
In the case of this study, use of δ15N and δ13C values fulfilled our requirements regarding differentiation of the feeding grounds, examination of feeding ecology and thus trophic magnification of Hg. Although, when the object of interest is a higher resolution of the movement of the fish [43] or if δ13C alone fails to distinguish spatial differences between habits or sources [44, 45], other isotopes such as sulphur (34S/32S) can be applied especially in estuary systems with a certain salinity gradient [44, 46, 47].
Conclusions
The study showed that the THg concentrations measured in associated aquatic systems may be affected by high mobility of perch, which could be an issue for consideration during Hg contamination monitoring events. The recent arrivals can change distribution of Hg concentrations measured at specific locations, especially more contaminated inland individuals can noticeably rise concentration values in the coastal areas close to estuaries. The home-range migration habits of fish species selected as a biological matrix for investigation of the environmental Hg concentrations and possibly other hazardous substances, are an important feature which has to be discussed more carefully for accurate conclusions on the environmental status of the studied areas. We highly recommend implementation of chemical markers, such as stable isotopes, for identification of mobile specimens when designing monitoring programs focused on Hg and other hazardous substances. All the three sampling stations showed considerable trophic magnification of Hg through the food chain. Therefore, we concluded that different feeding grounds in the frame of one interconnected system may have specific features, such as higher or lower TMF and also unique food items, such as freshwater O. limosus and marine species C. harengus membras or N. melanostomus. The model showed that trophic position of prey is not decisive regarding Hg accumulation rates, although Hg concentration measured in prey in conjunction with its consumption ratio serves as good explainer of the measured concentrations of the hazardous substance.
Availability of data and materials
The datasets obtained and analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- AAS:
-
Atomic absorption spectrophotometry
- AIC:
-
Akaike information criterion
- ANCOVA:
-
Analysis of covariance
- C:
-
Carbon
- 13C/12C:
-
Carbon stable isotope ratio
- δ 13C:
-
Carbon isotope value
- GAM:
-
Generalized additive model
- GF/F:
-
Glass fiber filter
- HELCOM:
-
The Baltic Marine Environment Protection Commission
- HEPP:
-
Hydroelectric power plant
- Hg:
-
Mercury
- LIAE:
-
Latvian Institute of Aquatic Ecology
- LOG:
-
Logarithmic
- N:
-
Nitrogen
- 15N/14N:
-
Nitrogen stable isotope ratio
- δ 15N:
-
Nitrogen isotope value
- SD:
-
Standard deviation
- SIAR:
-
Package “Stable Isotope Analysis in R”
- SPM:
-
Suspended particulate matter
- THg:
-
Total mercury
- US EPA:
-
United States Environmental Protection Agency
References
Mason RP, Fitzgerald WF, Morel FMM (1994) The biogeo-chemical cycling of elemental mercury: anthropogenic influences. Geochim Cosmochim Acta 58:3191–3198. https://doi.org/10.1016/0016-7037(94)90046-9
Chalkidis A, Jampaiah D, Aryana A, Wood CD, Hartley PG, Sabri YM, Bhargava SK (2020) Mercury-bearing wastes: Sources, policies and treatment technologies for mercury recovery and safe disposal. J Environ Manage 270:110945. https://doi.org/10.1016/j.jenvman.2020.110945
Kocman D, Horvat M, Pirrone N, Cinnirella S (2013) Contribution of contaminated sites to the global mercury budget. Environ Res 125:160–170. https://doi.org/10.1016/j.envres.2012.12.011
HELCOM (2016) HELCOM Monitoring Manual: Introduction. Available via https://helcom.fi/media/documents/Monitoring-Manual-general-Introduction-text.pdf. Accessed 1 Mar 2021.
OSPAR Commission (2008) Co-ordinated Environmental Monitoring Programme - Assessment Manual for contaminants in sediment and biota. Available via: https://www.ospar.org/documents?v=7115 Accessed 1 Mar 2021
HELCOM (2010) Hazardous substances in the Baltic Sea – An integrated thematic assessment of hazardous substances in the Baltic Sea. Baltic Sea Environment Proceedings 120B. Available via http://www.helcom.fi/Lists/Publications/BSEP120B.pdf. Accessed 19 Sept 2020.
'Commission Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs' (2006) Official Journal L 364, 5.
Polak-Juszczak L (2018) Distribution of organic and inorganic mercury in the tissues and organs of fish from the southern Baltic Sea. Environ Sci Pollut Res 25:34181–34189. https://doi.org/10.1007/s11356-018-3336-9
Jacobson P, Bergström U, Eklöf J (2019) Size-dependent diet composition and feeding of Eurasian perch (Perca fluviatilis) and northern pike (Esox lucius) in the Baltic Sea. Boreal Environ Res 24:137–153
Suhareva N, Aigars J, Poikane R, Jansons M (2020) Development of fish age normalization technique for pollution assessment of marine ecosystem, based on concentrations of mercury, copper, and zinc in dorsal muscles of fish. Environ Monitor Assess 192(5):279. https://doi.org/10.1007/s10661-020-08261-x
Järv L (2000) Migrations of the perch (Perca fluviatilis L.) in the coastal waters of western Estonia. Proc Estonian Acad Sci Biol Ecol 49(3):270–276
Gerlach G, Schardt U, Eckmann R, Meyer A (2001) Kin-structured subpopulations in Eurasian perch (Perca fluviatilis L.). Heredityv (Edinb) 86:213–221. https://doi.org/10.1046/j.1365-2540.2001.00825.x
Huxham M, Kimani E, Newton J, Augley J (2007) Stable isotope records from otoliths as tracers of fish migration in a mangrove system. J Fish Biol 70(5):1554–1567. https://doi.org/10.1111/j.1095-8649.2007.01443.x
MacKenzie KM, Palmer MR, Moore A, Ibbotson AT, Beaumont WR, Poulter DJ, Trueman CN (2011) Locations of marine animals revealed by carbon isotopes. Sci Rep 1:21. https://doi.org/10.1038/srep00021
Richert JE, Galván-Magaña F, Klimley AP (2015) Interpreting nitrogen stable isotopes in the study of migratory fishes in marine ecosystems. Mar Biol 162:1099–1110. https://doi.org/10.1007/s00227-015-2652-6
Mizutani H, Fukuda M, Kabaya T, Wada E (1990) Carbon isotope ratio of feathers reveals feeding behaviour of cormorants. Auk 107:400–437. https://doi.org/10.2307/4087626
Smith RJ, Hobson KA, Koopman HN, Lavigne DM (1996) Distinguishing between populations of fresh and salt-water harbor seals (Phoca vitulina) using stable-isotope ratios and fatty acids. Can J Fish Aquat Sci 53(2):272–279. https://doi.org/10.1139/f95-192
McCormack SA, Trebilco R, Melbourne-Thomas J, Blanchard JL, Fulton EA, Constable A (2019) Using stable isotope data to advance marine food web modelling. Rev Fish Biol Fisheries 29:277–296. https://doi.org/10.1007/s11160-019-09552-4
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Manko P (2016) Stomach content analysis in freshwater fish feeding ecology. Mydavateľstvo Prešovskej univerzity, Prešov. Available via https://www.researchgate.net/publication/312383934_Stomach_content_analysis_in_freshwater_fish_feeding_ecology. Accessed 9 June 2021.
Rumbold DG, Lange TR, Richard D, DelPizzo G, Hass N (2018) Mercury biomagnification through food webs along a salinity gradient down-estuary from a biological hotspot. Estuar Coast Shelf Sci 200:116–125. https://doi.org/10.1016/j.ecss.2017.10.018
U.S. EPA. (1998) "Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry," Revision 0. Available via: https://www.epa.gov/esam/epa-method-7473-sw-846-mercury-solids-and-solutions-thermal-decomposition-amalgamation-and. Accessed 1 Mar 2021.
Qi H, Coplen TB, Geilmann H, Brand WA, Böhlke JK (2003) Two new organic reference materials for δ13C and δ15N measurements and a new value for the δ13C of NBS 22 oil. Rapid Commun Mass Spectrom 17:2483–2487. https://doi.org/10.1002/rcm.1219
Bradley MA, Barst BD, Basu N (2017) A review of mercury bioavailability in humans and Fish. nt. J Environ Res Public Health 14(2):169. https://doi.org/10.3390/ijerph14020169
Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92(10):823–835. https://doi.org/10.1139/cjz-2014-0127
Fisk AT, Hobson KA, Norstrom RJ (2001) Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the North water Polynya marine food web. Environ Sci Technol 35:732–738. https://doi.org/10.1021/es001459w
Muto EY, Soares LSH, Sarkis JES, Hortellani MA, Petti MAV, Corbisier TN (2014) Biomagnification of mercury through the food web of the Santos continental shelf, subtropical Brazil. Mar Ecol Prog Ser 512:55–69. https://doi.org/10.3354/meps10892
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Zuur AF, Ieno EN (2016) A protocol for conducting and presenting results of regression-type analyses. Methods Ecol Evol 7:636–645. https://doi.org/10.1111/2041-210X.12577
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer-Verlag, New York. https://doi.org/10.1007/978-0-387-87458-6
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Semeniuk CAD, Magnhagen C, Pyle G (2016) Behaviour of perch. In: Couture P, Pyle G (eds) Biology of Perch. CRC Press, Boca Raton
Wang WX, Rainbow PS (2005) Trace metals in barnacles: the significance of trophic transfer. Sci China Life Sci 48:110–117. https://doi.org/10.1007/BF02889808
Kehrig HA (2011) Mercury and plankton in tropical marine ecosystems: A review. Oecol Aust 15(04):869–880. https://doi.org/10.4257/oeco.2011.1504.07
Le Croizier G, Schaal G, Gallon R, Fall M, Le Grand F, Munaron JM, Rouget ML, Machu E, Le Loc’h F, Laë R, De Morais LT (2016) Trophic ecology influence on metal bioaccumulation in marine fish: inference from stable isotope and fatty acid analyses. Sci Total Environ 573:83–95. https://doi.org/10.1016/j.scitotenv.2016.08.035
Jones HJ, Swadling KM, Tracey SR, Macleod CK (2013) Long term trends of Hg uptake in resident fish from a polluted estuary. Mar Pollut Bull 73(1):263–272. https://doi.org/10.1016/j.marpolbul.2013.04.032
Nfon E, Cousins IT, Järvinen O, Mukherjee AB, Verta M, Broman D (2009) Trophodynamics of mercury and other trace elements in a pelagic food chain from the Baltic Sea. Sci Total Environ 407(24):6267–6274. https://doi.org/10.1016/j.scitotenv.2009.08.032
Sun T, Wu H, Wang X, Ji C, Shan X, Li F (2020) Evaluation on the biomagnification or biodilution of trace metals in global marine food webs by meta-analysis. Environ Pollut. https://doi.org/10.1016/j.envpol.2019.113856
Almqvist G, Strandmark AK, Appelberg M (2010) Has the invasive round goby caused new links in Baltic food webs? Environ Biol Fish 89:79–93. https://doi.org/10.1007/s10641-010-9692-z
Puntila R, Strake S, Florin A-B, Naddafi R, Lehtiniemi M, Behrens JW, Kotta J, Oesterwind D, Putnis I, Ojaveer H, Ložys L, Uspenskiy A, Yurtseva A (2018) Abundance and distribution of round goby (Neogobius melanostomus): HELCOM Baltic Sea Environment Fact Sheet 2018. Available via: https://helcom.fi/wp-content/uploads/2020/06/BSEFS-Abundance-and-distribution-of-round-goby.pdf . Accessed 1 Mar 2021
Van Walleghem JL, Blanchfield PJ, Hrenchuk LE, Hintelmann H (2013) Mercury elimination by a top predator, Esox lucius. Environ Sci & Technol 47:4147–4154. https://doi.org/10.1021/es304332v
Brian F, Chumchal MM (2011) Sulfur stable isotope indicators of residency in estuarine fish. Limnol Oceanogr. https://doi.org/10.4319/lo.2011.56.5.1563
France R (1995) Critical examination of stable isotope analysis as a means for tracing carbon pathways in stream ecosystems. Can J Fish Aquat Sci 52(3):651–656
Jardine TD, Kidd KA, Polhemus JT, Cunjak RA (2008) An elemental and stable isotope assessment of water strider feeding ecology and lipid dynamics: synthesis of laboratory and field studies. Freshw Biol 53(11):2192–2205. https://doi.org/10.1111/j.1365-2427.2008.02044.x
Carr MK, Jardine TD, Doig LE, Jones PD, Bharadwaj L, Tendler B, Chételat J, Cott P, Lindenschmidt K-E (2017) Stable sulfur isotopes identify habitat-specific foraging and mercury exposure in a highly mobile fish community. Sci Total Environ 586:338–346. https://doi.org/10.1016/j.scitotenv.2017.02.013
Peterson BJ, Howarth RW, Garritt RH (1986) Sulfur and carbon isotopes as tracers of salt-marsh organic matter flow. Ecology 67:865–874. https://doi.org/10.2307/1939809
Acknowledgements
The authors would like to thank the staff of Latvian Institute of Aquatic Ecology Viktors Pērkons, Atis Labucis, PhD Māris Skudra and Miks Papirtis for taking care of sampling campaigns, operation of boat and sampling equipment, such as scientific gill nets and Wildco Petite Ponar Grab; Ieva Putna-Nīmane and Anete Fedorovska for taking part in zooplankton sampling and pre-treatment; as well as Mintauts Jansons for analysis of THg concentrations and management of analytical equipment. The authors would also like to thank Professor, Dr.chem. Arturs Vīksna, Lauma Buša, Māris Bērtiņš from the Laboratory of Analytical Chemistry at Faculty of Chemistry, University of Latvia for analysis of stable isotopes.
Funding
This study has received funding from the State Research Program EVIDEnT—“The value and dynamic of Latvia’s ecosystems under changing climate” and the project “Improving knowledge on the state of the marine environment” financed by European Maritime and Fisheries Fund 2014–2020, Project Nr.: 17-00-F06803-000001.
Author information
Authors and Affiliations
Contributions
NS: conceptualization, sampling, investigation, sample treatment, data interpretation and visualization, writing of and drafting the original version. JA: conceptualization, data interpretation, writing—review and editing; RP: sampling site selection, experimental analysis, writing—review and editing. JT: sampling, experimental analysis, editing and reviewing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Quality assurance with biota reference material ERM-CE278k and ERM-BB422 within current study; Table S2. Trophic levels found in the available literature; Table S3. Computed ratios of source contribution in the final mixture (perch dorsal muscles), data obtained by means of Bayesian mixing model SIAR; Table S4. List of collected organisms and measured total mercury (THg) concentration, carbon and nitrogen isotopic values; Table S5. Estimated regression parameters (intercept and slope values), standard errors, t-values and P-values for the Gaussian GAM presented in Eqs. (1, 2, 3).
Additional file 2.
Visualization of mercury (Hg) concentrations estimated for the Gaussian GAM for the maximum and minimum possible contribution of Clupea harengus membras to diet of perch.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suhareva, N., Aigars, J., Poikāne, R. et al. The influence of feeding ecology and location on total mercury concentrations in Eurasian perch (Perca fluviatilis). Environ Sci Eur 33, 82 (2021). https://doi.org/10.1186/s12302-021-00523-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-021-00523-w