Abstract
Background
Genetically modified glyphosate-tolerant cultivar varieties have been a commercial success widely known as Roundup Ready plants. As new glyphosate-tolerant varieties are introduced to satisfy agriculture demand, it is relevant to review the scientific evidence that documents the quality and safety of such biotechnology. Assessments of genetically modified glyphosate-tolerant plants are partly based on the reports from laboratory comparisons with non-modified plants (near-isogenic relatives). Such comparative testing is typically performed as analysis of plant material composition and in animal feeding studies. The material for testing is typically produced in test-fields set up as model environments. Most of this research is planned, performed and reported by researchers employed by biotech industry companies.
Perspective
The present paper aims to: (1) review 15 reports on compositional analyses of glyphosate-tolerant cultivars and 15 reports from animal feeding studies, (2) discuss recent data indicating glyphosate residue in Roundup Ready soybean, (3) outline recent developments of cultivars with increased tolerance to glyphosate.
Findings
The reviewed industry studies show methodological flaws: glyphosate-tolerant GM crops are designed for use with glyphosate herbicide. However, glyphosate herbicides are often not applied in test-study cultivation. In the studies where glyphosate herbicides were applied to growing plants, the produced plant material was not analyzed for glyphosate residues. This review has failed to identify industry studies that mention glyphosate residues in glyphosate-tolerant plants. This indicates that questions and evidence of importance for regulatory assessment have been systematically ignored. Independent research has investigated this issue and found that glyphosate-tolerant plants accumulate glyphosate residues at unexpected high levels. Glyphosate residues are found to have potential to affect plant material composition. Furthermore, these residues are passed on to consumers.
Conclusions
Industry studies are not sufficient for regulation. Despite decades of risk assessments and research in this field, specific unanswered questions relating to safety and quality aspects of food and feed from GM crops need to be addressed by regulators. Independent research gives important supplementary insight.
Similar content being viewed by others
Introduction
Recently proposed changes in the European Union (EU) legislative framework for assessment and approval of genetically modified cultivars (GM crops) could lead to a delegation of responsibility, from the European Food Safety Authority (EFSA) to regulatory authorities of individual EU member states [89]. Continuing challenges relating to assessments of applications for import or cultivation of GM crops accentuate the need for reliable and transparent evidence on GM crop quality and safety issues.
In a 2013 review of two decades of research on possible unintended compositional changes in GM crops, two senior scientists state that such GM crops have been subjected to a large number of analytical studies, which confirm compositional equivalence. They conclude that GM crops are safe and rhetorically ask; “How much uncertainty remains after 20 years of research?” [1].
I see that the authors have concluded that compositional equivalence is sufficiently established and I hear their argument stating that further safety studies of GM crops are unnecessary. However, I still propose to answer the rhetorical question presented by these senior scientist authors, of whom one is representing a major industrial producer of GM crops whilst the other is retired from the Food and Drug Administration of the United States.
Unresolved important uncertainties remain a concern regarding genetically modified crop quality and safety. One such specific issue will be reviewed here: the somewhat neglected fact that GM crops designed and modified to be tolerant to herbicides such as glyphosate, will be subjected to application of such chemicals in the field and, therefore, must be expected to have biological interaction with these herbicidal sprays.
Background
Glyphosate herbicides have chemical and physical qualities that facilitate penetration into the plant tissue and transportation within the plant, disrupting plant metabolism and killing the recipient by systemic action [2]. Glyphosate is an important chemical; it is a best-selling herbicide with an annual application in the order of 0.6–1.2 million tons globally [3, 4]. Glyphosate is used in farming, parks, gardening, forestry and wetland management [5]. Glyphosate is widely used as a desiccant to induce ripening in semi mature crops [6] and it has been found to have antibiotic qualities [7]. In the context of this review, it must be noted that the advent of glyphosate-tolerant crops has contributed to a sharp increase in global dispersal of this chemical [8].
Early findings justified that glyphosate was widely recognized as having relatively low environmental impact [9], low toxicity for field workers handling the chemical, and low toxicity for consumers ingesting residues of it through food [10]. However, in recent years such established assumptions on safety have come under revision, as glyphosate is found to have more subtle and complex effects than what has previously been acknowledged [11, 86]. Furthermore, although recent pesticide screenings of fruits, vegetables and other food in the EU have shown that a majority of the samples (55 %) do not contain traceable quantities of pesticide residues, still glyphosate stands out as a commonly detected pesticide in European food, present in approximately 8 % of the samples [12]. However, the vast majority of samples show concentrations well below the existing spacious acceptance levels. The Codex Alimentarius maximum residue levels (MRLs) for glyphosate in food and agriculture products span a wide range from 0.05 mg/kg in commodities such as banana, milk, eggs and animal meat, up to 500 mg/kg for commodities strictly intended for feed use, such as hay from grasses, alfalfa fodder and pea fodder (Table 1). MRLs for the most common crops which are commercialized in glyphosate-tolerant varieties range from 5 mg/kg (maize), to 20 mg/kg for soybean, 30 mg/kg for rape seed and 40 mg/kg for cotton seed. Notably, regulation in the EU and the USA define higher MRLs for several of these commodities. Despite the detected increasingly wide-spread occurrence of glyphosate residues in food, animal feed, water [5], air [13], human blood and human milk [14] and human urine [15, 80], it is not within the mandate of this review to evaluate whether the relatively high acceptance levels (MRLs) for glyphosate residues in food and feed are scientifically justified, however relevant the question may seem.
Herbicide-tolerant cultivars dominate agriculture
It has been estimated that an overwhelming 81 % majority of GM crops in cultivation are herbicide-tolerant varieties [16]. The majority of those herbicide-tolerant crops are Roundup Ready plant cultivars (RR crops) genetically modified to tolerate glyphosate herbicides such as the commercial product Roundup. The first such varieties were introduced in 1996 and rapidly gained popularity amongst farmers. Herbicide tolerance allows for post-emergence application and in principle eliminates the need for pre-plant tillage and manual weeding. This is an advantage which contributes to reduced soil erosion and reduced production expenses [16–18]. Despite challenges from increasing numbers of agriculture weeds that are resistant to glyphosate herbicide, glyphosate tolerant cultivars such as RR soy, RR corn, RR canola and RR cotton are still the most popular and widely grown genetically modified plant varieties [8]. Additional glyphosate-tolerant cultivars such as RR sugar beet, RR wheat and RR alfalfa are introduced as promising and potentially important crops [4]. Glyphosate tolerant plants thus form a dominant and increasing proportion of the biomass produced globally from industrial agriculture. This biomass is used for farm-animal feed, for bio-fuels and for important constituents in human food products.
Industry provides most data for risk assessment of GM crops
Regulatory assessments of applications for import and use of products from GM cultivars into the European Union/EEC area, and applications for open cultivation of such plants in Europe, are centrally processed by the European Food Safety Authority (EFSA) based on documentation submitted by applicants [19, 21, 22, 24]. As mentioned in the introduction, the recently proposed changes in EU regulation of GM crops could delegate this challenging responsibility to individual member states [89].
In a standing controversy over GMO safety, the EU approval process as conducted by EFSA has been claimed to be unsupportive of independent research findings [19, 20, 86].
Typically industry applications for import and/or cultivation contain three main categories of information; (1) biochemical information on the actual event including the structure and origin of transgenic construct, (2) information from compositional analysis where the GM cultivar in question is compared to near-isogenic mother lines or other comparators representative of unmodified varieties grown under similar conditions, and (3) results from feeding studies in test animals such as rodents or farm animals such as pigs and poultry. Implicitly, the GM material in such tests should be representative of the actual material intended for consumption.
European regulation defines guidelines for animal feeding studies and for compositional analysis, to determine whether food and feed from GM crops reliably has qualitative equivalence to that of conventional non-modified crops [21, 22].
The concept of substantial equivalence [23] is used by regulators and industry scientists to validate GM-crop quality. Comparative analysis of composition and comparative testing in animal feeding trials are still the two fundamental methods in use for assessment of substantial equivalence of products from herbicide-tolerant crops and other genetically modified biomass intended for consumption. Guidelines for such analysis and testing aim to ensure that the new varieties are as safe and nutritious as conventional plants. Therefore, such testing includes risk assessments which anticipate potentially adverse effects stemming from qualitative differences or undesirable constituents [23, 24]. The Food and Agriculture Organization (FAO) and World Health Organization (WHO) established the Codex Alimentarius commission in 1963 to develop harmonized international food standards, guidelines and codes of practice to protect the health of the consumers. The aim of the Codex regulation is to anticipate not only direct risk, but also indirect/unanticipated risk [25]. Thus, it is interesting to note that the Codex Alimentarius commission in 1999–2001 had protracted evaluations on the possibility of establishing specific and unique standards for herbicide residue levels in herbicide-tolerant GM crops [26]. The reports of this regulatory process document that this question was seen relevant at that time. However, the result of this process was a decision not to establish separate residue limits for herbicide-tolerant cultivars.
Although generally recognized as safe by regulators in the United States Food and Drug Administration [27], safety assessment of products from GM crops is a contested issue in Europe [19] and numerous other countries world-wide. Safety assessment is mostly based on testing performed by industry companies or by researchers working for such companies. Complex legal and commercial aspects of patented biotechnology products restrict independent researcher access to both such GM material (patented property of industry) and to data from development and testing, which is regarded as intellectual property and thus confidential [28, 29].
Review of published evidence
15 published reports from compositional analyses of plant material grown from glyphosate-tolerant cultivars and 15 published reports from tests of such material in animal feeding studies were extracted from peer-reviewed scientific journals (Tables 2, 3). The majority of these studies are found to be funded by biotech industry companies and are also designed and performed by researchers employed by these companies.
Analyses of composition
15 publications present results from comparative analyses in which specific glyphosate-tolerant plant material is compared to near-isogenic unmodified material or other relevant conventional plant material. 14 of the 15 published analyses are industry studies (Table 2). Of these, only 7 (50 %) specify that glyphosate herbicide was applied during cultivation. None of the 14 industry studies present data on glyphosate residues or give other indications that such analyses have been performed. Only one independent study reports glyphosate residues [30].
All 14 industry studies (in which glyphosate has not been quantified) find the various glyphosate-tolerant GM crops (soybean, corn and canola) to be compositionally equivalent to non-modified comparators. The one independent study (in which glyphosate has been quantified) finds significant differences in composition of glyphosate-tolerant soybean compared to non-modified varieties of soybean (Table 2).
Animal feeding studies
15 published reports from animal feeding studies with glyphosate-tolerant plant material are also seen to lack information on herbicide residues (Table 3). 6 of these 15 studies are performed by researchers with industry affiliation. The remaining nine studies are independent studies performed by researchers in government agencies, universities or other institutions recognized to be independent from the implicit financial issues associated with GM-crop commercialisation and production.
Of the six studies performed by industry, plant material for feed has been produced with application of glyphosate herbicide in three studies (50 %). In the nine independent studies, plant material for feed has been produced with application of glyphosate herbicide in five studies (56 %). Unfortunately, no information on dosage is given in any of these studies (100 %).
3 of the 9 independent studies report that relevant pesticide analysis have been performed (33 %). Of the six industry studies, only one (17 %) reports that pesticide analysis has been performed [31] (Table 3). However, although glyphosate herbicide is the only pesticide applied in the mentioned study, the subsequent analysis for pesticide residues includes numerous chemical compounds known as active ingredients in various other commercial pesticide formulations. Paradoxically, the analysis does not include glyphosate or the main metabolite of this chemical. Due to those obvious shortcomings of the pesticide analysis in the mentioned study, it is concluded that none of the six industry studies include analyses for relevant pesticides (0 %).
Based on the data from test animal performance and histology, seven studies find no significant effects from GM feed (produced from glyphosate-tolerant plant material). These seven studies include one of the independent studies and all six industry studies. The remaining eight independent studies find significant effects attributable to GM feed produced from glyphosate-tolerant plant material (Table 3).
In the eight studies reporting significant effects from GM feed, two studies relate these effects to residues of glyphosate. One of the studies indicates that test animal growth and reproduction decrease in correlation with increasing levels of glyphosate residues [32].
Discussion
30 published reports from studies of compositional analysis glyphosate-tolerant GM plant varieties and from feeding studies using glyphosate-tolerant GM plant varieties have been reviewed. These studies were performed in the years 1996–2015. A simple synthesis of available information on study design, methods and results shows that:
-
14 of 15 studies on composition and 6 of 15 animal feeding studies were performed by biotech industry companies.
-
16 of 30 studies (53 %), used material actually sprayed with glyphosate herbicide during cultivation. No information on dosage was given.
-
Only 4 of 30 studies (13 %) address the issue of glyphosate residues. None of these 4 studies were funded by industry.
-
Only 1 of 30 studies (3 %) has performed analysis and quantification of glyphosate residues.
These findings fundamentally challenge the basis for regulatory assumption of substantial equivalence between glyphosate-tolerant GM varieties and unmodified comparators. The findings are a strong argument for mandatory inclusion of pesticide analysis data in regulatory assessment of GM crop, notably in assessments of herbicide-tolerant crops. Two of the animal feeding studies performed by independent researchers [86, 87] used GM crop material as well as unmodified comparators supplied by industry. No analysis was performed to control the compositional quality of this material.
Thus, the literature review indicates that there are relatively few representative studies available for regulatory evaluation of scientific evidence on herbicide-tolerant crop quality and safety. Furthermore, it is found that the majority of available studies are presented as reports from compositional analyses and animal feeding studies, and predominantly performed either by biotech industry companies (with potentially conflicting interests in research outcome) or by subcontractors working for the biotech industry companies (Tables 2, 3). Society should expect the biotech industry companies to continue to conduct such studies to peer-review standard and the industry should continue to bear associated costs. However, it is evident that appropriate revisions of standards are needed, and supplementary studies by independent researchers should be encouraged. Published evidence on safety testing presented by the industry has generally been recognized by EFSA as sufficient for regulatory purpose, despite the fact that several potentially conflicting issues have been continuously raised by independent researchers [19, 20, 28, 33–35, 52, 86]. Such critique has also questioned both the principle of delegated self-control and the validity of industry methods. Some of this critique has led to temporary adjustments of protocols and changes in methodology. A review by independent scientists in 1999 [33] examined results of three initial industry tests that were published in 1996. These first industry tests claimed substantial equivalence of GM crops glyphosate-tolerant GTS 40-3-2 soybean [34–36] and seed from glyphosate-tolerant cotton [37] compared to unmodified isolines. However, the review noted that the industry reports were based on tests of glyphosate-tolerant material grown in artificial conditions without application of complimentary glyphosate herbicides. The GM crops thus produced, were seen to be “not representative” of the crops actually produced in agriculture [33]. Several industry researchers immediately acknowledged the necessity to change these specific approaches, and subsequent industry publications on quality of glyphosate-tolerant varieties of soy [38–41], maize [42], alfalfa [43] and cotton [44] specified that normal cultivation practice had been used in production, including prescription rate application of glyphosate via commercial glyphosate herbicides such as Roundup. One industry paper published immediately following the 1999 criticism even specifies in its title that glyphosate herbicides have been applied [45]. Despite this change of practice and the acknowledged need for realistic field conditions to produce material for evaluation, numerous subsequent tests have been published where again biomass from glyphosate-tolerant cultivars is used for comparison despite having been grown in artificial conditions without application of complimentary herbicides. Recently, 10 studies presented by industry applicants as evidence for regulatory approval of glyphosate-tolerant cultivars were reviewed [34, 35], and the author concludes that lack of relevant herbicide application is still a discrediting flaw in such studies. However, although this highlights one systematic flaw in studies currently accepted for regulatory purpose documentation, the unknown magnitude of herbicide residues must be recognized as a subsequent and not least important aspect.
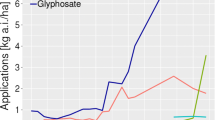
The relevance of testing for herbicide residues is highlighted by the findings of a recent study on composition of plant material [30] performed by independent researchers. The study reports high levels of glyphosate residues (Fig. 1) in glyphosate-tolerant soybean (Roundup Ready soy GTS 40-3-2). The study also finds that residues of glyphosate and the primary metabolite aminomethylphosphonic acid (AMPA) are correlated to differences in crop composition. In 2003 and 2004, independent research demonstrated that residues of glyphosate herbicides will accumulate in glyphosate-tolerant plant material [46, 47] but found lower quantities than the subsequent findings reported in 2014 [30]. Another recent report from tests performed in Argentina (by independent scientists working for the German NGO Test-Biotech) have reported findings of even higher levels of glyphosate residues in harvests of glyphosate-tolerant soy [65] (Fig. 1). These latest results indicate very high glyphosate residue levels up to 100 mg/kg in soybean and stand as an important indication which necessitates further sampling and analysis.
Recent data on glyphosate residues in glyphosate tolerant soybean. Data from analysis of samples from fields in Iowa, USA [30] and province of Salta, Argentina [65]. Residues are shown as detections of glyphosate and the primary metabolite, AMPA. Reference lines indicate maximum residue limit (MRL). Former European MRL of 0.1 mg/kg was raised 200-fold in 1999 to 20 mg/kg. US MRL at 20 mg/kg was raised to 40 mg/kg in 2014. Codex Alimentarius MRL for soybean is 20 mg/kg [90]
The results indicate a rise in glyphosate residue levels in recent decades. In 1999, a major producer of both glyphosate and GM crops declared that glyphosate residue levels of 5.6 mg/kg in glyphosate-tolerant soybean were considered to be extreme high values [30]. It seems apparent from Fig. 1 that such levels at present would be considered moderate or even low. To explain tendencies of rising residue levels it would be relevant to investigate actual application rates. Global production figures support the notion that very large quantities of glyphosate are being sold and dispersed.
It is interesting to note that several independent researchers have mentioned the specific question of glyphosate-residues in glyphosate-tolerant crops, asking for more data to clarify this issue [46, 47, 62]. The question has also been addressed in a review of concepts and controversies in EFSA environmental risk assessment of GM-crops; it was found that even in an environmental context more data on glyphosate residues is needed, as post-harvest biomass is potentially affecting soil biota and adjacent environments [19].
Studies of glyphosate-tolerant cultivar composition have identified differences in essential plant constituents, which have been attributed to in-plantae metabolic effects of glyphosate residues [48–50]. Such research indicates that glyphosate residues have negative effects on composition. Contrary to this, a recent review by authors from the United States Department of Agriculture [51] conclude that there is not sufficient evidence for claiming that glyphosate in glyphosate-tolerant crops a) significantly affects mineral composition or b) changes rhizosphere microbial community or c) increases susceptibility to disease from plant pathogens.
As a direct critique of the regulatory policies enforced by the European Food safety Authority EFSA, independent scientists have claimed that the present regime of industry self-control (autoregulation) is insufficient to provide necessary evidence and ensure the long-term interests of society. Industry studies therefore must be supplemented with additional, independent, research [19, 20, 52]. This, however, is not a view shared by researchers representing interests of biotech companies, who often participate in systematic opposition to any results questioning industry studies. It has been described as highly regrettable that independent scientific work is often attacked and discredited by concerted efforts of industry proponents and journal editors loyal to biotech sector interests [28]. A recent study [52] found clear evidence of double-standards in criteria for evaluation of safety studies on GMO cultivars such as herbicide-resistant plants. The authors document that evidence confirming safety is not exposed to the same intensive scrutiny as evidence indicating possible harm. This is paradoxical, as it should be evident that faulty findings in the first of these categories has potential for inflicting negative effects on consumer health. Faulty findings in the second category will not have the same implications, but may lead to exaggerated precaution, which can be conflicting in relation to commercial interests.
Evidence has emerged during the compilation of this review, which to a certain degree confirms the claims of double-standards: One of the industry studies reviewed here serves as a noteworthy example of malpractice. The study [31] was published by journal Food and Chemical Toxicology. The scientists authoring the study were employees of commercial companies Pioneer Hi-bred and DuPont. They conducted a safety study on DP-356Ø43-5 glyphosate-tolerant soybean by testing cultivated material in a feeding study using rats. According to the methods chapter of the study, the tested DP-356Ø43-5 glyphosate-tolerant soybean was sprayed with glyphosate herbicide. Glyphosate herbicide was the only pesticide used in the strictly controlled production on parallel fields of; (a) glyphosate-tolerant soy (sprayed) and (b) unmodified soy (not sprayed). The irregular aspect relates to the fact that a wide array of subsequent tests for pesticides was performed in the produced soy materials, screening these for a variety of active ingredient chemicals. And, although glyphosate-herbicide was specified to be the only pesticide applied in the strictly controlled test-plot cultivation, an analysis for glyphosate residues was omitted. Instead the cultivated material was analyzed for numerous herbicide ingredients that were fundamentally irrelevant. This published study should be seen as an example supporting the arguments demanding revision of the regulatory framework mandating self-control of biotech industry products. Furthermore, given the recent heightening of qualitative requirements for such studies, which in its utmost consequence is seen as retractions of publications, I nominate the mentioned study [31] as a prime candidate for editorial re-evaluation.
Other reviews of published testing
Four recent reviews of data on GM crops in agriculture [53–56] present evidence confirming herbicide-tolerant cultivar equivalence, as compared to non-modified comparators. None of these reviews mention herbicide residues or their potentially conflicting nature in relation to concept of substantial equivalence. Contrary to this, three reviews by independent scientists approach the role of herbicide residues in GM crops or present indications of toxicity. In one of these [57] the authors review several contested safety assessment studies and speculate whether adverse effects reported in animal testing in 2002 [58], in 2004 [59] and in 2009 [60] could be attributable to pesticide residues contained in the tested GM-crop material. Another recent review [61] concludes that parts of published evidence in assessments of health risks of GMO foods are general indications of toxicity.
The regulatory challenges relating to oversight and development of standards for investigating herbicide residues in herbicide-resistant crops are largely ignored by industry, by most independent researchers and by regulatory authorities. However, a few specific aspects have been reviewed and important recommendations have been presented [62]. Such recommendations include specific measures, notably the concept of supervised field trials, which is seen as an important potential improvement of the current system of industry self-control and scientific autonomy.
The future of herbicide-tolerant crops
Commercially advanced herbicide-tolerant cultivars are popular amongst stakeholder investors and farmers engaged in agro-industrial production of maize and soybean in countries of North- and South America. From a database listing GM crop varieties pending regulatory approval [63] it seems that a majority of these GM crops are either herbicide-tolerant varieties or varieties with stacked events which include herbicide tolerance.
Some new varieties have herbicide-tolerance traits which are selected from microorganisms systematically bred in environments with high glyphosate concentrations [64]. Traditional first-generation glyphosate-tolerant crops, such as the GTS-40-3-2 soybean which still dominates global production, are only 45–50 times more tolerant than unmodified varieties (the glyphosate dose inducing LC50-outcome in GTS-40-3-2 is about 50× that of unmodified soy). Obviously, this physiological vulnerability can be perceived as a deficiency, subsequently limiting continuous spiraling increase of dosage as main strategy against hard weed.
By using new sources of transgenes and gene-stacks with combinations of several transgenes conveying multi-pathway tolerance to specific active ingredients, second-generation cultivars are seen as having significantly improved tolerance to specific herbicides or combinations of herbicides. This development should be seen primarily as a method paving the way for escalating application of herbicide. It seems that in the on-going struggle to eradicate resistant weeds, farmers rely heavily on solutions offered by commercial producers of herbicides. A main strategy seems to be developments that allow for higher dosage of herbicides such as glyphosate.
It is recommended that such developments should be met by regulatory initiative to ensure necessary regulatory oversight of inevitable and expectable secondary consequences, such as compositional changes and combinatorial effects with other plant- or pesticidal compounds. These potential changes must be monitored in analysis of representative material, which can be taken as samples from the actual agro-ecological production systems.
The present maximal residue limits (MRLs) allow for relative high concentrations of herbicide residues. In Brazil in 2004 the MRL in soybean was increased from 0.2 to 10 mg/kg: a 50-fold increase, but only for glyphosate tolerant soy. In Europe, the MRL for glyphosate in soybean was raised by a factor 200 from 0.1 to 20 mg/kg in 1999 [66] and the same MRL of 20 mg/kg was adopted by the US based on recommendations of the Codex Alimentarius Commission. In 2013, the MRL tolerance levels for glyphosate residues in US soybean were raised from 20 to 40 mg/kg (Fig. 1). The increases coincide with industry development of new GM varieties with stronger tolerance to glyphosate. In these cases the MRL values appear to have been adjusted pragmatically in response to actual observed, or expected, increases in the content of residues in glyphosate-tolerant soybeans. In this context it would be appropriate to collect and review more of the existing data on glyphosate residues in glyphosate-tolerant crops. However relevant such a question may be, it cannot be satisfactorily answered here due to the fact that only sparse published information exists on this issue.
Despite the limited number of analyses for glyphosate residues in glyphosate tolerant crops, the few recent tests reported [30, 65] indicate surprisingly high levels of glyphosate residues. Such findings should fundamentally challenge regulatory assumption of substantial equivalence between glyphosate-tolerant varieties and their unmodified comparators.
Substantial equivalence
The principle of substantial equivalence is fundamental for assessment of genetically modified plants, justifying some explanation. Substantial equivalence is a concept developed by OECD in 1991–1993 [23], establishing that a novel food, for example one derived from genetic modification or engineering, should be considered the same as (and as safe as) a conventional food, if it demonstrates the same characteristics and composition as the conventional food [67]. In 1997, the European Commission regulated its policy on novel foods (from GM plants) stating that food and feed from such plants are expected not to “present a danger for the consumer”, or “mislead the consumer”, or “differ from foods or food ingredients which they are intended to replace to such an extent that their normal consumption would be nutritionally disadvantageous for the consumer” [68] The regulation goes on to state that “[this policy…] shall apply to foods or food ingredients […] which, on the basis of the scientific evidence available […] are substantially equivalent to existing foods or food ingredients as regards their composition, nutritional value, metabolism, intended use and the level of undesirable substances contained therein” [68]. This allows a discussion on the qualitative evaluation of substances which vary from benign to harmful. It should be evident that pesticide residues belong in the category “undesirable substances”.
Post-market monitoring
European Union legislation [69] specifies framework for post-market monitoring of GM plant material, to ensure traceability of individual feed-lots entering the European common market. This is important, as only such traceability through proper labeling will ensure that possible adverse effects from specific harvests or specific batches of feed can be identified. At present the USA, which is the largest market for genetically modified food for human consumption, has a lack of such traceability. In the USA, this situation has been established through commercial and political influence. Contrary to this, European legislation accommodates traceability of feed for industrial scale production of farmed animals, such as pigs, poultry and cattle. This traceability, however, is not enforced at present. It has been claimed that such a deductive approach to material quality of GM crops would be unfeasible [22]. Contrary to this it can be argued that labeling and traceability should be used systematically in enforced post-market monitoring. Especially as this systematic approach allows for efficient identification of possible adverse effects from novel feed ingredients following large-scale introduction. In guidance documents for risk assessment of food and feed from GM plants, EFSA has specifically stated the need for post-market monitoring of “undesirable substances” [70] thus flagging a clearly defined regulatory intension. Based on the findings on potentially high residue levels reported here, it is recommended that EFSA gives priority to implementing the existing regulation.
Conclusion
Of 30 reviewed studies on composition and feed quality of glyphosate-tolerant GM crop material, only half of the studies use material produced with application of glyphosate herbicide. Only one of the 30 studies has analyzed the material for glyphosate residues.
Application of representative dosage of herbicides as well as subsequent analysis of herbicide residues is missing in industry testing of glyphosate-tolerant GM crops. This implies that central data from compositional analysis, animal feeding studies and overall risk assessment performed by industry and submitted to national and international regulatory bodies as evidence as safety, is not representative of the materials actually produced by farmers and delivered onto the commercial market. In part the scientific evidence produced by industry is found to have unacceptable standard for regulatory purpose. Such evidence should be disregarded and demands for new evidence should be brought forward.
Published data on glyphosate residues in glyphosate-tolerant crops are sparse. The findings presented here suggest that this could be an issue with important implications.
Scientific evidence produced by biotech industry companies should be supplemented with data from independent research. Alternatively, the risk assessments and analyses performed by industry should be competently supervised to ensure both transparency and an overall satisfactory standard of testing.
This leads to a recommendation to EFSA, FAO and OECD, hereby presenting scientific argumentation for necessary measures.
Regular revision of regulatory framework and safety measures is needed to secure future quality of important food and feed material. Such action is fundamental for safeguarding coherence, relevance and public trust.
References
Herman R, Price WD. Unintended compositional changes in genetically modified (GM) crops: 20 years of research. J Agric Food Chem. 2013;. doi:10.1021/jf400135r.
Amrhein N, Schab J, Steinrücken HC. The mode of action of the herbicide glyphosate. Naturwissenschaften. 1980;67:356–7.
Pollak P. Fine chemicals: the industry and the business. Wiley; 2011.
Szeḱács A, Darvas B. Forty years with glyphosate. In: Mohammed Nagib Hasaneen MN, editor. Herbicides—properties, synthesis and control of weeds. InTech; 2012.
Cuhra M, Traavik T, Bøhn T. Clone- and age-dependent toxicity of a glyphosate commercial formulation and its active ingredient in daphnia magna. Ecotoxicology. 2013;22:251–62.
Haalck F, Reinken B. Sikkation, ein Grund zum Fragen. 2010. http://www.schattenblick.de/infopool/umwelt/redakt/umko0005.html. Accessed 28 May 2015.
US 7771736 B2. Glyphosate formulations and their use for the inhibition of 5-enolpyruvylshikimate-3-phosphate synthase. 2002. http://www.google.com/patents/US7771736. Accessed 28 May 2015.
Benbrook CM. Impacts of genetically engineered crops on pesticide use in the US—the first sixteen years. Environ Sci Eur. 2012;24:1–13.
Giesy JP, Dobson S, Solomon KR. Ecological risk assessment for Roundup herbicide. Rev Environ Contam Toxicol. 2000;167:35–120.
Williams GM, Kroes R, Munro IC. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 2000;31:117–65.
Samsel A, Seneff S. Glyphosate, pathways to modern diseases II: celiac sprue and gluten intolerance. Interdiscip Toxicol. 2014;. doi:10.2478/intox-2013-0026.
EFSA. The 2013 European Union report on pesticide residues in food. EFSA J. 2015;. doi:10.2903/j.efsa.2015.4038.
Majewski MS, Coupe RH, Foreman WT, Capel PD. Pesticides in Mississippi air and rain: a comparison between 1995 and 2007. Environ Toxicol Chem. 2014;33:1283–93.
Krüger M, Schledorn P, Schrödl W, Hoppe HW, Lutz W, et al. Detection of glyphosate residues in animals and humans. J Environ Anal Toxicol. 2014;. doi:10.4172/2161-0525.1000210.
Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect. 2004;112:321–6.
Bonny S. Herbicide-tolerant transgenic soybean over 15 years of cultivation: pesticide use, weed resistance, and some economic issues. The case of the USA. Sustainability. 2011;3:1302–22.
Duke SO, Powles SB. Glyphosate: a once-in-a-century herbicide. Pest Manag Sci. 2008;64:319–25.
James C. A global overview of biotech (GM) crops: adoption, impact and future prospects. GM Crops. 2010;. doi:10.4161/gmcr.1.1.9756.
Hilbeck A, Meier M, Römbke J, Jänsch S, Teichmann H, Tappeser B. Environmental risk assessment of genetically modified plants-concepts and controversies. Environ Sci Eur. 2011;. doi:10.1186/2190-4715-23-13.
Dolezel M, Miklau M, Hilbeck A, Otto M, Heissenberger A, Tappeser B, Gaugitsch H. Scrutinizing the current practice of the environmental risk assessment of GM maize applications for cultivation in the EU. Environ Sci Eur. 2011;23:1–15.
Kuiper HA, Kleter GA, Noteborn HPJM, Kok EJ. Assessment of the food safety issues related to genetically modified foods. Plant J. 2001;27:503–28.
Kok EJ, Keijer J, Kleter GA, Kuiper HA. Comparative safety assessment of plant-derived foods. Regul Toxicol Pharm. 2008;50:98–113.
OECD. Safety evaluation of foods derived by modern biotechnology. Concepts and principles. France: Organisation for Economic Co-operation and Development OECD Paris; 1993.
EFSA. Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 2010;8:1–111.
Haslberger A. Codex guidelines for GM foods include the analysis of unintended effects. Nat Biotechnol. 2003;21:739–40.
FAO. Feasibility of establishing MRLs for genetically modified crops and metabolite residues. The Hague: Codex Alimentarius commission. Food and Agriculture Organization of the United Nations; 2001.
US FDA. Policy brief in the federal register (57 FR 22984) FDA statement of policy: foods derived from new plant varieties. 1992. http://www.fda.gov/food/biotechnology/default.html. Accessed 28 May 2015.
Waltz E. Battlefield. Nature. 2009;461:27–32.
Nielsen K. Biosafety data as confidential business information. PLoS Biol. 2013;11(3):e1001499.
Bøhn T, Cuhra M, Traavik T, Sanden M, Fagan J, Primicerio R. Compositional differences in soybeans on the market: glyphosate accumulates in Roundup Ready GM soybeans. Food Chem. 2014;153:207–15.
Appenzeller LM, Munley SM, Hoban D, Sykes GP, Malley LA, Delaney B. Subchronic feeding study of herbicide-tolerant soybean DP-356Ø43-5 in Sprague-Dawley rats. Food Chem Toxicol. 2008;46:2201–13.
Cuhra M, Dando M, Traavik T, Primicerio R, Holderbaum D, Bøhn T. Glyphosate residues in Roundup-Ready soybean impair daphnia magna life-cycle. J Agric Chem Environ. 2015;4:24–36.
Millstone E, Brunner E, Mayer S. Beyond substantial equivalence. Nature. 1999;401:525–6.
Hammond BG, Vicini JL, Hartnell GF, Naylor MW, Knight CD, Robinson EH, Fuchs RL, Padgette SR. The feeding value of soybeans fed to rats, chickens, catfish and dairy cattle is not altered by incorporation of glyphosate tolerance. J Nutr. 1996;126:717–27.
Viljoen C. Letter to the editor. Food Chem Toxicol. 2013;59:809–10.
Padgette SR, Taylor NB, Nida DL, Bailey MR, MacDonald J, Holden LR, Fuchs RL. The composition of glyphosate-tolerant soybean seeds is equivalent to that of conventional soybeans. J Nutr. 1996;126:702–16.
Nida DL, Patzer S, Harvey P, Stipanovic R, Wood R, Fuchs RL. Glyphosate-tolerant cotton: the composition of the cottonseed is equivalent to that of conventional cottonseed. J Agric Food Chem. 1996;44:1967–74.
Cromwell GL, Lindemann MD, Randolph JH, Parker GR, Coffey RD, Laurent KM, Armstrong CL, Mikel WB, Stanisiewski EP, Hartnell GF. Soybean meal from roundup ready or conventional soybeans in diets for growing-finishing swine. J Anim Sci. 2002;80:708–15.
Harrigan GG, Ridley WP, Riordan SG, Nemeth MA, Sorbet R, Trujillo WA, Breeze ML, Schneider RW. Chemical composition of glyphosate-tolerant soybean 40-3-2 grown in Europe remains equivalent with that of conventional soybean (Glycine max L.). J Agric Food Chem. 2007;55:6160–8.
Lundry DR, Ridley WP, Meyer JJ, Riordan SG, Nemeth MA, Trujillo WA, Breeze ML, Sorbet R. Composition of grain, forage, and processed fractions from second-generation glyphosate-tolerant soybean, MON 89788 Is equivalent to that of conventional soybean (Glycine max L.). J Agric Food Chem. 2008;56:4611–22.
McCann MC, Liu K, Trujillo WA, Dobert RC. Glyphosate-tolerant soybeans remain compositionally equivalent to conventional soybeans (Glycine max L.) during three years of field testing. J Agric Food Chem. 2005;53:5331–5.
McCann MC, Trujillo WA, Riodan SG, Sorbet R, Bogdanova NN, Sidhu RS. Comparison of the forage and grain composition from insect-protected and glyphosate-tolerant MON 88017 corn to conventional corn (Zea mays L.). J Agric Food Chem. 2007;55:4034–42.
McCann MC, Rogan GJ, Fitzpatrick S, Trujillo WA, Sorbet R, Hartnell GF, Riodan SG, Nemeth MA. Glyphosate-tolerant alfalfa is compositionally equivalent to conventional alfalfa (Medicago sativa L.). J Agric Food Chem. 2006;54:7187–92.
Ridley WP, Sidhu RS, Pyla PD, Nemeth MA, Breeze ML, Astwood JD. Comparison of the nutritional profile of glyphosate-tolerant corn event NK603 with that of conventional corn (Zea mays L.). J Agric Food Chem. 2002;50:7235–43.
Taylor NB, Fuchs RL, MacDonald J, Shariff AR, Padgette SR. Compositional analysis of glyphosate-tolerant soybeans treated with glyphosate. J Agric Food Chem. 1999;47:4469–73.
Arregui MC, Lenardon A, Sanchez D, Maitre MI, Scotta R, Enrique S. Monitoring glyphosate residues in transgenic glyphosate-resistant soybean. Pest Manag Sci. 2004;60:163–6.
Duke SO, Rimando AM, Pace PF, Reddy KN, Smeda RJ. Isoflavone, glyphosate, and aminomethylphosphonic acid levels in seeds of glyphosate-treated, glyphosate-resistant soybean. J Agric Food Chem. 2003;51:340–4.
Zobiole LHS, Oliveira RS Jr, Kremer RJ, Constantin J, Yamada T, Castro C, Oliveira FA, Oliveira A Jr. Effect of glyphosate on symbiotic N2 fixation and nickel concentration in glyphosate-resistant soybeans. Appl Soil Ecol. 2010;44:176–80.
Zobiole LHS, Oliveira RS, Visentainer JV, Kremer RJ, Bellaloui N, Yamada T. Glyphosate affects seed composition in glyphosate-resistant soybean. J Agric Food Chem. 2010;58:4517–22.
Zobiole LHS, Kremer RJ, Oliveira RS Jr, Constantin J. Glyphosate affects chlorophyll, nodulation and nutrient accumulation of “second generation” glyphosate-resistant soybean (Glycine max L.). Pestic Biochem Physiol. 2011;99:53–60.
Duke SO, Lydon J, Koskinen WC, Moorman TB, Chaney RL, Hammerschmidt R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J Agric Food Chem. 2012;60:10375–97.
Wickson F, Bøhn T, Wynne B, Hilbeck A, Funtowicz S. Science-based risk assessment requires careful evaluation of all studies. Nat Biotechnol. 2013;31:1077–8.
Delaney B. Strategies to evaluate the safety of bioengineered foods. Int J Toxicol. 2007;26:389–99.
Harrigan GG, Lundry D, Drury S, Berman K, Riordan SG, Nemeth MA, Ridley WP, Glenn KC. Natural variation in crop composition and the impact of transgenesis. Nat Biotechnol. 2010;28:402–4.
Ricroch A, Bergé JB, Kuntz M. Evaluation of genetically engineered crops using transcriptomic, proteomic, and metabolomic profiling techniques. Plant Physiol. 2011;155:1752–61.
Snell C, Berheim A, Bergé JB, Kuntz M, Pascal G, Paris A, Ricroch A. Assessment of the health impact of GM plant diets in long term and multigenerational animal feeding trials: a literature review. Food Chem Toxicol. 2011;. doi:10.1016/j.fct.2011.11.048.
Domingo JL, Bordonaba JG. A literature review on the safety assessment of genetically modified plants. Environ Int. 2011;37:734–42.
Malatesta M, Caporaloni C, Gavaudan S, Rocchi MBL, Serafini S, Tiberi C, Gazzanelli G. Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean. Cell Struct Funct. 2002;27:173–80.
Vecchio L, Cisterna B, Malatesta M, Martin TE, Biggiogera M. Ultrastructural analysis of testes from mice fed genetically modified soybean. Eur J Histochem. 2004;48:448–54.
Vendemois JS, Roullier F, Cellier D, Seralini GE. A comparison of the effects of GM corn varieties on mammalian health. Int J Biol Sci. 2009;5:706–26.
Dona A, Arvanitoyannis IS. Health risks of genetically modified foods. Crit Rev Food Sci Nutr. 2009;49:164–75.
Kleter GA, Unsworth JB, Harris CA. The impact of altered herbicide residues in transgenic herbicide-resistant crops on standard setting for herbicide residues. Pest Manag Sci. 2011;67:1193–210.
ISAAA. GM Approval Database. International Service for the Acquisition of Agri-biotech Applications (ISAAA) 2014. http://www.isaaa.org/gmapprovaldatabase. Accessed 28 May 2015.
Cao G, Liu Y, Liu G, Wang J, Wang G. Draft genome sequence of Pseudomonas strain P818, isolated from glyphosate-polluted soil. Genome Announc. 2013;1:e01079-13.
Then C. High levels of residues from spraying with glyphosate found in soybeans in Argentina. TestBiotech background report 2013. 22 Oct 2013.
Samsel A, Seneff S. Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: pathways to modern diseases. Entropy. 2013;15:1416–63.
Womach J. Agriculture: a glossary of terms, programs and laws. CRS Report for Congress. 2005. http://www.cnie.org/NLE/CRSreports/05jun/97-905.pdf. Accessed 28 May 2015.
European Commission. Regulation (EC) No 258/97 concerning novel foods and novel food ingredients. Bruxelles: European Commission; 1997.
European Commission. Regulation 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC. 2003. http://europa.eu/legislation_summaries/environment/nature_and_biodiversity/l21170_en.htm. Accessed 28 May 2015.
EFSA. Guidance on the post-market environmental monitoring (PMEM) of genetically modified plants. EFSA J. 2011;9:2316.
Sidhu RS, Hammond BG, Fuchs RL, Mutz JN, Holden LR, George B, Olson T. Glyphosate-tolerant corn: the composition and feeding value of grain from glyphosate-tolerant corn is equivalent to that of conventional corn (Zea mays L.). J Agric Food Chem. 2000;48:2305–12.
Sidhu RS, Brown S. Petition for determination of nonregulated status for MON 88017 corn. (http://www.aphis.usda.gov/brs/aphisdocs/04-12501p.pdf. Accessed 28 May 2015.
Obert JC, Ridley WP, Schneider RW, Riordan SG, Nemeth MA, Trujillo WA, Breeze ML, Sorbet R, Astwood JD. The composition of grain and forage from glyphosate tolerant wheat MON 71800 is equivalent to that of conventional wheat (Triticum aestivum L.). J Agric Food Chem. 2004;52:1375–84.
Berman KH, Harrigan GG, Riordan SG, Nemeth MA, Hanson C, Smith M, Sorbet R, Zhu E, Ridley WP. Compositions of forage and seed from second-generation glyphosate-tolerant soybean MON 89788 and insect-protected soybean MON 87701 from Brazil are equivalent to those of conventional soy bean (Glycine max). J Agric Food Chem. 2010;58:6270–6.
Delaney B, Appenzeller LM, Roper JM, Mukerji P, Hoban D, Sykes GP. Thirteen week rodent feeding study with processed fractions from herbicide tolerant (DP-Ø73496-4) canola. Food Chem Toxicol. 2014;66:173–84.
Brake DG, Evenson DP. A generational study of glyphosate-tolerant soybeans on mouse fetal, postnatal, pubertal and adult testicular development. Food Chem Toxicol. 2004;42:29–36. doi:10.1016/j.fct.2003.08.003.
Zhu Y, Li D, Wang F, Yin J, Jin H. Nutritional assessment and fate of DNA of soybean meal from Roundup Ready or conventional soybeans using rats. Arch Anim Nutr. 2004;58:295–310.
Healy C, Hammond B, Kirkpatrick J. Results of a 13-week safety assurance study with rats fed grain from corn rootworm-protected, glyphosate-tolerant MON 88017 corn. Food Chem Toxicol. 2008;46:2517–24.
Malatesta M, Boraldi F, Annovi G, Baldelli B, Battistelli S, Biggiogera M, Quaglino D. A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochem Cell Biol. 2008;130:967–77.
German Federal Institute for Risk Assessment BFR. Glyphosate in urine—concentrations are far below the range indicating a potential health hazard. 2013; BfR Opinion No. 014/2013. http://www.brf.bund.de.
Séralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS. Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ Sci Eur. 2014;. doi:10.1186/s12302-014-0014-5.
Carman JA, Vlieger HR, Ver Steeg LJ, Sneller VE, Robinson GW, Clinch-Jones CA, Haynes JI, Edwards JW. A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J Organic Syst. 2013;8:38–54.
Cuhra M, Traavik T, Bøhn T. Life cycle fitness differences in Daphnia magna fed Roundup-Ready soybean or conventional soybean or organic soybean. Ecotoxicology. 2013;. doi:10.1111/anu.12199.
Taylor M, Hartnell G, Lucas D, Davis S, Nemeth M. Comparison of broiler performance and carcass parameters when fed diets containing soybean meal produced from glyphosate-tolerant (MON 89788), control, or conventional reference soybeans. Poult Sci. 2007;86:2608–14.
Guyton KZ, Loomis D, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;. doi:10.1016/S1470-2045(15)70134-8.
Robinson C, Holland N, Leloup D, Muilerman H. Conflicts of interest at the European Food Safety Authority erode public confidence. J Epidemiol Community Health. 2013;. doi:10.1136/jech-2012-202185.
Bakke-McKellep AM, Koppang EO, Gunnes G, Sanden M, Hemre GI, Landsverk T, Krogdahl Å. Histological, digestive, metabolic, hormonal and some immune factor responses in Atlantic salmon, Salmo salar L., fed genetically modified soybeans. J Fish Dis. 2007;. doi:10.1111/j.1365-2761.2007.00782.x.
Sissener NH, Sanden M, Bakke AM, Krogdahl Å, Hemre GI. A long term trial with Atlantic salmon (Salmo salar L.) fed genetically modified soy; focusing general health and performance before, during and after the parr–smolt transformation. Aquaculture. 2009;294:108–17.
European Commission. Online resources on genetically modified organisms: commission proposes changes allowing member states freedom to restrict or prohibit use of Authorised GMOs. http://www.ec.europa.eu/food/plant/gmo/new/idex_en.htm. Accessed 24 June 2015.
Codex Alimentarius pesticide details: 158 glyphosate. FAO/WHO Food Standards database. http://www.codexalimentarius.net/pestres/data/pesticides/details.html?d-16497-o=2&id=158&d-16497-s=3. Accessed 24 June 2015.
Acknowledgements
The work is funded by the Norwegian Research Council, project 84107 Miljø-2015. The author is grateful for constructive and challenging comments and improvements given by anonymous reviewers.
Compliance with ethical guidelines
Competing interests The author declares that he has no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cuhra, M. Review of GMO safety assessment studies: glyphosate residues in Roundup Ready crops is an ignored issue. Environ Sci Eur 27, 20 (2015). https://doi.org/10.1186/s12302-015-0052-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-015-0052-7