Abstract
Background
Previous observational studies have demonstrated inconsistent and inconclusive results of changes in the intestinal microbiota in patients with obesity and metabolic disorders. We performed a systematic review to explore evidence for this association across different geography and populations.
Methods
We performed a systematic search of MEDLINE (OvidSP) and Embase (OvidSP) of articles published from Sept 1, 2010, to July 10, 2021, for case–control studies comparing intestinal microbiome of individuals with obesity and metabolic disorders with the microbiome of non-obese, metabolically healthy individuals (controls). The primary outcome was bacterial taxonomic changes in patients with obesity and metabolic disorders as compared to controls. Taxa were defined as “lean-associated” if they were depleted in patients with obesity and metabolic disorders or negatively associated with abnormal metabolic parameters. Taxa were defined as “obesity-associated” if they were enriched in patients with obesity and metabolic disorders or positively associated with abnormal metabolic parameters.
Results
Among 2390 reports screened, we identified 110 full-text articles and 60 studies were included. Proteobacteria was the most consistently reported obesity-associated phylum. Thirteen, nine, and ten studies, respectively, reported Faecalibacterium, Akkermansia, and Alistipes as lean-associated genera. Prevotella and Ruminococcus were obesity-associated genera in studies from the West but lean-associated in the East. Roseburia and Bifidobacterium were lean-associated genera only in the East, whereas Lactobacillus was an obesity-associated genus in the West.
Conclusions
We identified specific bacteria associated with obesity and metabolic disorders in western and eastern populations. Mechanistic studies are required to determine whether these microbes are a cause or product of obesity and metabolic disorders.
Similar content being viewed by others
Introduction
Obesity-related metabolic disorders, including type 2 diabetes (T2DM), cardiovascular diseases, and non-alcoholic fatty liver disease (NAFLD), affect 13% of the population and result in 2.8 million deaths each year [1, 2], and are a significant socioeconomic burden to society. Pathophysiology of obesity and metabolic disorders is multi-factorial, and currently, therapies are limited. The role of intestinal microbiota in patients with obesity and metabolic disorders have been extensively studied in the past decade. Humanized mouse models showed that the microbiome in obese subjects appeared to be more efficient in harvesting energy from the diet and may thereby contribute to the pathogenesis of obesity [3, 4]. However, observational studies reported inconsistent and inconclusive changes of intestinal microbiota in patients with obesity and metabolic disorders [5]. For instance, the Firmicutes and Bacteriodetes ratio (F/B ratio) is not a reproducible marker across human cohorts [6].
Microbial-based therapies such as probiotics aiming to reshape the gut microbial ecosystem have been increasingly explored in the treatment of obesity-related metabolic disorders [7, 8]. Traditional probiotics, primarily consisting of Lactobacillus and Bifidobacterium have been shown to elicit weight loss in subjects with obesity yet the effect sizes were small with large variations of efficacy among different studies [9]. Emerging evidence showed that Akkermansia muciniphila was depleted in patients with obesity-related metabolic disorders. These results have led to mechanistic studies and clinical trials to test its efficacy in the management of obesity and metabolic disorders [10].
Age, geography, and dietary patterns largely affect the gut microbiome [11,12,13]. The gut microbiota of vegetarians was dominated by Clostridium species [14] whereas subjects who mainly consumed fish and meat had high level of F. prausnitzii [15]. In recent years, the prevalence of childhood obesity has increased sharply. However, only limited data has issued the function and structure of gut microbiota in children and adolescents with obesity [16].
We have therefore conducted a systematic review of case–control studies evaluating the microbiota in patients with obesity and metabolic disorders compared to lean, healthy controls to summarize the current evidence in the relationship between individual members of the microbiota and obesity. We aimed to identify novel candidates as live biotherapeutics to facilitate the treatment of obesity and metabolic disorders.
Materials and methods
Search strategy
This systematic review was performed in accordance with the PRISMA 2009 guidelines [17]. We performed a systematic search of MEDLINE (OvidSP) and Embase (OvidSP) of articles published from Sept 1, 2010 to July 10, 2021 to identify case-control studies comparing gut microbiota in patients with obesity and metabolic disorder and non-obese, metabolically healthy controls. Search strategy is shown in the Appendix.
Study selection and patient population
Studies were included if they were (1) case–control studies comparing gut microbiota in patients with obesity and metabolic disorders and non-obese, metabolically healthy individuals (controls); (2) intestinal microbiota was assessed by next-generation sequencing (NGS; 16s rRNA amplicon or shotgun metagenomic sequencing); and (3) obesity was defined based on body mass index (BMI) ≥ 30kg/m2 and metabolic disorders including type 2 diabetes mellitus, non-alcoholic fatty liver disease, cardiovascular disease, and metabolic syndrome were diagnosed according to respective guidelines (Table 1). Studies from all age groups were included. Studies were excluded if they were (1) case reports, reviews, meta-analyses, re-analysis of public datasets, or conference abstracts, (2) without data for individual bacterial groups, (3) not in English, and (4) not a case–control design. Studies of genetic-associated obesity such as Prader–Willi syndrome were also excluded.
Study outcomes
The primary outcome was the bacterial taxonomic changes in patients with obesity and metabolic disorders compared to non-obese, metabolically healthy controls. Secondary outcomes included the changes in bacteria diversity and F/B ratio, subgroup analysis of microbiota changes in adults and children with obesity and metabolic disorders, and in Eastern and Western populations. Data on microbiota community composition were extracted from each study. Taxa were defined as “lean-associated” if they were depleted in patients with obesity and metabolic disorders or negatively associated with abnormal metabolic parameters such as high body mass index (BMI), elevated fasting plasma glucose and elevated serum cholesterol. Taxa were defined as “obesity-associated” if they were enriched in patients with obesity and metabolic disorders or positively associated with abnormal metabolic parameters. Taxon at each level (phylum, class, order, family, genus) was only counted once for each study (i.e., if a genus was both depleted in obesity and negatively associate with fat mass in the same study, it was only counted once).
Eligibility assessment and data extraction
Two authors (JW, HW) independently reviewed studies and excluded based on titles, abstracts, or both to lessen the selection bias and then reviewed selected studies with full text for complete analysis. JW extracted data from studies and entered it into a designated spreadsheet. HW checked the accuracy of this process. The data were re-checked when there was a discrepancy. XZ arbitrated if the discrepancy cannot be resolved by consensus and discussion. The data collected included the following: participant characteristics, including age group, country, types of metabolic disorders, number of patients; types of specimens, microbiota assessment method, microbiome diversity, and Firmicutes/Bacteroides ratio.
Quality assessment
The Newcastle-Ottawa Scale was applied to assess the quality of included studies. The Newcastle-Ottawa Scale consists of 3 domains (maximum 9 stars); selection (is the case definition adequate, representativeness of the cases, selection of controls, definition of controls); comparability (comparability of baseline characteristics); and exposure (ascertainment of exposure, same method of ascertainment for cases and controls, attrition rate).
Results
Study characteristics
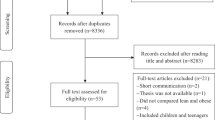
Overall, 2390 citations were retrieved; 2280 were excluded based on title, abstract, and the availability of full text; 110 articles were subsequently fully reviewed. After further review, 50 full-text articles were rejected (Fig. 1). The final analysis included 60 studies (Table 1). Of these, 44 studies assessed the gut microbiota in adults and 16 in infants, children, and adolescents. Ethnicity of subjects consisted of Asian, Black, Caucasian, Hispanic, or Latino. Fifty-eight out of 60 (96.7%) studies evaluated intestinal microbiota in stool samples and two studies assessed the microbiota in duodenal biopsies. Thirty-two studies involved patients with obesity [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48, 76], ten involved patients with T2DM [49,50,51,52,53,54,55,56,57,58], eleven involved patients with NAFLD or non-alcoholic steatohepatitis (NASH) [59,60,61,62,63,64,65,66,67,68, 75], and seven involved patients with metabolic syndrome [69,70,71,72,73,74, 77]. General characteristics and diagnostic criteria for obesity and metabolic disorders in each study were summarized in Table 1.
Microbiome assessment methods
Of the 58 studies assessing stool microbiome, 50 studies assessed the gut microbiota by using 16S ribosomal RNA (rRNA) gene sequencing, six used shotgun metagenomic sequencing and two studies applied both 16s rRNA and shotgun metagenomic sequencing. Both studies assessing biopsy microbiome applied 16S rRNA sequencing.
Primary outcomes
At the phylum level, significant changes of phyla Firmicutes, Bacteroidetes, and Proteobacteria were most reported in obese, metabolic diseased subjects compared with controls. Among 60 studies included, 22 studies reported significant changes in Firmicutes with 15 studies showing phylum Firmicutes were obesity-associated and 7 showing it was lean-associated [18, 21, 23, 28, 29, 32, 34, 42, 43, 45,46,, 46, 4850, 53,54,55, 59, 62, 63, 68, 69, 71]; 20 studies reported significant changes in Bacteroidetes with 8 studies showing it was obesity-associated and 12 showing it was lean-associated [20, 23, 29, 31, 32, 35, 37, 43, 46, 55, 57, 59, 61,62,63, 68,68,, 69, 71, 74, 75]. Fifteen studies reported significant change in Proteobacteria with 13 studies showing it was obesity-associated and 2 showing it was lean-associated [19, 20, 22, 29, 31, 32, 45, 46, 55, 59, 61, 65, 68, 69, 71]. Studies consistently reported that Fusobacteria as obesity-associated taxa (n = 5) [18, 20, 22, 32, 61], Actinobacteria was a lean-associated taxa (n = 7) [20, 23, 32, 45, 62, 68, 69] and Tenericutes was lean-associated (n = 4) [20, 22, 48, 77] (Table 2). The details on the differential levels of taxon in each eligible study are shown in Supplementary table 1.
At lower taxonomic levels, studies consistently reported the class Bacilli, Gammaproteobacteria and family Coriobacteriaceae to be obesity-associated. Controversial results were reported for class Clostridia, family Lachnospiraceae, Rikenellaceae, and Ruminococcaceae (Supplementary table 2). At the genus level, Alistipes, Akkermansia, Bifidobacterium, Desulfovibrio, and genera in the Clostridium cluster IV (Faecalibacterium, Eubacterium, Oscillospira, Odoribacter) were the most reported lean-associated genera, while Prevotella, Lactobacillus, Blautia, Escherichia, Succinivibrio, and Fusobacterium were the most reported obesity-associated genera. Significant change in genera Ruminococcus, Coprococcus, Dialister, Bacteroides, Clostridium and Roseburia were reported but results were controversial (Table 3).
Secondary outcomes
Forty (67%) studies provided alpha diversity of the gut microbiota. Among them, 18 reported significant reduction in diversity while four reported significant increase of alpha diversity in obesity and metabolic disorders compared with controls. The remaining studies (n = 18) found no significant difference in alpha diversity between both groups. In addition, 11 studies demonstrated significant difference in β-diversity [20, 23, 27, 28, 32, 40, 47, 55, 58, 66, 69], while 10 studies showed no significant difference in β-diversity between patients with obesity and metabolic disorders and controls [24, 26, 38, 49, 50, 57, 65, 70, 74, 79]. Twenty-two (37%) studies reported Firmicutes/Bacteroidetes (F/B) ratio [51,52,53,54, 56,57,58,59,60,61,62,63,64,65,66,67,68, 71,72,73,74,75]. Among them, eight studies reported significant increase [34,35,36, 39, 48, 52, 59, 75] and three studies reported a significant decreased in F/B ratio [33, 41, 44]. Eleven studies reported no significant change in F/B ratio in patients with obesity and metabolic disorders compared with controls (Supplementary Table 3) [37, 42, 46, 53, 54, 60,61,62,63, 67, 68].
Difference of microbiota between adult and childhood obesity
The trend for most microbial changes in adult and childhood obesity were consistent. Studies reported Actinobacteria as lean-associated, while Proteobacteria and Firmicutes as obesity-associated in both adults and childhood obesity. However, discrepancies were observed for several genera. Three studies in adults consistently reported that Fusobacterium was obesity-associated, but controversial results were found in children [18, 20, 22, 32, 61, 77]. Moreover, more studies reported that Dorea [39, 46, 49, 77] and Ruminococcus [39, 44, 49, 69] were obesity-associated in adults, while more studies reported them to be lean-associated in children [19, 68]. Three studies consistently reported that Turicibacter was lean-associated in adults [44, 66, 69], but one study reported it to be obesity-associated in children [20]. Notably, three studies in adults reported that the genus Bifidobacterium was lean-associated [22, 57, 58], while controversial results were found in children (3 lean-associated and 2 obesity-associated) [19,20,21, 38, 68]. These findings suggested that microbiota in childhood obesity and metabolic disorders were more heterogeneous compared with adults.
Difference of microbiota between the East and the West
Large discrepancies in gut microbiome in obesity and metabolic disorders were observed in studies from the East and the West. Four studies exclusively consisting of populations in the West reported that the Family Coriobacteriaceae was obesity-associated [27, 38, 53, 71] whereas none in the East reported significant change of this bacterial family between obese subjects and controls. Four studies in the East reported that the family Ruminococcaceae was lean-associated [22, 60, 61, 63], but conflicting results were found in studies from the West (2 lean-associated and 2 obesity-associated) [27, 36, 43, 68]. At the genus level, four studies reported that Prevotella was lean-associated in the East (3 lean-associated and 1 obesity-associated) [19, 20, 26, 61], while other studies from the West have reported it to be obesity-associated (2 lean-associated and 5 obesity-associated) [38, 55, 67, 68, 72, 73, 75]. Three studies reported that Ruminococcus was lean-associated in the East [20, 63, 67], but most studies reported it to be obesity-associated in the West (1 lean-associated and 5 obesity-associated) [23, 39, 44, 49, 62, 69]. Similar findings were observed for Roseburia (3 lean-associated in the east [30, 63, 66], 1 lean-associated and 2 obesity-associated in the west [53, 68, 69]). Notably, the common genus Lactobacillus was repeatedly reported to be obesity-associated in the West (1 lean-associated and 4 obesity-associated) [19, 38, 44, 46, 57]. Controversial results for Lactobacillus were also reported in the East (2 lean-associated and 2 obesity-associated) [21, 59, 60, 63].
Quality of the evidence
The Newcastle Ottawa Scale showed that all 60 studies provided an adequate explanation in the definition and selection method for patients with obesity and metabolic disorders (Table 4). Fifty-five (91.7%) of 60 studies did the same process for controls. Twenty (33.3%) and 27 (45%) studies demonstrated comparable data of sex and age in patients with obesity / metabolic disorders and controls.
Discussion
To our knowledge, this is the most comprehensive systematic review in microbiota and obesity and metabolic disorders, as we extracted the data of each available bacterial group using the lowest taxonomic level based on NGS of each included study. We believe that the findings reflect the best available current evidence demonstrating the relationship between individual bacterial taxa and obesity or metabolic disorders.
Proteobacteria was the most consistently reported obesity-associated phylum. Several members of Proteobacteria, such as Proteus mirabilis and E. coli, were potential drivers of inflammation in the gastrointestinal tract [7, 80, 81]. Low-grade inflammation is a risk factor for developing metabolic diseases including atherosclerosis, insulin resistance, and diabetes mellitus [82]. Besides stool microbiota, obese subjects with T2DM also showed a high bacterial load with an increase in Enterobacteriaceae in plasma, liver, and omental adipose tissue microbiota [83].
Lactobacillus was reported to be an obesity-associated taxon and abundance was higher in the stool of patients with obesity and metabolic diseases. This food-derived probiotic genus showed relative low prevalence and abundance in the commensal gut microbiota [52]. Previous clinical trials of Lactobacillus, alone or in combination with Bifidobacterium, showed variable efficacy in weight loss in patients with obesity [9]. These inconsistent results indicated that the underlying mechanisms of Lactobacillus (at least some of its species) in the treatment of metabolic disorders warrant further investigation. Other commensal bacteria such as Bifidobacterium spp., Alistipes spp., and Akkermansia that constitute a large proportion of the gut microbiota were frequently observed to be higher in healthy individuals than obese, metabolically affected subjects. These species might therefore exert a more durable beneficial effect for the consideration in managing obesity compared with Lactobacillus.
Akkermansia muciniphila (Actinobacteria phylum), a species identified by NGS, was one of the most commonly reported lean-associated bacteria in obesity and metabolic diseases. A. muciniphila was reported to help modulate the gut lining which could promote gut barrier function and prevent inflammation caused by the “leaky” gut [84]. A clinical trial demonstrated that supplementation with A. muciniphila could reduce body weight and decrease the level of blood markers for liver dysfunction and inflammation in obese insulin-resistant volunteers [10]. Another proof-of-concept study showed that supplementation with five strains including A. muciniphila was safe and associated with improved postprandial glucose control [85]. These findings highlight the potential of specific live biotherapeutics in weight control in subjects with obesity and metabolic diseases.
Other genera that were consistently reported to be more abundant in lean healthy individuals than obese subjects were Alistipes (Bacteroidetes phylum) and Faecalibacterium (Firmicutes phylum). Alistipes could produce small amounts of short-chain fatty acids (SCFA, acetic, isobutyric, isovaleric, and propionic acid) [86] while Faecalibacterium is one of the major butyrate producers in the human gut [87, 88]. SCFA have anti-inflammatory properties [89] and may promote weight loss through the release of glucagon-like peptide 1 that promotes satiety and the activation of brown adipose tissue via the gut–brain neural circuit [90, 91]. Butyrate could activate the GPR43-AKT-GSK3 signaling pathway to increase glucose metabolism by liver cells and improve glucose control in diabetes mice [92]. They could also inhibit the expression of PPARγ, increase fat oxidation in skeletal muscle mitochondria, and reduce lipogenesis in high-fat diet (HFD) mouse model [93].
We have identified several genera, including Bifidobacterium, Roseburia, Prevotella, and Ruminococcus, that were consistently reported to be lean-associated exclusively in subjects from the East. Bifidobacterium spp. are widely used probiotics proven to be safe and well-tolerated and exhibited a significant effect in lowering serum total cholesterol both in mice and in humans [94]. Roseburia is another major butyrate-producing genus of the human gut [95]. R. intestinalis could maintain the gut barrier function through upregulation of the tight junction protein [96]. Supplementation of R. intestinalis and R. hominis could ameliorate alcoholic fatty liver disease in mice [97]. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon [98]. Prevotella copri (Bacteroidetes phylum) was found to improve aberrant glucose tolerance syndromes and enhance hepatic glycogen storage in animals via the production of succinate [99]. However, a recent study also showed that the prevalence of P. copri exacerbated glucose tolerance and enhanced insulin resistance which occur before the development of ischemic cardiovascular disease and type 2 diabetes [100].
Only limited human studies in the current review reported an increased ratio of F/B in obesity. An increased ratio of F/B was shown in studies of the high-fat diet mouse model [6]. No taxon distinction was found to be specific for any type of metabolic disease. This was in line with a recent study that showed obesity, but not type 2 diabetes, was associated with notable alterations in microbiome composition [58].
The strength of this study is that we applied a robust method of grouping various types of disease-microbiome associations into “lean, metabolically healthy state” or “obese, metabolically diseased state.” Despite various metabolic disorders may affect the gut microbiota in different manners, the inter-study variation often supersedes the intra-study variation between disease and control groups [101]. Overall, the most striking observation is the lack of consistency in results between studies. This probably relates to the limitations of the studies included in this review. Also, it relies on the striking stability and individuality of adult microbiota, changing over time. Heterogeneity between studies is often a problem in systematic reviews. Several different methods were used to assess the microbiota, which makes it difficult to compare results between studies and likely contributes to the differences in results. While the standardization of study protocol (sample storage, DNA extraction, sequencing, analysis methods, and stringent subject recruitment criteria) could potentially result in comparable data between studies, this remains a big challenge across different regions. Moreover, we excluded studies that used species- or group-specific primers for microbiota assessment because such methods could only capture certain bacterial groups. This limits the total number of studies included. For robust microbiota results that are comparable among studies, there need to be efforts for standardization of sample storage, DNA extraction, sequencing, and analysis methods among groups undertaking gut microbiota studies. Finally, longitudinal studies would allow for a more robust association of changes in the microbiota to changes in obesity and metabolic disorders.
Conclusions
This systematic review identified consistent evidence for several lean-associated genera that may have therapeutic potential for obesity and metabolic diseases. Besides A. muciniphila, species from genera Faecalibacterium, Alistipes, and Roseburia might also harbor therapeutic potentials against obesity and metabolic diseases. These results provided a guide for the future development of certain bacteria into live biotherapeutics that may be helpful for the management of obesity and metabolic disorders. Further in-vitro and in-vivo research are needed to elucidate their role in the management of obesity and metabolic diseases.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- OB:
-
Obesity
- T2DM:
-
Type 2 diabetes
- NAFLD:
-
Non-alcoholic fatty liver disease
- NGS:
-
Next-generation sequencing
- BMI:
-
Body mass index
- F/B:
-
Firmicutes/Bacteroidetes
- NASH:
-
Non-alcoholic steatohepatitis
- OMS:
-
Obese with metabolic syndrome
- SCFA:
-
Short-chain fatty acids
- HFD:
-
High-fat diet
References
Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014;62:96–102. https://doi.org/10.1016/j.ypmed.2014.02.003.
WHO. Obesity and overweight, World Health Organization. 2017. http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 12 Jan 2018.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. https://doi.org/10.1038/nature05414.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. https://doi.org/10.1038/nature07540.
Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow SATE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. 2018;2018:4095789. https://doi.org/10.1155/2018/4095789.
Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe. 2019;26(2):265–72.e4.
Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, et al. Effects of Fecal Microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol. 2020;18(4):855–63.e2. https://doi.org/10.1016/j.cgh.2019.07.006.
Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. https://doi.org/10.1371/journal.pmed.1003051.
Borgeraas H, Johnson LK, Skattebu J, Hertel JK, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2018;19(2):219–32. https://doi.org/10.1111/obr.12626.
Depommier C, Everard A, Druart C, Plovier H, van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–103. https://doi.org/10.1038/s41591-019-0495-2.
Nam YD, Jung MJ, Roh SW, Kim MS, Bae JW. Comparative analysis of Korean human gut microbiota by barcoded pyrosequencing. PLoS One. 2011;6(7):e22109. https://doi.org/10.1371/journal.pone.0022109.
Deschasaux M, Bouter KE, Prodan A, et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat Med. 2018;24(10):1526.
Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222.
Hayashi H, Sakamoto M, Benno Y. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol Immunol. 2002;46(12):819–31. https://doi.org/10.1111/j.1348-0421.2002.tb02769.x.
Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72(2):1027–33. https://doi.org/10.1128/AEM.72.2.1027-1033.2006.
Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta TA, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3(1):36. https://doi.org/10.1186/s40168-015-0101-x.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339(1):b2535. https://doi.org/10.1136/bmj.b2535.
Andoh A, Nishida A, Takahashi K, Inatomi O, Imaeda H, Bamba S, et al. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J Clin Biochem Nutr. 2016;59(1):65–70. https://doi.org/10.3164/jcbn.15-152.
Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7-18 years old children from the American Gut Project. Pediatr Obes. 2019;14(4):e12480. https://doi.org/10.1111/ijpo.12480.
Chen X, Sun H, Jiang F, Shen Y, Li X, Hu X, et al. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;2020(1):8317. https://doi.org/10.7717/peerj.8317.
Da Silva CC, Monteil MA, Davis EM. Overweight and obesity in children are associated with an abundance of Firmicutes and Reduction of Bifidobacterium in their gastrointestinal microbiota. Child Obes (Print). 2020;16(3):204–10. https://doi.org/10.1089/chi.2019.0280.
Gao R, Zhu C, Li H, Yin M, Pan C, Huang L, et al. Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity. 2018;26(2):351–61. https://doi.org/10.1002/oby.22088.
Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. A study of the correlation between obesity and intestinal flora in school-age children. Sci Rep. 2018;8(1):14511. https://doi.org/10.1038/s41598-018-32730-6.
Haro C, Rangel-Zuniga OA, Alcala-Diaz JF, et al. Intestinal microbiota is influenced by gender and body mass index. PLoS One. 2016;11(5):e0154090. https://doi.org/10.1371/journal.pone.0154090.
Houttu N, Mokkala K, Laitinen K. Overweight and obesity status in pregnant women are related to intestinal microbiota and serum metabolic and inflammatory profiles. Clin Nutr. 2018;37(6):1955–66. https://doi.org/10.1016/j.clnu.2017.12.013.
Hu H-J, Park S-G, Jang HB, Choi MG, Park KH, Kang JH, et al. Obesity alters the microbial community profile in Korean adolescents. PloS One. 2015;10(7):e0134333. https://doi.org/10.1371/journal.pone.0134333.
Kaplan RC, Wang Z, Usyk M, Sotres-Alvarez D, Daviglus ML, Schneiderman N, et al. Gut microbiome composition in the Hispanic Community Health Study/Study of Latinos is shaped by geographic relocation, environmental factors, and obesity. Genome Biol. 2019;20(1):219. https://doi.org/10.1186/s13059-019-1831-z.
Liu R, Hong J, Feng Q, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–68. https://doi.org/10.1038/nm.4358.
Lopez-Contreras BE, Moran-Ramos S, Villarruel-Vazquez R, et al. Composition of gut microbiota in obese and normal-weight Mexican school-age children and its association with metabolic traits. Pediatr Obes. 2018;13(6):381–8. https://doi.org/10.1111/ijpo.12262.
Lv Y, Qin X, Jia H, Chen S, Sun W, Wang X. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. Br J Nutr. 2019;122(9):986–95. https://doi.org/10.1017/S0007114519001909.
Mendez-Salazar EO, Ortiz-Lopez MG, Granados-Silvestre MDLA, Palacios-Gonzalez B, Menjivar M. Altered gut microbiota and compositional changes in firmicutes and proteobacteria in Mexican undernourished and obese children. Front Microbiol. 2018;9:2494.
Nardelli C, Granata I, D’ Argenio V, et al. Characterization of the Duodenal mucosal microbiome in obese adult subjects by 16S rRNA sequencing. Microorganisms 2020; 8, 4, 485, https://doi.org/10.3390/microorganisms8040485.
Blasco G, Moreno-Navarrete JM, Rivero M, Pérez-Brocal V, Garre-Olmo J, Puig J, et al. The gut metagenome changes in parallel to waist circumference, brain iron deposition, and cognitive function. J Clin Endocrinol Metab. 2017;102(8):2962–73. https://doi.org/10.1210/jc.2017-00133.
Davis SC, Yadav JS, Barrow SD, Robertson BK. Gut microbiome diversity influenced more by the Westernized dietary regime than the body mass index as assessed using effect size statistic. MicrobiologyOpen. 2017;6(4):e00476. https://doi.org/10.1002/mbo3.476.
Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, et al. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One. 2015;10(4):e0124599. https://doi.org/10.1371/journal.pone.0124599.
Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2015;14(1):311. https://doi.org/10.1186/s12866-014-0311-6.
Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15(1):100. https://doi.org/10.1186/s12876-015-0330-2.
Nirmalkar K, Murugesan S, Pizano-Zarate ML, et al. Gut microbiota and endothelial dysfunction markers in obese Mexican children and adolescents. Nutrients. 2018;10(12):2009. https://doi.org/10.3390/nu10122009.
Ottosson F, Brunkwall L, Ericson U, Nilsson PM, Almgren P, Fernandez C, et al. Connection between BMI-related plasma metabolite profile and gut microbiota. J Clin Endocrinol Metab. 2018;103(4):1491–501. https://doi.org/10.1210/jc.2017-02114.
Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, et al. A taxonomic signature of obesity in a large study of American adults. Sci Rep. 2018;8(1):9749. https://doi.org/10.1038/s41598-018-28126-1.
Ppatil D, Pdhotre D, Gchavan S, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37(4):647–57. https://doi.org/10.1007/s12038-012-9244-0.
Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes. 2014;38(12):1525–31. https://doi.org/10.1038/ijo.2014.46.
Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19(1):95–105. https://doi.org/10.1111/1462-2920.13463.
Vieira-Silva S, Falony G, Belda E, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581(7808):310–5. https://doi.org/10.1038/s41586-020-2269-x.
Ville A, Levine E, Zhi D, Lararia B, Wojcicki JM. Alterations in the gut microbiome at 6 months of age in obese Latino infants. J Am Coll Nutr. 2020;39(1):47–53. https://doi.org/10.1080/07315724.2019.1606744.
Yasir M, Angelakis E, Bibi F, Azhar EI, Bachar D, Lagier JC, et al. Comparison of the gut microbiota of people in France and Saudi Arabia. Nutr Diabetes. 2015;5(4):e153. https://doi.org/10.1038/nutd.2015.3.
Yun Y, Kim HN, Kim SE, Heo SG, Chang Y, Ryu S, et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017;17(1):151. https://doi.org/10.1186/s12866-017-1052-0.
Zacarias MF, Collado MC, Gomez-Gallego C, et al. Pregestational overweight and obesity are associated with differences in gut microbiota composition and systemic inflammation in the third trimester. PloS One. 2018;13(7):e0200305. https://doi.org/10.1371/journal.pone.0200305.
Allin KH, Tremaroli V, Caesar R, et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61(4):810–20. https://doi.org/10.1007/s00125-018-4550-1.
Barengolts E, Green SJ, Eisenberg Y, et al. Gut microbiota varies by opioid use, circulating leptin and oxytocin in African American men with diabetes and high burden of chronic disease. PLoS One. 2018;13(3):e0194171.
Leite AZ, Rodrigues NC, Gonzaga MI, et al. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and Bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 2017;8:1107.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. https://doi.org/10.1038/nature11450.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. https://doi.org/10.1038/nature12198.
Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. https://doi.org/10.1371/journal.pone.0009085.
Ahmad A, Yang W, Chen G, Shafiq M, Javed S, Ali Zaidi SS, et al. Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS One. 2019;14(12):e0226372. https://doi.org/10.1371/journal.pone.0226372.
Koo SH, Chu CW, Khoo JJC, Cheong M, Soon GH, Ho EXP, et al. A pilot study to examine the association between human gut microbiota and the hostʼs central obesity. JGH Open. 2019;3(6):480–7. https://doi.org/10.1002/jgh3.12184.
Sroka-oleksiak A, Mlodzinska A, Bulanda M, et al. Metagenomic analysis of duodenal microbiota reveals a potential biomarker of dysbiosis in the course of obesity and type 2 diabetes: a pilot study. J Clin Med. 2020;9(2):369. https://doi.org/10.3390/jcm9020369.
Thingholm LB, Ruhlemann MC, Koch M, et al. Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe. 2019;26(2):252–64.e10.
Zhao Y, Zhou J, Liu J, Wang Z, Chen M, Zhou S. Metagenome of gut microbiota of children with nonalcoholic fatty liver disease. Front Pediatr. 2019;7:518. https://doi.org/10.3389/fped.2019.00518.
Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5(1):8096. https://doi.org/10.1038/srep08096.
Shen F, Zheng R-D, Sun X-Q, Ding W-J, Wang X-Y, Fan J-G. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16(4):375–81. https://doi.org/10.1016/S1499-3872(17)60019-5.
Sobhonslidsuk A, Chanprasertyothin S, Pongrujikorn T, Kaewduang P, Promson K, Petraksa S, et al. The association of gut microbiota with nonalcoholic steatohepatitis in Thais. Biomed Res Int. 2018;2018:9340316–8. https://doi.org/10.1155/2018/9340316.
Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L, et al. Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci Rep. 2016;6(1):32002. https://doi.org/10.1038/srep32002.
Li F, Sun G, Wang Z, Wu W, Guo H, Peng L, et al. Characteristics of fecal microbiota in non-alcoholic fatty liver disease patients. Sci China Life Sci. 2018;61(7):770–8. https://doi.org/10.1007/s11427-017-9303-9.
Nistal E, Saenz de Miera LE, Ballesteros PM, et al. An altered fecal microbiota profile in patients with non-alcoholic fatty liver disease (NAFLD) associated with obesity. Rev Esp Enferm Dig. 2019;111(4):275–82. https://doi.org/10.17235/reed.2019.6068/2018.
Yun Y, Kim HN, Lee EJ, Ryu S, Chang Y, Shin H, et al. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS One. 2019;14(3):e0213692. https://doi.org/10.1371/journal.pone.0213692.
Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol. 2015;91(2):1–9. https://doi.org/10.1093/femsec/fiu002.
Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57(2):601–9. https://doi.org/10.1002/hep.26093.
Chavez-Carbajal A, Nirmalkar K, Perez-Lizaur A, et al. Gut microbiota and predicted metabolic pathways in a sample of Mexican women affected by obesity and obesity plus metabolic syndrome. Int J Mol Sci. 2019;20(2):438. https://doi.org/10.3390/ijms20020438.
De La Cuesta-Zuluaga J, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. Int J Obes. 2018;42(3):424–32. https://doi.org/10.1038/ijo.2017.281.
Gallardo-Becerra L, Cornejo-Granados F, Garcia-Lopez R, et al. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb Cell Factories. 2020;19(1):61. https://doi.org/10.1186/s12934-020-01319-y.
Gozd-Barszczewska A, Koziol-Montewka M, Barszczewski P, Mlodzinska A, Huminska K. Gut microbiome as a biomarker of cardiometabolic disorders. Ann Agric Environ Med. 2017;24(3):416–22. https://doi.org/10.26444/aaem/75456.
Kashtanova DA, Tkacheva ON, Doudinskaya EN, Strazhesko I, Kotovskaya Y, Popenko A, et al. Gut microbiota in patients with different metabolic statuses: Moscow study. Microorganisms. 2018;6(4):98. https://doi.org/10.3390/microorganisms6040098.
Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, et al. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benefic Microbes. 2017;8(4):545–56. https://doi.org/10.3920/BM2016.0184.
Feinn R, Kravetz AM, Galuppo B, et al. Effect of gut microbiota and PNPLA3 rs738409 variant on nonalcoholic fatty liver disease (NAFLD) in obese youth. J Clin Endocrinol Metab. 2020;105(10):e3585. https://doi.org/10.1210/clinem/dgaa382.
Li R, Liang X, Su M, Lai KP, Chen J, Huang X. Integrated omics analysis reveals the alteration of gut microbe-metabolites in obese adults. Brief Bioinforma. 2021;22(3):bbaa165. https://doi.org/10.1093/bib/bbaa165.
Yuan X, Chen R, Zhang Y, Lin X, Yang X, McCormick KL. The role of the gut microbiota on the metabolic status of obese children. Microb Cell Factories. 2021;20(1):53. https://doi.org/10.1186/s12934-021-01548-9.
Gomez-Acebo I, Dierssen-Sotos T, de Pedro M, et al. Epidemiology of non-steroidal anti-inflammatory drugs consumption in Spain. The MCC-Spain study. BMC Public Health. 2018;18(1):1134.
Manosalva AGL, Yntema T, Chen L, Garmaeva S, Hu S, Koster M, et al. Omeprazole-induced dysbiosis impacts bile acid metabolism in mice and humans. Atherosclerosis. 2019;287:e120. https://doi.org/10.1016/j.atherosclerosis.2019.06.349.
Rhodes JM. The role of Escherichia coli in inflammatory bowel disease. Gut. 2007;56(5):610–2. https://doi.org/10.1136/gut.2006.111872.
Zhang J, Hoedt EC, Liu Q, et al. Elucidation of Proteus mirabilis as a key bacterium in Crohnʼs disease inflammation. Gastroenterology. 2021;160(1):317–30.e11.
Ellulu MS, Patimah I, Khazaʼai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–63. https://doi.org/10.5114/aoms.2016.58928.
Anhe FF, Jensen BAH, Varin TV, et al. Type 2 diabetes influences bacterial tissue compartmentalisation in human obesity. Nat Metab. 2020;2(3):233–42. https://doi.org/10.1038/s42255-020-0178-9.
Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179–84. https://doi.org/10.1126/science.aaw7479.
Perraudeau F, McMurdie P, Bullard J, Cheng A, Cutcliffe C, Deo A, et al. Improvements to postprandial glucose control in subjects with type 2 diabetes: a multicenter, double blind, randomized placebo-controlled trial of a novel probiotic formulation. Bmj Open Diab Res Ca. 2020;8(1):e001319. https://doi.org/10.1136/bmjdrc-2020-001319.
Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. 2020;11:906. https://doi.org/10.3389/fimmu.2020.00906.
Walker AW, Duncan SH, Louis P, Flint HJ. Phylogeny, culturing, and metagenomics of the human gut microbiota. Trends Microbiol. 2014;22(5):267–74. https://doi.org/10.1016/j.tim.2014.03.001.
De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149(1):73–80. https://doi.org/10.1016/j.ijfoodmicro.2011.03.003.
Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. https://doi.org/10.1186/s40168-019-0704-8.
Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–91. https://doi.org/10.1038/nrendo.2015.128.
Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67(7):1269–79. https://doi.org/10.1136/gutjnl-2017-314050.
Zhang WQ, Zhao TT, Gui DK, Gao CL, Gu JL, Gan WJ, et al. Sodium butyrate improves liver glycogen metabolism in type 2 diabetes mellitus. J Agric Food Chem. 2019;67(27):7694–705. https://doi.org/10.1021/acs.jafc.9b02083.
den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–408. https://doi.org/10.2337/db14-1213.
Xiao JZ, Kondo S, Takahashi N, Miyaji K, Oshida K, Hiramatsu A, et al. Effects of milk products fermented by Bifidobacterium longum on blood lipids in rats and healthy adult male volunteers. J Dairy Sci. 2003;86(7):2452–61. https://doi.org/10.3168/jds.S0022-0302(03)73839-9.
Duncan SH, Hold GL, Barcenilla A, Stewart CS, Flint HJ. Roseburia intestinalis sp. nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int J Syst Evol Microbiol. 2002;52(Pt 5):1615–20. https://doi.org/10.1099/00207713-52-5-1615.
Hiippala K, Jouhten H, Ronkainen A, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(8):988. https://doi.org/10.3390/nu10080988.
Seo B, Jeon K, Moon S, et al. Roseburia spp. abundance associates with alcohol consumption in humans and its administration ameliorates alcoholic fatty liver in mice. Cell Host Microbe. 2020;27(1):25.
Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. Isme j. 2012;6(8):1535–43. https://doi.org/10.1038/ismej.2012.4.
De Vadder F, Kovatcheva-Datchary P, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016;24(1):151–7. https://doi.org/10.1016/j.cmet.2016.06.013.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–81. https://doi.org/10.1038/nature18646.
Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. MBio. 2016;7(4):e01018-16.
Acknowledgements
Not applicable.
Funding
It was funded by InnoHK, The Government of Hong Kong, Special Administrative Region of the People’s Republic of China.
Author information
Authors and Affiliations
Contributions
All authors contributed to the analysis and interpretation of data, the writing and critical revision of manuscript at all stages of development. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
Francis Chan and Siew Ng are co-founders and in the board of GenieBiome Ltd. Siew Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and Abbvie and a speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie, and Takeda. She has received research grants from Olympus, Ferring, and Abbvie. Francis Chan has served as an advisor and lecture speaker for Eisai Co. Ltd., AstraZeneca, Pfizer Inc., Takeda Pharmaceutical Co., and Takeda (China) Holdings Co. Ltd. XU Zhilu is an employee of GenieBiome Ltd. All other authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Table 1. Differentially abundant taxa at each taxonomic level in patients with obesity and metabolic diseases reported in individual studies. Supplementary Table 2. Differentially abundant taxa at class, order, and family level in obesity / metabolic diseases. Supplementary Table 3. Microbiota diversity and F/B Ratio in Obesity / metabolic diseases.
Appendix. Searching strategy
Appendix. Searching strategy
1 obese.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
2 obesity.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
3 overweight.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
4 microbiota.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
5 microbiome.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
6 fecal.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
7 faecal.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
8 gut.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
9 intestinal.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
10 1 or 2 or 3
11 4 or 5
12 6 or 7 or 8 or 9
13 10 and 11 and 12
14 metagenomics.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
15 metagenomic.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
16 16s.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
17 14 or 15 or 16
18 13 and 17
19 remove duplicates from 18
20 metabolic disease.mp. [mp=ti, ab, hw, tn, ot, dm, mf, dv, kw, fx, dq, nm, kf, ox, px, rx, an, ui, sy]
21 10 or 20
22 11 and 12 and 21
23 17 and 22
24 remove duplicates from 23
25 limit 24 to full text
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
XU, Z., JIANG, W., HUANG, W. et al. Gut microbiota in patients with obesity and metabolic disorders — a systematic review. Genes Nutr 17, 2 (2022). https://doi.org/10.1186/s12263-021-00703-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12263-021-00703-6