Abstract
Background
Early identification of autism spectrum disorder (ASD) provides an opportunity for early intervention and improved developmental outcomes. The use of electroencephalography (EEG) in infancy has shown promise in predicting later ASD diagnoses and in identifying neural mechanisms underlying the disorder. Given the high co-morbidity with language impairment, we and others have speculated that infants who are later diagnosed with ASD have altered language learning, including phoneme discrimination. Phoneme learning occurs rapidly in infancy, so altered neural substrates during the first year of life may serve as early, accurate indicators of later autism diagnosis.
Methods
Using EEG data collected at two different ages during a passive phoneme task in infants with high familial risk for ASD, we compared the predictive accuracy of a combination of feature selection and machine learning models at 6 months (during native phoneme learning) and 12 months (after native phoneme learning), and we identified a single model with strong predictive accuracy (100%) for both ages. Samples at both ages were matched in size and diagnoses (n = 14 with later ASD; n = 40 without ASD). Features included a combination of power and nonlinear measures across the 10‑20 montage electrodes and 6 frequency bands. Predictive features at each age were compared both by feature characteristics and EEG scalp location. Additional prediction analyses were performed on all EEGs collected at 12 months; this larger sample included 67 HR infants (27 HR-ASD, 40 HR-noASD).
Results
Using a combination of Pearson correlation feature selection and support vector machine classifier, 100% predictive diagnostic accuracy was observed at both 6 and 12 months. Predictive features differed between the models trained on 6- versus 12-month data. At 6 months, predictive features were biased to measures from central electrodes, power measures, and frequencies in the alpha range. At 12 months, predictive features were more distributed between power and nonlinear measures, and biased toward frequencies in the beta range. However, diagnosis prediction accuracy substantially decreased in the larger, more behaviorally heterogeneous 12-month sample.
Conclusions
These results demonstrate that speech processing EEG measures can facilitate earlier identification of ASD but emphasize the need for age-specific predictive models with large sample sizes to develop clinically relevant classification algorithms.
Similar content being viewed by others
Background
The past decade has witnessed a dramatic increase in the prevalence of autism spectrum disorder (ASD), a neurodevelopmental disorder characterized by deficits in social communication and repetitive and restrictive behaviors [1]. The CDC estimates that one in 54 children has an ASD diagnosis [2], up from the one in 88 prevalence reported about a decade ago [3]. Currently, ASD is diagnosed using behavioral measures, so a diagnosis cannot be made until toddlerhood or later when behavioral symptoms are reliably observable [4]. However, there is strong support for the assertion that early intervention leads to better intellectual and behavioral outcomes [5, 6]. Therefore, a central focus for the field has been to develop objective, biological markers to facilitate earlier detection, and subsequent intervention of ASD.
Neuroimaging measures provide strong candidate tools for early identification as they can be obtained from the newborn period onwards. For example, several recent studies have used magnetic resonance imaging (MRI) data collected in infancy to predict ASD diagnoses [7, 8]. However, MRI has several drawbacks, including expense and participant restrictions, making it a less feasible general screening tool. Electroencephalography (EEG), on the other hand, may prove to be a more scalable tool, given its low cost and ease of acquisition in awake and sleeping infants without participant restrictions. Moreover, EEG is known to be sensitive to brain-related changes in ASD before behavioral symptoms are observable [9,10,11,12,13]. Initial efforts to predict ASD diagnoses using baseline (i.e., resting-state) EEG early in life have shown promise [14,15,16,17]. However, diagnostic prediction using EEG recorded during tasks related to ASD symptoms has yet to be attempted and may outperform prior baseline EEG-based classification.
Language is frequently delayed or impaired in ASD [18,19,20,21,22], which may result from atypical peak synaptic sensitivity [23] and cortical excitatory and inhibitory imbalance [24] that disrupt neural circuits typically involved in language development (e.g., altered sensitive period dynamics). Therefore, focusing on the brain’s electrical activity during a language processing task may facilitate improved diagnostic prediction accuracy relative to baseline conditions, and provide insights into the neurobiology of language processing deficits within ASD. Notably, EEG has been used to measure differences in language processing in children with ASD who are older than 12 months [25,26,27], suggesting EEG is sensitive to atypical neural processing of language stimuli in ASD.
Perceptual narrowing of phoneme discrimination is a critical first stage in language acquisition [28]. Very young infants can discriminate between native and non-native phonemes better than adults, but they lose this ability over the first year of life as their phoneme perception is tuned to the language(s) experienced in daily life during this sensitive period of learning [29]. However, there is evidence that phoneme discrimination may develop differently in infants with ASD, thereby impacting language development [30,31,32]. This study focused on the phoneme learning sensitive period over the first year of life as a potential source of early indicators of subsequent ASD diagnosis.
There were two overarching goals of the present study. First, we aimed to evaluate whether EEG data collected during a language phoneme task at either 6 or 12 months of age in infants with familial risk for ASD can accurately predict later ASD diagnosis. We utilized EEG data collected from high familial risk infant siblings as part of a prospective longitudinal study, where diagnosis of ASD was determined at 2‑3 years of age. Though power analysis of EEG is most common, nonlinear measures can capture dynamical properties of the brain that power analysis is not able to quantify. For example, entropies evaluate the regularity and stability of patterns within the EEG signal, the fractal dimension measures self-similarity of a signal across multiple scales, and the Hurst exponent and detrended fluctuation analysis calculate long-term autocorrelation. Beyond capturing nonlinear patterns generated from a nonlinear system (i.e., the brain), these measures have exhibited sensitivity to changes in the brain’s balance of neural excitation and inhibition [33], and the ability to index transitions to epileptic seizures [34]. The excitatory-inhibitory balance of neural circuits undergoes critical developmental shifts during the first year of life with significant effects on neuroplasticity [35], including those posited to support language development, and each of these mechanisms are thought to be disrupted in ASD [32]. This prior evidence suggests that nonlinear dynamics are core features of healthy brain function and may relate to several fundamental neurodevelopmental processes over the first year of life. Nonlinear measures of adult EEG have accurately classified other clinical conditions, including depression [36,37,38], schizophrenia [39,40,41], and epilepsy [42,43,44]. Our lab previously found that these measures computed from resting-state EEG are useful in predicting ASD outcome [16], and we now aim to improve predictive capacity by evaluating these measures on language processing-related data. Second, given that expected perceptual narrowing of phoneme discrimination occurs between 6 and 12 months of age, we aimed to compare the EEG features most predictive of diagnosis and determine whether there are developmental differences in which features are most important during versus after the language phoneme learning period.
Methods
Study design and participant demographics
Participants were recruited to Boston Children’s Hospital to participate in a longitudinal study of infant siblings of children with ASD. Institutional review board approval was obtained from Boston University and Boston Children’s Hospital (# X06-08-0374). All infants had a gestational age greater than 36 weeks, no history of seizures, prenatal drug exposure, hearing impairment, or known genetic mutation involved in neurodevelopment. Infants were designated high risk (HR) for ASD based on the confirmed ASD clinical diagnosis of an older sibling. 104 HR infants were enrolled in the longitudinal study and ASD outcome was determined using the Autism Diagnostic Observation Schedule (ADOS) in conjunction with a clinical best estimate. For infants meeting criteria on the ADOS or coming within 3 points of clinical diagnosis cutoffs, a Licensed Clinical Psychologist reviewed scores and video recordings and provided a best estimate clinical judgment of ASD diagnosis.
Three sets of EEG data were evaluated in predictive models: a 6-month sample, full 12-month sample, and matching 12-month sample. At 6-months, EEGs from 54 HR infants were analyzed (14 HR-ASD, 40 HR-noASD). A “matching” 12-month dataset was curated to assess longitudinal changes in ASD prediction at the different ages. 13 HR-ASD and 24 HR-noASD participants contributed data at both timepoints. The single HR-ASD participant in the 6-month cohort who did not contribute data at 12 months was replaced by an HR-ASD, demographically matched participant. All 40 HR-noASD 12-month samples were included in the matched dataset, resulting in the same sample size as that of the 6-month dataset. Additional prediction analyses were performed on all EEGs collected at 12 months; this larger sample included 67 HR infants (27 HR-ASD, 40 HR-noASD). Demographic and data quality information of each outcome group is presented in Table 1. Fisher’s exact test was used to evaluate differences of demographic information between groups. The 12-month HR-ASD group had significantly lower mean maternal education than the HR-noASD group (p = 0.004). No other significant demographic differences were observed.
Behavioral assessments
The Mullen Scales for Early Learning (MSEL) and ADOS were administered at each data collection visit of the longitudinal study, including 6 and 12 months. The MSEL provides an index of ability in domains including language, cognition, and motor development.
EEG paradigm
A subset of an oddball phoneme speech task was used for the present analysis, namely, only EEG data recorded during the standard (most frequent) English phoneme (a voiced, unaspirated dental/da/). Each trial consisted of the auditory stimulus played over 300 ms and followed by a variable interstimulus interval between 1000 and 1200 ms [31].
EEG data acquisition and processing
EEG data were acquired in a dimly lit, sound-attenuated, electrically shielded room. A research assistant was present in the room to ensure that the infant remained calm and still during the language paradigm by blowing bubbles or presenting toys if the infant became distracted or fussy. Assistants did not engage in social interaction with the infant during task completion. EEG data were collected with either a 64-channel Geodesic Sensor Net or a 128-channel Hydrocel Geodesic Sensor Net (Electrical Geodesics, Inc. (EGI), Eugene, OR, USA), using a 0.1-Hz high-pass analog filter and online rereferencing to the vertex (channel Cz) through NetStation software (EGI, Eugene, OR, USA). Impedances were kept below 100 KΩ in accordance with the connected DC-coupled amplifier (Net Amps 200 or Net Amps 300, Electrical Geodesics, Inc.). Data were sampled at either 250 or 500 Hz.
EEG data were exported from NetStation to MATLAB format (R2017A). Files were batch processed using the Harvard Automated Processing Pipeline for EEG (HAPPE) within the Batch Electroencephalography Automated Processing Platform (BEAPP) software [45, 46].
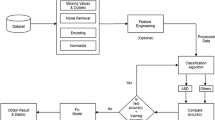
Data were 1 Hz digital high-pass and 100 Hz low-pass filtered, downsampled to 250 Hz (if needed), and run through the HAPPE module using a spatially distributed subset of channels (Fig. 1). Default HAPPE artifact-rejection settings were used as they were optimized for this dataset prior to HAPPE’s original release. Namely, HAPPE artifact removal steps included bad channel identification, electrical line noise removal via Cleanline multitapering approach, artifact removal through wavelet-enhanced ICA and through a second ICA decomposition with automated component rejection above 50% artifact probability via the Multiple Artifact Rejection Algorithm [47, 48]. Bad channels were then interpolated and EEG data were re-referenced to the average reference and mean signal detrended.
Two EEG nets were used in the study: the 128-channel EGI HydroCel Geodesic Sensor Net (version 1.0) presented on the left and the 64-channel EGI Geodesic Sensor Net (version 2.0) presented on the right. The 10-20 montage channels evaluated in this study are highlighted in blue, and HAPPE channels included in preprocessing steps are highlighted in yellow
EEG data decomposition
The middle 20 s of the longest stretch of consecutive standard phoneme (English “da”) presentations in each file were selected for analysis to maximize the number of participants included while ensuring nonlinear measures could be calculated with fidelity. EEG data from 10‑20 montage channel equivalents (18 channels) for each net type were then decomposed into frequency sub-bands using a discrete wavelet transform and a coarse-graining procedure (see Supplementary Materials for description).
EEG measures
Eleven different nonlinear measures and power were computed for each waveband from each of the 18 electrodes for each participant (Table 2).
Feature selection strategies
Across models, three distinct feature selection methods were evaluated to prevent model overfitting: selection based on (1) the features most correlated with autism outcomes (Pearson correlation coefficient), (2) the features with most significant F ratio of mean square variances by group (F test), and (3) the features selected using recursive feature elimination (RFE) with cross validation based on a linear support vector machine. Each selection method was restricted to selecting 20 features.
Classification strategies
We evaluated multiple prediction models that varied in classification approach, including a support vector machine (SVM) with radial basis function, a Gaussian Naïve Bayes algorithm, linear discriminant analysis, and a k-nearest neighbors model where k = 7, the square root of the sample size of the 6-month and matching 12-month datasets. All other models were trained using default parameters from the Python open source package scikit-learn.
Previous studies have found that including test samples in feature selection biases prediction accuracies [52], so we employed nested leave-one-out cross validation to evaluate the validity of model performance. Five metrics were used to evaluate model performance: accuracy, sensitivity, specificity, positive predictive value, and negative predictive value. Given the imbalanced nature of the sample, permutation testing was used to assess the significance of observed prediction accuracy. A null distribution of predictive accuracy was generated by repeating the following procedure over 1000 iterations: diagnostic labels were shuffled and cross validation analysis using features selected with true labels was performed.
Characterizing selected features across 6 and 12 months
To determine the frequency of feature characteristics (i.e., channel, frequency band, measure) selected during the nested leave-one-out procedure (Fig. 1), the 20 features selected over each of the 54 iterations for 6-month and matching 12-month datasets were collated over the characteristic categories, summed, and then divided by the total iteration count. Significant differences between diagnostic groups were evaluated with a student’s t test with Bonferroni correction for multiple comparisons.
Results
Autism prediction at 6 and 12 months
Various prediction models drawing from three feature selection methods and four machine learning classifiers were evaluated for prediction accuracy of future autism diagnosis using either 6-month- or 12-month-matched EEG datasets (each with 40 HR-noASD and 14 HR-ASD participants). The SVM classifier with features selected by the Pearson correlation ranking method achieved 100% diagnostic prediction accuracy for both ages while other classification attempts were more variable (Table S2). Predictive accuracy using true labels with the SVM classifier was significantly better than chance as determined by the null distribution generated with random labels for both timepoints (6-month z-score 7.35, p < 0.0001; 12-month z-score 7.5, p < 0.0001).
Predictive features at 6 and 12 months
The nature and spatial distribution of features selected in the successful 6- and 12-month predictive models were extracted in order to compare EEG features most predictive of ASD diagnosis either during or after perceptual narrowing of phoneme discrimination. Importantly, the same feature selection method and machine learning algorithm (Pearson correlation coefficient feature selection and an SVM classifier) achieved 100% predictive accuracy for both the 6-month- and 12-month-matched datasets, allowing for direct comparison of the features selected at each iteration of nested cross validation across the two ages.
Figure 2 shows the selection rates of features by channel, measure, and frequency. At 6 months, features were selected largely from central and left of the midline locations (Fig. 2A), and power was the most frequently selected measure (Fig. 2B). Five of the 12 measures (power, approximate entropy, Hurst exponent, Lempel-Ziv complexity, and permutation entropy) were consistently selected across iterations. Within iterations, power was most frequently selected. Additionally, while almost all frequency bands were selected at each iteration, sub-bands with frequency ranges below 16 Hz were more frequently selected (Fig. 2C). At 12 months, selected channels changed most in the left hemisphere, with increased left lateralization (i.e., shifts away from midline) and representation from especially dense frontal and temporo-parietal scalp regions (Fig. 2D). Seven of the 12 measures were selected in at least 80% of the iterations, and half of the selected measures in each iteration were power or Lyapunov exponent computed at different wavebands and channels (Fig. 2E). While measures related to all frequency bands were consistently chosen across iterations, the average count per iteration of features related to the 15.6‑31.2 Hz range (largely canonical Beta frequencies) was nearly double at any other frequency range (Fig. 2F).
Information about features most correlated with autism diagnostic outcome for nearly overlapping 6- and 12-month analyses (n = 54). The bottom row visualizes the values for the 12-month dataset (middle row) minus the 6-month dataset (top row). A, D, G Average number of features selected from each channel. Color indicates number of features selected from a given channel. B, E, H Average count of each EEG measure across iterations (orange) and percentage of iterations that each measure was selected at least once (blue). C, F, I Average count of each wavelet across iterations (orange) and percentage of iterations that each wavelet was selected at least once (blue)
We next compared the mean value of each of the 20 most frequently selected features (see the “Methods” section: the “Characterizing selected features across 6 and 12 months” section) between ASD outcome groups (Table 3). After correcting for multiple comparisons, only approximate entropy computed from the F3 electrode in the delta range (1‑4 Hz) was significantly different between the two groups at 6 months (HR-ASD: mean 0.815 ± 0.08; HR-noASD: mean 0.76 ± 0.037; p < 0.0025). In contrast, at 12 months of age, the mean values measured for seven of the 20 features most commonly chosen across iterations were significantly different between groups such that HR-ASD infants had consistently higher values for each of these features than HR-noASD infants. Significant features across model iterations were also those that were most often chosen during feature selection (Fig. 2E): Lyapunov exponent, Hurst exponent, sample entropy, and power. Lempel-Ziv complexity was the only measure selected in all iterations that was not significantly different between groups after Bonferroni correction.
Autism prediction at 12 months: full sample
We next evaluated the diagnosis prediction accuracy using all available 12-month participants and found a considerable decrease in almost all evaluation metrics (Table S2), including the combination of Pearson correlation coefficient feature selection and SVM classifier which dropped from 100 to 7% accuracy when the sample was expanded. Only one of the 12 classification schemes—F test feature selection with SVM algorithm—resulted in accuracy marginally above chance (61.2%) at the severe expense of sensitivity (3.7%), the measure evaluating the percentage of infants with ASD who were predicted correctly.
Given this discrepancy, we assessed whether there were behavioral differences in the HR-ASD participants between full and matched 12-month samples that could provide possible explanation for the differences in brain-based classification accuracy (Table 4). We found that 12-month-old participants who also participated at the 6-month timepoint had significantly lower severity scores on the 36-month ADOS compared to 12-month-old participants who only contributed data at the 12-month timepoint. Additionally, features that were significantly different between 12-month HR-noASD and longitudinal HR-ASD participants poorly discriminated between HR-noASD and cross sectional HR-ASD participants (Fig. 3).
Feature distributions for features most significantly different between the longitudinal 12-month classification analyses (n = 54). Features are listed and emboldened in Table 3. Kernel density estimates are color coded by group: blue for HR-noASD (n = 40); orange for longitudinal HR-ASD (n = 14); and green for cross sectional HR-ASD (n = 13)
Discussion
This study evaluated multiple classification schemes to predict ASD diagnosis in high-familial risk infants using language-task EEG data. Specifically, EEG was evaluated at 6 or 12 months of age, timepoints that span a critical early language-learning period in development. One hundred percent diagnostic classification accuracy was achieved using the Pearson correlation coefficient feature selection with the SVM classifier regardless of whether infants were within the critical period of language phoneme learning (6 months) or after (12 months). However, the features selected to achieve the 100% prediction rate differed between the two ages both in measure type and spatial distribution. Importantly, overall performance across models tested was highly variable and notably reduced when the sample demographics and size changed. Although we used robust statistical methods to limit overfitting in small samples, these constrained models may be unable to fit the variability present in ASD. The implications of these results in the search for early neuroimaging biomarkers of ASD diagnosis are discussed below.
Developmental shift in predictive features
The longitudinal nature of the study provides the opportunity to assess whether predictive EEG features change across the course of a language-learning critical period over the first year of life. Several differences were identified. At 6 months, power from frequencies below 16 Hz dominated as the most common measures selected; in contrast, at 12 months, additional nonlinear measures were more consistently selected with a shift toward high frequencies, suggesting that nonlinear measures of high frequency signal better define binary ASD diagnostic outcomes based on language task EEG at 1 year of life. This trend is consistent with previous longitudinal studies that observe power differences between high- and low-risk infants emerging before 6 months but often dissipating by 12 months [9, 11, 14]. While power was the most common feature type selected at 6-months, few features were statistically different between HR-noASD and HR-ASD groups at this age after correcting for multiple comparisons. In contrast, at 12 months, significant differences between outcome groups were identified in 7 of the 20 most common features (Table 3). This suggests that different classification strategies are utilized at the two ages, with a combination of different power features taken together at 6 months and independent contributions of a range of power and nonlinear features at 12 months. McIntosh and colleagues previously used EEG to demonstrate that neural variability (measured with entropy) increases with neurodevelopment [53]. Therefore, the inclusion of more nonlinear measures versus power at 12 months compared to 6 months in the present study may indicate that these novel measures are more attuned to capturing variability inherent to development at the end of the first year of life, in line with previous research. Additionally, over the first year of life, the balance between excitation and inhibition in neural circuitry changes profoundly, so the sensitivity of entropy to excitation/inhibition dynamics [34] may advocate for their relevance in early-life development, consistent with the successful classification results dependent on entropy and other nonlinear measures.
We also observed differences between developmental time points topographically, with a shift leftwards from central-midline locations, including increased involvement of the temporal and lateral frontal scalp sites at 12 months (Fig. 2G). Power measures computed from the left-frontal F7 channel were particularly predictive as F7 power from all five frequency ranges was identified as 5 of the 20 most commonly selected features at 12 months (Table 3), and F7 power between 1 and 15.6 Hz was significantly increased in the HR-ASD group compared to HR-noASD. This shift may indicate that infants who are later diagnosed with ASD show atypical activity in the network of left-lateralized regions involved in typical language perception by 12 months of age [54,55,56,57,58]. Our EEG-related findings corroborate MRI findings of atypical activation related to passive auditory stimuli in adults and children with ASD over similar cortical areas [59, 60]. The window between 6 and 12 months is especially important for developing language ability, and the observed shift in the scalp sites included in successful prediction of ASD mirrors the developmental shifts in neural circuitry for language processing with development (e.g., left lateralization).
Variable classification performance
Our evaluation of machine learning models identified a single model with high accuracy for matched 6- and 12-month datasets. However, the nested leave-one-out cross validation results varied greatly across different feature selection method and machine learning algorithm combinations. Performance variability may be attributed to the small size of the datasets, which risk overfitting or underfitting to the training data despite our efforts to minimize the dimensionality of the data before classification. Similarly sized studies using MRI data have previously demonstrated sensitivity lower than 90% with near-perfect specificity [7, 8]. These other studies only presented results from a single classification scheme, so the variability across classification schemes for MRI data is unknown.
The decrease in accuracy from 100% with the matched 12-month dataset to 7% with the full 12-month dataset using a SVM classifier suggests an inability to effectively separate the two diagnostic outcome classes after the HR-ASD group was expanded. Behavioral phenotypes of the HR-ASD infants added to complete the full 12-month analysis were more variable and severe than those who participated at both ages. It is possible that enrollment bias influenced the sample characteristics in that high-risk families enrolling at a later age may have had increased concerns about ASD related to observed symptoms. We postulate that the inclusion of more HR-ASD samples that had more similar feature distributions to the HR-noASD group resulted in increased feature overlap across outcome classes, preventing accurate hyperboundary separation (Fig. 3). It may also be that additional HR-ASD samples increased the heterogeneity of that group, essentially creating a continuum distribution of features across both groups. The result would be the same: A discriminating hyperboundary between groups would be difficult to find. Our 12-month results also suggest that more complex modeling will be required at this age to appropriately account for the full range of heterogeneity in ASD at the brain and behavioral levels. Simpler classification approaches might still perform well with an increased 6-month sample size if EEG measures tend to be less variable and better stratified between the ASD diagnostic outcomes at this younger age.
The effect of heterogeneity and sample size on prediction
Autism is a heterogeneous disorder—its defining categories are broad and encompass a spectrum of symptom severity. Our observed decline in classification accuracy with increased sample size and important shifts in sample phenotypes (including ADOS severity) highlights factors that must be considered by the greater autism research community for future diagnosis prediction efforts. This within-study classification distinction serves as a case study of poor generalizability to a larger sample.
The HR-ASD infants who participated in both 6- and 12-month timepoints had significantly different ADOS severity scores at 36 months than the HR-ASD infants who joined the study at 12 months. On all five behavioral measures evaluated in Table 4, the 12-month-only HR-ASD group had higher ADOS scores and lower MSEL scores (corresponding to overall lower indexes of development). We hypothesize that the inclusion of a more severe and variable dataset reduced the accuracy of classification since we were predicting ASD as a binary diagnosis. Using resting-state EEG from the same infant-sibling dataset in this analysis, we have previously observed a similar lower accuracy in a 12-month sample, although a similar size sample at 9 months had high accuracy. As discussed in our previous paper, this may be a real neurodevelopmental trend reflecting the cross-over of neurodevelopmental trajectories of infants who do and do not go on to develop ASD [15, 16]. Alternatively, this finding may correspond with previous meta-analyses of brain-disorder prediction field that have found decreasing accuracies reported as sample sizes—and, importantly, heterogeneity within the sample—increase [61,62,63]. Overall, more sample data are needed in order to more completely represent the brain activity differences that arise in ASD, which would also permit the use of more complex models that may more appropriately account for the variability and complex associations between brain activity and diagnosis.
Limitations and future directions
We acknowledge several limitations of the current study in addition to the discussed challenges of sample size and heterogeneity. First, our focus on infants with familial risk of autism may not generalize to other ASD-risk groups or to the general population. Second, the specificity of our findings to ASD (versus other comorbid conditions) is unknown. Further testing across clinical populations (e.g., global developmental delay without ASD or isolated language delay) is needed to understand whether EEG could also be used to predict comorbidities with significant impact on functional outcomes. This study determines ASD outcome at age 3, which is appropriate for assessing ASD but not for many other developmental conditions that emerge across early childhood. Therefore, questions about comorbidity call for the extension of longitudinal studies to track participants beyond 3 years to capture a more complete clinical description of participants. Third, our sample was not diverse ethnically, racially, or in income level. Predictive analyses require not only large sample sizes but also must include infants from diverse populations in order to improve clinical applicability to the general population. These results suggest that collaboration across samples is critical to moving forward in developing early predictive models.
Future studies of early predictive markers of ASD and other neurodevelopmental disorders need to be acutely aware of participant age, given the dramatic developmental changes in predictive feature profiles over the 6-month age window in the study. Moreover, given the variability of behavioral measures within the ASD outcome group, future studies should consider distinguishing different subpopulations of ASD grouped by biological presentations or phenotype profiles at the behavioral level as opposed to only binary diagnosis.
Conclusions
These results demonstrate that speech processing EEG measures may facilitate earlier identification of ASD. However, different nonlinear and power measures were predictive of ASD outcomes depending on developmental age with respect to early language learning. Overall, these findings emphasize the need for age-specific predictive models with large sample sizes and the challenge of discriminating diagnostic differences in highly heterogeneous populations.
Availability of data and materials
Python and Jupyter Notebook scripts used for computation of nonlinear measures and machine learning analysis are available. The datasets of preprocessed nonlinear and power measures analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADOS:
-
Autism Diagnostic Observation Schedule
- ASD:
-
Autism spectrum disorder
- BEAPP:
-
Batch EEG automated processing platform
- EEG:
-
Electroencephalography
- EGI:
-
Electrical Geodesics, Inc
- HAPPE:
-
Harvard automated preprocessing pipeline for EEG
- HR:
-
High risk
- MARA:
-
Multiple Artifact Rejection Algorithm
- MRI:
-
Magnetic resonance imaging
- MSEL:
-
Mullen Scales of Early Learning
- SVM:
-
Support vector machine
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed; 2013.
United States. Autism and developmental disabilities monitoring network surveillance year 2008 principal investigators. MMWR Surveill Summ. 2012;61:1–19.
Maenner M, Shaw K, Baio J. Prevalence of autism spectrum disorder am ong children aged 8 years—autism and developmental disabilities monitoring network. Morbidity and Mortality Weekly Report, Surveillance Summaries. 2002.
Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in Autism. Mol Psychiatry. 2017;22:1–10 Nature Publishing Group.
Cohen H, Amerine-Dickens M, Smith T. Early intensive behavioral treatment: replication of the UCLA model in a community setting. J Dev Behav Pediatr. 2006;27. https://doi.org/10.1097/00004703-200604002-00013.
Howlin P, Charman T. Autism spectrum disorders: interventions and outcome. In: The SAGE Handbook of Developmental Disorders. London: SAGE Publications Ltd; 2011. p. 307–28.
Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017;9. https://doi.org/10.1126/scitranslmed.aag2882.
Cody Hazlett H, Gu H, Munsell BC, Hyung Kim S, Styner M, Wolff JJ, et al. Early brain development in infants at high risk for autism spectrum disorder HHS Public Access. Nature. 2017;542:348–51.
Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS One. 2012;7. https://doi.org/10.1371/journal.pone.0039127.
Wilkinson CL, Levin AR, Gabard-Durnam LJ, Tager-Flusberg H, Nelson CA. Reduced frontal gamma power at 24 months is associated with better expressive language in toddlers at risk for autism. Autism Res. 2019;12:1211–24.
Gabard-Durnam L, Tierney AL, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Alpha asymmetry in infants at risk for autism spectrum disorders. J Autism Dev Disord. 2015;45:473–80.
Orekhova EV, Elsabbagh M, Jones EJ, Dawson G, Charman T, Baron-Cohen S, et al. EEG hyper-connectivity in high-risk infants is associated with later autism. J Neurodev Disord. 2014;6:40.
Righi G, Tierney AL, Tager-Flusberg H, Nelson CA. Functional connectivity in the first year of life in infants at risk for autism spectrum disorder: an EEG study. PLoS One. 2014;9. https://doi.org/10.1371/journal.pone.0105176.
Gabard-Durnam LJ, Wilkinson C, Kapur K, Tager-Flusberg H, Levin AR, Nelson CA. Longitudinal EEG power in the first postnatal year differentiates autism outcomes. Nat Commun. 2019;10. https://doi.org/10.1038/s41467-019-12202-9.
Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9:18.
Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep. 2018;8. https://doi.org/10.1038/s41598-018-24318-x.
Dickinson A, Daniel M, Marin A, Gaonkar B, Dapretto M, McDonald NM, et al. Multivariate neural connectivity patterns in early infancy predict later autism symptoms. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:59–69.
Billstedt E, Gillberg C, Gillberg C. Autism after adolescence: population-based 13- to 22-year follow-up study of 120 individuals with autism diagnosed in childhood. J Autism Dev Disord. 2005;35:351–60.
Magiati I, Tay XW, Howlin P. Cognitive, language, social and behavioural outcomes in adults with autism spectrum disorders: a systematic review of longitudinal follow-up studies in adulthood. Clin Psychol Rev. 2014;34:78–86 Elsevier Inc.
Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. J Child Psychol Psychiatry. 2009;50:1235–45.
Howlin P, Mawhood L, Rutter M. Autism and developmental receptive language disorder—a follow-up comparison in early adult life. II: Social, Behavioural, and Psychiatric Outcomes. J Child Psychol Psychiatry. 2000;41:561–78.
Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, et al. Patterns of growth in verbal abilities among children with autism spectrum disorder. J Consult Clin Psychol. 2007;75:594–604.
Berger JM, Rohn TT, Oxford JT. Autism as the early closure of a neuroplastic critical period normally seen in adolescence. Biol Syst Open Access. 2012;02. https://doi.org/10.4172/2329-6577.1000118.
Gao R, Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med. 2015;15:146–67.
Ferri R, Elia M, Agarwal N, Lanuzza B, Musumeci SA, Pennisi G. The mismatch negativity and the P3a components of the auditory event-related potentials in autistic low-functioning subjects. Clin Neurophysiol. 2003;114:1671–80.
Martineau J, Garreau B, Roux S, Lelord G. Auditory evoked responses and their modifications during conditioning paradigm in autistic children. J Autism Dev Disord. 1987;17:525–39.
Bidet-Caulet A, Latinus M, Roux S, Malvy J, Bonnet-Brilhault F, Bruneau N. Atypical sound discrimination in children with ASD as indicated by cortical ERPs. J Neurodev Disord. 2017;9:13.
Werker JF, Lalonde CE. Cross-language speech perception: initial capabilities and developmental change. Dev Psychol. 1988;24(5):672–83.
Polka L, Werker JF. Developmental changes in perception of nonnative vowel contrasts. J Exp Psychol Hum Percept Perform. 1994;20:421–35.
Minagawa-Kawai Y, Naoi N, Kikuchi N, Yamamoto JI, Nakamura K, Kojima S. Cerebral laterality for phonemic and prosodic cue decoding in children with autism. NeuroReport. 2009;20:1219–24.
Seery AM, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Atypical lateralization of ERP response to native and non-native speech in infants at risk for autism spectrum disorder. Dev Cogn Neurosci. 2013;5:10–24.
Leblanc JJ, Fagiolini M. Autism: a critical period disorder? Neural Plasticity. 2011. https://doi.org/10.1155/2011/921680. Hindawi Publishing Corporation.
Liang Z, Wang W, Sun X, Li D, Voss LJ, Sleigh JW, et al. EEG entropy measures in anesthesia. Front Comput Neurosci. 2015;9:16.
Li J, Yan J, Liu X, Ouyang G. Using permutation entropy to measure the changes in EEG signals during absence seizures. PLoS One. 2018;13:e0193691.
Werker JF, Hensch TK. Critical periods in speech perception: new directions. 2015;66:173–96. https://doi.org/10.1146/annurev-psych-010814-015104.
Hosseinifard B, Moradi MH, Rostami R. Classifying depression patients and normal subjects using machine learning techniques and nonlinear features from EEG signal. Comput Methods Prog Biomed. 2013;109:339–45.
Acharya UR, Sudarshan VK, Adeli H, Santhosh J, Koh JEW, Puthankatti SD, et al. A novel depression diagnosis index using nonlinear features in EEG signals. Eur Neurol. 2015;74:79–83.
Bachmann M, Päeske L, Kalev K, Aarma K, Lehtmets A, Ööpik P, et al. Methods for classifying depression in single channel EEG using linear and nonlinear signal analysis. Comput Methods Prog Biomed. 2018;155:11–7.
Sabeti M, Katebi S, Boostani R. Entropy and complexity measures for EEG signal classification of schizophrenic and control participants. Artif Intell Med. 2009;47:263–74.
Boostani R, Sadatnezhad K, Sabeti M. An efficient classifier to diagnose of schizophrenia based on the EEG signals. Expert Syst Appl. 2009;36:6492–9.
Zhao Q, Hu B, Liu L, Ratcliffe M, Peng H, Zhai J, et al. An EEG based nonlinearity analysis method for schizophrenia diagnosis. BioMed. 2012;2012:136–42 Proceedings of the 9th IASTED International Conference on Biomedical Engineering.
Bosl WJ, Loddenkemper T, Nelson CA. Nonlinear EEG biomarker profiles for autism and absence epilepsy. Neuropsychiatr Electrophysiol. 2017;3:1.
Sathyanarayana A, el Atrache R, Jackson M, Alter AS, Mandl KD, Loddenkemper T, et al. Nonlinear analysis of visually normal EEGs to differentiate benign childhood epilepsy with centrotemporal spikes (BECTS). Sci Rep. 2020;10:1–12.
Fergus P, Hussain A, Hignett D, Al-Jumeily D, Abdel-Aziz K, Hamdan H. A machine learning system for automated whole-brain seizure detection. Appl Comput Inform. 2016;12:70–89.
Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, Levin AR. The Harvard automated processing pipeline for electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front Neurosci. 2018;12:97.
Levin AR, Leal ASM, Gabard-Durnam LJ, O’Leary HM. BEAPP: The batch electroencephalography automated processing platform. Front Neurosci. 2018:12. https://doi.org/10.3389/fnins.2018.00513.
Winkler I, Brandl S, Horn F, Waldburger E, Allefeld C, Tangermann M. Robust artifactual independent component classification for BCI practitioners. J Neural Eng. 2014;11. https://doi.org/10.1088/1741-2560/11/3/035013.
NITRC: CleanLine: Tool/Resource Info. 2012. Retrieved February 6, 2021, from https://www.nitrc.org/projects/cleanline
Schölzel C. Nonlinear measures for dynamical systems. 2019.
Ruffini G, Ibañez D, Kroupi E, Gagnon JF, Montplaisir J, Postuma RB, et al. Algorithmic Complexity of EEG for Prognosis of Neurodegeneration in Idiopathic Rapid Eye Movement Behavior Disorder (RBD). Ann Biomed Eng [Internet]. 2019;47:282–96. [cited 2021 Jan 11]. Springer New York LLC.
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nature Methods [Internet]. Nature Research. 2020;17:261–72. [cited 2021 Jan 11].
Kassraian-Fard P, Matthis C, Balsters JH, Maathuis MH, Wenderoth N. Promises, pitfalls, and basic guidelines for applying machine learning classifiers to psychiatric imaging data, with autism as an example. Frontiers in Psychiatry [Internet]. Frontiers Media S.A. 2016;7:177. [cited 2021 Jan 11].
McIntosh AR, Kovacevic N, Itier RJ. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput Biol. 2008;4:e1000106.
Ortiz-Mantilla S, Hämäläinen JA, Realpe-Bonilla T, Benasich AA. Oscillatory dynamics underlying perceptual narrowing of native phoneme mapping from 6 to 12 months of age. J Neurosci. 2016;36:12095–105.
Cantiani C, Piazza C, Mornati G, Molteni M, Riva V. Oscillatory gamma activity mediates the pathway from socioeconomic status to language acquisition in infancy. Infant Behav Dev. 2019;57:101384.
Musacchia G, Ortiz-Mantilla S, Choudhury N, Realpe-Bonilla T, Roesler C, Benasich AA. Active auditory experience in infancy promotes brain plasticity in Theta and Gamma oscillations. Dev Cogn Neurosci. 2017;26:9–19.
Dehaene-Lambertz G. The human infant brain: a neural architecture able to learn language. Psychon Bull Rev. 2017;24:48–55.
Sket GM, Overfeld J, Styner M, Gilmore JH, Entringer S, Wadhwa PD, et al. Neonatal white matter maturation is associated with infant language development. Front Hum Neurosci. 2019:13. https://doi.org/10.3389/fnhum.2019.00434.
Lai G, Schneider HD, Schwarzenberger JC, Hirsch J. Speech stimulation during functional MR imaging as a potential indicator of autism. Radiology. 2011;260:521–30.
Liu J, Tsang T, Jackson L, Ponting C, Jeste SS, Bookheimer SY, et al. Altered lateralization of dorsal language tracts in 6-week-old infants at risk for autism. Dev Sci. 2019;22. https://doi.org/10.1111/desc.12768.
Arbabshirani MR, Plis S, Sui J, Calhoun VD. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. NeuroImage. 2017;145:137–65.
Varoquaux G. Cross-validation failure: small sample sizes lead to large error bars. NeuroImage. 2018;180:68–77 Academic Press Inc.
Schnack HG, Kahn RS. Detecting neuroimaging biomarkers for psychiatric disorders: sample size matters. Front Psychiatry. 2016;7. https://doi.org/10.3389/fpsyt.2016.00050.
Acknowledgements
We thank all of the families who participated in this study. We also thank Dr. Casey Lew-Williams for his thoughtful feedback and support of this project. Finally, we thank the entire ISP study team for their role in data collection and study coordination.
Funding
Support for this work was provided by The National Institutes of Health (R01-DC010290 to HTF and CAN; R21 DC 08637 to HTF; 1T32MH112510 to CLW; 1K23DC017983), Autism Speaks (1323 to HTF), Simons Foundation (137186 to CAN), and the Princeton Fifty-Five Fund for Senior Thesis Research (FP).
Author information
Authors and Affiliations
Contributions
C.A.N. and H.T.F. designed the longitudinal study. L.G.-D. and C.L.W. processed the electrophysiological data. F.C.P., C.L.W., L.G.-D., and W.B. designed and carried out the analyses. F.C.P. and W.B. authored the code. F.C.P. drafted the paper, and all authors provided critical revisions. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board approval was obtained from Boston Children’s Hospital (IRB#X06-08-0374) prior to starting the study. Written, informed consent was obtained from all caregivers prior to their children’s participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Information.
Provides additional descriptions of (1) Wavelet decomposition and coarse-graining procedure, (2) Classification strategies. Supplemental Table provides comprehensive evaluation metrics for machine learning classification results the three data sets.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Peck, F.C., Gabard-Durnam, L.J., Wilkinson, C.L. et al. Prediction of autism spectrum disorder diagnosis using nonlinear measures of language-related EEG at 6 and 12 months. J Neurodevelop Disord 13, 57 (2021). https://doi.org/10.1186/s11689-021-09405-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11689-021-09405-x