Abstract
We report the efficiency enhancement in dye-sensitized solar cells (DSSCs) using Er3+/Yb3+-co-doped Y2O3 (i.e., Y2O3:Er3+/Yb3+) phosphor nanoparticles, prepared by a simple and cost-effective urea-based homogeneous precipitation method, for efficient near-infrared (NIR) sunlight harvesting. Under the light excitation at a wavelength of 980 nm, the as-prepared samples exhibited strong upconversion emissions at green and red visible wavelengths. To investigate the influence of Y2O3:Er3+/Yb3+ nanoparticles on the photovoltaic performance of DSSCs, the phosphor nanoparticles were incorporated into titanium dioxide films to form a composite photoelectrode. For the resulting DSSCs, the increased power conversion efficiency (PCE) of 6.68 % was obtained mainly by the increased photocurrent of J SC = 13.68 mA/cm2 due to the light harvesting enhancement via the NIR-to-visible upconversion process (cf., PCE = 5.94 %, J SC = 12.74 mA/cm2 for the reference DSSCs without phosphor nanoparticles), thus, indicating the PCE increment ratio of ~12.4 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
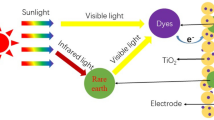
Dye-sensitized solar cells (DSSCs), which can convert solar energy into electric energy by the photovoltaic effect, have been intensively studied as a promising candidate for the next-generation photovoltaic devices because of their simple structure, good stability, low manufacturing cost, and eco-friendly feature [1–5]. Over the past few years, although significant progress in DSSCs has been achieved, the power conversion efficiency (PCE) is still not satisfied compared to the silicon-based solar cells, which limits their further applications [6–8]. As is well known, the PCE of the DSSCs is strongly dependent on the light absorption ability of dyes such as N3, N-719, N-749, etc., which usually absorb energy from the relatively narrow sunlight spectrum in the visible wavelength range of 400–800 nm [3, 8]. Therefore, if the near-infrared (NIR) light (>800 nm) which comprises nearly 50 % in the sunlight could be converted into the visible light and reabsorbed by dyes, the PCE of DSSCs would be further improved.
Up to date, enormous methods have been carried out to improve the performances of solar cells, by incorporating with nanowire particles and upconverting materials [9–11]. In particular, the introduction of upconverting nanoparticles into DSSCs devices was considered as an alternative method to improve the efficiency of DSSCs. Since they can convert the low energy photons (NIR light) into the high energy ones (visible light), the enhanced solar energy generation of DSSCs can be obtained due to the increased visible light absorption in dyes [12–14]. Demopoulos et al. reported that the PCE enhancement by 10 % was achieved using β-NaYF4:Er3+/Yb3+ nanoplatelets as the upconverting layer in DSSCs [13]. In addition, Wu et al. also showed the potential application of the Er3+/Yb3+-co-doped TiO2 upconverting nanoparticles in DSSC, exhibiting a boosted PCE of 7.05 % (i.e., PCE = 6.41 % for the pristine DSSC) [15]. Nevertheless, these obtained results are still far away from the practical application, so more efforts are required.
Recently, rare-earth (RE) ions doped nanomaterials were intensively investigated, and it was revealed that the luminescent properties of these RE ions doped nanomaterials can be modified by adjusting the size, shape, and phase of particles [16–18]. Among these nanomaterials, yttrium trioxide (Y2O3) is widely used as the optical host material owing to its high melting point, high thermal stability, and low toxicity [19, 20]. Furthermore, according to the Raman spectra, the Y2O3 has low phonon energy as low as ~600 cm−1 [21], which results in the high probability of the radiative transition. From this, it is expected that strong upconversion (UC) emissions could be obtained in RE ions doped Y2O3 material system. On the other hand, Er3+ ions, as a member of trivalent RE ions, have drawn considerable attention due to their unique green and red emissions corresponding to (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions, respectively. Moreover, Yb3+ ions are usually co-doped with Er3+ ions, as a sensitizer, to improve the luminescent properties because of their strong absorption in the NIR wavelength region and efficient energy transfer (ET) from the Yb3+ to Er3+ ions [22]. Also, the light scattering properties can be affected by the nanoparticles with the size ranging from 200–1000 nm [2, 13]. In this work, the upconverting Er3+/Yb3+-co-doped Y2O3 (abbreviated as Y2O3:Er3+/Yb3+) nanoparticles were prepared by a urea-based homogeneous precipitation method and their structural and optical properties were investigated. After incorporating the Y2O3:Er3+/Yb3+ into TiO2 nanocrystalline films to form a composite photoelectrode in DSSCs, for the fabricated devices, the current density-voltage (J-V) characteristics and the incident photon to current conversion efficiency (IPCE) spectra were explored.
Methods
Sample Preparation
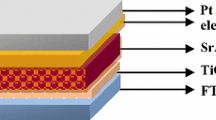
To obtain the optimum UC emission property [23], both the Er3+ and Yb3+ ion concentrations were fixed at 1 mol%, and the Y2O3:Er3+/Yb3+ nanoparticles were successfully synthesized via a facile and simple urea-based homogeneous precipitation method, followed by appropriate thermal treatment. Briefly, stoichiometric amounts of yttrium nitrate hexahydrate (Y(NO3)3 · 6H2O, 99.8 %), erbium nitrate pentahydrate (Er(NO3)3 · 5H2O, 99.9 %), and ytterbium nitrate pentahydrate (Yb(NO3)3 · 5H2O, 99.9 %) were weighted and dissolved in 200 ml of deionized (DI) water to form a transparent solution. After that, moderate urea was added, and the mixed solution was sealed in a beaker, and then it was heated at 80 °C for 3 h under vigorous mechanical stirring. Subsequently, the precursor was centrifuged and washed with DI water and alcohol for several times to remove the remained ions. Finally, the precipitate was sintered at 800 °C for 3 h, thus, yielding the Y2O3:Er3+/Yb3+ nanoparticles. For the fabrication of DSSCs, the cleaned fluorine doped tin oxide (FTO)-deposited glass substrates were used. Firstly, TiO2 colloids (PST-18NR) were coated on the FTO surface to form a TiO2 film with a thickness of ~5 μm by a doctor-blade method, and then the samples were sintered at 500 °C for 2 h. The TiO2 colloids (PST-400C) mixed with 1 wt% Y2O3:Er3+/Yb3+ nanoparticles were subsequently screen-printed on the TiO2 film, which creates a 5-μm-thick TiO2 + Y2O3:Er3+/Yb3+ layer. Afterwards, the as-prepared film was soaked in the N-719 dye solution (3 × 10−4M in ethanol) for 24 h. For comparison, a dye-sensitized TiO2 film without Y2O3:Er3+/Yb3+ nanoparticles was also prepared. Meanwhile, the platinum (Pt) counter electrode was prepared on the FTO glass using Pt paste (Dyesol, counter PT-1), followed by heating at 500 °C for 2 h. Lastly, the DSSC devices were assembled by an injection of electrolyte (Dyesol, electrolyte HPE) and a sealing process with the help of a hot press.
Characterization
The phase structure of the fabricated nanophosphor samples was analyzed by using an X-ray diffractometer (XRD; Mac Science, M18XHF-SRA) with Cu Kα (λ = 1.5402 Å) radiation, and the JADE software was applied to analyze the XRD data. The structural morphology was observed by using a transmission electron microscope (TEM; JEM-2100 F, JEOL). The room-temperature UC spectrum was checked by using a fluorescence spectrophotometer (Ocean optics USB 4000) under the excitation of a laser light at a wavelength (λ) of 980 nm with a pump power of 660 mW. The optical transmission and reflection spectra of dye-sensitized photoanodes with and without Y2O3:Er3+/Yb3+ phosphor nanoparticles were characterized by using a UV–vis-NIR spectrophotometer (Cary 5000, Varian). The J-V curves were measured by using a photocurrent system consisting of a solar simulator (ABET, SUN 3000) with a 1000 W Xe short-arc lamp and a source meter (Keithley 2400). The IPCE spectra from 300 to 800 nm were evaluated by using a 300 W xenon arc lamp as the light source coupled to a monochromator (TLS-300× xenon light source, Newport) with an optical power meter (2935-c, Newport).
Results and Discussion
From the XRD pattern in Fig. 1a, it was observed that the diffraction peaks were consistent with the standard pattern (JCPDS#41-1105) and no impurity phases were detected. This means that the powders with a pure cubic phase of Y2O3 were synthesized and the RE (Er3+, Yb3+) ions were well diffused into the Y2O3 host lattice. In Fig. 1b, the TEM image confirmed that the as-prepared Y2O3:Er3+/Yb3+ nanoparticles have sphere-like morphologies with the size range of 250–300 nm which is larger than those of the sample prepared by a coprecipitation method (70 nm) [23]. Furthermore, from the high-resolution TEM (HR-TEM) image in Fig. 1c, the interplanar distance between the adjacent lattice planes was estimated to be about 0.294 nm, which is well matched with the (222) plane of the Y2O3. In Fig. 1d, the selected area electron diffraction (SAED) pattern displayed bright dots and rings. This indicates that the samples are relatively well crystallized. As shown in Fig. 1f, the elemental mapping image taken from the area in the scanning transmission electron microscope (STEM) image of Fig. 1e demonstrates that the constituent materials of Y, O, Er, and Yb are uniformly distributed.
\
Figure 2a, depicts the UC emission spectrum of the Y2O3:Er3+/Yb3+ nanoparticles. Under the light excitation at λ ex = 980 nm, the fabricated samples exhibited a strong yellow UC emission that can be seen by the naked eye as shown in the inset of Fig. 2a. As shown in Fig. 2a, it is clear that the UC spectrum consists of three distinct bands, that is, two green emissions at λ = 510–542 nm and 542–570 nm and strong red emission at λ = 625–700 nm. The former is attributed to the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions, respectively and the latter is ascribed to the 4 F9/2 → 4I15/2 transition [19, 24]. These results give a reasonable agreement with the previous reports on Er3+-doped materials [22, 25]. Here, it is noteworthy that these emission bands are located in the absorption wavelength range of the N-719 dye (i.e., λ = 300–800 nm). Thus, the use of Y2O3:Er3+/Yb3+ nanoparticles in N-719 dye-based photovoltaic devices would increase the photocurrents due to the enhanced light absorption, except for the sunlight, caused by their green and red light emissions converted from the lights at NIR wavelengths, and thus, the improved device performance of DSSCs could be achieved.
a UC spectrum of Y2O3:Er3+/Yb3+ nanoparticles (red solid line) under 980 nm light excitation and b schematic energy level diagram of Yb3+ and Er3+ ions and proposed UC mechanism in Y2O3:Er3+/Yb3+ nanoparticles under 980 nm light excitation. The inset of a shows the photograph taken in the darkness by using a digital camera
The energy level diagram of Er3+ and Yb3+ ions including possible UC processes is illustrated in Fig. 2b. Under the light excitation at λ ex = 980 nm, the Yb3+ ions are excited from the ground sate to the 2F5/2 level and they drop back. Thus, the energy is transferred to the adjacent Er3+ ions, resulting in the population of 4I11/2 level. Then, the multiphonon relaxation (MPR) process occurs, and part of the 4I11/2 level decays to the 4I13/2 level. Meanwhile, the Yb3+ ions absorb the second photon energy, and again, the energy is transferred to the adjacent Er3+ ions, and the 4F9/2 and 4F7/2 levels are populated. Subsequently, the electrons relax to the 2H11/2, 4S3/2, and 4F9/2 levels due to the non-radiative (NR) process. As a result, the strong green and red UC emissions are observed due to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions, respectively.
To investigate the light-scattering behaviors of the Y2O3:Er3+/Yb3+ nanoparticles in DSSCs, the absorption (i.e., 1-R-T) of the dye-sensitized photoanodes with the Y2O3:Er3+/Yb3+ nanoparticles was extracted by the measured total reflection (R) and transmission (T) spectra in Fig. 3. As can be seen in Fig. 3a, there were differences in both the total transmission and reflection between the dye-sensitized photoanodes consisting of the TiO2 and the TiO2 + Y2O3:Er3+/Yb3+. Especially, the transmission was further decreased. From these results, as shown in the absorption spectra of Fig. 3b, it can be observed that the dye-sensitized photoanode consisting of the TiO2 + Y2O3:Er3+/Yb3+ exhibited the relatively higher absorption spectrum compared to one consisting of only TiO2 over a wide wavelength region of 350–750 nm. This indicates the enhancement of the light-scattering property in the dye-sensitized photoelectrodes by incorporating the Y2O3:Er3+/Yb3+ nanoparticles into the TiO2 photoelectrode, and thus, it can lead to the higher photocurrents in DSSCs due to the enhancement in light absorption.
To investigate the influence of the UC emission phosphors on the photovoltaic performance of DSSCs, the Y2O3:Er3+/Yb3+ nanoparticles were introduced into the TiO2 photoelectrode. Figure 4 shows (a) the J-V curves and (b) the IPCE spectra of the DSSCs with and without the Y2O3:Er3+/Yb3+ nanoparticles. The device characteristics (i.e., short-circuit current density; J SC, open-circuit voltage; V OC, fill factor; FF, PCE) of the corresponding DSSC devices are summarized in the inset of Fig. 4a. The corresponding schematic diagram of the DSSC with the Y2O3:Er3+/Yb3+ nanoparticles is also shown. As shown in Fig. 4a, the DSSC without the Y2O3:Er3+/Yb3+ nanoparticles exhibited the photovoltaic characteristics of J SC = 12.74 mA/cm2, V OC = 0.75 V, FF = 62.06 % and PCE = 5.94 %. In contrast, by employing the Y2O3:Er3+/Yb3+ nanoparticles in the DSSC, for all the photovoltaic characteristics, the increased values of J SC = 13.68 mA/cm2, V OC = 0.76 V, FF = 64.32 %, and PCE = 6.68 % were measured, which leads to the significant PCE increment ratio of ~12.4 %. This is mainly because a considerable enhancement (i.e., increment ratio of ~7.4 %) in the J SC is caused by the efficient UC emissions of Er3+ ions from the NIR light to the visible light as well as the enhanced light-scattering properties [26, 27]. The V OC also increased slightly. According to previous reports [27, 28], the V OC corresponds to the energy difference between the electronic Fermi level of TiO2 film and the redox potential of I−/I3 −, i.e., V OC = E F(TiO2) − E redox. It is well known that when RE ions are doped and substituted into the Ti4+ sites in the TiO2, a p-type doping effect appears, resulting in the increment of the Fermi level of the TiO2 [29]. Thus, the V OC was enhanced. Using the Y2O3:Er3+/Yb3+ nanoparticles, the increased photocurrents by the light-scattering effect can be also verified by the IPCE data. As can be seen in Fig. 4b, the DSSC with the Y2O3:Er3+/Yb3+ nanoparticles showed a higher IPCE spectrum compared to the DSSC without Y2O3:Er3+/Yb3+ nanoparticles over an entire spectra range of 350–750 nm. This is well matched with the aforementioned absorption properties in Fig. 3b.
Conclusions
In summary, the upconverting Y2O3:Er3+/Yb3+ phosphor nanoparticles were synthesized and introduced into the TiO2 photoelectrode of DSSCs. Under the excitation of the NIR (λ ex = 980 nm) light, the strong green and red UC emissions, corresponding to the (2H11/2, 4S3/2) → 4I15/2 and 4F9/2 → 4I15/2 transitions, respectively, were observed with the light-scattering effect over a wide wavelength range of 350–750 nm. For the DSCCs incorporated with the Y2O3:Er3+/Yb3+ nanoparticles, the enhanced photovoltaic performance was achieved, indicating the increase in the PCE value from 5.94 to 6.68 % (i.e., PCE increment ratio of ~12.4 %). These results can provide a better insight into the phosphor nanoparticles with the NIR sunlight-upconverting functions into the visible lights as well as the light-scattering effect for high-performance dye-sensitized photovoltaic devices.
References
O’Regan B, Grätzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353:737–40.
Kawamura G, Ohmi H, Tan WK, Lockman Z, Muto H, Matsuda A. Ag nanoparticle-deposited TiO2 nanotube arrays for electrodes of dye-sensitized solar cells. Nanoscale Res Lett. 2015;10:219.
Liang L, Liu Y, Bu C, Guo K, Sun W, Huang N. Highly uniform, bifunctional core/double-shell-structured β-NaYF4:Er3+, Yb3+ @SiO2@TiO2 hexagonal sub-microprisms for high-performance dye sensitized solar cell. Adv Mater. 2013;25:2174–80.
Zhou Z, Wang J, Nan F, Bu C, Yu Z, Liu W, et al. Upconversion induced enhancement of dye sensitized solar cell based on core-shell structured β-NaYF4:Er3+, Yb3+@SiO2 nanoparticles. Nanoscale. 2014;6:20525–5055.
Huang X, Han S, Huang W, Liu X. Enhancing solar cell efficiency: the search for luminescent materials as spectral converters. Chem Soc Rev. 2013;42:173–201.
Shan G, Demopoulos GP. Near-infrared sunlight harvesting in dye-sensitized solar cells via the insertion of an upconverter-TiO2 nanocomposite layer. Adv Mater. 2010;22:4373–7.
Yuan C, Chen G, Li L, Damasco JA, Ning Z, Xing H, et al. Simultaneous multiple wavelength upconversion in a core-shell nanoparticle for enhanced near infrared light harvesting in a dye-sensitized solar cell. ACS Appl Mater Interfaces. 2014;6:18018–25.
Ramasamy P, Kim J. Combined plasmonic and upconversion rear reflector for efficient dye-efficient dye-sensitized solar cells. Chem Commun. 2014;50:879–81.
Chen C, Ban D, Helander MG, Lu ZH, Poole P. Near-infrared inorganic/organic optical upvonverter with an external power efficiency of >100%. Adv Mater. 2010;22:4900–4.
Ko SH, Lee D, Kang HW, Nam KH, Yeo JY, Hong SJ, et al. Nanoforest of hydrothermally grown hierarchical ZnO nanowires for a high efficiency dye-sensitized solar cell. Nano Lett. 2011;11:666–71.
Wang X, Yan X. Ultraviolet and infrared photo-excited synergistic effect in Er3+-doped YbF4 phosphors. Opt Lett. 2011;36:4353–5.
Ramasamy P, Manivasakan P, Kim J. Upconversion nanophosphors for solar cell applications. RSC Adv. 2014;4:34873–95.
Shan GB, Assaaoudi H, Demopoulos G. Enhanced performance of dye-sensitized solar cells by utilization of an external, bifunctional layer consisting of uniform β-NaYF4:Er3+/Yb3+ nanoplatelets. ACS Appl Mater Interfaces. 2011;3:3239–43.
Liang L, Liu Y, Zhao X. Double-shell β-NaYF4:Yb3+, Er3+/SiO2/TiO2 submicroplates as a scattering and upconverting layer for efficient dye-sensitized solar cell”. Chem Commum. 2013;49:958–3960.
Xie G, Wei Y, Fan L, Wu J. Application of doped rare-earth doped oxide TiO2:(Tm3+, Yb3+) in dye-sensitized solar cell. J Phys Conf Ser. 2012;339:012010.
Feng X, Sayle DC, Wang ZL, Paras MS, Santora B, Sutorik AC, et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science. 2006;312:1504–8.
Wang X, Bu Y, Xiao Y, Kan C, Lu D, Yan X. Size and shape modifications, phase transition, and enhanced luminescence of fluoride nanocrystals induced by doping. J Mater Chem C. 2013;1:3158–66.
Yang L, Li Y, Yu S, Hao J, Zhong J, Chu PK. Phase transformation and size tuning in controlled-growth of nanocrystals via self-seeded nucleation with preferential thermodynamic stability. Chem Commun. 2011;47:12544–6.
Du P, Luo L, Yue Q, Li W. The simultaneous realization of high- and low-temperature thermometry in Er3+/Yb3+-co-doped Y2O3 nanoparticles. Mater Lett. 2015;143:209–11.
Wu J, Xie G, Lin J, Lan Z, Huang M, Huang Y. Enhancing photoelectrical performance of dye-sensitized solar cell by doping with europium-doped yttria rare-earth oxide. J Power Sources. 2010;195:6937–40.
Abrashev MV, Todorov ND, Geshev J. Raman spectra of R2O3 (R-rare-earth) sesquioxides with C-type bixbytie crystal structure: a comparative study. J Appl Phys. 2014;116:103508.
Du P, Yu JS. Effect of molybdenum on upconversion emission and temperature sensing properties in Na0.5Bi0.5TiO3:Er/Yb ceramics. Ceram Int. 2015;41:6710–4.
Lu P, Hou Y, Tang A, Wu H, Teng F. Upconversion multicolor tuning: red to green emission from Y2O3:Er, Yb nanoparticles by calcination. Appl Phys Lett. 2013;102:233103.
Zhou N, Qiu P, Wang K, Fu H, Gao G, He R, et al. Shape-controllable synthesis of hydrophilic NaLuF4:Yb, Er nanocrystals by a surfactant-assistant two-phase system. Nanoscale Res Lett. 2013;8:518.
Lojpur VM, Ahrenkiel PS. Dramićanin: color-tunable up-conversion emission in Y2O3:Yb3+, Er3+ nanoparticles prepared by polymer complex solution method. Nanoscale Res Lett. 2013;8:131.
Zou Z, Wang J, Nan F, Bu C, Yu Z, Liu W, et al. Upconversion induced enhancement of dye sensitized solar cells based on core-shell structured β-NaYF4:Yb3+/Er3+@SiO2 nanoparticles. Nanoscale. 2014;6:20525–2055.
Wang J, Wu J, Lin J, Huang M, Huang Y, Lan Z, et al. Application of Y2O3:Er3+ nanorods in dye-sensitized solar cells. ChemSusChem. 2012;5:1307–12.
Grätzel M. Photoelectrochemical cells. Nature. 2001;414:338–44.
Schlichthörl G, Huang SY, Sprague J, Frank AJ. Band edge movement and recombination kinetics in dye-sensitized nanocrystalline TiO2 solar cell: a study by intensity modulated photovoltage spectroscopy. J Phys Chem B. 1997;101:8141–55.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2014–069441).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PD prepared and characterized the nanoparticles. JHL and SMC synthesized the DSSCs devices. PD and JSY cowrote the manuscript. JWL participated in revising the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Du, P., Lim, J.H., Leem, J.W. et al. Enhanced Photovoltaic Performance of Dye-Sensitized Solar Cells by Efficient Near-Infrared Sunlight Harvesting using Upconverting Y2O3:Er3+/Yb3+ Phosphor Nanoparticles. Nanoscale Res Lett 10, 321 (2015). https://doi.org/10.1186/s11671-015-1030-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-1030-0