Abstract
Background
Studies investigating the association between migraine and dementia have reported inconsistent findings. This study aimed to evaluate whether patients with migraine have an increased risk of dementia compared to individuals without migraine.
Methods
We obtained data from the 2002–2019 Korean National Health Insurance Health Screening Cohort. Non-migraine controls were selected using a 1:1 risk-set matching with a time-dependent propensity score. The main outcome was the development of all-cause dementia, and the secondary outcome was the development of each cause of dementia (Alzheimer’s, vascular, mixed or other specified, and unspecified dementia). The incidence rate of dementia was calculated using Poisson regression, and the association between migraine and dementia was evaluated using Cox proportional hazards regression.
Results
Among 88,390 participants, 66.1% were female, and the mean baseline age was 55.3 ± 9.4 years. During the study period, dementia cases were identified in 4,800 of the 44,195 patients with migraine and 3,757 of the 44,915 matched controls. The incidence rate of dementia was 139.6 (95% confidence interval [CI], 135.7–143.5) and 107.7 (95% CI, 104.3–111.1) cases per 10,000 person-years in patients with migraine and matched controls, respectively. Patients with migraine had a 1.30 (hazard ratio [HR], 1.30; 95% CI, 1.25–1.35), 1.29 (HR, 1.29; 95% CI, 1.23–1.35), 1.35 (HR, 1.35; 95% CI, 1.19–1.54), 1.36 (HR, 1.36; 95% CI, 1.00–1.83), and 1.30 (HR, 1.30; 95% CI, 1.17–1.45) times higher risk of developing all-cause dementia, Alzheimer’s dementia, vascular dementia, mixed or other specified dementias, and unspecified dementia than their matched controls, respectively.
Conclusion
Our results suggest that migraine is associated with an increased risk of subsequent dementia. Further research is warranted to confirm these findings and to reveal the underlying mechanisms.
Similar content being viewed by others
Background
Migraine is a common primary headache disorder characterized by episodic disabling headaches and is often accompanied by focal neurological symptoms called aura [1]. Migraine affects approximately 15% of the general population worldwide and ranks second among the top causes of disability [2, 3]. While migraine is most prevalent in young and middle-aged adults, dementia, another leading cause of disability, primarily affects the geriatric population [4].
Previous studies investigating the association between migraine and the risk of dementia have shown inconsistent results. Several cohort studies have reported an increased risk of all-cause dementia, Alzheimer’s dementia (AD), or vascular dementia (VaD) in patients with migraine compared to non-migraine individuals, while others did not find such relationships [5,6,7,8]. Moreover, in two studies, an association between migraine and the risk of dementia was observed only in women [9, 10]. In addition, studies on the relationship between migraine and cognitive function have shown inconsistent findings [11,12,13]. The heterogeneity of the results among studies may be attributed to differences in their designs. Specifically, the identification of patients with migraine (incident cases or retrospectively evaluated at baseline), duration of follow-up (5–24 years), the age distribution of the study population, and outcomes (all-cause dementia, AD, or VaD) varied among the studies.
Considering the long preclinical stage of AD and the gap between the prevalent age of migraine and dementia, a sufficient follow-up period should be evaluated to ensure the longitudinal relationship between the two disorders [14]. Additionally, since each cause of dementia (e.g., AD and VaD) has a different pathophysiology, separate analyses investigating the association between migraine and each type of dementia should be performed. A sufficient sample size is required to secure the statistical power to perform separate analyses for each type of dementia. Another research interest is migraine aura, a known risk factor for various conditions, including ischemic stroke and myocardial infarction [15,16,17]. Islamoska et al. reported that migraine with aura (MA) was associated with an increased risk of dementia in a Danish cohort study [7]. However, there is still insufficient evidence for the association between MA, migraine without aura (MO), and dementia among the Asian population.
Therefore, this study aimed to investigate the risk of developing all-cause dementia, AD, VaD, and other specified and unspecified dementias after migraine diagnosis over a 16-year follow-up period using Korean population-based data. To assess the temporal context of the association between migraine and dementia, additional analyses were performed according to the age at migraine diagnosis and follow-up duration. Finally, based on the findings of previous studies, we evaluated whether the presence of aura or sex affects the association between migraine and dementia.
Methods
Study subjects and data sources
We used data from the 2002–2019 Korea National Health Insurance Service Health Screening Cohort (NHIS-HEALS). All Korean citizens aged ≥ 40 years are eligible for the biennial general health screening program. The NHIS-HEALS comprised 514,866 general health screening participants aged between 40 and 79 years in 2002, equating to a 10% simple random sample of the target population [18]. The data were provided by the National Health Insurance Service (NHIS), which covers 97% of the Korean population. Since the NHIS also manages the healthcare claims of the remaining 3% of the Korean population, the medical aid program beneficiaries, the NHIS database contains the medical records of the entire Korean population. The NHIS-HEALS includes anonymized participant information (sex, age, health insurance premium decile determined by household income, residential areas, medical records, and health screening database). All participants were followed up until their loss of eligibility due to death or emigration [18].
Migraine cohort
The migraine cohort was constructed as follows: First, individuals with at least two diagnoses of migraine (International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10] G43) during the study period were assigned to the migraine cohort. Next, patients with medical records of migraine from January 1, 2002, to December 31, 2003 (a two-year washout period), were excluded. The migraine cohort was further subdivided into the MA group (ICD-10 code G43.0), MO group (ICD-10 code G43.1), and unspecified group (none of the above).
Identification of dementia cases
The primary outcome was the incidence of all-cause dementia. The secondary outcomes were each cause of dementia, including AD (ICD-10 codes G30 or F00), VaD (ICD-10 code F01), other specified dementias (ICD-10 codes F02, G31.00, G31.82), and unspecified dementia (ICD-10 code F03). Individuals were classified as dementia patients if they had at least two ambulatory visits or one hospital admission for dementia and did not have any medical records of dementia before December 31, 2003.
Risk-set matching with propensity score
This study tried to mimic the prospective study design and overcome the inherent limitations of the retrospectively constructed NHIS-HEALS through the risk-set matching method using time-dependent propensity score [19, 20]. The control group was selected from individuals at risk of migraine, considering other potential confounders such as age, sex, socioeconomic status, comorbidities and lifestyle factors.
First, we calculated the hazard component for being patients with migraine represented as a time-dependent propensity score, using a Cox proportional-hazard model with January 1, 2004 as baseline (after the 2002–2003 washout period) and migraine diagnosis as an event [21]. The baseline characteristics of the participants used for propensity score matching were collected over two years before the baseline (2002–2003). Age (continuous variable), sex, household income level (medical aid beneficiary or decile for NHIS enrollees), residential area (urban or rural), registered disability, past medical history (stroke, diabetes mellitus, hypertension, and depression; individuals with more than two outpatient visits or one admission based on ICD-10 codes), smoking status (never smoker, ex-smoker, current smoker, or unspecified), BMI (< 25 or ≥ 25), and alcohol consumption (none, ≤ 7, 7–14, or > 14 units per week) were included as covariates.
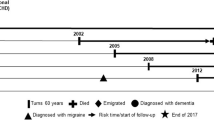
Second, each patient with migraine was matched to individuals of the same age, sex, baseline household income level, and at risk of migraine at the index date (the date of criteria for migraine cohort were fulfilled). The index dates of the control individuals were set the same as the index dates of their matched patients with migraine. We repeated this risk-set matching sequentially for all migraine patients [22]. To mimic the design of a prospective study, risk-set matching was performed independently of the future diagnosis of migraine. In other words, because the control individuals were those who had not yet developed migraine (at risk of migraine) at the time of matching, they had the possibility of developing migraine during follow-up. Therefore, most individuals who had been assigned to the migraine cohort before matching were included as patients with migraine in the main analysis, but a minority were included as controls for other patients who developed migraine before them.
Subsequently, a 1:1 matching on time-dependent propensity score was performed for each risk set using a nearest-neighbor matching algorithm with a maximum difference of hazard components between patients with migraine and control individuals of < 0.1 [19, 23]. The matched patient-control set was removed from the subsequent risk set to generate a non-overlapping sample. The 1:1 propensity score matching was repeated until no more patients with migraine remained in the risk set.
Statistical analyses
The balance of baseline characteristics between the migraine and control groups was assessed with a standardized difference; if the absolute value of the standardized difference was less than 0.1, the distribution of covariates was considered balanced [24]. We used the Kaplan–Meier method and stratified log-rank test to evaluate the cumulative incidence of dementia in patients with migraine and matched controls. We calculated the incidence rate (IR, the number of dementia cases per 10,000 person-years) of dementia and the 95% confidence interval (CI) using a generalized estimating equation with a Poisson distribution. The effect size was estimated as the hazard ratio (HR) using the Cox proportional hazards model. Subgroup analyses according to age at migraine diagnosis, follow-up duration, sex, and presence of aura were also performed. In some cases, the first date of migraine diagnosis during study period might be indicative of an active period of migraine (instead of new migraine onset), particularly among patients who were initially diagnosed with migraine at an older age. Therefore, we performed additional sensitivity analyses that investigated the association between migraine and dementia (all-cause, AD, VaD, mixed or other specified, and unspecified dementia) among individuals with first migraine diagnosis at age < 60 years. Moreover, same analyses were performed with five years of washout period (instead of two years) to exclude more active period of previously diagnosed migraine. Statistical significance was defined as a two-tailed p-value of < 0.05. All analyses were performed using the SAS Enterprise Guide software (version 7.1; SAS Institute, Cary, NC, USA) and R (version 4.1.3; Vienna, Austria; Rproject.org/).
Results
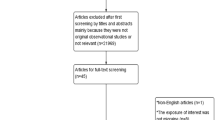
Between January 1, 2002, and December 31, 2019, 54,870 individuals met the inclusion criteria for the migraine cohort. Of these, 10,675 individuals were excluded because they had medical records for migraine (n = 6,234) or dementia during the washout period (n = 42), developed dementia before migraine diagnosis (n = 1,472), were included as controls for other patients with migraine (n = 2,745), or showed insufficient matching results (n = 182). The final study sample comprised 44,195 patients with migraine and 44,195 matched controls (Fig. 1). The mean follow-up time was 7.84 years (maximum, 16 years), and 692,867 person-years were generated. There were 8,557 patients with new-onset dementia during the 16-year follow-up period. In the baseline year of 2004, 66.1% of the study subjects were female, and the mean age was 55.3 (± 9.4) years. The standardized differences for all covariates were < 0.1 (Table 1).
The cumulative incidence of dementia during the entire study period showed a statistically significant difference between the migraine and control cohorts (p < 0.001, stratified log-rank test; Fig. 2).
Among the 44,195 patients with migraine, 4,800 developed dementia over 343,871 person-years (IR, 139.6 per 10,000 person-years; 95% CI, 135.7–143.5), while 3,757 developed dementia over 348,995 person-years (IR, 107.7 per 10,000 person-years; 95% CI, 104.3–111.1) among the 44,195 control subjects. Patients with migraine were 1.30 times more likely to develop dementia than their matched controls (HR, 1.30; 95% CI, 1.25–1.35). Patients with migraine showed 1.42 times (HR, 1.42; 95% CI, 1.34–1.51), 1.26 times (HR, 1.26; 95% CI, 1.14–1.40), and 1.16 times (HR, 1.16; 95% CI, 1.05–1.28) higher risk of developing dementia than their matched controls at 0–5, 6–10, and > 10 years after migraine diagnosis, respectively. However, the magnitudes of association between migraine and risk of dementia were similar between sexes, between groups by age at migraine diagnosis, and among groups by the presence of aura (Table 2).
Patients with migraine had 1.29 times (HR, 1.29; 95% CI, 1.23–1.35), 1.35 times (HR, 1.35; 95% CI, 1.19–1.54), 1.36 times (HR, 1.36; 95% CI, 1.00–1.83), and 1.30 times (HR, 1.30; 95% CI, 1.17–1.45) higher risk of developing AD, VaD, mixed or other specified dementias, and unspecified dementia than their matched controls, respectively (Table 3).
Sensitivity analyses showed that the association between migraine and all-cause dementia was significant when only migraine cases before the age of 60 were included. Patients with migraine also showed higher incidence of each type of dementia in sensitivity analyses. However, statistical significance was observed only for AD in analyses with 2-year washout period whereas VaD and unspecified dementia showed significant relations to migraine in analyses wtih 5-year washout period (Supplementary Table S1, S2).
Discussion
In this study, we found that patients with migraine exhibited an increased risk of developing all-cause dementia, AD, VaD, and mixed or other specified dementias, and unspecified dementia compared to non-migraine-matched controls. Although the mechanism of this association remains largely unknown, there are several possible explanations for the pathways linking migraine and dementia.
First, migraine is associated with an increased risk of myocardial infarction and ischemic stroke, which are known risk factors for AD or VaD [15, 25]. Second, studies have found that migraine is related to structural and functional brain changes, including alterations in cerebral blood flow, increased white matter hyperintensities, subclinical infarct-like lesions, and brain volume changes [26,27,28].
Other possible mechanisms linking migraine and dementia include alterations in the cortisol-hippocampal pathway, inflammation, increased amyloid plaque formation, deficits in nerve growth factors due to comorbid depression, and chronic pain-related changes in the memory network structure of brain [29,30,31].
In the subgroup analyses, patients with migraine showed a higher risk of developing dementia than their matched controls, regardless of sex, age at migraine diagnosis, and follow-up duration. However, the magnitude of the association was prominent in patients diagnosed with migraine less than five years ago and attenuated with longer follow-up periods, indicating that a reverse causation (e.g., early diagnosis of dementia after consulting neurologists for migraine treatment) or a shared underlying cause between migraine and dementia may exist. Nevertheless, our findings provide evidence of longitudinal relationship between migraine and dementia development, since there was an increased risk of dementia in patients who had been diagnosed with migraine > 10 years ago or those who were diagnosed with migraine before the age of 60 years (when dementia was less prevalent).
Although the magnitudes of the association were found to be similar between MA and MO, more than half of the patients with migraine did not have information on aura. Moreover, since MA is known to have a higher risk of cardiovascular and cerebrovascular events, or structural abnormalities in the brain than MO, it is difficult to deny the theoretical relevance between MA and dementia with our findings alone [7]. Therefore, the impact of aura on dementia should be re-investigated with an accurate evaluation of migraine aura. Furthermore, future studies need to explore whether the characteristics of migraine attacks, such as severity or frequency, affect the development of dementia.
Contrary to our findings, most previous cohort studies did not find a statistically significant association between migraine and the risk of VaD [5, 32, 33]. The non-significant association may be attributed to the small sample size and lack of statistical power, because those studies showed consistent trends toward an increased risk of VaD in patients with migraine. Using a large sample with a nationwide cohort that provided sufficient statistical power, our study found that migraine was associated with an increased risk of VaD and AD. Moreover, we emulated the effect of prospective studies and reduced the immortal time bias in retrospective claims-based data using the risk-set matching method.
However, certain limitations of this study should be considered when interpreting its findings. First, several variables that could affect the development of dementia, such as educational level or baseline cognitive function, were not available for NHIS-HEALS. However, previous studies reported that educational level was not associated with migraine, or that individuals with higher educational levels were more prevalent with migraine [34, 35]. Additionally, we selected control individuals with the same baseline household income level as patients with migraine, and educational level was strongly correlated with income in South Korea [36]. Thus, in our study, educational level as a confounder had a minimal effect on the relationship between migraine and dementia. Second, the overall age of migraine onset among subjects in this study was greater than the peak prevalent age (35–39 years) of migraine because NHIS-HEALS comprised individuals aged 40–79 years in 2002. Therefore, a portion of the migraine cohort might comprise patients with active period of migraine instead of incident migraine cases. Although sensitivity analyses showed similar results to those of main analysis, findings for each type of dementia were not robust due to a small number of events. Third, because a large proportion of participants did not have information on presence of aura, we cannot draw a robust conclusion about the role of migraine aura on development of PD despite the evidence presented in previous studies and the biologically plausible mechanism. Last, the diagnosis of migraine, dementia, and other comorbidities may be inaccurate, owing to the inherent limitations of claim data. Therefore, to improve diagnostic validity and reduce false-positive cases, we required at least two diagnoses for migraine and at least one hospital admission or two outpatient visits for dementia and other comorbidities. Nevertheless, further studies involving longer follow-up durations (> 30 years), the entire adult population and detailed information regarding migraine are required to enhance the generalizability of our findings.
Conclusion
In conclusion, in the present study, patients with migraine had an increased risk of developing all-cause dementia, AD, VaD, and other dementias compared with their risk-set matched controls. However, further studies are warranted to generalize our findings and elucidate the underlying pathophysiological mechanisms linking migraine and dementia.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because the NHIS exclusively allows authorized persons to access data in a separate space.
Abbreviations
- AD:
-
Alzheimer’s dementia
- VaD:
-
Vascular dementia
- MA:
-
Migraine with aura
- MO:
-
Migraine without aura
- NHIS-HEALS:
-
Korea National Health Insurance Service Health Screening Cohort
- NHIS:
-
National Health Insurance Service
- ICD-10:
-
International Statistical Classification of Diseases and Related Health Problems, 10th revision
- BMI:
-
Body mass index
- IR:
-
Incidence rate
- CI:
-
Confidence interval
- HR:
-
Hazards ratio
References
Ferrari MD, Goadsby PJ, Burstein R, Kurth T, Ayata C, Charles A et al (2022) Migraine. Nat Rev Dis Primers 8(1):2. https://doi.org/10.1038/s41572-021-00328-4
Ashina M, Katsarava Z, Do TP, Buse DC, Pozo-Rosich P, Özge A et al (2021) Migraine: epidemiology and systems of care. Lancet 397(10283):1485–1495
Stovner LJ, Nichols E, Steiner TJ, Abd-Allah F, Abdelalim A, Al-Raddadi RM et al (2018) Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 17(11):954–976
Nichols E, Szoeke CE, Vollset SE, Abbasi N, Abd-Allah F, Abdela J et al (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 18(1):88–106
Morton RE, St. John PD, Tyas SL, (2019) Migraine and the risk of all-cause dementia, Alzheimer’s disease, and vascular dementia: a prospective cohort study in community-dwelling older adults. Int J Geriatr Psychiatry 34(11):1667–1676
Hagen K, Stordal E, Linde M, Steiner TJ, Zwart J-A, Stovner LJ (2014) Headache as a risk factor for dementia: a prospective population-based study. Cephalalgia 34(5):327–335
Islamoska S, Hansen ÅM, Wang H-X, Garde AH, Andersen PK, Garde E et al (2020) Mid-to late-life migraine diagnoses and risk of dementia: a national register-based follow-up study. J Headache Pain 21(1):1–12
George KM, Folsom AR, Sharrett AR, Mosley TH, Gottesman RF, Hamedani AG et al (2020) Migraine headache and risk of dementia in the atherosclerosis risk in communities neurocognitive study. Headache 60(5):946–953
Lee S-Y, Lim J-S, Oh DJ, Kong IG, Choi HG (2019) Increased risk of neurodegenerative dementia in women with migraines: A nested case–control study using a national sample cohort. Medicine 98(7):e14467
Kostev K, Bohlken J, Jacob L (2019) Association between migraine headaches and dementia in more than 7,400 patients followed in general practices in the United Kingdom. J Alzheimers Dis 71(1):353–360
Martins IP, Maruta C, Alves PN, Loureiro C, Morgado J, Tavares J et al (2020) Cognitive aging in migraine sufferers is associated with more subjective complaints but similar age-related decline: a 5-year longitudinal study. J Headache Pain 21(1):1–12
Pellegrino Baena C, Goulart AC, Santos IdS, Suemoto CK, Lotufo PA et al (2018) Migraine and cognitive function: baseline findings from the Brazilian longitudinal study of adult health: ELSA-Brasil. Cephalalgia 38(9):1525–1534
Wen K-x, Nguyen N, Hofman A, Ikram M, Franco O (2016) Migraine is associated with better cognition in the middle-aged and elderly: the rotterdam study. Eur J Neurol 23(10):1510–1516
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer Dement 7(3):280–292
Adelborg K, Szépligeti SK, Holland-Bill L, Ehrenstein V, Horváth-Puhó E, Henderson VW, et al (2018) Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 360:k96
Kurth T, Rist PM, Ridker PM, Kotler G, Bubes V, Buring JE (2020) Association of migraine with aura and other risk factors with incident cardiovascular disease in women. JAMA 323(22):2281–2289
Peng K-P, Chen Y-T, Fuh J-L, Tang C-H, Wang S-J (2017) Migraine and incidence of ischemic stroke: a nationwide population-based study. Cephalalgia 37(4):327–335
Seong SC, Kim Y-Y, Park SK, Khang YH, Kim HC, Park JH et al (2017) Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open 7(9):e016640
Rosenbaum PR (2020) Modern algorithms for matching in observational studies. Ann Rev Stat Appl 7:143–176
Li YP, Propert KJ, Rosenbaum PR (2001) Balanced risk set matching. J Am Statl Assoc 96(455):870–882
Lu B (2005) Propensity score matching with time-dependent covariates. Biometrics 61(3):721–728
Kim SH, Jeong SH, Kim H, Park E-C, Jang S-Y (2022) Development of open-angle glaucoma in adults with seropositive rheumatoid arthritis in Korea. JAMA Netw Open 5(3):e223345–e223345
Jang S-Y, Yang D-S, Cha Y-H, Yoo H-J, Kim K-J, Choy W-S (2020) Suicide in elderly patients with hip fracture: a South Korean nationwide cohort study. J Bone Joint Surg Am 102(12):1059–1065
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stati Med 28(25):3083–3107
von Cederwald BF, Josefsson M, Wåhlin A, Nyberg L, Karalija N (2022) Association of cardiovascular risk trajectory with cognitive decline and incident dementia. Neurology 98(20):e2013–e2022
Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM et al (2012) Structural brain changes in migraine. JAMA 308(18):1889–1896
Hamedani AG, Rose KM, Peterlin BL, Mosley TH, Coker LH, Jack CR et al (2013) Migraine and white matter hyperintensities: the ARIC MRI study. Neurology 81(15):1308–1313
Bashir A, Lipton RB, Ashina S, Ashina M (2013) Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology 81(14):1260–1268
Byers AL, Yaffe K (2011) Depression and risk of developing dementia. Nat Rev Neurol 7(6):323–331
Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener H et al (2005) Gray matter decrease in patients with chronic tension type headache. Neurology 65(9):1483–1486
Wang J, Xu W, Sun S, Yu S, Fan L (2018) Headache disorder and the risk of dementia: a systematic review and meta-analysis of cohort studies. J Headache Pain 19(1):1–8
Lee H-J, Yu H, Myeong SG, Park K, Kim D-K (2021) Mid-and late-life migraine is associated with an increased risk of all-cause dementia and Alzheimer’s disease, but not vascular dementia: a nationwide retrospective cohort study. J Pers Med 11(10):990
Tzeng N-S, Chung C-H, Lin F-H, Yeh C-B, Huang S-Y, Lu R-B et al (2017) Headaches and risk of dementia. Am J Med Sci 353(3):197–206
Queiroz LP, Peres M, Piovesan E, Kowacs F, Ciciarelli M, Souza J et al (2009) A nationwide population-based study of migraine in Brazil. Cephalalgia 29(6):642–649
Rist PM, Kurth T (2013) Migraine and cognitive decline: a topical review. Headache 53(4):589–598
Organization for Economic Cooperation and Devleopment (2021) Education at Glance 2021: OECD Indicators. OECD Publishing Paris. https://doi.org/10.1787/b35a14e5-en
Acknowledgements
None.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, South Korea (grant number: HI20C1130).
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
KH was involved in concept and design of the study, acquisition, and analysis of data, drafting and revision of the manuscript and figures, and statistical analysis. S-IJ was involved in concept and design of the study, acquisition and analysis of data, critical revision of the manuscript for important intellectual content, supervision, and providing of administrative, technical, and material support. SHJ acquired and analyzed the data. S-YJ and SHK did the statistical analysis. E-CP supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Yonsei University Health System (IRB No: 4–2022-0480) and adhered to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file1
: Table S1. Association between migraine and risk of dementia among individuals who diagnosed migraine before age of 60. Table S2. Association between migraine and risk of dementia among with five years of washout period among individuals who diagnosed migraine before age of 60.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hurh, K., Jeong, S.H., Kim, S.H. et al. Increased risk of all-cause, Alzheimer’s, and vascular dementia in adults with migraine in Korea: a population-based cohort study. J Headache Pain 23, 108 (2022). https://doi.org/10.1186/s10194-022-01484-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-022-01484-y