Abstract
Background
Headache is one of the most common symptoms after concussion, and mild traumatic brain injury (mTBI) is a risk factor for chronic migraine (CM). However, there remains a paucity of data regarding the impact of mTBI on migraine-related symptoms and clinical course.
Methods
Of 2161 migraine patients who participated in the American Registry for Migraine Research between February 2016 and March 2020, 1098 completed questions assessing history of TBI (50.8%). Forty-four patients reported a history of moderate to severe TBI, 413 patients reported a history of mTBI. Patients’ demographics, headache symptoms and triggers, history of physical abuse, allodynia symptoms (ASC-12), migraine disability (MIDAS), depression (PHQ-2), and anxiety (GAD-7) were compared between migraine groups with (n = 413) and without (n = 641) a history of mTBI. Either the chi-square-test or Fisher’s exact test, as appropriate, was used for the analyses of categorical variables. The Mann-Whitney test was used for the analyses of continuous variables. Logistic regression models were used to compare variables of interest while adjusting for age, gender, and CM.
Results
A significantly higher proportion of patients with mTBI had CM (74.3% [307/413] vs. 65.8% [422/641], P = 0.004), had never been married or were divorced (36.6% [147/402] vs. 29.4% [187/636], P = 0.007), self-reported a history of physical abuse (24.3% [84/345] vs. 14.3% [70/491], P < 0.001), had mild to severe anxiety (50.5% [205/406] vs. 41.0% [258/630], P = 0.003), had headache-related vertigo (23.0% [95/413] vs. 15.9% [102/640], P = 0.009), and difficulty finding words (43.0% [174/405] vs. 32.9% [208/633], P < 0.001) in more than half their attacks, and headaches triggered by lack of sleep (39.4% [155/393] vs. 32.6% [198/607], P = 0.018) and reading (6.6% [26/393] vs. 3.0% [18/607], P = 0.016), compared to patients without mTBI. Patients with mTBI had significantly greater ASC-12 scores (median [interquartile range]; 5 [1–9] vs. 4 [1–7], P < 0.001), MIDAS scores (42 [18–85] vs. 34.5 [15–72], P = 0.034), and PHQ-2 scores (1 [0–2] vs. 1 [0–2], P = 0.012).
Conclusion
Patients with a history of mTBI are more likely to have a self-reported a history of physical abuse, vertigo, and allodynia during headache attacks, headaches triggered by lack of sleep and reading, greater headache burden and headache disability, and symptoms of anxiety and depression. This study suggests that a history of mTBI is associated with the phenotype, burden, clinical course, and associated comorbid diseases in patients with migraine, and highlights the importance of inquiring about a lifetime history of mTBI in patients being evaluated for migraine.
Similar content being viewed by others
Background
Traumatic brain injury (TBI) results from an external mechanical force to the brain [1]. The United States Centers for Disease Control and Prevention estimates that 1.4–3.8 million TBIs occur each year in the United States, with the majority being considered “mild” in severity [2]. According to the International Classification of Headache Disorders 3rd edition (ICHD-3), post-traumatic headache (PTH) is defined as headache that begins or substantially worsens within 7 days of a trauma or injury to the head and/or neck [3]. Persistent PTH (i.e. PTH that has been present for longer than 3 months) is more often seen after mild traumatic brain injury (mTBI) than after moderate to severe TBI [4,5,6]. It is estimated that 19 to 28% of patients with mTBI will still have headache at 1 year after the trauma [7, 8].
Pre-existing migraine is a risk factor for developing persistent PTH [9]. Yet, the effect of TBI on headache features, associated symptoms, triggers, and comorbidities among patients with migraine is not well known. Likewise, a history of head or neck injury is also a risk factor for migraine chronification [10]. Moreover, headache is one of the most common post-concussive symptoms, occurring up to 80%% of patients [11], and more than 60% have headache features that are consistent with those seen in migraine or probable migraine [12].
This study aimed to investigate the effect of mTBI history in migraine patients enrolled in the American Registry for Migraine Research (ARMR) [13] by examining differences in migraine-related symptoms, clinical attributes, and associated disability among patients diagnosed with migraine with and without a history of mTBI.
Methods
Design and setting
The ARMR, established by the American Migraine Foundation, is a multicenter, longitudinal patient registry that collected participants’ clinical data, imaging, and biospecimens. Patients were recruited and enrolled from ARMR sites, which are specialty headache clinics in the United States [13]. Institutional Review Board approvals were obtained from each of the enrolling sites, and all participants completed an informed consent process. Headache specialists assigned one or more ICHD-3 diagnoses to each patient, which were entered into ARMR by a clinician or research staff member. According to ARMR inclusion criteria, participants had to be at least 18 years of age, able and willing to provide informed consent, and able to access a computer or other electronic device with an internet connection. No a priori statistical power calculation was conducted. The sample size of the data for this research analysis was based on the available data, collected via questionnaires completed by patients at the time they enrolled into ARMR between February 1, 2016 to March 6, 2020.
Study population /inclusion and exclusion criteria
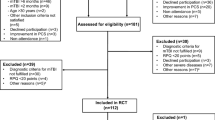
Of 2218 patients who participated in the ARMR study, and were assigned an ICHD migraine diagnosis between February 2016 and March 2020, 57 (2.6%) individuals were excluded due to having one or more headache diagnoses in addition to migraine: headache attributed to trauma or injury to the head and/or neck (i.e. PTH) (n = 50) or headache attributed to cranial and/or cervical vascular disorder (n = 7). Among the remaining 2161 patients, 1098 (50.8%) completed the questions about history of TBI. The question was “Have you ever had a head injury? A head injury may be a hit to the head that caused headaches, dazed feeling, mental fogginess, lightheadedness, blurred vision, dizziness or vomiting.” and participants could answer by two alternatives Yes/ No. Forty-four patients with moderate/severe TBI were excluded. In accordance with ICHD-3 criteria, moderate/severe TBI was defined as TBI with loss of consciousness exceeding 30 min or post-traumatic amnesia exceeding 24 h. Twenty-five excluded patients had loss of consciousness for more than 30 min and 25 had post-traumatic amnesia for more than 24 h (6 patients had both symptoms). The patients were divided into 2 subgroups: the mTBI group (n = 413) who had a history of mTBI, and the non-TBI group (n = 641) who did not have a history of any TBI (Fig. 1).
Flow-chart summarizing inclusion and exclusion of patients
aThe first numbers indicate the ICHD-3 diagnosis code. bThe last numbers indicate the number of patients excluded because of having that headache or facial pain type. ARMR: American Registry for Migraine Research, TBI: traumatic brain injury, mTBI: mild traumatic brain injury.
Data extraction
Data extracted from the ARMR database included the clinician’s assigned ICHD-3 headache diagnosis, and patient-reported age, gender, race, education level, marital status, employment status, household income, history of any type of abuse, and history of physical abuse. TBI characteristics included: severity of TBI, worsening headache or new headache after the TBI, duration of new/worsening headache within 7 days after TBI, and time elapsed between most recent TBI and enrollment. Headache-related symptoms included: headache triggers, average monthly headache frequency over the last 3 months, duration of headache history (years since first headache), headache intensity using a Numeric Rating Scale (NRS) [14, 15], how often the headache-related symptoms (nausea, vomiting, dizziness, vertigo, sensitivity to light, sensitivity to noise, and sensitivity to smell, difficulty finding words) were occurred during headache attack, subjective cognitive impairment during migraine attacks (Mig-SCog) score [16], and Allodynia Symptom Checklist-12 (ASC-12) scores [17]. Disability data included: Migraine Disability Assessment Scale (MIDAS) score [18], Work Productivity and Activity Impairment due to headache (WPAI) scores [19], and Patient-Reported Outcomes Measurement Information System- Pain Interference (PROMIS-PI) score [20]. Coexisting headache diagnoses extracted include: Migraine with aura (MwA) and Medication-Overuse Headache (MOH). Psychiatric comorbidity data extracted included: Patient Health Questionnaire-2 (PHQ-2) score [21], General Anxiety Disorder-7 (GAD-7) score [22], and self-reported history of post-traumatic stress disorder (PTSD).
NRS is a commonly used scale in which a person rates their pain from 0 (no pain) to 10 (worst pain) [14, 15]. The Mig-SCog score measures subjective cognitive impairment during migraine attacks [16]. The ASC-12 assesses cutaneous allodynia symptoms during headache attacks [17]. The MIDAS questionnaire quantifies headache-related disability over the prior 3-month period [18]. WPAI evaluates disability and impact on work productivity due to a specific health problem amongst those who are employed [19]. Based on answers about the past 7 days, four different scores are calculated (WPAI-%Absenteeism; percent work time missed due to headache, WPAI-%Presenteeism; percent impairment while working due to headache, WPAI-%Total Work Productivity Impairment; percent overall work impairment due to headache, and WPAI-%Total Activity Impairment; percent activity impairment due to headache). The PROMIS-PI Adult Short Form 6b measures pain interference on functioning and quality of life over the past 7 days. PROMIS-PI raw scores can be converted to standardized T scores by using conversion tables available at http://www.nihpromis.org/, so that the scores can be compared with the score of the United States general population and used as a normal distribution [20]. The PHQ-2 score is a valid brief tool for detecting depressive disorders over the last 2 weeks [21]. GAD-7 assesses the severity of general anxiety disorder symptoms [22].
Statistical analysis
Descriptive statistics are presented as mean ± standard deviation, median (interquartile range: [IQR]), or number (percentage). Patient characteristics were compared using the chi-square test for categorical variables. Fisher’s exact tests were used for comparing headache triggers, since some categories included a count of fewer than 5, which is not suitable for chi-square-test comparison. Welch’s t-test was performed for PROMIS-PI-T scores. The Mann-Whitney test was used for the other continuous variables because a series of Shapiro-Wilk-tests revealed that all the continuous variables in this study were not normally distributed. In the analysis, missing data were handled using pairwise deletion.
To assess differences in gender between mTBI group and non-TBI group, we excluded transgender patients, since the number of transgender patients was small (n = 1 for mTBI group and 2 for non-TBI group). Similarly, because the numbers of patients identifying as race categories other than white were also small, we thus combined all patients who did not identify as white into one category for purposes of analysis. To assess the difference between two groups in age at onset of headache, the Log-rank test was performed. To describe the characteristics of migraine patients with mTBI regardless of having Chronic migraine (CM), logistic regression models adjusted for age, gender, and CM were performed. P-values < 0.05 on likelihood ratio tests were considered significant. Data were analyzed using Graph Pad PRISM for Windows (version 5.04, Graph Pad Software, Inc., CA, USA), JMP for Windows (version 11.2.0 m SAS Institute Inc., NC, USA) and R statistical computing program (version 3.2.2, https://www.r-project.org/).
Results
Sociodemographics
Sociodemographics and diagnoses are shown in Table 1. The mean age was 46 ± 13 years. Participants were predominantly female (n = 929; 88.1%), white (n = 975; 94.1%), with a college or graduate degree (n = 955; 94.2%), employed full- or part-time (n = 620; 60.3%), and married or living with domestic partner (n = 703; 67.8%). The most common household income category was between $50,000 and $99,999 (n = 306; 31.6%). The proportion of patients who reported being married or in a domestic partnership in the mTBI group was significantly lower than that of the non-TBI groups (63.4% [255/402] vs. 70.6% [449/636], P = 0.016). There was no significant difference in age, gender, race, educational level, employment status, or household income between the two groups. These results were consistent with those of the logistic regression model adjusted for age, gender, and CM.
Characteristics of TBI among mTBI group
Of 413 patients in mTBI group, 268 (64.8%) of patients had headaches prior to their head injury, while 139 (33.7%) of them did not have headaches prior to their head injury (missing data = 6). The latest TBI event occurred an average of 18.3 ± 16.0 (median [IQR]; 14.4 [4.7–28.5]) years before enrollment into ARMR. Despite not having a clinician-assigned ICHD diagnosis of PTH, one hundred and forty-five patients reported that they had new/worsening headache within 7 days of their TBI (Table 2).
Headache phenotype and associated symptoms
Amongst our study population, 27.3% (288/1054) had MwA, 69.2% (729/1054) had CM, and 6.3% (66/1054) had MOH as one of the registered multiple diagnoses. Among the total sample, 47.7% (503/1054) of patients had nausea, 7.0% (74/1054) had vomiting, 41.0% (432/1054) had headache with non-vertigo dizziness, 18.7% (197/1053) had vertigo-type dizziness, 74.8% (788/1053) had sensitivity to light, 71.1% (748/1053) had sensitivity to noise, 48.9% (513/1052) had sensitivity to smell, and 36.8% (382/1050) had difficulty finding words in half or more of their headache attacks.
The proportion of patients with a CM diagnosis in the mTBI group was significantly higher than in the non-TBI group (74.3% [307/413] vs. 65.8% [422/641]; p = 0.004). These were no significant differences between two groups in the proportion of having MwA (27.8% [115/413] vs. 27.0% [173/641], P = 0.761) and MOH (6.8% [28/413] vs. 5.9% [38/641], P = 0.578). Patients in the mTBI group were more likely to have non-vertigo dizziness (P = 0.006), vertigo (P = 0.010), and difficulty finding words (P < 0.001) compared to the patients in non-TBI group. Patients in the mTBI group had significantly greater allodynia scores (median [IQR] 5 [1,2,3,4,5,6,7,8,9] vs. 4 [1,2,3,4,5,6,7], P < 0.001] compared to the patients in the non-TBI group. The patients in the mTBI group had significantly higher Mig-SCog scores (8.9 ± 4.9 vs. 7.5 ± 4.7, P < 0.001) compared to the non-TBI group. There were no significant differences between two groups for nausea (P = 0.159), vomiting (P = 0.445), sensitivity to light (P = 0.096), sensitivity to noise (P = 0.433), and sensitivity to smell (P = 0.415). After adjusting for age, gender, and CM, significant differences were consistent for dizziness (P = 0.003), vertigo (P = 0.009), allodynia score (P < 0.001), difficulty finding words (P < 0.001), and Mig-Scog score (P < 0.001) (Table 3).
Headache triggers
Headache triggers are summarized in Table 4. About one third of all migraine patients had headaches that were always triggered by lack of sleep (35.3% [353/1000]), stress (30.8% [308/1000]), and weather change (29.8% [298/1000]). Patients in the mTBI group were more likely to describe that their headaches were always triggered by lack of sleep (39.4% [155/393] vs. 30.9% [198/607], P = 0.032), bright lights (21.4% [84/393] vs. 15.4% [99/607], P = 0.045), and reading (6.6% [26/393] vs. 2.8% [18/607], P = 0.007) compared to non-TBI patients in the initial analysis. After adjusting for age, gender, and CM diagnosis, only lack of sleep (P = 0.018) and reading (P = 0.016) maintained a significant difference between the mTBI and non-TBI groups. There were no significant differences between the two groups in other triggers.
Headache features and disability
Headache features and disability measures are shown in Table 5, including: headache frequency, duration of headache history, age at onset of headache, NRS and scores on MIDAS, WPAI, and PROMIS-PI-T. Among the total population, median headache days per month was 12.0 (IQR; 6.0–25.0), median duration of headache history was 19.3 (IQR; 7.4–31.8) years, median age at onset of headache was 20 (IQR; 13–34), median NRS was 6 (IQR; 5–7), and the mean PROMIS-PI-T score of the total patient sample was 63.2 ± 7.0. MIDAS scores of the TBI group were significantly higher than those of the non-TBI group (median [IQR]; 42 [18–85] vs. median 34.5 [15–72], P = 0.010). The patients in the mTBI group had significantly higher WPAI-%Absenteeism (12.8 ± 22.2 vs. 8.7 ± 17.2, P = 0.012) compared to the non-TBI group. After adjusting for age, gender, and CM, significant differences remain between groups with and without history of mTBI in MIDAS scores (P = 0.035), and WPAI-%Absenteeism (P = 0.031).
There were no significant differences between two groups in WPAI-%Presenteeism (39.6 ± 25.5 vs. 36.5 ± 25.2, P = 0.385), WPAI-%Total Work Productivity Impairment (40.0 ± 27.1 vs. 36.4 ± 26.3, P = 0.140), WPAI-%Activity Impairment due to migraine (44.4 ± 26.0 vs. 40.4 ± 27.1, P = 0.148), or PROMIS-PI-T scores (63.6 ± 7.1 vs. 62.9 ± 7.0, P = 0.101). Further, there was no significant difference between groups with and without history of mTBI in headache frequency (median [IQR]; 13.0 days [6.0–25.3] vs. 11.2 [5.7–23.7], P = 0.283], duration of headache history (median [IQR]; 20.1 years [8.2–34.4] vs. 18.7 [7.2–30.7], P = 0.132), age at onset of headache (23.5 ± 14.2 vs. 24.9 ± 14.5, Hazard Ratio (developing headache per unit period) [Confidence Interval (CI)]; 1.12 [0.97–1.30], P = 0.116), and NRS (median [IQR]; 6 [5–7] vs. 6 [IQR; 5–7], P = 0.857).
History of abuse and psychiatric disorders
In total, 36.8% (308/836, missing data = 218) of migraine patients self-reported a history of abuse in their lifetime, in particular physical abuse history (18.4% [154/836], missing data = 218). PTSD was seen in 26.2% (122/465, missing data = 589) of the total population. A higher proportion of patients in the mTBI group self-reported a history of any type of abuse (47.0% [162/345] vs. 29.7% [146/491], P < 0.001), including the physical abuse subtype (24.3% [84/345] vs. 14.3% [70/491], P < 0.001). The patients in the mTBI group also had significantly higher PHQ-2 (P = 0.034) scores and a greater proportion had mild to severe GAD-7 grades (P = 0.003). A greater proportion of patients with a history of mTBI self-reported a history of PTSD (33.3% [68/204] vs. 20.7% [54/261], P = 0.002) compared to the non-TBI group. After adjusting for age, gender, and CM, significant differences between groups remain for history of abuse (P < 0.001), history of physical abuse (P < 0.001), PHQ-2 scores (P = 0.012), GAD-7 scores (P = 0.003), and history of PTSD (P = 0.002) (Table 6).
Discussion
Migraine severity and related disability were greater in those with a history of mTBI
Within our study population, 38% of patients with migraine reported a history of mTBI, which occurred a median of 14.4 (IQR; 4.7–28.5) years before enrollment into ARMR. This proportion is higher than expected in the general population (19.1% lifetime prevalence of mTBI) [23]. Further, when compared with non-TBI patients, a significantly greater proportion of patients with a history of mTBI had CM. While, TBI has been reported as an independent risk factor for CM [24, 25], the occurrence of chronic daily headache is not necessarily related to the proximity of the TBI [4].
The patients in the mTBI group had significantly greater headache-related disability (higher MIDAS scores) over the prior 3 months and more work productivity impairment due to headache in the past 7 days (WPAI-%Absenteeism), compared to those without a history of mTBI. This underscores the likelihood that a history of mTBI is associated with a more severe clinical phenotype of migraine that may persist for many years after the TBI event, regardless of having a CM diagnosis.
Remote effect of mTBI on migraine
While a history of mTBI did not appear to significantly affect the age of migraine onset, this study found that even a long-ago history of TBI (median 14.4 years) might have a durable effect on migraine, including severity, disability, and associated features such as vertigo, cognitive dysfunction, allodynia, and psychological comorbidities. These remote effects are compatible with previous reports. A prior study following veterans with deployment-related TBI for 4–11 years after injury reported more frequent and severe headaches compared to the age-, sex-, and race-matched veterans without TBI [26]. Moreover, the vast majority of these veterans (89%) experienced PTH with a phenotype of migraine. The authors concluded that there was no improvement in headache prevalence or frequency over time, suggesting that the process related to headache causation initiated by the TBI either remained active or produced permanent changes in brain function allowing the headaches to continue for a protracted time. This is further supported by emerging data in blood-based biomarkers, showing persistent elevation of neurofilament light chain, up to 22 years after an initial mTBI [27]. Such findings raise the possibility of persistent neuroinflammation and pathological cascades long after mTBI. In addition to structural and biochemical changes in the brain, TBI-induced psychiatric disorders such as depression, anxiety, and PTSD may also continuously increase the severity of migraine [28,29,30]. Overall, these results suggest that, while a history of mTBI may not affect the onset of migraine, but that there are measurable remote effects chronification and severity.
Headache-associated symptoms differ in those with a history of mTBI compared to those without
Consistent with prior studies [31], our study found that the patients in the mTBI group reported having significantly more vestibular dysfunction, including dizziness and vertigo after adjusting for age, gender, and CM. Another study reported that half of TBI patients have vestibular complaints at 5 years after their TBI [32], and some authors have noted an association between vestibular symptoms and headache in blast-exposed soldiers, hypothesizing this to be suggestive of a “migraine-like” headache phenotype. The authors speculated that this association might suggest a potential migraine biology underlying the vestibular and balance dysfunction following concussion [33].
Allodynia has been reported in an estimated 35% of military members with PTH and 42–46% of civilians with PTH [34,35,36], leading to the hypothesis that mTBI may increase the risk for allodynia [37, 38] and CM through altered descending modulation of trigeminal sensory processing [39]. The estimated frequency of allodynia among people with migraine is perhaps more variable (15.1% to 69.7%) [40], largely depending on chronicity, with a higher prevalence in CM than in episodic migraine patients [41, 42]. In our study, patients in the mTBI group were more likely to report significant headache-associated allodynia (higher ASC-12 scores), compared to those in the non-TBI group. Interestingly, this was true even after adjusting for CM, suggesting a potential independent impact of TBI history on the manifestation of headache-associated allodynia. As with vertigo and dizziness, the presence of allodynia in a patient presenting with a CM phenotype should elicit queries of a TBI history.
It is well known that migraine attacks are associated with poorer cognitive performance than headache-free periods, consistent with cognitive difficulties subjectively reported during attacks. Most clinic-based studies report worse cognitive performance in people with migraine compared to those without [43]. In particular, verbal skills (auditory comprehension, reading, aphasia screening, verbal reasoning, vocabulary, and phoneme detection) of people with migraine were reported to be mildly impaired [44]. It is also known that cognitive impairment is a common and disabling consequence of TBI [45], and mTBI has been demonstrated to be an independent risk factor for dementia [46, 47]. Prolonged cognitive impairment is also described in patients with PTH, particularly in those with a history of headache prior to the TBI event [48].
Our findings further support these observations, with higher Mig-SCog scores and greater difficulty finding words reported by the mTBI group, compared to the patients in the non-TBI group (Tables 3 and 5). These results suggest that people with migraine and a history of mTBI, regardless of whether they have CM, should be assessed for persistent cognitive dysfunction, and support ongoing investigations into the extent to which people with migraine who have a history of mTBI have an elevated risk of later-life cognitive impairment.
Triggers of headache in mTBI group
Lack of sleep and reading were the most notable headache triggers reported by the mTBI group in our study, despite the fact that the mTBI event occurred a median of 14.4 years prior to study participation. In general, sleep deprivation is a common, well-recognized trigger of migraine. One of the largest retrospective surveys reported sleep disturbance as a trigger in 49.8% of migraineurs [49]. On the other hand, reading as a trigger (or precipitating) factor of migraine has been reported in 4–18%. The proportion of migraine patients with migraine attacks induced by reading was not significantly different from that of tension-type headaches induced by reading, in the previous studies [50, 51]. Reading may trigger headache after TBI via oculomotor dysfunction [52], verbal dysfunction, or cognitive dysfunction [45] after TBI. The TBI was reported to result in long-lasting oculomotor dysfunction [53], as well as verbal or cognitive dysfunction [54].
Physical abuse and psychological comorbidities
Anxiety and depression are widely recognized comorbidities in migraine, and serve as risk factors for CM [55, 56]. Further, mTBI can independently provoke or aggravate anxiety and depression [57, 58]. Finally, a history of trauma and diagnosis of post-traumatic stress disorder (PTSD) is highly associated with both comorbid mood disorders [59] and migraine [43]. Given this background, it is perhaps not surprising that PTSD is more prevalent in patients with migraine than in the general population (14–25% vs. 1–12%), and is even more prevalent in patients with CM compared with patients with episodic migraine (43% vs. 9%), despite a similar frequency and prevalence of trauma exposure between the groups [43]. One study reported that 60% of migraine patients with PTSD had physical or sexual abuse as a traumatic life event and 42% of people with migraine reported physical or sexual assault [60]. In our study, the migraineurs in the mTBI group had significantly higher PHQ-2 scores and GAD-7 grades compared to the non-TBI group in initial analysis, which remained significant after adjusting for diagnosis of CM. Further, about a quarter of migraine patients in this sample reported having PTSD, with a significantly higher proportion of migraine patients in the mTBI group reported having PTSD compared to the non-TBI group. In summary, these findings are consistent with previous reports, and extend the body of data that support the complex relationship between affective symptom burden and migraine, migraine chronicity, and history of trauma.
A prior study found that among mTBI patients who were hospitalized, the most common reported cause of the injury was vehicle (55–58%), followed by fall (23–24%), and violence (5–10%) [61]. Meanwhile, another study found that mTBI outpatients who presented to a headache center reported that the cause of the injury was a fall (42–45%), followed by motor vehicle accident (18–24%) [36, 62]. These discrepancies in the cause of mTBI are explained by the severity of the injury, such as the tendency for traffic accidents to result in serious injuries compare to falls. Although the ARMR questionnaires did not ask directly about the cause of the TBI, the higher proportion of self-reported abuse history in the mTBI group than in the non-TBI group indicate that some of the TBIs could be due to abuse. TBI by abuse is more likely to be repetitive than TBI by motor vehicle accidents [63, 64]. Patients with mTBI suffer more PTH than their moderate-to-severe counterparts [65], and repeated TBI worsens the long-term prognosis of PTH [66]. While the nature of the reported physical abuse was not investigated in this study, it is notable that our sample was predominately female. Domestic violence occurs in approximately 1 in every 4 women in the United States, and up to 94% of injuries women sustain from abuse are to the neck and head [67,68,69]. Thus, given the prevalence of migraine in women, and the high rate of domestic violence and repetitive head injuries these women sustain, a history of domestic violence and physical abuse should be a part of the clinical evaluation of women with migraine. Moreover, future research should focus on the relationship between physical abuse, head injuries, and their impact on the clinical course of migraine.
Limitations
In general, the strengths and limitations of the ARMR as a data source have been previously described [13]. In brief, since ARMR patients are enrolled from specialty headache centers, there is an overrepresentation of chronic migraine within the sample. Further, the overwhelming majority of participants within the sample are white, and thus results cannot necessarily be generalized to the overall US population. Additionally, the findings of this analyses should be interpreted as exploratory, given the potential for type I error inflation due to multiple comparisons.
Additionally, in the mTBI group, 145 patients reported “new onset or worsening headache within 7 days of their injury,” and thus may have a history consistent with the ICHD-based diagnosis of persistent PTH with a migraine or CM phenotype that was not coded by providers within the ARMR database. It is possible that when headaches have been present for a long time, clinicians may not ask about inciting/triggering events, such as mTBI, that led to worsening headache patterns, reflecting a systematic bias to overlook PTH when TBI is relatively remote or is overlaid on a history of migraine. In our sample, the effect of mTBI on migraine onset or headache worsening may be underestimated, since about 65% of the mTBI group reported a history of headaches before the most recent trauma reported in ARMR, and thus information about initial head injury and its relationship to headache onset was lacking. Most of the patients have a long history of headache and the TBI event occurred on median 14.4 years before enrollment into ARMR, making recall bias and recall error a limitation. The other limitation is that there is no detailed information on the frequency and intensity of headaches during the 14 years between the TBI event and the study enrollment. Future longitudinal studies are needed to better understand the natural history of migraine relative to recurrent TBI.
Conclusion
About 38% of patients with migraine in our study had a history of mTBI, which occurred a median of over 14 years before enrollment. A history of mTBI was found to be associated with increased headache frequency, a diagnosis of chronic migraine, and greater headache-related disability, higher rates of cognitive dysfunction, reading/lack of sleep as a headache trigger, vertigo/dizziness, allodynia, and higher rates of anxiety and depression symptoms. The biological mechanisms underlying the increased prevalence of these features merit further exploration. A greater proportion of migraine patients with a history of mTBI reported a history of abuse, especially physical abuse. This study shows the necessity of inquiring about a lifetime history of TBI in patients being evaluated for migraine. People with migraine who have sustained prior TBI may represent a more severe phenotype and some patients with CM may actually represent persistent PTH with a CM phenotype. Since patients with persistent PTH are often clinically regarded as more resistant to conventional migraine treatments, these results raise the question as to whether this subgroup of patients, thought to have treatment-resistant CM, may actually have persistent PTH. This potential association will require further study.
Key findings
● In the American Registry for Migraine Research patient population, 38% of patients with migraine had a history of mTBI.
● A history of mTBI is associated with increased diagnosis of chronic migraine, headache frequency, vertigo/dizziness, allodynia, reading/lack of sleep as a trigger, headache-related disability, cognitive dysfunction, anxiety, and depression.
● Symptoms eliciting a history of prior mTBI should be an integral part of the evaluation of patients with migraine, especially in those with a severe phenotype including chronic migraine.
● The extent to which a subgroup of patients diagnosed with CM in clinical practice actually has persistent PTH should be a focus of future research.
Availability of data and materials
The datasets used and/or analysed during the study ae available from the corresponding author with approval of the ARMR on reasonable request.
Abbreviations
- ARMR:
-
The American registry for migraine research
- ASC-12:
-
Allodynia symptom checklist-12
- CM:
-
Chronic migraine
- GAD-7:
-
General anxiety disorder-7
- ICHD-3:
-
The international classification of headache disorders 3rd edition
- IQR:
-
Interquartile range
- MIDAS:
-
Migraine disability assessment scale
- MIG-SCog:
-
Subjective cognitive impairment during migraine attacks
- MOH:
-
Medication-overuse headache
- mTBI:
-
Mild traumatic brain injury
- MwA:
-
Migraine with aura
- NRS:
-
Numeric rating scale
- PHQ-2:
-
Patient health questionnaire-2
- PROMIS-PI:
-
Patient-reported outcomes measurement information system- pain Interference
- PTH:
-
Post-traumatic headache
- PTSD:
-
Post-traumatic stress disorder
- TBI:
-
Traumatic brain injury
- WPAI:
-
Work productivity and activity impairment due to headache
References
Labastida-Ramírez A, Benemei S, Albanese M, et al (2020) Persistent post-traumatic headache: a migrainous loop or not? The clinical evidence. J Headache Pain 21:55. https://doi.org/https://doi.org/10.1186/s10194-020-01122-5, 1
Laker SR (2011) Epidemiology of concussion and mild traumatic brain injury. PM R 3:S354–S358. https://doi.org/https://doi.org/10.1016/j.pmrj.2011.07.017, 10 Suppl 2
(2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211. https://doi.org/https://doi.org/10.1177/0333102417738202
Couch JR, Bearss C (2001) Chronic daily headache in the posttrauma syndrome: relation to extent of head injury. Headache 41:559–564. https://doi.org/https://doi.org/10.1046/j.1526-4610.2001.041006559.x, 6
Lahz S, Bryant RA (1996) Incidence of chronic pain following traumatic brain injury. Arch Phys Med Rehabil 77:889–891. https://doi.org/https://doi.org/10.1016/s0003-9993(96)90275-0, 9
Yamaguchi M (1992) Incidence of headache and severity of head injury. Headache 32:427–431. https://doi.org/https://doi.org/10.1111/j.1526-4610.1992.hed3209427.x, 9
Barker-Collo S, Theadom A, Starkey N, Kahan M, Jones K, Feigin V (2018) Factor structure of the Rivermead post-concussion symptoms questionnaire over the first year following mild traumatic brain injury. Brain Inj 32:453–458. https://doi.org/https://doi.org/10.1080/02699052.2018.1429659, 4
Hartvigsen J, Boyle E, Cassidy JD, Carroll LJ (2014) Mild traumatic brain injury after motor vehicle collisions: what are the symptoms and who treats them? A population-based 1-year inception cohort study. Arch Phys Med Rehabil 95:S286–S294. https://doi.org/https://doi.org/10.1016/j.apmr.2013.07.029, 3
Chan TLH, Woldeamanuel YW (2020) Exploring naturally occurring clinical subgroups of post-traumatic headache. J Headache Pain 21:12. https://doi.org/https://doi.org/10.1186/s10194-020-1080-2, 1
Scher AI, Midgette LA, Lipton RB (2008) Risk factors for headache chronification. Headache 48:16–25. https://doi.org/https://doi.org/10.1111/j.1526-4610.2007.00970.x, 1
Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA (1981) Disability caused by minor head injury. Neurosurgery 9(3):221–228
Lucas S, Hoffman JM, Bell KR, Walker W, Dikmen S (2012) Characterization of headache after traumatic brain injury. Cephalalgia 32:600–606. https://doi.org/https://doi.org/10.1177/0333102412445224, 8
Schwedt TJ, Digre K, Tepper SJ, Spare NM, Ailani J, Birlea M, Burish M, Mechtler L, Gottschalk C, Quinn AM, McGillicuddy L, Bance L, Dumkrieger G, Chong CD, Dodick DW (2020) The American registry for migraine research: research methods and baseline data for an initial patient cohort. Headache 60:337–347. https://doi.org/https://doi.org/10.1111/head.13688, 2
Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S, European Palliative Care Research Collaborative (EPCRC). (2011) Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manag 41:1073–1093. https://doi.org/https://doi.org/10.1016/j.jpainsymman.2010.08.016, 6
Lines CR, Vandormael K, Malbecq W (2001) A comparison of visual analog scale and categorical ratings of headache pain in a randomized controlled clinical trial with migraine patients. Pain 93:185–190. https://doi.org/https://doi.org/10.1016/s0304-3959(01)00315-3, 2
Gil-Gouveia R, Martins IP (2019) Cognition and cognitive impairment in migraine. Curr Pain Headache Rep 23:84. https://doi.org/https://doi.org/10.1007/s11916-019-0824-7, 11
Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, Serrano D, Stewart WF, American Migraine Prevalence Prevention Advisory Group (2008) Cutaneous allodynia in the migraine population. Ann Neurol 63:148–158. https://doi.org/https://doi.org/10.1002/ana.21211, 2
Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN (2000) Validity of the migraine disability assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 88:41–52. https://doi.org/https://doi.org/10.1016/S0304-3959(00)00305-5, 1
Vo P, Fang J, Bilitou A, Laflamme AK, Gupta S (2018) Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. J Headache Pain 19:82. https://doi.org/https://doi.org/10.1186/s10194-018-0907-6, 1
Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS (2010) Development of a PROMIS item bank to measure pain interference. Pain 150:173–182. https://doi.org/10.1016/j.pain.2010.04.025, 1
Kroenke K, Spitzer RL, Williams JBW (2003) The patient health Questionnaire-2: validity of a two-item depression screener. Med Care 41:1284–1292. https://doi.org/https://doi.org/10.1097/01.MLR.0000093487.78664.3C, 11
Swinson RP (2006) The GAD-7 scale was accurate for diagnosing generalised anxiety disorder. Evid Based Med 11:184. https://doi.org/https://doi.org/10.1136/ebm.11.6.184, 6
Corrigan JD, Yang J, Singichetti B, Manchester K, Bogner J (2018) Lifetime prevalence of traumatic brain injury with loss of consciousness. Inj Prev 24:396–404. https://doi.org/https://doi.org/10.1136/injuryprev-2017-042371, 6
Viana M, Bottiroli S, Sances G, Ghiotto N, Allena M, Guaschino E, Nappi G, Tassorelli C (2018) Factors associated to chronic migraine with medication overuse: a cross-sectional study. Cephalalgia 38:2045–2057. https://doi.org/https://doi.org/10.1177/0333102418761047, 14
Hoem Nordhaug L, Vik A, Hagen K, et al (2016) Headaches in patients with previous head injuries: a population-based historical cohort study (HUNT). Cephalalgia 36:1009–1019. https://doi.org/https://doi.org/10.1177/0333102415618948, 11
Couch JR, Stewart KE (2016) Headache prevalence at 4-11 years after deployment-related traumatic brain injury in veterans of Iraq and Afghanistan wars and comparison to controls: a matched case-controlled study. Headache 56:1004–1021. https://doi.org/https://doi.org/10.1111/head.12837, 6
Guedes VA, Kenney K, Shahim P, Qu BX, Lai C, Devoto C, Walker WC, Nolen T, Diaz-Arrastia R, Gill JM, the CENC Multisite Observational Study Investigators (2020) Exosomal neurofilament light: a prognostic biomarker for remote symptoms after mild traumatic brain injury? Neurology 94:e2412–e2423. https://doi.org/https://doi.org/10.1212/WNL.0000000000009577, 23
Yilmaz T, Roks G, de Koning M, Scheenen M, van der Horn H, Plas G, Hageman G, Schoonman G, Spikman J, van der Naalt J (2017) Risk factors and outcomes associated with post-traumatic headache after mild traumatic brain injury. Emerg Med J 34:800–805. https://doi.org/https://doi.org/10.1136/emermed-2015-205429, 12
Varner C, Thompson C, de Wit K, Borgundvaag B, Houston R, McLeod S (2021) Predictors of persistent concussion symptoms in adults with acute mild traumatic brain injury presenting to the emergency department. CJEM 23:365–373. https://doi.org/https://doi.org/10.1007/s43678-020-00076-6, 3
Guglielmetti M, Serafini G, Amore M, Martelletti P (2020) The relation between persistent post-traumatic headache and PTSD: similarities and possible differences. Int J Environ Res Public Health 17:. https://doi.org/https://doi.org/10.3390/ijerph17114024, 11
Savola O, Hillbom M (2003) Early predictors of post-concussion symptoms in patients with mild head injury. Eur J Neurol 10:175–181. https://doi.org/https://doi.org/10.1046/j.1468-1331.2003.00552.x, 2
Berman JM, Fredrickson JM (1978) Vertigo after head injury--a five year follow-up. J Otolaryngol 7(3):237–245
Hoffer ME, Balaban C, Gottshall K, Balough BJ, Maddox MR, Penta JR (2010) Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol 31:232–236. https://doi.org/https://doi.org/10.1097/MAO.0b013e3181c993c3, 2
Metti A, Schwab K, Finkel A, Pazdan R, Brenner L, Cole W, Terrio H, Scher AI (2020) Posttraumatic vs nontraumatic headaches: a phenotypic analysis in a military population. Neurology 94:e1137–e1146. https://doi.org/https://doi.org/10.1212/WNL.0000000000008935, 11
Levy D, Gruener H, Riabinin M, Feingold Y, Schreiber S, Pick CG, Defrin R (2020) Different clinical phenotypes of persistent post-traumatic headache exhibit distinct sensory profiles. Cephalalgia 40:675–688. https://doi.org/https://doi.org/10.1177/0333102419896368, 7
Ashina H, Iljazi A, Al-Khazali HM, et al (2020) Persistent post-traumatic headache attributed to mild traumatic brain injury: deep phenotyping and treatment patterns. Cephalalgia 40:554–564. https://doi.org/https://doi.org/10.1177/0333102420909865, 6
Cortez MM, Millsap L, Rea NA, Sciarretta C, Brennan KC (2021) Photophobia and allodynia in persistent post-traumatic headache are associated with higher disease burden Cephalalgia 3331024211010304. https://doi.org/https://doi.org/10.1177/03331024211010304
Hanna JJ, Chong CD, Dumkrieger GM, Ross KB, Schwedt TJ (2020) Sensory hypersensitivities in those with persistent post-traumatic headache versus migraine. Cephalalgia Rep 3:2515816320942191. https://doi.org/https://doi.org/10.1177/2515816320942191
Ashina H, Porreca F, Anderson T, Amin F.M., Ashina M., Schytz H.W., Dodick D.W. (2019) Post-traumatic headache: epidemiology and pathophysiological insights. Nat Rev Neurol 15:607–617. https://doi.org/https://doi.org/10.1038/s41582-019-0243-8, 10
Park S-P, Seo J-G, Lee W-K (2015) Osmophobia and allodynia are critical factors for suicidality in patients with migraine. J Headache Pain 16:529. https://doi.org/https://doi.org/10.1186/s10194-015-0529-1, 1
Baykan B, Ekizoglu E, Karli N, Kocasoy-Orhan E, Zarifoglu M, Saip S, Siva A, Ertas M (2016) Characterization of Migraineurs having allodynia: results of a large population-based study. Clin J Pain 32:631–635. https://doi.org/https://doi.org/10.1097/AJP.0000000000000301, 7
Lovati C, Mariotti C, Giani L, D’Amico D, Sinelli A, de Angeli F, Capiluppi E, Bussone G, Mariani C (2013) Central sensitization in photophobic and non-photophobic migraineurs: possible role of retino nuclear way in the central sensitization process. Neurol Sci 34 Suppl 1:S133–S135. https://doi.org/https://doi.org/10.1007/s10072-013-1369-x
Minen MT, Begasse De Dhaem O, Kroon Van Diest A, et al (2016) Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry 87:741–749. https://doi.org/https://doi.org/10.1136/jnnp-2015-312233, 7
Vuralli D, Ayata C, Bolay H (2018) Cognitive dysfunction and migraine. J Headache Pain 19:109. https://doi.org/https://doi.org/10.1186/s10194-018-0933-4, 1
McInnes K, Friesen CL, MacKenzie DE, et al (2017) Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One 12:e0174847. https://doi.org/https://doi.org/10.1371/journal.pone.0174847, 4
Wang H-K, Lin S-H, Sung P-S, Wu MH, Hung KW, Wang LC, Huang CY, Lu K, Chen HJ, Tsai KJ (2012) Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry 83:1080–1085. https://doi.org/https://doi.org/10.1136/jnnp-2012-302633, 11
Fann JR, Ribe AR, Pedersen HS, Fenger-Grøn M, Christensen J, Benros ME, Vestergaard M (2018) Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry 5:424–431. https://doi.org/https://doi.org/10.1016/S2215-0366(18)30065-8, 5
Giza CC, Kutcher JS, Ashwal S, et al (2013) Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the guideline development Subcommittee of the American Academy of neurology. Neurology 80:2250–2257. https://doi.org/https://doi.org/10.1212/WNL.0b013e31828d57dd
Kelman L (2007) The triggers or precipitants of the acute migraine attack. Cephalalgia 27:394–402. https://doi.org/https://doi.org/10.1111/j.1468-2982.2007.01303.x, 5
Haque B, Rahman KM, Hoque A, Hasan ATMH, Chowdhury RN, Khan SU, Alam MB, Habib M, Mohammad QD (2012) Precipitating and relieving factors of migraine versus tension type headache. BMC Neurol 12:82. https://doi.org/https://doi.org/10.1186/1471-2377-12-82, 1
Spierings EL, Ranke AH, Honkoop PC (2001) Precipitating and aggravating factors of migraine versus tension-type headache. Headache 41:554–558. https://doi.org/https://doi.org/10.1046/j.1526-4610.2001.041006554.x, 6
Thiagarajan P, Ciuffreda KJ, Ludlam DP (2011) Vergence dysfunction in mild traumatic brain injury (mTBI): a review. Ophthalmic Physiol Opt 31:456–468. https://doi.org/https://doi.org/10.1111/j.1475-1313.2011.00831.x, 5
Mani R, Asper L, Khuu SK (2018) Deficits in saccades and smooth-pursuit eye movements in adults with traumatic brain injury: a systematic review and meta-analysis. Brain Inj 32:1315–1336. https://doi.org/https://doi.org/10.1080/02699052.2018.1483030, 11
Mac Donald CL, Barber J, Jordan M, Johnson AM, Dikmen S, Fann JR, Temkin N (2017) Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol 74:821–829. https://doi.org/https://doi.org/10.1001/jamaneurol.2017.0143, 7
Pearl TA, Dumkrieger G, Chong CD, Dodick DW, Schwedt TJ (2020) Impact of depression and anxiety symptoms on patient-reported outcomes in patients with migraine: results from the American registry for migraine research (ARMR). Headache 60:1910–1919. https://doi.org/https://doi.org/10.1111/head.13911, 9
Ashina S, Serrano D, Lipton RB, Maizels M, Manack AN, Turkel CC, Reed ML, Buse DC (2012) Depression and risk of transformation of episodic to chronic migraine. J Headache Pain 13:615–624. https://doi.org/https://doi.org/10.1007/s10194-012-0479-9, 8
Cole WR, Bailie JM (2016) Neurocognitive and psychiatric symptoms following mild traumatic brain injury. In: Laskowitz D, Grant G (eds) Translational research in traumatic brain injury. CRC Press/Taylor and Francis Group, Boca Raton (FL)
Stein MB, Jain S, Giacino JT, Levin H, Dikmen S, Nelson LD, Vassar MJ, Okonkwo DO, Diaz-Arrastia R, Robertson CS, Mukherjee P, McCrea M, Mac Donald CL, Yue JK, Yuh E, Sun X, Campbell-Sills L, Temkin N, Manley GT, and the TRACK-TBI Investigators, Adeoye O, Badjatia N, Boase K, Bodien Y, Bullock MR, Chesnut R, Corrigan JD, Crawford K, Diaz-Arrastia R, Dikmen S, Duhaime AC, Ellenbogen R, Feeser VR, Ferguson A, Foreman B, Gardner R, Gaudette E, Giacino JT, Gonzalez L, Gopinath S, Gullapalli R, Hemphill JC, Hotz G, Jain S, Korley F, Kramer J, Kreitzer N, Levin H, Lindsell C, Machamer J, Madden C, Martin A, McAllister T, McCrea M, Merchant R, Mukherjee P, Nelson LD, Noel F, Okonkwo DO, Palacios E, Perl D, Puccio A, Rabinowitz M, Robertson CS, Rosand J, Sander A, Satris G, Schnyer D, Seabury S, Sherer M, Stein MB, Taylor S, Toga A, Temkin N, Valadka A, Vassar MJ, Vespa P, Wang K, Yue JK, Yuh E, Zafonte R (2019) Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI study. JAMA Psychiatry 76:249–258. https://doi.org/https://doi.org/10.1001/jamapsychiatry.2018.4288, 3
Teicher MH, Samson JA (2013) Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170:1114–1133. https://doi.org/https://doi.org/10.1176/appi.ajp.2013.12070957, 10
Peterlin BL, Tietjen GE, Brandes JL, Rubin SM, Drexler E, Lidicker JR, Meng S (2009) Posttraumatic stress disorder in migraine. Headache 49:541–551. https://doi.org/https://doi.org/10.1111/j.1526-4610.2009.01368.x, 4
Lucas S, Hoffman JM, Bell KR, Dikmen S (2014) A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia 34:93–102. https://doi.org/https://doi.org/10.1177/0333102413499645, 2
Kjeldgaard D, Forchhammer H, Teasdale T, Jensen RH (2014) Chronic post-traumatic headache after mild head injury: a descriptive study. Cephalalgia 34:191–200. https://doi.org/https://doi.org/10.1177/0333102413505236, 3
Alosco ML, Tripodis Y, Baucom ZH, Mez J, Stein TD, Martin B, Haller O, Conneely S, McClean M, Nosheny R, Mackin S, McKee AC, Weiner MW, Stern RA (2020) Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 95:e793–e804. https://doi.org/https://doi.org/10.1212/WNL.0000000000010040, 7
Deans KJ, Minneci PC, Lowell W, Groner JI (2013) Increased morbidity and mortality of traumatic brain injury in victims of nonaccidental trauma. J Trauma Acute Care Surg 75:157–160. https://doi.org/https://doi.org/10.1097/TA.0b013e3182984acb, 1
Mares C, Dagher JH, Harissi-Dagher M (2019) Narrative review of the pathophysiology of headaches and photosensitivity in mild traumatic brain injury and concussion. Can J Neurol Sci 46:14–22. https://doi.org/https://doi.org/10.1017/cjn.2018.361, 1
Mullally WJ (2017) Concussion. Am J Med 130:885–892. https://doi.org/https://doi.org/10.1016/j.amjmed.2017.04.016, 8
Arosarena OA, Fritsch TA, Hsueh Y, Aynehchi B, Haug R (2009) Maxillofacial injuries and violence against women. Arch Facial Plast Surg 11:48–52. https://doi.org/https://doi.org/10.1001/archfacial.2008.507, 1
Wu V, Huff H, Bhandari M (2010) Pattern of physical injury associated with intimate partner violence in women presenting to the emergency department: a systematic review and meta-analysis. Trauma Violence Abuse 11:71–82. https://doi.org/https://doi.org/10.1177/1524838010367503, 2
Valera EM, Joseph A-LC, Snedaker K, Breiding MJ, Robertson CL, Colantonio A, Levin H, Pugh MJ, Yurgelun-Todd D, Mannix R, Bazarian JJ, Turtzo LC, Turkstra LS, Begg L, Cummings DM, Bellgowan PSF (2021) Understanding traumatic brain injury in females: a state-of-the-art summary and future directions. J Head Trauma Rehabil 36:E1–E17. https://doi.org/https://doi.org/10.1097/HTR.r0000000000000652, 1
Acknowledgements
The authors gratefully acknowledge the American Registry for Migraine Research (ARMR) for the use of registry data to conduct this research. In addition, we would like to thank the patients and clinicians who participated in this registry.
Funding
This study is part of the ARMR, established by American Migraine Foundation.
Author information
Authors and Affiliations
Contributions
Conception and design; RI, DD, MT, and TS. Acquisition of data; RI and GD. Analysis and interpretation of data; RI, DD, MT, GD, and TS. Drafting the manuscript; RI, DD, MT, GD, and TS. Revising the manuscript for intellectual content; MC, KCB, and KD. Final approval of the completed manuscript; RI, DD, MT, GD, MC, KCB, KD, and TS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethic approval was give by the Mayo Clinic Institutional Review Board.
Consent for publication
Not applicable.
Competing interests
Ryotaro Ishii has nothing to disclose.
David W. Dodick reports the following conflicts within the past 12 months: Consulting: AEON, Amgen, Clexio, Cerecin, Cooltech, Ctrl M, Allergan, Alder, Biohaven, GSK, Linpharma, Lundbeck, Promius, Eli Lilly, eNeura, Novartis, Impel, Satsuma, Theranica, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Praxis, Revance, Equinox. Honoraria: CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, MJH Lifesciences, Miller Medical Communications, Southern Headache Society (MAHEC), WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Research Support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock Options/Shareholder/Patents/Board of Directors: Ctrl M (options), Aural analytics (options), ExSano (options), Palion (options), Healint (Options), Theranica (Options), Second Opinion/Mobile Health (Options), Epien (Options/Board), Nocira (options), Matterhorn (Shares/Board), Ontologics (Shares/Board), King-Devick Technologies (Options/Board), Precon Health (Options/Board). Patent 17189376.1–1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis.
Meesha Trivedi has nothing to disclose.
Gina Dumkrieger has received research support from Amgen.
Melissa Cortez served as a consultant for Eli Lilly, and has received research funding from Amgen Early Investigator Award in Migraine Research and National Institutes of Health (1K23NS105920, 1R21HD100897).
KC Brennan served as an advisory board for Allergan/Abbvie.
Kathleen Digre serves on the Board of Directors for the American Headache Society and is supported in part by an Unrestricted Grant from Research to Prevent Blindness, New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah.
Todd Schwedt has received personal compensation for serving as a consultant or advisory board member within the past 12 months from Abbvie/Allergan, Biohaven, Click Therapeutics, Eli Lilly, Equinox, Lundbeck, and Novartis. He has stock options in Aural Analytics and Nocira. He has received research funding from: Amgen, American Migraine Foundation, Arizona State University, Henry Jackson Foundation, National Institutes of Health, Patient Centered Outcomes Research Institute, and U.S. Department of Defense. He serves on the Board of Directors for the American Headache Society and the International Headache Society.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ishii, R., Schwedt, T.J., Trivedi, M. et al. Mild traumatic brain injury affects the features of migraine. J Headache Pain 22, 80 (2021). https://doi.org/10.1186/s10194-021-01291-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-021-01291-x